Summary
Paroxysmal nocturnal hemoglobinuria (PNH) is characterized by intravascular hemolysis, venous-thrombosis, and bone marrow failure. Seventeen patients with debilitating PNH, including 8 who were HLA-alloimmunized, underwent a reduced intensity allogeneic hematopoietic cell transplant (HCT). All received cyclophosphamide/fludarabine +/− ATG followed by a G-CSF mobilized HCT from an HLA-matched relative. GPI-negative neutrophils were detectable following engraftment, but disappeared completely at a median 100 days after transplant. With a median follow-up of nearly 6 years, 15patients (87.8%) survive, all without any evidence of PNH, transfusion-independent, off anticoagulation. Allogeneic reduced-intensity (RI) HCT remains a curative therapeutic option for PNH patients who are not candidates for eculizumab treatment.
Keywords: Paroxysmal nocturnal hemoglobinuria, reduced-intensity, hematopoietic cell transplant, survival, fludarabine
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a non-malignant clonal disorder of hematopoietic stem cells characterized by a somatic mutation in the PIG-A gene(1). Due to a defect in the glycosylphosphatidylinositol (GPI) anchor, PNH stem cells and their progeny lack GPI-anchored surface proteins, some of which (i.e. CD55 and CD59) protect erythrocytes from complement-mediated lysis(2). Patients with PNH may manifest with a variety of symptoms including recurrent intravascular hemolysis, venous thrombosis(3), and hematopoietic failure(4), all of which shorten survival compared to healthy age-matched controls(5, 6). Eculizumab, a monoclonal antibody to C5a, is highly effective in preventing both hemolysis and thrombosis associated with this disorder(7). However, this agent requires life-long therapy and may be unaffordable for many patients. Following treatment, low levels of persistent extravascular hemolysis as a consequence of complement C3 opsonization can occur leading to the persistent need for erythrocyte transfusions in a minority of eculizumab-treated patients(8). Furthermore, although eculizumab can be effective in controlling intravascular hemolysis in PNH patients with bone marrow failure, it does not improve hematopoiesis with these patients requiring additional therapy such as immunosuppression with ATG or allogeneic HCT. Although allogeneic bone marrow transplantation can be curative for PNH, the procedure has historically been associated with high rates of rejection and regimen-related mortality(9, 10). This is likely the consequence of these patients being heavily transfused and having a high incidence of HLA alloimmunization. Further, historical preparative regimens were often more myeloablative than they were immunosuppressive, and patients typically were transplanted with bone marrow grafts, which contained lower numbers of CD34+ cells and T-cells, all factors that may have increased the odds of graft rejection. Previously we reported that fludarabine-based RI-HCT could be used to reduce the risk of graft-rejection in heavily-transfused and human leukocyte antigen (HLA)-alloimmunized patients. Further, we described that T-cells engrafting after RI-HCT can immunologically eradicate PNH through a graft-vs.-marrow effect. Here we report long-term outcome on the use of this approach in 17 patients with severe, debilitating PNH who underwent a RI-HCT from an HLA-matched relative.
Methods
Eligibility for transplantation included a diagnosis of PNH with one or more of the following: 1) transfusion-dependence; 2) a history of thrombotic events; or 3) recurrent debilitating hemolytic crises. Patients were eligible for treatment on this research trial and were included in this analysis if by definition(11) they had classic PNH, or clinical or subclinical PNH (PNH clone of at least 5%) in the setting of other bone marrow failure conditions. Seventeen consecutive patients with PNH underwent a peripheral blood allogeneic RI-HCT at the National Heart, Lung and Blood Institute on IRB approved protocol 99-H-0050.Bone marrow failure, patient preference or drug unavailability precluded the use of eculizumab in all patients. Patients received a T-cell replete G-CSF mobilized peripheral blood HCT from a 6/6 HLA-matched relative following conditioning with intravenous cyclophosphamide 60 mg/kg/d (days -7 and -6) and fludarabine 25 mg/m2/d (days -5 to -1). To prevent graft-rejection, patients with a significant transfusion history had equine antithymocyte globulin (40mg/kg/day days -5 to -2) added to the conditioning regimen (n= 14). Graft-versus-host disease (GVHD) prophylaxis consisted of initially of cyclosporine (CSA) administered alone (n=1) or with mycophenolate mofetil (MMF) 1 gram by mouth twice daily (n=4). Because an analysis of a similar transplant regimen for hematological malignancies demonstrated superiority of combined CSA/methotrexate over CSA/MMF for reducing acute GVHD(12), the last 12 PNH patients transplanted on study received CSA combined with methotrexate 5mg/m2/d given on days +1, +3 and +6. On day 0, an un-manipulated G-CSF mobilized peripheral blood allograft (target 5 × 106 CD34+ cells/kg recipient weight) from an HLA identical (6/6) relative was infused. All recipients received their grafts from an HLA-matched sibling with the exception of one patient who received an allograft from her HLA-matched mother (Patient #4). Peripheral blood samples were collected on post-transplant days 15, 30, 45, 60, 100 and monthly thereafter if necessary to enumerate the percentage of PNH granulocytes and erythrocytes and to quantitate the percentage donor chimerism in myeloid and T-cell lineages(13). Complete donor chimerism was defined as the first time to ≥ 95% donor-derived cells in peripheral blood. Patients receiving systemic anticoagulation before transplantation continued to be anticoagulated post-transplant for 3–6 months after GPI-negative neutrophils became undetectable in the blood as a protocol-specified safety requirement to avoid thrombotic events in this high-risk cohort. Eculizumab was not used in any patients to reduce the risk of peri-transplant hemolysis. Acute GVHD was graded and staged prospectively using criteria from the 1994 Consensus Conference on Acute GVHD Grading. The diagnosis of clinical features of chronic GVHD was determined prospectively and classified into limited or extensive based on the Revised Seattle Classification. Overall survival was estimated by the Kaplan-Meier method censoring at the last follow-up. The probabilities of development of acute and chronic GVHD, and transplant-related mortality (TRM) were estimated using the cumulative incidence methods and compared using Gray’s test, where death without the specified event was considered a competing risk. Analysis was performed using the R statistical programming software and its cmprsk package (www.r-project.org).
Results
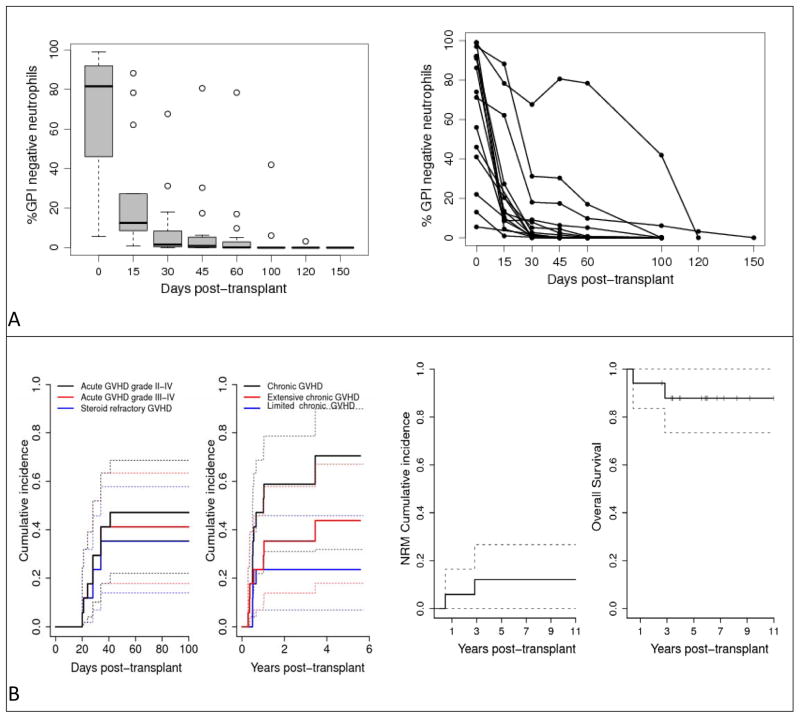
Seventeen patients with PNH who had a median age of 31 (range 20–42 years) underwent transplantation (Table 1). The median percentage of GPI-negative neutrophils pre-transplant was 81.6% (range 5.5%–99%). Indications for transplant included: 10patientswith PNH-associated bone marrow failure including 2 with cytogenetic evidence for evolution to myelodysplastic syndrome; 3 with thrombotic events; and 4 with recurrent debilitating hemolysis including 2 with erythrocyte transfusion-dependence. Eight patients (47%) were HLA-alloimmunized prior to transplantation with a median 76.5% (range 45%–100%) panel reactive antibody. Allografts contained a median6.7 × 106cells/kg (range 3.1 to 21.1 × 106) CD34+ cells and 2.5 × 108cells/kg CD3+ cells (range 1.4 to 4.3 × 108). All patients engrafted with no graft-rejection or late graft-failure. Neutrophil and platelet recovery occurred at a median 14 days (range 9 to 18) and 12 days (range 5 to 15) respectively. Chimerism analysis revealed sustained donor engraftment occurred in both myeloid (CD14+/CD15+) and T-cell (CD3+) lineages in all 17 patients; the median time to achievement of full donor myeloid and T-cell chimerism was 15 and 30 days respectively. Sequential peripheral blood flow cytometry analysis revealed evidence for donor immune-mediated eradication of PNH (Figure 1A); although GPI-negative neutrophils were detectable early in all patients after transplant, these populations declined and disappeared in all patients at a median 100 days (range 30 to 150) post-transplant. The cumulative incidence of grade 2–4 acute GVHD was 47.1% (n=8) and the cumulative incidence of chronic GVHD was 70.6% (n=11). The sample of patients is too small to draw conclusions on the effect of GVHD prophylaxis regimens on the incidence. No thrombotic events occurred post-transplant. Two patients died, one from complications related to acute GVHD (day 169) and one from complications of a perforated peptic ulcer 2.8 years later. With a median follow-up of nearly 6 years (range 2.6–11 years), 15 patients (87.8%) survive without any evidence of PNH, transfusion-independent and off anticoagulation (Figure 1B).
Table 1.
Patient characteristics, clinical features and transplant outcomes (SAA = severe aplastic anemia; MDS = myelodysplastic syndrome; TD = transfusion-dependent on red cells and/or platelets; ATG = antithymocyte globulin; CSA = cyclosporine; MMF = mycophenolate mofetil; MTX = methotrexate; SR = steroid-refractory; IST = immunosuppressive therapy; TRM = transplant related mortality; IPSS = International Prognostic Scoring System; Int-1 = Intermediate-1).
| Patient | Age at transplant |
Sex | Diagnosis | Clinical Features | Pre-transplant HLA- alloimmunization (%PRA) |
Pre- transplant %GPI- negative neutrophils |
Equine ATG with conditioning |
GVHD Prophylaxis |
Acute GVHD |
Chronic GVHD |
Follow- up (years) |
Discontinuation of IST (years) |
Survival Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | M | PNH | Hemolysis and thrombosis (portal vein), TD | Yes (46%) | 92.0 | Yes | CSA | None | Extensive | 11.0 | 8.6 | Alive |
| 2 | 25 | F | PNH | Hemolysis and cytopenia, TD | Yes (68%) | 97.0 | Yes | CSA+MMF | None | Limited | 9.2 | 1.0 | Alive |
| 3 | 35 | M | PNH | Hemolysis and cytopenia, TD | No | 92.0 | Yes | CSA+MMF | Grade 3–4, SR | Extensive | 2.8 | On IST at last follow-up | Dead |
| 4 | 27 | F | PNH | Hemolysis and cytopenia, TD | Yes(45%) | 13.0 | Yes | CSA+MMF | Grade 3–4 | Limited | 8.2 | 3.1 | Alive |
| 5 | 35 | M | PNH | Hemolysis and thrombosis (portal vein) | No | 92.0 | Yes | CSA+MMF | None | None | 2.6 | Not applicable | Alive |
| 6 | 31 | M | PNH/SAA | Cytopenia, TD | No | 91.0 | Yes | CSA+MTX | Grade 3–4, SR | Extensive | 7.3 | 5.5 | Alive |
| 7 | 42 | F | PNH/MDS | Cytopenia and IPSS Int-1MDS t(1;13), TD | Yes (98%) | 86.2 | Yes | CSA+MTX | None | Extensive | 6.7 | 5.1 | Alive |
| 8 | 21 | M | PNH | Cytopenia, TD | Yes (95%) | 22.0 | Yes | CSA+MTX | Grade 3–4, SR | Limited | 6.0 | 2.2 | Alive |
| 9 | 34 | M | PNH | Hemolysis and thrombosis (cerebrovascular) | No | 99.0 | No | CSA+MTX | None | Extensive | 5.9 | On IST at last follow-up | Alive |
| 10 | 32 | M | PNH | Hemolysis | No | 74.0 | No | CSA+MTX | Grade 3–4, SR | Extensive | 5.9 | On IST at last follow-up | Alive |
| 11 | 34 | F | PNH | Hemolysis and cytopenia, TD | Yes (85%) | 56.0 | Yes | CSA+MTX | Grade 3–4, SR | Not applicable | 169 (days) | Not applicable | Dead(acute GVHD TRM) |
| 12 | 33 | M | PNH | Hemolysis, TD | Yes (100%) | 99.0 | Yes | CSA+MTX | None | None | 5.6 | Not applicable | Alive |
| 13 | 23 | M | PNH/MDS | Cytopenia and IPSS Int-1 MDS del13q, TD | Yes (55%) | 5.5 | Yes | CSA+MTX | Grade 3–4, SR | Extensive | 3.4 | On IST at last follow-up | Alive |
| 14 | 20 | M | PNH/SAA | Cytopenia, TD | No | 46.0 | Yes | CSA+MTX | None | None | 3.4 | Not applicable | Alive |
| 15 | 24 | M | PNH/SAA | Cytopenia, TD | No | 81.6 | Yes | CSA+MTX | None | Limited | 4.0 | 2.1 | Alive |
| 16 | 37 | M | PNH/SAA | Hemolysis, TD | No | 41.0 | Yes | CSA+MTX | None | None | 3.9 | Not applicable | Alive |
| 17 | 20 | M | PNH | Hemolysis | No | 71.2 | No | CSA+MTX | Grade 2 | None | 3.3 | Not applicable | Alive |
Figure 1.
(A) Summary of GPI-negative neutrophil eradication.(B) Transplant outcomes in 17 patients with PNH.
Discussion
Although allogeneic HCT can be curative for PNH, there is a substantial risk of mortality(9, 14, 15)and graft-rejection with conventional myeloablative BMT. Data from a pilot trial evaluating RI-HCT for PNH provided the first evidence that engrafting donor T-cells could eradicate abnormal recipient stem cells through a graft-versus-marrow effect, obviating the need for potentially toxic myeloablative conditioning(16). The current report published here reflects the largest single-institution experience to date of RI peripheral-blood HCT for PNH. Although the optimal transplant paradigm for this population has not been defined, the excellent outcome and long-term survival observed in our cohort suggest this approach can be used to overcome the high risk of graft-rejection that has historically contributed to the poor outcome associated with conventional BMT for PNH. Despite nearly half the patients in this series being HLA-alloimmunized, all achieved sustained donor engraftment with none experiencing graft-rejection, graft-failure, or relapse of their PNH. The profound recipient lympho-depletion achieved with fludarabine/cyclophosphamide and ATG conditioning combined with the transplantation of an allograft containing high numbers of donor CD34+ cells and T-cells likely led to rapid donor T-cell engraftment that eradicated both PNH-type recipient stem cells and host T-cells that mediate graft rejection.
Although the incidence of both acute and chronic GVHD were high in this series, only one patient died from early transplant related complications, with long-term survival being excellent at 88% with almost 6 years median follow-up. Furthermore, chronic GVHD resolved completely in 7/11(63%) patients allowing discontinuation of all immunosuppressive therapy at a median 3.1 years (range 1 to 8.6) post-transplant. Previously, we and others have presented in vitro and in vivo data showing PNH cells are sensitive to immunological eradication by allo-reactive donor T-cells(17, 18). The long-term follow-up data presented here provide definitive evidence that PNH can be cured by this immunological effect. Further, we show these immune-mediated remissions are durable, are not associated with the recurrence of PNH, allow patients to discontinue anticoagulation without recurrent thrombosis, and result in excellent long-term survival in patients who historically would be at high risk for treatment failure and death with a conventional allogeneic BMT.
Recently, the largest retrospective analysis published to date on HCT in 211 patients with PNH transplanted at 83 different EBMT centers using registry data suggested inferior survival compared with non-transplanted matched cases(10). The only predictor of transplant outcome identified in that study was the indication for transplant, with recipients who experienced thrombotic events having the worst outcome with only a 57% 5-year survival. In contrast to that analysis, our prospective study involved a cohort of PNH patients treated at a single center who all received the same fludarabine-based reduced intensity transplant approach, which was tailored specifically for use in bone marrow failure syndromes associated with a high-risk of graft rejection. Of note, all three patients in our analysis who had thrombosis as an indication for transplant had long-term survival. However, the relatively small size of our patient cohort and their excellent long-term survival precluded us from conducting a multivariate investigation of disease-specific predictors of outcome. To date, there is no prospective intent-to-treat study comparing transplantation versus eculizumab in the management of patients with PNH. Eculizumab’s high efficacy and established safety has precluded such a study given the risk of mortality associated with stem cell transplantation. Despite eculizumab’s inarguable success as being a highly effective therapy for PNH, there remains a subset of patients who cannot be managed lifelong with this drug due to either lack of access, unwillingness to be committed to life-long treatment, or the persistent need for erythrocyte transfusion despite therapy. Moreover, in patients with PNH and bone marrow failure, improvement in hematopoiesis does not occur with this therapy. Eculizumab, which is one of the world’s most expensive medications, is prohibitively expensive for patients without medical insurance. Although the cost of allogeneic transplantation can vary substantially depending on the transplant approach utilized and the presence or absence of post-transplant complications, a recent study estimated a median cost of about $200,000 U.S. dollars for the first 100 days of care post-allogeneic HCT(19). Therefore, HCT as a curative treatment modality that obviates the need for a life-long therapy for PNH costing $400,000 per patient year, should be appreciated as being remarkably cost-effective(20). The 15 patients in our study who were cured of PNH who have long-term survival represent a $6,000,000 annual cost avoidance, with the potential total savings on this drug being extraordinary when one considers the expected longevity of this relatively young cohort (median age 31 years). In the context of our long-term follow-up study showing excellent survival and a high probability of cure, these financial considerations further support fludarabine-based RI-HCT as being a viable treatment option for selected PNH patients.
In this study that utilized G-CSF mobilized peripheral blood progenitor cells as the graft source, there was an unacceptably high incidence of chronic GVHD. Subsequent studies have clearly demonstrated the superiority in reduced incidence of GVHD with bone marrow as the preferred graft source in other bone marrow failure conditions(21). T-cell depletion following peripheral blood HCT using a variety of different in vitro and in vivo strategies has demonstrated success in lowering the incidence of chronic-GVHD following peripheral blood HCT for severe aplastic anemia(22–24), although in some cases the risk of graft rejection has been excessively high(25). To reduce the risk of rejection in allogeneic HCT for PNH and aplastic anemia associated with complete ex vivo T-cell depletion or uncontrolled in vivo T-cell depletion, we are exploring a strategy whereby high numbers of mobilized CD34+ selected peripheral blood cells are combined with non-mobilized T-cells at a T-cell dose that matches cell numbers typically infused following a bone marrow harvest (ClinicalTrials.gov Identifier: NCT01174108).
Acknowledgments
Funding
This work was supported by the Intramural Research Program of the NHLBI at the NIH.
Footnotes
Author’s contributions
J.P., P.S., and A.A.S., collected data. X.T., and N.G., performed the statistical analysis. J.P., C.R., L.C., S.G., T.D., S.V., J.B., P.S., N.S.Y., and R.W.C., were all involved in patient recruitment, treatment and clinical care. H.K. and D.S. were involved in stem cell collection and processing. J.P., X.T., and R.W.C. wrote the paper. All the authors reviewed the paper and approved the final version.
Conflict of interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyata T, Yamada N, Iida Y, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330:249–255. doi: 10.1056/NEJM199401273300404. [DOI] [PubMed] [Google Scholar]
- 2.Ham TH, Dingle JH. Studies on Destruction of Red Blood Cells. Ii. Chronic Hemolytic Anemia with Paroxysmal Nocturnal Hemoglobinuria: Certain Immunological Aspects of the Hemolytic Mechanism with Special Reference to Serum Complement. J Clin Invest. 1939;18:657–672. doi: 10.1172/JCI101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunewald M, Siegemund A, Grunewald A, et al. Plasmatic coagulation and fibrinolytic system alterations in PNH: relation to clone size. Blood Coagul Fibrinolysis. 2003;14:685–695. doi: 10.1097/00001721-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Maciejewski JP, Sloand EM, Sato T, Anderson S, Young NS. Impaired hematopoiesis in paroxysmal nocturnal hemoglobinuria/aplastic anemia is not associated with a selective proliferative defect in the glycosylphosphatidylinositol-anchored protein-deficient clone. Blood. 1997;89:1173–1181. [PubMed] [Google Scholar]
- 5.de Latour RP, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112:3099–3106. doi: 10.1182/blood-2008-01-133918. [DOI] [PubMed] [Google Scholar]
- 6.Socie G, Mary JY, de Gramont A, et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet. 1996;348:573–577. doi: 10.1016/s0140-6736(95)12360-1. [DOI] [PubMed] [Google Scholar]
- 7.Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350:552–559. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 8.Hill A, Rother RP, Arnold L, et al. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010;95:567–573. doi: 10.3324/haematol.2009.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saso R, Marsh J, Cevreska L, et al. Bone marrow transplants for paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1999;104:392–396. doi: 10.1046/j.1365-2141.1999.01195.x. [DOI] [PubMed] [Google Scholar]
- 10.Peffault de Latour R, Schrezenmeier H, Bacigalupo A, et al. Allogeneic stem cell transplantation in paroxysmal nocturnal hemoglobinuria. Haematologica. 2012;97:1666–1673. doi: 10.3324/haematol.2012.062828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan R, Hedlin H, Goodwin R, et al. Reduced Incidence of Acute Graft Versus Host Disease (GVHD) and Transplant-Related Mortality (TRM) with the Addition of Short-Course Mini-Dose Methotrexate (MTX) to Cyclosporine (CSA) as GVHD Prophylaxis Following Nonmyeloablative Hematopoietic Stem Cell Transplantation (NST) ASH Annual Meeting Abstracts. 2007;110:2990. [Google Scholar]
- 13.Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–3241. [PubMed] [Google Scholar]
- 14.Bemba M, Guardiola P, Garderet L, et al. Bone marrow transplantation for paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1999;105:366–368. [PubMed] [Google Scholar]
- 15.Santarone S, Bacigalupo A, Risitano AM, et al. Hematopoietic stem cell transplantation for paroxysmal nocturnal hemoglobinuria: long-term results of a retrospective study on behalf of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) Haematologica. 2010;95:983–988. doi: 10.3324/haematol.2009.017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan R, Takahashi Y, McCoy JP, et al. Overcoming graft rejection in heavily transfused and allo-immunised patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br J Haematol. 2006;133:305–314. doi: 10.1111/j.1365-2141.2006.06019.x. [DOI] [PubMed] [Google Scholar]
- 17.Antin JH, Ginsburg D, Smith BR, Nathan DG, Orkin SH, Rappeport JM. Bone marrow transplantation for paroxysmal nocturnal hemoglobinuria: eradication of the PNH clone and documentation of complete lymphohematopoietic engraftment. Blood. 1985;66:1247–1250. [PubMed] [Google Scholar]
- 18.Takahashi Y, McCoy JP, Jr, Carvallo C, et al. In vitro and in vivo evidence of PNH cell sensitivity to immune attack after nonmyeloablative allogeneic hematopoietic cell transplantation. Blood. 2004;103:1383–1390. doi: 10.1182/blood-2003-04-1281. [DOI] [PubMed] [Google Scholar]
- 19.Majhail NS, Mau LW, Denzen EM, Arneson TJ. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large national private claims database. Bone Marrow Transplant. 2013;48:294–300. doi: 10.1038/bmt.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy RM. Rare diseases mean big profits. Fortune. 2012;166:24. [PubMed] [Google Scholar]
- 21.Bacigalupo A, Socie G, Schrezenmeier H, et al. Bone marrow versus peripheral blood as the stem cell source for sibling transplants in acquired aplastic anemia: survival advantage for bone marrow in all age groups. Haematologica. 2012;97:1142–1148. doi: 10.3324/haematol.2011.054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novitzky N, Thomas V, du Toit C, McDonald A. Reduced-intensity conditioning for severe aplasia using fludarabine and CY followed by infusion of ex vivo T-cell-depleted grafts leads to excellent engraftment and absence of GVHD. Bone Marrow Transplant. 2009;43:779–785. doi: 10.1038/bmt.2008.390. [DOI] [PubMed] [Google Scholar]
- 23.Elhasid R, Arush MB, Zaidman I, et al. Safe and efficacious allogeneic bone marrow transplantation for nonmalignant disorders using partial T cell depletion and no posttransplantation graft-versus-host-disease prophylaxis. Biol Blood Marrow Transplant. 2007;13:329–338. doi: 10.1016/j.bbmt.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 24.de la Rubia J, Cantero S, Sanz GF, et al. Transplantation of CD34+ selected peripheral blood to HLA-identical sibling patients with aplastic anaemia: results from a single institution. Bone Marrow Transplant. 2005;36:325–329. doi: 10.1038/sj.bmt.1705067. [DOI] [PubMed] [Google Scholar]
- 25.Urbano-Ispizua A, Rozman C, Pimentel P, et al. The number of donor CD3(+) cells is the most important factor for graft failure after allogeneic transplantation of CD34(+) selected cells from peripheral blood from HLA-identical siblings. Blood. 2001;97:383–387. doi: 10.1182/blood.v97.2.383. [DOI] [PubMed] [Google Scholar]