Abstract
Background
The incidence of systemic nonalbicans Candida (especially C. glabrata) infections is increasing dramatically in intensive care units, but relatively little is known about the pathogenesis or host defenses associated with these life threatening infections.
Materials and methods
The course of systemic C. glabrata infection was assessed as the fungal burden in the kidneys and livers of mice sacrificed 1, 8, and 15 days after intravenous C. glabrata. Sixteen hours before each sacrifice, half of the mice were injected intraperitoneally with intact viable or nonviable E. coli cells, or with E. coli lipopolysaccharide (LPS), or with tumor necrosis factor (TNF)-α. To clarify the effect of LPS and TNF-α on phagocytosis, resident (unstimulated) mouse peritoneal macrophages were harvested, cultivated ex vivo, and some cultures were treated with LPS or TNF-α prior to 30 min incubation with C. glabrata.
Results
Compared to mice injected with vehicle, each agent (intact E. coli cells or E. coli LPS or TNF-α) was consistently associated with decreased numbers of tissue C. glabrata, and some of these decreases were significant (P<.05). Compared to untreated macrophages, phagocytosis of C. glabrata was increased with LPS-treated macrophages (P<.01), and phagocytosis was also increased in the presence of TNF-α (P<.01).
Conclusion
E. coli LPS and TNF-α may participate in host defense against C. glabrata by a mechanism involving increased macrophage phagocytosis, suggesting that stimulation of inflammatory cytokines may facilitate host clearance of C. glabrata.
Keywords: Candida glabrata, Escherichia coli, lipopolysaccharide, tumor necrosis factor-α, macrophage
INTRODUCTION
Systemic fungal infections are a major cause of morbidity and mortality in the critically ill, and a large proportion of infections occur in critical care units, including neonatal intensive care units [1]. Despite advances in antifungal therapy, the incidence of fungemia is rising [2–4]. The vast majority of fungal isolates are Candida spp., now the fourth most common cause of nosocomial blood stream infections [1, 2, 5]. Risk factors include prolonged antimicrobial therapy, glucocorticosteroids, peritoneal dialysis, hemodialysis, chemotherapy, radiation therapy, immunosuppression, mechanical ventilation, parenteral nutrition, high fungal colonization, neutropenia, bladder catheterization, intravascular catheters, complicated abdominal surgery, and length of stay in the intensive care unit [2–6]. Systemic candidiasis has a poor outcome. For example, in a large tertiary care hospital, crude and attributable mortality was 61% and 49%, respectively [7]; and mortality in medical and surgical patients was 85% and 45.2%, respectively, for an overall mortality of 60.8% [3].
Historically, C. albicans is the most prevalent Candida sp. in clinical specimens, but the incidence of non-albicans Candida is increasing [1–2, 4–6, 8]. C. glabrata is now the second most frequently isolated Candida sp. [1, 2, 4, 5], a phenomenon often attributed to use of azole antifungal agents [6] but this hypothesis is controversial. Of the more than 80 species of Candida, only C. glabrata exists solely as budding yeast, although hyphal elements have been observed in response to nitrogen starvation [9].
Compared to C. albicans, little is known about the epidemiology and pathogenesis of C. glabrata [10]. In intravenously (IV) inoculated mice, C. glabrata is not as lethal as C. albicans. Brieland et al. [11] observed 100% mortality following ≥5 × 106 C. albicans while all mice survived ≤108 C. glabrata. However, C. glabrata and C. albicans fungemia appear similarly lethal in the clinical setting [2, 10]. In fact, Blot et al. [12] noted that patients with systemic C. glabrata had a somewhat higher mortality than those with systemic C. albicans, an observation attributed to the advanced age of patients with C. glabrata. Bacterial infection may play a role in candida infections. In 31 patients infected with Candida sp., all had bacterial infections and eight (26%) had gram-negative enterobacteria [4]. Hederwick et al. [13] noted that bacterial infection may be a risk factor for yeast colonization.
There is experimental evidence that C. albicans and Escherichia coli can act as copathogens. Burd et al. [14] reported 100% mortality in mice injected with C. albicans along with viable E. coli or E. coli lipopolysaccharide (LPS), while mice injected with C. albicans, E. coli, or LPS alone had 3%, 20%, and 21% mortality, respectively. Klaerner et al. [15] noted 83% mortality when C. albicans and E. coli were injected together at concentrations that did not elicit mortality as single agents. In mice injected with nonlethal doses of viable E. coli or E. coli LPS, C. albicans-associated mortality was augmented by viable E. coli and by E. coli LPS; mice injected with E. coli or LPS had increased numbers of C. albicans in kidney tissue, suggesting that LPS was responsible for enhanced growth of C. albicans in tissue, and that this factor was responsible for increased lethality [16]. Thus, viable E. coli, as well as E. coli LPS, enhance systemic C. albicans virulence in mice.
Experiments were designed to clarify the effect of E. coli, and its LPS, on systemic C. glabrata in mice. Results were unexpected and indicated that E. coli and its LPS might have a protective effect. Because LPS is a potent stimulator of tumor necrosis factor (TNF)-α [17], experiments were designed to clarify the effect of TNF-α on C. glabrata infection, and results suggested that this inflammatory cytokine may play a role in host defense against C. glabrata.
MATERIALS AND METHODS
Microbes and mice
C. glabrata ATCC 15126 was obtained from the American Type Culture Collection (Manassas, VA, USA). Streptomycin-resistant E. coli M21 is a rodent isolate. For experiments, overnight cultures were washed, and diluted in Hank’s balanced salt solution (HBSS). Microbial concentrations were determined by hemocytometer (C. glabrata) or densitometry (E. coli) and confirmed by quantitative culture on appropriate agar media. Female 18 to 22 g Swiss-Webster mice were purchased from Harlan Sprague-Dawley, Indianapolis, IN, USA. The University of Minnesota Institutional Animal Care and Use Committee approved all experimental protocols.
Effect of LPS, intact E. coli, and TNF-α on persistence of systemic C. glabrata
Speilberg et al. [18] noted that mice injected in the tail vein with C. albicans die of progressive sepsis, verifying the clinical relevance of this model. Mice were injected IV (tail vein) with 108 (107.7–8.4) C. glabrata suspended in 0.1 ml HBSS. (This is a nonlethal injection based on a preliminary experiment where five mice were injected with 109, 108, or 107 C. glabrata, with 24 hr mortality [40%] only in mice injected with 109 C. glabrata.) At selected time periods after IV C. glabrata, mice were sacrificed by cervical dislocation, and kidneys and livers were aseptically excised and quantitatively cultured [19]. Numbers of tissue microbes were converted to log10 and analyzed by Mann-Whitney (two groups), or by Kruskal-Wallis followed by Mann-Whitney post hoc (more than two groups). Data are presented as the avg ± SE and statistical significance was P≤.05. Microbes were identified by standard techniques [20], including observing rose colored colonies on CHROM agar Candida (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA), characteristic of C. glabrata [21].
Experiments were designed to clarify the effects of parenteral E. coli LPS, nonviable and viable E. coli whole cells, and TNF-α on persistence of C. glabrata in the kidneys and livers of mice. Mice injected IV with C. glabrata were randomly selected to be sacrificed 1, 8, and 15 days later. In the first protocol, half the mice selected to be sacrificed at each time point were injected intraperitoneally (IP), at 16 hr before sacrifice, with 100 µg LPS (E. coli 0111:B4, List Biological Laboratories, Campbell, CA, USA) suspended in 0.5 ml sterile saline, and control mice received sterile saline. This time point was chosen because parenteral LPS induces inflammatory cytokines (including TNF-α) that peak 2 to 4 hours after LPS injection in mice [22]. In a second protocol, LPS was replaced with 5 × 108 heat killed (60°C, 1 hr) E. coli. In a third protocol, LPS was replaced with 108 viable E. coli. The doses of LPS and E. coli elicited only occasional mortality, although characteristic morbidity (ruffled fur and turpor) was consistently evident. In a fourth protocol, LPS was replaced with 5 µg recombinant murine TNF-α (R&D Systems, Minneapolis, MN, USA) suspended in phosphate buffered saline containing 0.1% bovine serum albumin, and control mice received vehicle. This dose of TNF-α elicits a febrile response in mice within 4 hr after IP injection [23].
Effect of LPS on in vitro phagocytosis of C. glabrata by mouse macrophages
To assess the effect of LPS on the ability of macrophages to interact with C. glabrata in vitro, untreated mice were sacrificed, injected IP with 3 ml of HBSS containing 10 µg/ml heparin, resident peritoneal exudate cells were collected, washed, and resuspended in Dulbecco’s minimal essential medium supplemented with 15% heat-inactivated fetal calf serum and 4 mM L-glutamine. These cells (5 × 105/well) were transferred to 24-well dishes containing 12-mm sterile glass coverslips, and incubated 2 hr at 37°C in 5% CO2. Nonadherent cells were washed off and the supernatant contained minimal numbers of cells, indicating that each well contained approximately 4–5 × 105 adherent cells. These adherent cells were >90% macrophages, verified by reactivity to a rat anti-mouse antibody (MCA497GA, Serotec Ltd., Oxford, UK) that recognizes the F4/80 antigen on mouse macrophages. Macrophages were then incubated overnight in tissue culture medium, with and without 100 µg/ml LPS. To verify LPS-induced macrophage activation, expression of the MARCO receptor (macrophage receptor with collagenous structure) was analyzed according to manufacturer’s directions using rat anti-mouse MARCO conjugated to fluorescein (MCA1849F, Serotec). Resident mouse peritoneal macrophages express MARCO, but expression of MARCO is increased in response to LPS, and up-regulation can be used as an indicator of macrophage responsiveness to LPS [24]. Fluorescent intensities were quantified (Metamorph software, Optical Insights LLC, Tucson, AZ, USA). For each treatment, the average fluorescent intensity was calculated (after subtracting background) from 20 random areas containing macrophages. After overnight incubation with LPS, 106 C. glabrata was added to each well of the 24-well dish and incubated 30 min at 37°C. Macrophages were washed, stained with Wright Giemsa, and numbers of macrophage-associated C. glabrata were quantified by light microscopy. Results represent the average of at least 225 macrophages per treatment, with individual data points collected from groups of 15 randomly selected, consecutive macrophages and expressed as the avg (± SE) number of yeast cells per macrophage. Data were analyzed using a t-test. Reproducibility was verified by repeating the experiment on three separate days. In replicate experiments, the intracellular location of C. glabrata was verified using immunofluorescence. Following incubation with C. glabrata, macrophages were rinsed, incubated 30 min in HBSS supplemented with 5% normal goat serum, incubated 60 min at room temperature with a polyclonal rabbit antiserum raised against C. glabrata and diluted 1:1000 in HBSS with 5% goat serum, fixed 15 min at room temperature with 1% paraformaldehyde, incubated 30 min at room temperature with goat anti-rabbit IgG conjugated to cyanine-3 (Jackson ImmunoReserach Laboratories, Inc., West Grove, PA, USA), mounted in SlowFade (Molecular Probes, Eugene, OR, USA) and viewed with epifluorescent microscopy. Because the primary antibody does not penetrate the macrophage membrane, only extracellular C. glabrata react with the secondary antibody and fluoresce red. Representative images were collected with Metamorph software, permitting an overlay of phase contrast and fluorescent images.
A similar protocol was used to study the effect of TNF-α on macrophage phagocytosis of C. glabrata. Modifications included the addition of 3 × 105 (rather than 5 × 105) peritoneal exudate cells, and 5 × 105 (rather than 106) yeast cells per well. Varying concentrations of TNF-α were added to the macrophages along with the yeast inoculum. These concentrations were based on plasma levels of TNF-α following IP injection of LPS into mice [22]. Results represent the average of at least 800 macrophages per TNF-α concentration, with individual data points collected from groups of 100 randomly selected, consecutive macrophages and expressed as the avg (± SE) number of yeast cells per macrophage. Reproducibility was verified by repeating the experiment on three separate days. Differences between TNF-α concentrations were analyzed by a one-way analysis of variance followed by Fisher’s post hoc, and simple regression was used to assess the dose response. For all statistical analyses in this study, the significance level was P<.05.
RESULTS
Effect of LPS on systemic C. glabrata
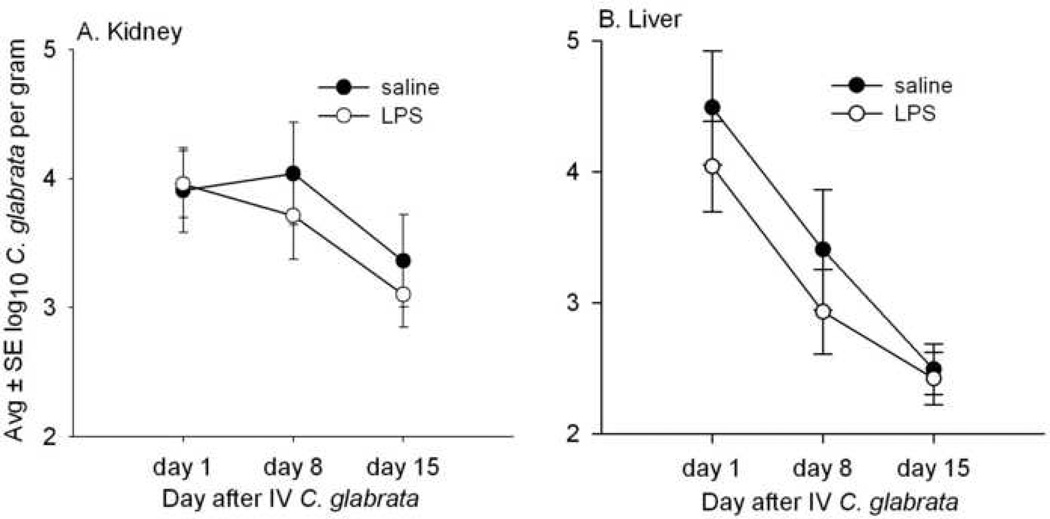
Figures 1A and 1B present the course of systemic infection in mice sacrificed 1, 8, and 15 days after 108 IV C. glabrata. These data show that, compared to mice injected with saline, mice given IP LPS 16 hr before each sacrifice had no significant differences in the numbers of C. glabrata recovered from the kidneys and liver. In this protocol, a total of 45 mice were treated with IP saline before sacrifice and 45 were given LPS. There were two deaths in LPS-treated mice, one on day 1 and one on day 8.
FIG. 1.
Concentrations of viable C. glabrata in the kidney (A) and liver (B) of mice sacrificed 1, 8, and 15 days after IV injection of 108 C. glabrata, and also injected IP with saline or 100 µg/ml LPS 16 hr before each sacrifice. The numbers of C. glabrata in the kidney and liver were consistently similar to or less in LPS- compared to saline-treated mice, although none of these differences were statistically significant. Each time point represents 14 to 15 mice.
Effect of nonviable E. coli on systemic C. glabrata
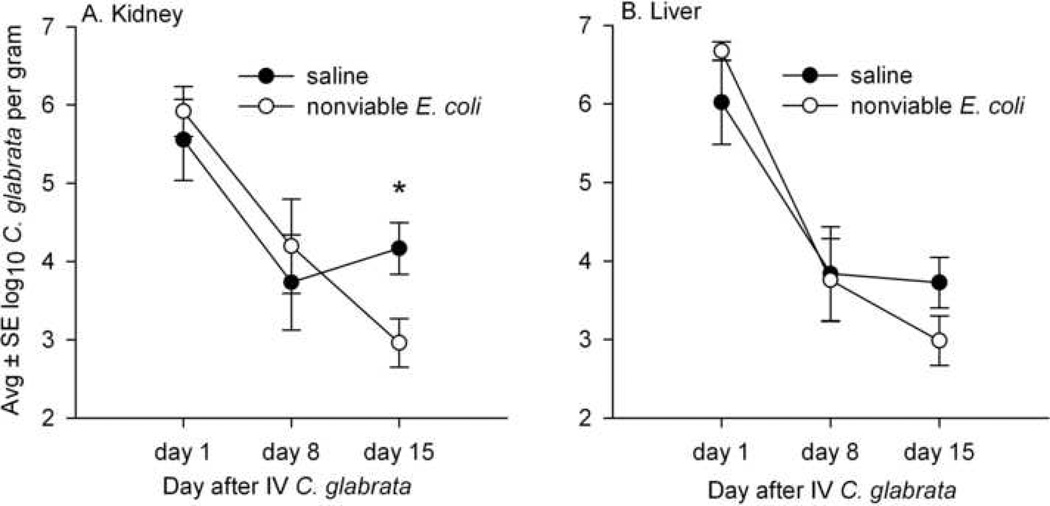
Figures 2A and 2B compare the numbers of C. glabrata in the kidneys and livers of mice sacrificed on days 1, 8, and 15 after IV C. glabrata and also injected IP with either saline or 5 × 108 nonviable E. coli 16 hr before each sacrifice time point. A total of 30 mice were given IP saline before sacrifice and 30 were given nonviable E. coli, and there were three deaths in each group. C. glabrata was the only microbe isolated from mouse tissue and no bacteria were recovered. In mice with long-standing (15 day) systemic C. glabrata, the numbers of C. glabrata recovered from kidney and liver were decreased in mice given nonviable E. coli, compared to mice given saline, and this difference was significant (P<.05) for kidney tissue.
FIG. 2.
Concentrations of viable C. glabrata in the kidney (A) and liver (B) of mice sacrificed 1, 8, and 15 days after IV injection of 108 C. glabrata, and also injected IP with saline or nonviable E. coli 16 hr before each sacrifice. On day 15, the numbers of C. glabrata in the kidney and liver were decreased in mice treated with E. coli compared to mice treated with saline, and this difference was significant only in the kidney (*P<.05) and close to significance in the liver (P=0.1). Each time point represents 8–10 mice.
Effect of viable E. coli on systemic C. glabrata
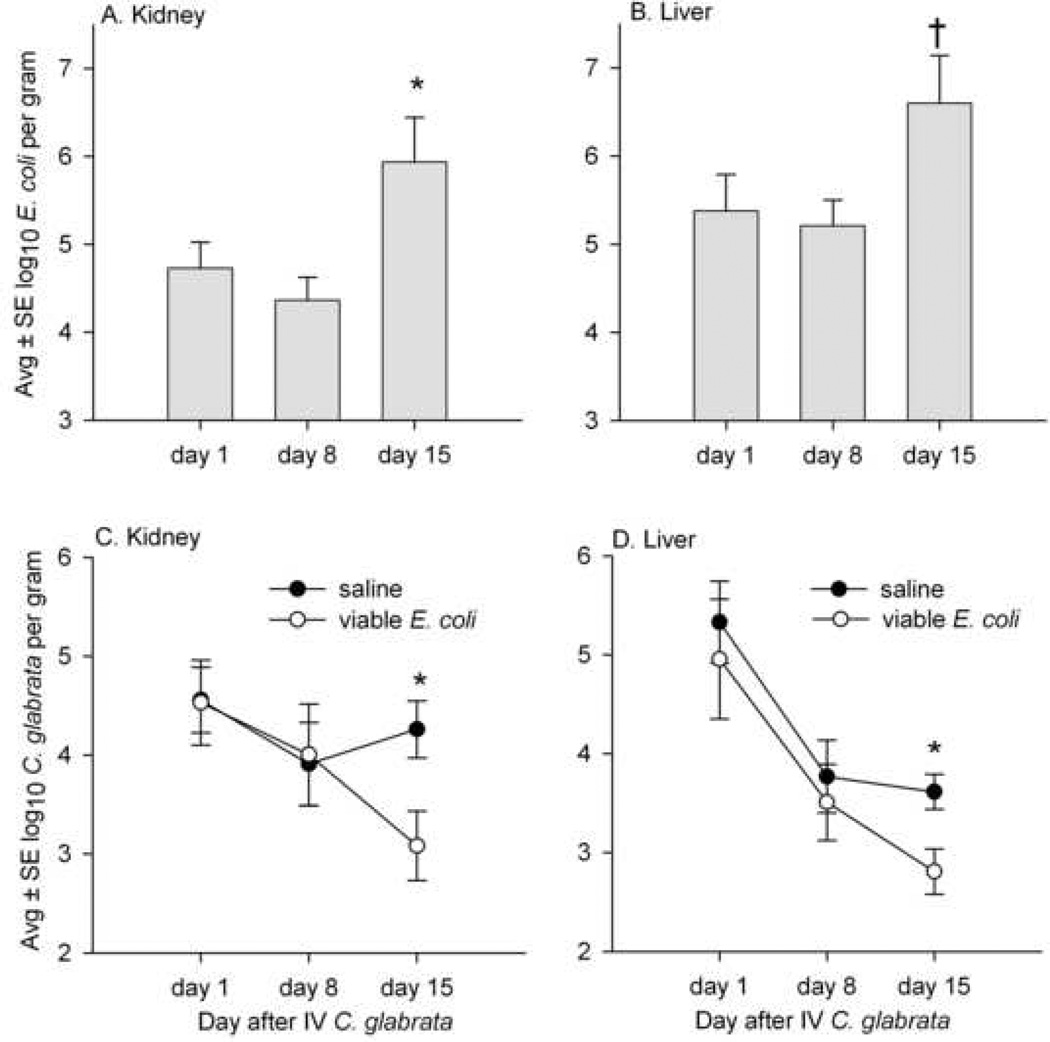
In the next experiment, mice were injected IP with 108 viable (rather than nonviable) E. coli 16 hr prior to sacrifice on days 1, 8, and 15 after IV C. glabrata. Figures 3A and 3B present the numbers of E. coli recovered from the kidney and liver of these mice, with E. coli recovery greatest in mice with long-standing (15 day) C. glabrata infection. A total of 30 mice were treated with IP saline before sacrifice and 30 were given viable E. coli. There was one death in saline-treated mice and four deaths in mice given viable E. coli, and this difference was not significant (P = 0.9). In mice with long-standing (15 day) systemic C. glabrata infection, the numbers of C. glabrata in the kidney and liver were decreased in mice given viable E. coli, and this difference was significant (P<.05) for both kidney and liver tissue (Figs. 3C and 3D).
FIG. 3.
Concentrations of viable E. coli and C. glabrata in the kidneys and livers of mice sacrificed on days 1, 8, and 15 after IV injection of 108 C. glabrata, and with saline or 108 viable E. coli injected IP 16 hr before each sacrifice. A and B show that the numbers of E. coli recovered from the kidney and liver on day 15 were increased compared to days 1 and 8 (* and †, increased compared to days 1 and 8 at P<.05 and P<.01, respectively). C and D show that, on day 15, the numbers of C. glabrata in the kidney and liver were decreased in mice treated with E. coli compared to mice treated with saline, and these differences were significant (*P<.05). Each time point represents 9 to 10 mice given IP saline and 7 to 10 mice given IP E. coli.
Effect of TNF-α on systemic C. glabrata
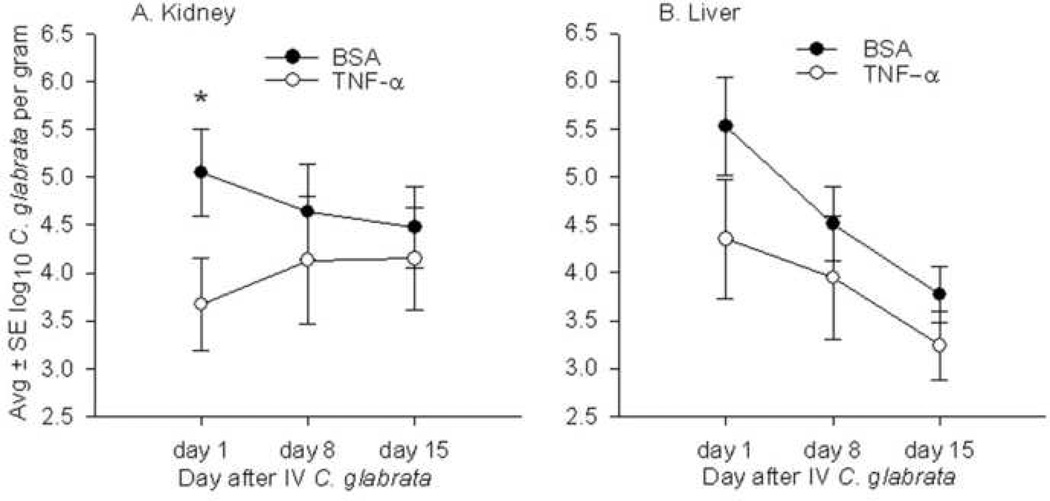
To investigate the effect of TNF-α in clearance of systemic C. glabrata, mice were injected IP with 5 µg TNF-α 16 hr prior to sacrifice on days 1, 8, and 15 after IV C. glabrata. Mice given TNF-α had fewer numbers of C. glabrata recovered from their kidneys and livers compared to control mice (Fig. 4). However, this difference was statistically significant only in kidney tissue and approached significance (P=0.1) in the liver of mice sacrificed 1 day after IV C. glabrata. Of 24 mice given TNF-α and 30 mice given vehicle, there was one death in the vehicle-treated group.
FIG. 4.
Concentrations of C. glabrata in the kidney (A) and liver (B) of mice sacrificed on days 1, 8, and 15 after IV injection of 108 C. glabrata, and with 0.1% bovine serum albumin (BSA) or 5 µg TNF-α injected IP 16 hr before each sacrifice. Compared to mice injected with BSA, the numbers of C. glabrata in the kidney and liver were consistently decreased at all time points in mice injected with TNF-α., but his difference was significant (*P<.05) only in the kidney tissue of mice sacrificed on day 1. Each time point represents 9 or 10 mice given BSA and 8 mice given TNF-α.
In vitro phagocytosis of C. glabrata by LPS-stimulated mouse peritoneal macrophages
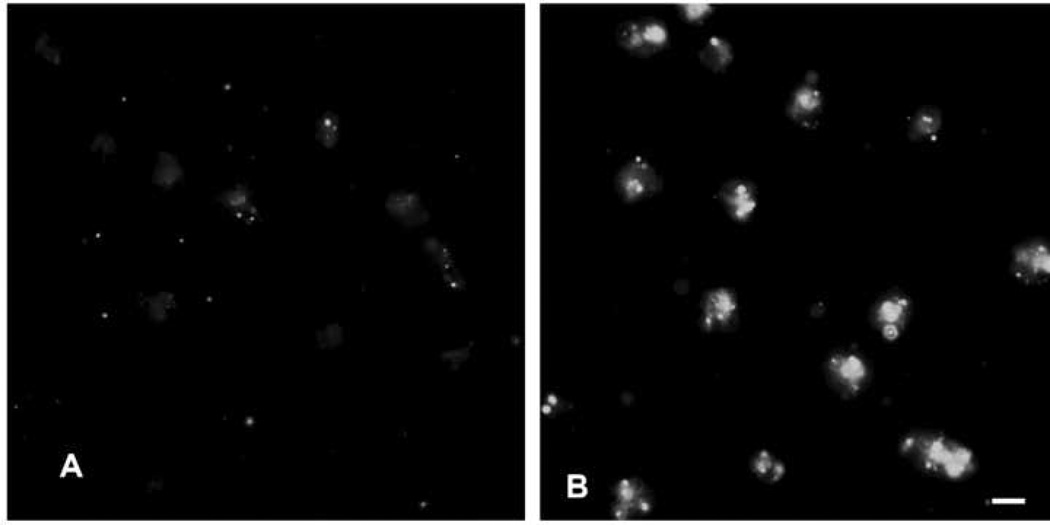
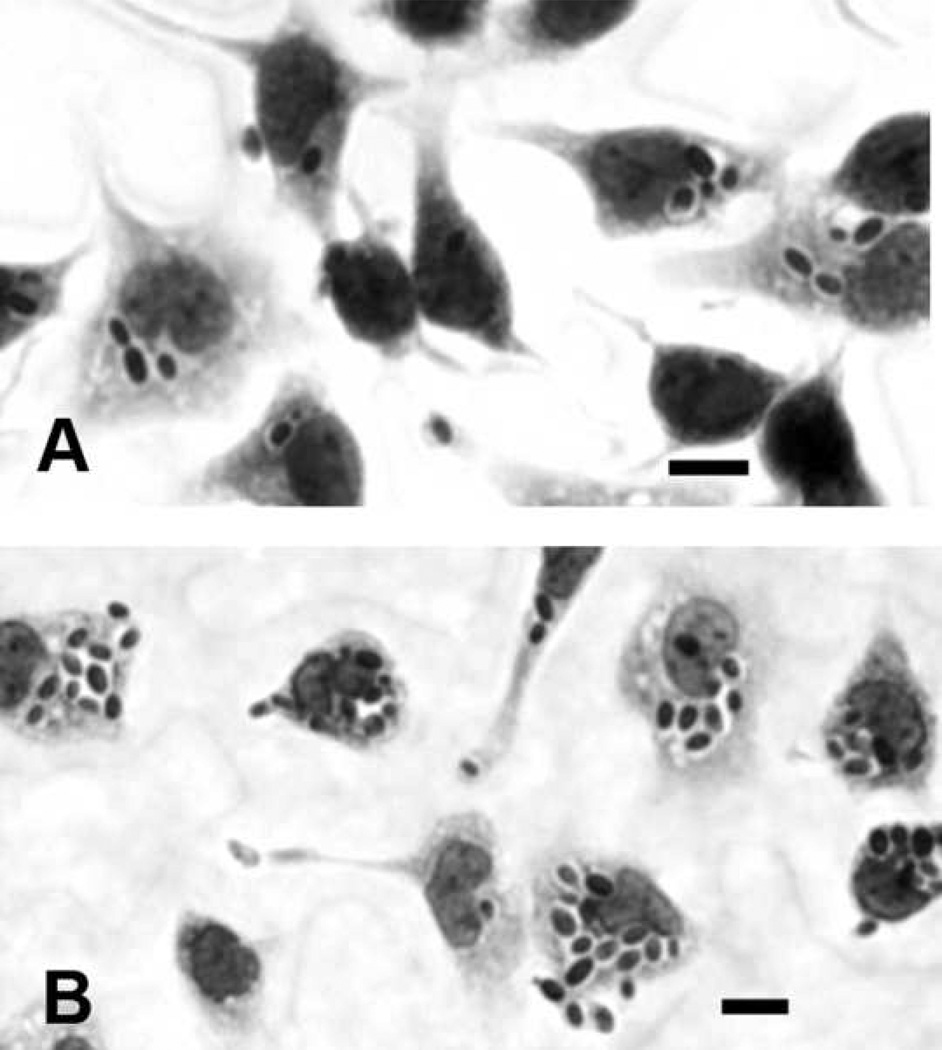
To investigate if LPS-induced macrophage activation might play a role in clearance of C. glabrata, resident mouse peritoneal macrophages were harvested, treated overnight with LPS, and then incubated 30 min with C. glabrata. LPS resulted in up-regulation of the macrophage receptor MARCO (687 ± 91 versus 2664 ± 115 fluorescent intensity units for control and treated macrophages, respectively, P<.01), verifying LPS-induced macrophage activation (Fig. 5). LPS was associated with increased numbers (P<.01) of macrophage-associated C. glabrata, i.e., 3.7 ± 0.2 versus 6.4 ± 0.4 C. glabrata per control and LPS-treated macrophage, respectively. Figure 6 shows representative Wright-Giemsa stains of control and LPS-treated macrophages incubated with C. glabrata, where many macrophage-associated C. glabrata appear surrounded by halos characteristic of an intracellular location. Following immunofluorescent staining of these cultures, only an occasional yeast cell reacted with the red fluorescent conjugate, verifying the intracellular location of nearly all (>99%) yeast cells (not shown).
FIG. 5.
Fluorescent microscopy of (A) control macrophages and (B) macrophages incubated overnight with 100 µg/ml LPS prior to incubation with rat anti-mouse MARCO conjugated to fluorescein, showing LPS-induced expression of MARCO. Scale bar = 10 microns
FIG. 6.
Representative Wright-Giemsa stains of C. glabrata incubated 30 min with control (A) and LPS-stimulated (B) macrophages, showing increased numbers of C. glabrata associated with LPS stimulated macrophages. Scale bars = 10 microns
Effect of TNF-α on in vitro phagocytosis of C. glabrata by mouse peritoneal macrophages
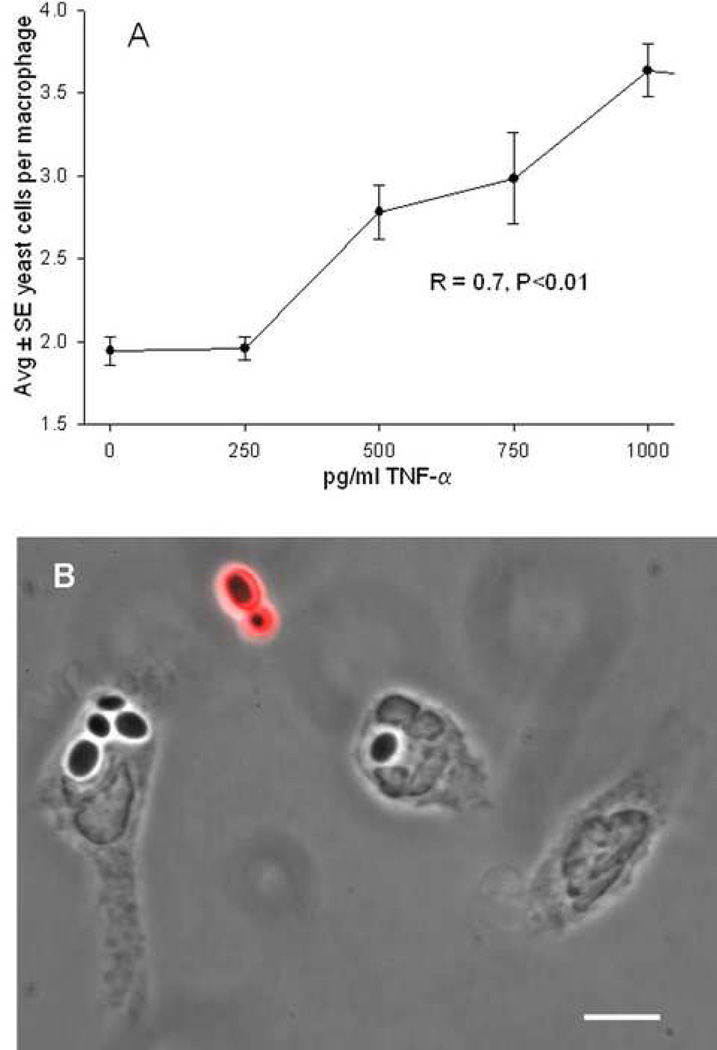
Because it is well-known that LPS stimulates macrophages to secrete TNF-α, varying concentrations of TNF-α were added to mouse peritoneal macrophages at the onset of incubation with C. glabrata. Numbers of yeast cells per macrophage were then quantified from Wright Giemsa stained cultures. Figure 7A indicates that TNF-α facilitated C. glabrata internalization in a dose dependent manner. Figure 7B is an immunofluorescent micrograph showing that macrophage-associated C. glabrata did not fluoresce red and were in the intracellular location.
FIG. 7.
A, Effect of varying concentrations of TNF-α on internalization of C. glabrata following 30 min incubation with mouse macrophages, showing a dose response in the effect of TNF-α. With exception of 250 pg/ml, all concentrations of TNF-α show increased internalization compared to 0 pg/ml (P<.01). B, Overlay of a phase contrast and a fluorescent image containing macrophages incubated with C. glabrata in the presence of 500 pg/ml TNF-α, where only extracellular yeast fluoresce red confirming the intracellular location of C. glabrata. Scale bar = 6 microns.
DISCUSSION
The aim of this study was to clarify the effects of E. coli and its LPS on host defense against systemic C. glabrata. The course of systemic infection was monitored as the fungal burden in the kidney and liver of mice sacrificed 1, 8, and 15 days after IV C. glabrata, with E. coli or its LPS injected the day before each sacrifice. Although rarely statistically significant, a consistent finding was that E. coli, and its LPS, was associated with decreased numbers of tissue C. glabrata. This finding was most dramatic in mice with long standing (15 day) C. glabrata infection, where the kidney fungal burden was significantly decreased in mice given both viable and nonviable E. coli (compared to controls), and a similar decrease was noted in the liver of mice given viable E. coli (Fig. 3). This finding was surprising because others have reported that C. albicans and E. coli (and its LPS) act as copathogens in mice, resulting in increased mortality as well as increased growth of C. albicans in the kidney [14–16].
Because it is well-known that LPS stimulates macrophages to secrete the inflammatory cytokine TNF-α [17], we tested the effect of parenteral TNF-α on the tissue burden of mice with systemic C. glabrata. Mice given TNF-α had lower numbers of C. glabrata in their kidneys and livers, compared to control mice, and this difference was significant in the kidneys of mice sacrificed one day after IV C. glabrata (Fig. 4). Taken as a whole, these data indicated that TNF-α played a role in decreasing the fungal burden in mouse tissue following IV C. glabrata. These observations are compatible with Brieland et al. [11] who noted increased levels of TNF-α and interferon-γ in mice after IV C. glabrata, and anti-TNF-α was associated with increased numbers of kidney C. glabrata. Based on the collective results from our in vivo experiments, we speculated that LPS might play a role in activating macrophages to interact with C. glabrata. Consistent with this hypothesis, LPS-stimulated macrophages had increased internalization of C. glabrata (Fig. 6). Although others have reported that C. glabrata can be phagocytosed by macrophages [25–27], we can now add that LPS appears to stimulate phagocytosis.
A number of investigators have studied the role of TNF-α in C. albicans infections, and these results are compatible with our findings with C. glabrata. For example, there is evidence that C. albicans stimulates macrophages to secrete TNF-α [28], which in turn promotes macrophage phagocytosis of C. albicans [29]. Both TNF-α and interleukin-6 are produced in mice with systemic C. albicans [30] and mannoprotein from C. albicans stimulates dendritic cells to release these inflammatory cytokines [31]. Vonk et al. [32] reported that both TNF-α and lymphotoxin-α (members of the TNF superfamily) were important for host defense (likely involving neutrophil phagocytosis of C. albicans) in experimental intraabdominal abscesses in mice. And, anti-TNF-α increased the numbers of kidney C. albicans in systemically infected mice, a phenomenon not noted with neutropenic mice, again suggesting that neutrophils are important for host defense against C. albicans [22]. Louie et al. [33] noted that TNF-α was produced during systemic C. albicans infection and that anti-TNF-α increased mortality as well as the tissue fungal burden of C. albicans. Thus, TNF-α can play a protective role in host defense against systemic C. albicans infection, and this phenomenon likely involves neutrophils. Based on this literature, the effect of TNF-α on C. glabrata-neutrophil interactions should be addressed in future studies.
Although not often statistically significant, our general observation that E. coli and its LPS decreased the tissue burden of C. glabrata in mice was in contrast to reports that E. coli and its LPS increase mortality and tissue fungal burden in mice with systemic C. albicans [14–16]. It seems reasonable to expect that if TNF-α plays a role in ameliorating systemic C. albicans infection, then E. coli and its LPS (known to induce TNF-α) should ameliorate systemic C. albicans infection. The reason for this apparent paradox with C. albicans is beyond the scope of the present study, and may be related to the fact that C. albicans readily undergoes morphogenesis in vivo, while C. glabrata does not.
In summary, data from the present study indicated that concomitant infection with E. coli (or its LPS) might aid host defense against systemic C. glabrata by a mechanism involving LPS-induced macrophage activation, likely involving macrophage secretion of TNF-α, although direct evidence for this scenario was not provided by these experiments. In an elegant study, Murray et al. [29] linked TNF-α secretion and C. albicans phagocytosis by macrophages, where TNF-α traffics from the Golgi to the recycling endosome, where a vesicle-associated membrane protein mediates delivery of TNF-α to the cell surface at the site of phagocytic cup formation, and where fusion of the membrane protein at the cup simultaneously facilitates release of TNF-α expanding the macrophage membrane facilitating phagocytosis. Based on the data herein, a similar study with C. glabrata would be timely. Nonetheless, data from the present study suggested that inflammatory cytokines, such as TNF-α, might be investigated as potential therapeutic agents, either alone or as adjuvants with traditional antifungal agents.
ACKNOWLEDGEMENTS
This work was supported by the USA Public Health Service and National Institutes of Health grant R01 GM059221 and The March of Dimes Birth Defect Foundation grant #6-FY04-53. We wish to acknowledge the excellent technical assistance of Robb Garni, Melody Shepherd, and Milena Sivertson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 3rd Annual Academic Surgical Congress (Association for Academic Surgery), Huntington Beach, CA, February 12–15, 2008.
REFERENCES
- 1.Rangel-Frausto M, Wiblin T, Blumberg HM, Saiman L, Patterson J, Rinaldi M, Pfaller M, Edwards JE, Jr, Jarvis W, Dawson J, Wenzel RP. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin Infect Dis. 1999;29:253. doi: 10.1086/520194. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE. A perspective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37:634. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 3.Charles PE, Doise JM, Quenot JP, Aube H, Dalle F, Chavanet P, Nilesi N, Aho LS, Portier H, Blettery B. Candidemia in critically ill patients: difference of outcome between medical and surgical patients. Intensive Care Med. 2003;29:2162. doi: 10.1007/s00134-003-2002-x. [DOI] [PubMed] [Google Scholar]
- 4.Peres-Bota D, Rodriguez-Villalobos H, Dimopoulos G, Melot C, Vincent J-L. Potential risk factors for infection with Candida spp. in critically ill patients. Clin Microbiol Infect. 2004;10:550. doi: 10.1111/j.1469-0691.2004.00873.x. [DOI] [PubMed] [Google Scholar]
- 5.Wisplinghoff H, Bischoff T, Tallent SM, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial blood stream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 6.Snydman DR. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest. 2003;123:500S. doi: 10.1378/chest.123.5_suppl.500s. [DOI] [PubMed] [Google Scholar]
- 7.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 8.Lipsett PA. Surgical critical care: fungal infections in surgical patients. Crit Care Med. 2006;S215 doi: 10.1097/01.CCM.0000231883.93001.E0. [DOI] [PubMed] [Google Scholar]
- 9.Csank C, Haynes K. Candida glabrata displays pseudohyphal growth. FEMS Microbiol Lett. 2000;189:115. doi: 10.1111/j.1574-6968.2000.tb09216.x. [DOI] [PubMed] [Google Scholar]
- 10.Fidel PL, Jr, Vazquez JA, Sobel JD. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12:80. doi: 10.1128/cmr.12.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brieland J, Essig D, Jackson C, Frank D, Loebenberg D, Menzel F, Arnold B, DiDomenico B, Hare R. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect Immun. 2001;69:5046. doi: 10.1128/IAI.69.8.5046-5055.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blot S, Vandewoude K, Hoste E, Poelaert J, Colardyn F. Outcomes in critically ill patients with candidal fungaemia: Candida albicans vs. Candida glabrata. J Hosp Infect. 2001;47:308. doi: 10.1053/jhin.2000.0918. [DOI] [PubMed] [Google Scholar]
- 13.Hederwick SA, Lyons MJ, Liu M, Vazquez JA, Kauffman CA. Epidemiology of yeast colonization in the intensive care unit. Eur J Clin Microbiol Infect Dis. 2000;19:663. doi: 10.1007/s100960000348. [DOI] [PubMed] [Google Scholar]
- 14.Burd RS, Raymond CS, Dunn DL. Endotoxin promotes synergistic lethality during concurrent Escherichia coli and Candida albicans infection. J Surg Res. 1992;52:537. doi: 10.1016/0022-4804(92)90125-j. [DOI] [PubMed] [Google Scholar]
- 15.Klaerner HG, Uknis ME, Acton RD, Dahlberg PS, Carlone-Jambor C, Dunn DL. Candida albicans and Escherichia coli are synergistic pathogens during experimental microbial peritonitis. J Surg Res. 1997;70:161. doi: 10.1006/jsre.1997.5110. [DOI] [PubMed] [Google Scholar]
- 16.Akagawa G, Abe S, Yamaguchi H. Mortality of Candida albicans-infected mice is facilitated by superinfection of Escherichia coli or administration of its lipopolysaccharide. J Infect Dis. 1995;171:1539. doi: 10.1093/infdis/171.6.1539. [DOI] [PubMed] [Google Scholar]
- 17.Goetz FW, Planas JV, MacKenzie S. Tumor necrosis factors. Developmental & Comparative Immunology. 2004;28:487. doi: 10.1016/j.dci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis. 2005;192:336. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 19.Bendel CM, Hess DJ, Garni RM, Henry-Stanley M, Wells CL. Comparative virulence of Candida albicans yeast and filamentous forms in orally and intravenously inoculated mice. Crit Care Med. 2003;31:501. doi: 10.1097/01.CCM.0000049954.48239.A1. [DOI] [PubMed] [Google Scholar]
- 20.Winn W, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, Woods G, editors. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 6th ed. Philadelphia: Lippincott, Williams & Wilkins; 2006. [Google Scholar]
- 21.Odds FC, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32:1923. doi: 10.1128/jcm.32.8.1923-1929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 23.Sundgren-Anderson A, Ostlund P, Bartfai T. IL-6 is essential in TNF-α induced fever. Am J Physiol. 1998;275:R2028. doi: 10.1152/ajpregu.1998.275.6.R2028. [DOI] [PubMed] [Google Scholar]
- 24.van der Laan LJW, Dopp EA, Haworth R, Pikkarainen T, Kangas M, Elomaa O, Dijkstra CD, Gordon S, Tryggvason K, Kraal G. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J Immunol. 1999;162:939. [PubMed] [Google Scholar]
- 25.Bopp LH, Baltch AL, Ritz WJ, Michelsen PB, Smith RP. Antifungal effect of voriconazole on intracellular Candida glabrata, Candida krusei and Candida parapsilosis in human monocyte-derived macrophages. J Med Microbiol. 2006;55:865. doi: 10.1099/jmm.0.46393-0. [DOI] [PubMed] [Google Scholar]
- 26.Baltch AL, Bopp LH, Smith RP, Ritz WJ, Carlyn CJ, Michelsen PB. Effects of voriconazole, granulocyte-macrophage colony-stimulating factor, and interferon gamma on intracellular fluconazole-resistant Candida glabrata and Candida krusei in human monocyte-derived macrophages. Diag Microbiol Infect Dis. 2005;52:299. doi: 10.1016/j.diagmicrobio.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Garcha UK, Brummer E, Stevens DA. Synergy of fluconazole with human monocytes or monocyte-derived macrophages for killing of Candida species. J Infect Dis. 1995;172:1620. doi: 10.1093/infdis/172.6.1620. [DOI] [PubMed] [Google Scholar]
- 28.van der Graff CAA, Netea MG, Verschueren I, van der Meer JWM, Kullberg BJ. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun. 2005;73:7458. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- 30.Steinshamn S, Waage A. Tumor necrosis factor and interleukin-6 in Candida albicans infection in normal and granulocytopenic mice. Infect Immun. 1992;60:4003. doi: 10.1128/iai.60.10.4003-4008.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kielian TL, Blecha F. CD14 and other recognition molecules for lipopolysaccharide: a review. Immunopharmacology. 1995;29:187. doi: 10.1016/0162-3109(95)00003-c. [DOI] [PubMed] [Google Scholar]
- 32.Vonk AG, Netea MG, van Krieken JH, van der Meer JWM, Kullberg BJ. Delayed clearance of intraabdominal abscesses caused by Candida albicans in tumor necrosis factor-α- and lymphotoxin-α-deficient mice. J Infect Dis. 2002;186:1815. doi: 10.1086/345818. [DOI] [PubMed] [Google Scholar]
- 33.Louie A, Baltch AL, Smith RP, Franke MA, Ritz WJ, Singh JK, Gordon MA. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun. 1994;62:2761. doi: 10.1128/iai.62.7.2761-2772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]