Abstract
Chronic smoking in alcohol dependence is associated with abnormalities in brain morphology and metabolite levels in large lobar regions (e.g. frontal lobe). Here, we evaluated if these abnormalities are specifically apparent in several cortical and select subcortical components of the extended brain reward system (BRS), a network that is critically involved in the development and maintenance of all forms of addictive disorders. We studied 33 non-smoking and 43 smoking alcohol-dependent individuals (ALC) with 1 week of abstinence and 42 non-smoking Controls. At 1.5 Tesla, we obtained regional measures of cortical thickness and N-acetylaspartate (NAA; a surrogate marker of neuronal integrity) concentration in major components of the BRS as well as the corresponding measures throughout the cortex. Smoking ALC and non-smoking ALC demonstrated decreased thickness compared with Controls in the dorsolateral prefrontal cortex (DLPFC), insula, orbitofrontal cortex (OFC), the total BRS, total frontal cortex and global cortex. Smoking ALC had significantly decreased thickness compared to non-smoking ALC in the ACC, insula, the total BRS and total frontal cortex. Smoking ALC had also lower NAA concentrations than both non-smoking ALC and Controls in the DLPFC, insula, superior corona radiata and the total BRS. Alcohol consumption and common medical and psychiatric co-morbidities did not mediate differences between smoking and non-smoking ALC. This dual modality magnetic resonance (MR) study indicated that chronic smoking in ALC was associated with significant cortical thinning and NAA abnormalities in anterior brain regions that are implicated in the development and maintenance of addictive disorders.
Keywords: Alcohol dependence, brain reward system, cigarette smoking, cortical thickness, N-acetylaspartate, nicotine
INTRODUCTION
Accumulating evidence from clinical neuroscience research strongly suggests that neurobiological abnormalities of the extended brain reward system (BRS) are implicated in the development and maintenance of all forms of addictive disorders (Baler & Volkow 2006; Kalivas & O’Brien 2008; Makris et al. 2008b; Koob & Volkow 2010; Volkow et al. 2011). The BRS is a collection of discrete and overlapping cortical-cortical and cortical-subcortical circuits that interact to form the biological substrate for reward/saliency, motivation/drive, conditioning/habits and inhibitory control/executive functions (Volkow et al. 2010, 2011; Potenza et al. 2011). Recent human in vivo neuro-imaging methods have begun to identify morphological and biochemical abnormalities localized within the BRS of those with alcohol use disorders (AUD) and other addictive disorders (Volkow et al. 2008; Wrase et al. 2008; Makris et al. 2008a,b; Rando et al. 2011; Durazzo et al. 2010c).
The orbitofrontal cortex (OFC), dorsal prefrontal cortex (DLPFC), anterior cingulate cortex (ACC) and insula are major cortical components of the BRS (Kalivas & Volkow 2005; Baler & Volkow 2006; Makris et al. 2008b), and subcortical components include the cerebellar vermis and corona radiata (Durazzo et al. 2010c). The cortical components of the BRS, and their afferent and/or reciprocal connections with subcortical nuclei and other cortical regions, subserve the following reward-related processes and behaviors: OFC and insula: integration of interoceptive information, evaluation of stimulus saliency and representation of reward magnitude (Fellows 2007; Paulus 2007; Rolls & Grabenhorst 2008); OFC and ACC: self-monitoring, regulation of emotional and affective tone and behavior (Bush, Luu & Posner 2000; Bush et al. 2002; Kringelbach & Rolls 2004; Rolls 2004); DLPFC: adjustment of goal-directed activity based on current environmental contingencies and anticipated future consequences (Mega & Cummings 1994; Eslinger, Grattan & Geder 1995; Petrides 2005). It is important to consider that while each of these cortical regions is involved in unique aspects of reward-related processes, emotion and neurocognition (Cummings 1998; Baxter et al. 2008; Rudebeck et al. 2008; Buckley et al. 2009), there is considerable overlap and redundancy in their contributions to these functions. This highlights that neurocognition, emotion and behavior as the result of dynamic interactions among multiple reciprocally interconnected brain regions (Gazzaley & D’Esposito 2007). Collectively, these neocortical and paralimbic regions are critical for the integration of interoceptive and external information. Their interconnectivity with the striatum and limbic system enables the ‘top-down’ inhibitory control of affect, cognition and behavioral responses through modulation of activity in the striatum and limbic system (Baler & Volkow 2006; Crews & Boettiger 2009; Gazzaley & D’Esposito 2007; Kalivas 2009; Volkow et al. 2010; Potenza et al. 2011). Although the cerebellum and corona radiata are not traditional components of the extended BRS, there is a clear justification for their inclusion in this network. The cerebellum is involved in aspects of learning and memory, working memory, executive skills and reward processing (Martin-Solch et al. 2001; Sullivan 2003; Sullivan et al. 2003; Anderson et al. 2006; Olbrich et al. 2006; Paul et al. 2007). The corona radiata is white matter (WM) comprised of projection fibers (e.g. corticothalamic, corticostriatal, corticopontine) and afferents from subcortical gray matter (GM) that link frontal, parietal and temporal cortical regions with subcortical nuclei involved in executive skills, impulse control, emotional regulation and reward processing (Mega & Cummings 1994; Cummings 1995, 1998; Makris et al. 1999; Saint-Cyr 2003; ; Aralasmak et al. 2006 Schmahmann et al. 2007). Thus, the above regions and their interconnectivity with other cortical and subcortical nuclei/regions (e.g. ventral and dorsal striatum, dorsomedial thalamus) form independent and overlapping circuits that constitute the neurobiological substrate that enables adaptive and appropriate goal-related behavior based on drives, motivations, emotions, current environmental contingencies and consequences of previous behavior.
It is well established that the layered cortical cellular architecture demonstrates a modular or columnar organization, which is oriented perpendicular to the cortical surface (Innocenti & Vercelli 2010). Cortical thickness is related to the number or density of cells in a column (Rakic 1988) and is associated with neurocognition in healthy controls (Walhovd et al. 2006; Choi et al. 2008; Dickerson et al. 2008) and cocaine abusers (Makris et al. 2008a). Cortical thickness appears to represent a metric that is genetically and phenotypically distinct from cortical surface area and volume (Panizzon et al. 2009; Kremen et al. 2010; Winkler et al. 2010) and is suggested to serve as a proxy marker of the integrity of cortical cytoarchitecture (Makris et al. 2007). Cortical thickness is reputed to be more sensitive to neurodegenerative processes than cortical volumes (Hutton et al. 2009), and its application to addictive disorders may increases the ability to detect more subtle alcohol/substance-related morphological abnormalities.
N-acetylaspartate (NAA) is considered to be a surrogate marker of neuronal integrity (Vion-Dury et al. 1994; Sullivan 2000), with decreased concentrations related to neuronal loss, atrophied dendrites, reduced dendritic spine density, axonal injury or derangements of neurometabolism (De Stefano, Matthews & Arnold 1995; Hugg et al. 1996; Schuff et al. 2001; Baslow & Guilfoyle 2007). Significantly decreased NAA concentrations are observed in multiple brain regions of those with AUD as well as chronic cigarette smokers (Durazzo & Meyerhoff 2007; Durazzo, Meyerhoff & Nixon 2010b). Higher regional NAA level is also associated with better neurocognition in various neurodegenerative diseases, AUD and normal controls (Jung et al. 1999; Schuff et al. 2006; Durazzo & Meyerhoff 2007).
The adverse consequences of AUD on neurobiology and neurocognition are well documented (Durazzo & Meyerhoff 2007; Rourke & Grant 2009). It is widely recognized that pre-morbid and/or co-morbid participant characteristics can promote considerable variability in the pattern and magnitude of neurobiological and neurocognitive abnormalities demonstrated in AUD (Durazzo & Meyerhoff 2007; Durazzo et al. 2008). Chronic cigarette smoking is most common comorbidity in AUD, with a prevalence of approximately 60 to 90 percent (Room 2004; Durazzo & Meyerhoff 2007), and is associated with multiple neurobiological abnormalities, particularly in anterior brain regions (Durazzo & Meyerhoff 2007; Durazzo et al. 2010b). In previous magnetic resonance neuro-imaging studies, we found that treatment-seeking alcohol-dependent individuals (ALC), in the early phase of abstinence, demonstrated significantly lower frontal, temporal and medial temporal lobe volumes (Gazdzinski, Durazzo & Meyerhoff 2005a; Cardenas et al. 2007; Gazdzinski et al. 2008) and abnormal metabolite concentrations in the frontal and parietal lobes (Durazzo et al. 2004, 2006) relative to controls. In these studies, smoking ALC consistently demonstrated the greatest abnormalities as well as diminished recovery relative to non-smoking ALC. Therefore, chronic smoking appears to contribute to the considerable heterogeneity observed in the pattern and magnitude of neurobiological abnormalities observed in AUD. However, these proton magnetic resonance imaging (MRI) and spectroscopic imaging (1H MRSI) studies examined brain volumes and metabolite levels at the lobar level (e.g. total frontal GM, total frontal WM). The extent to which the greater volume and metabolite abnormalities are also apparent in the BRS of these smoking ALC is unknown.
Accordingly, the goal of this study was to interrogate the integrity of cortical and subcortical components of the BRS involved in ‘top-down’ inhibitory control/ executive functions in smoking and non-smoking ALC near the inception of treatment, by comparing these groups with non-smoking, light-drinking controls on measures of regional cortical thickness and NAA concentration. The dual MR modalities applied in this study enabled concurrent assessment of the macrostructural and neuronal integrity of multiple BRS components. To our knowledge, previous AUD research has not reported concurrent measurements of regional cerebral cortical thickness and NAA concentration. We predicted that: (1) smoking ALC demonstrate lower NAA levels and thinner cortices in the BRS than both non-smoking ALC and controls; and (2) the reductions of cortical thickness and NAA levels in smoking ALC are of greater magnitude in the BRS than in the frontal cortex and the global cerebral cortex.
MATERIALS AND METHODS
Participants
Data from 76 outpatient participants (four females) obtained from a larger cohort recruited from the VA Medical Center Substance Abuse Day Hospital and the Kaiser Permanente Chemical Dependence Recovery Program in San Francisco were used in analyses. All participants provided written informed consent prior to study according to the Declaration of Helsinki and underwent procedures that were approved by the University of California San Francisco and the San Francisco VA Medical Center. All treatment-seeking participants met the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV) criteria for alcohol dependence (97% with physiological dependence) at study enrollment. Primary inclusion criteria for the alcohol-dependent participants were fluency in English, DSM-IV diagnosis of alcohol dependence or alcohol abuse at the time of enrollment, consumption of greater than 150 standard alcohol-containing drinks (i.e. 13.6 g of ethanol per drink) per month for at least 8 years prior to enrollment for men, or consumption of greater than 80 drinks per month for at least 6 years prior to enrollment for women. The alcohol-dependent participants were actively involved in stabilization/early recovery outpatient treatment at the time of the baseline assessment. Light-drinking, non-smoking controls (Controls; n = 42; five females) were recruited from the local community and had no history of any DSM-IV Axis I disorder or biomedical conditions know or suspected to adversely influence brain neurobiology. Participants were between 28 and 66 years of age. In the Control cohort, 79% of Controls were Caucasian, 7% African American and Asian, 5% Latino and 3% Pacific Islander. In the ALC cohort, 75% were Caucasian, 12% African American, 7% Latino, 3% Native American and 3% Pacific Islander. Controls and ALC did not differ significantly on frequency of ethnicity. See Table 1 for group demographic data.
Table 1.
Group demographics, alcohol and cigarette use histories, self-report questionnaires and co-morbidity frequency. Mean (SD).
| Measure | Controls (n = 42) | nsALC (n = 33) | sALC (n = 43) |
|---|---|---|---|
| Age (years) | 44.6 (9.1) | 52.0 (9.7) | 50.1 (8.9) |
| Education (years) | 16.3 (2.8) | 14.3 (2.3) | 13.5 (2.0) |
| AMNART | 119 (7) | 113 (9) | 113 (9) |
| Percent Caucasian | 79 | 73 | 76 |
| Number of days abstinent | NA | 8 (4) | 7 (6) |
| 1-year average drinks/month | 16 (17) | 346 (180) | 406 (153) |
| Lifetime average drinks/month | 16 (14) | 173 (107) | 281 (153) |
| Percent with medical co-morbidity | NA | 52 | 56 |
| Percent with substance use disorder co-morbidity | NA | 21 | 18 |
| Percent with psychiatric co-morbidity | NA | 42 | 42 |
| FTND | NA | NA | 5.4 (2.1) |
| Cigarettes per day | NA | NA | 20.0 (9.6) |
| Pack years | NA | NA | 30.0 (20.5) |
| Smoking duration (years) | NA | NA | 23.5 (11.9) |
| Beck Depression Inventory | 3.7 (1.8) | 13.7 (9.5) | 14.9 (9.7) |
| STAI-state | 33.1 (6.9) | 46.0 (10.4) | 49.8 (12.7) |
| Body mass index | 25.8 (4.4) | 30.1 (5.1) | 25.4 (4.7) |
AMNART = American National Adult Reading Test; FTND = Fagerstrom Tolerance Test for Nicotine Dependence; NA = not applicable; nsALC = nonsmoking alcohol-dependent participant; sALC = smoking alcohol-dependent participant; STAI = State-Trait Anxiety Inventory.
Exclusion criteria for all ALC participants were history of the following: dependence on any substance other than alcohol or nicotine in the 5 years immediately prior to enrollment, any intravenous drug use in the 5 years immediately prior to enrollment in the study, current opioid agonist therapy, intrinsic cerebral masses, HIV/ AIDS, cerebrovascular accident, brain aneurysm, arteriovenous malformations, peripheral vascular disease, myocardial infarction, uncontrolled chronic hypertension (systolic > 180 mmHg and/or diastolic > 120 mmHg), type-1 diabetes or insulin-dependent conditions, moderate or severe chronic obstructive pulmonary disease, non-alcohol-related seizures, significant exposure to known neurotoxins (e.g. toluene, carbon tetra-chloride), demyelinating and neurodegenerative diseases, clinically documented Wernicke–Korsakoff syndrome, alcohol-induced persisting dementia, penetrating head trauma and closed head injury resulting in documented loss of consciousness for more than 10 minutes. Exclusion criteria also included history of schizophrenia-spectrum disorders, bipolar disorder, dissociative disorders, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder (with or without agoraphobia) and major depression with mood-incongruent psychotic symptoms. Hepatitis C, type-2 diabetes, hypertension, unipolar mood disorder (major depression and/or substance-induced mood disorder) were permitted in the ALC cohort given their high prevalence in AUD (Parekh & Klag 2001; Mertens et al. 2003, 2005; Stinson et al. 2005; Hasin et al. 2007). Participants were urinetested for illicit substances before all assessments (i.e. cannabinoids, opiates, phencyclidine, cocaine and amphetamines), and no participant tested positive for any of these substances.
Baseline assessment
For ALC, clinical and MR procedures were conducted approximately 7 ± 4 days after their last alcoholic drink. Structural MRI data were obtained from 42 Controls, 33 non-smoking ALC and 43 smoking ALC. Quantitative 1H MRSI data were obtained in a subset of 33 Controls, 25 non-smoking ALC and 36 smoking ALC. In the smaller 1H MRSI sample, 96% of participants had complete structural MRI data.
Clinical measures
Participants completed the Structured Clinical Interview for DSM-IV Axis I Disorders, Version 2.0 (SCID-I/P (First et al. 1998) and semi-structured interviews for lifetime alcohol consumption [Lifetime Drinking History; (Sobell et al. 1988; Sobell & Sobell 1992)] and substance use (in-house questionnaire assessing substance type, and quantity and frequency of use)]. From the Lifetime Drinking History, average number of alcoholic drinks per month over 1 year prior to enrollment, average number of drinks per month over lifetime, lifetime years of regular drinking (i.e. years in which the participant consumed at least one alcoholic drink per month), age of onset and duration of heavy drinking (i.e. drinking more than 100 drinks per month in males and 80 drinks per month in females) were calculated. Pre-morbid verbal intelligence was estimated with the American National Adult Reading Test [AMNART (Grober & Sliwinski 1991)]. Participants also completed standardized questionnaires assessing depressive [Beck Depression Inventory, BDI (Beck 1978)], and anxiety symptomatology [(State-Trait Anxiety Inventory, form Y-2, STAI (Spielberger et al. 1977)], and nicotine dependence via the Fagerstrom Tolerance Test for Nicotine Dependence (Fagerstrom, Heatherton & Kozlowski 1991). These measures were typically completed within 1 day of the MR procedures.
Magnetic resonance acquisition and analyses
Image acquisition
Structural imaging
A volumetric magnetization-prepared rapid gradient echo was acquired with repetition time (TR)/echo time (TE)/inversion time (TI) = 9.7/4/300 ms, 15° flip angle, 1×1 mm2 in-plane resolution and 1.5-mm-thick coronal partitions oriented perpendicular to the main long axes of bilateral hippocampi as seen on sagittal scout MRI. Additionally, a double spinecho sequence TR/TE/TI = 2500/20/80 ms with 1×1 mm2 in-plane resolution and 50 contiguous 3.0-mm-thick axial slices was also acquired. See Gazdzinski and colleagues (Gazdzinski et al. 2005b) for detailed information on the MR acquisition methods.
1H MRSI
Participants completed multi-slice 1H MRSI (TR/TI/TE = 1800/165/25 ms) with lipid inversion to suppress intense lipid signal. Absolute quantitation for NAA and other metabolites was completed. The three 15-mm-thick slices of the 1H MRSI data set were spatially aligned to the double spin-echo sequence and covered supraventricular, subcortical GM and WM regions and the superior half of the cerebellum (including the vermis) in the axial plane. The field-of-view and number of phase encoding steps were chosen to yield spectra of nominal voxel size of 0.8 × 0.8 × 1.5 cm3 or approximately 1 ml. See Meyerhoff and colleagues (Meyerhoff et al. 2004) for details on MRSI data acquisition.
Image processing
Freesurfer
The publicly available Freesurfer (v4.5) volumetric segmentation and cortical surface reconstruction methods (Dale, Fischl & Sereno 1999; Fischl, Sereno & Dale 1999; Fischl & Dale 2000; Fischl et al. 2004) were used to obtain regional measures of cortical thickness in millimeters. Spatial normalization to a template cortical surface allowed automatic parcellation of the cortical surfaces into 34 anatomical regions of interest (ROI) per hemisphere, and thickness measures were obtained for all 34 bilateral ROIs (see Fischl & Dale 2000 for method reliability and Fischl et al. 2004 for technical details). The anatomically labeled Freesurfer regions that were used to form the BRS ROIs were as follows: ACC—rostral and caudal; dorsal/DLPFC—rostral and caudal middle frontal and superior frontal gyri; OFC—medial and lateral; insula—standard Freesurfer label. For the ACC, DLPFC and OFC, an average thickness was calculated from the individual anatomical labels that constituted each region. There were no significant within-group-differences for hemisphere (i.e. no significant lateralization); therefore, thickness values for bilateral regions were combined and an average calculated for each BRS ROI. Average thickness measures were also computed for the total BRS, the total frontal cortex and for all cortical regions to obtain a measure of global cerebral cortical thickness.
1H MRSI
The metabolite concentration maps of the three 1H MRSI slices with a zero-filled 64 × 64 digital resolution were displayed together with the corresponding co-aligned brain anatomy as seen on a proton density-weighted MRI using SITOOLS (Soher et al. 1998), a program that has been extensively used to process 1H MRSI data in our laboratory (e.g. Durazzo et al. 2004, 2006; Meyerhoff et al. 2004). For detailed information on criteria used to evaluate spectral quality, see Wiedermann et al. (2001) and Meyerhoff et al. (2004). The BRS ROIs for which average metabolite concentrations were calculated from spectral data were the ACC, DLPFC, insula, cerebellar vermis, superior corona radiata (SCR) of the frontal WM. The OFC did not yield a sufficient number of spectra of acceptable quality to be included in analyses. See Durazzo and colleagues (Durazzo et al. 2010c) for details regarding the parcellation of the ACC, DLPFC, cerebellar vermis and SCR. Approximately 10 cases were randomly chosen from each region of this data set and independently parcellated by a technician who was trained in the procedure. The agreement of labeling of the boundaries across regions was greater than 97%. The DLPFC, ACC, insula and vermis voxels contained greater than 45%, 40%, 40% and 50% GM, respectively. The SCR contained only voxels with more than 70% WM. See Durazzo and colleagues (Durazzo et al. 2010c) for details regarding the tissue contributions (i.e. GM, WM and cerebrospinal fluid) for each 1H MRSI voxel and for spatial positioning of 1H MRSI slices. For examples of representative spectra, see Meyerhoff and colleagues (Meyerhoff et al. 2004).
The number of voxels used to calculate the average metabolite concentrations in each of the BRS ROIs was not significantly different among smoking ALC, nonsmoking ALC and Controls. There were no within group metabolite concentration differences for the left and right hemispheres, so the metabolite concentrations for ROIs were based on bilateral averages. Average NAA concentration was also separately calculated for the total BRS and for cortical GM from the frontal, parietal and temporal lobes. There were an insufficient number of spectra passing quality control from GM of the occipital lobe to be included in data analyses. Finally, a ‘global’ measure of cortical NAA was computed by averaging the lobar NAA concentration from frontal, parietal and temporal cortical GM.
Data analyses
Separate multivariate analysis of covariance (MANCO-VAs) were used to examine for group differences among the seven cortical thickness measures (ACC, DLPFC, OFC, insula, total BRS, total frontal cortex and global cortex) and eight NAA measures (ACC, DLPFC, insula, SCR, cerebellar vermis, total BRS, total frontal cortex and global cortex). MANCOVAs were controlled for age, intracranial volume (ICV) and body mass index, given their potential associations with cortical thickness and/or NAA concentration (Kochunov et al. 2007, 2010; Im et al. 2008; Haga et al. 2009; Hutton et al. 2009; Gazdzinski et al. 2010a,b). In secondary analyses, AMNART and education were added as covariates to assess the influence of these factors on regional thickness and NAA measures. Significant MANCOVAs (P < 0.05) were followed-up with univariate tests and pairwise t-tests comparing Controls, non-smoking ALC and smoking ALC. Given the significantly higher lifetime average drinks per month in smoking ALC compared to non-smoking ALC (see Table 1), all pairwise comparisons among these groups for thickness and NAA measures were controlled for lifetime average drinks per month. Although smoking and non-smoking ALC did not significantly differ on the frequency of medical, psychiatric and substance abuse co-morbidities, in secondary analyses, pairwise comparisons between smoking and non-smoking ALC were controlled for these co-morbidities to determine if they mediated the group differences. Alpha level for pairwise t-tests for cortical thickness measures was adjusted for multiple comparisons according to the seven thickness measures and the average intercorrelations among these measures for all groups combined (r=0.73) (Sankoh, Huque & Dubey 1997). The adjusted alpha level for these pairwise t-tests was P ≤ 0.029. For NAA measures, alpha level for pairwise t-tests was adjusted for multiple comparisons according to the eight NAA measures and the average intercorrelations among these measures for all groups combined (r = 0.62). The adjusted alpha level for these pairwise t-tests was P ≤ 0.024. Effect sizes for pairwise comparisons were calculated via Cohen’s d (Cohen 1988). Groups were compared on ICV with a separate univariate analysis as this measure may reflect the influence of potential pre-morbid factors on brain function in those with AUD (Schottenbauer et al. 2007). Associations between outcome measures, alcohol and cigarette consumption in the ALC group were examined with Spearman’s rho. Associations between cortical thickness and NAA levels in the ACC, DLPFC, insula and total frontal cortex (regions where both thickness and NAA levels were measured) were evaluated with partial correlations controlling for GM content of the 1H MRSI voxels for each region.
RESULTS
Demographic, alcohol and cigarette consumption variables
Controls were significantly younger (P = 0.016) than non-smoking ALC and had a greater number of years of formal education (P < 0.001) than both smoking and non-smoking ALC. Non-smoking ALC had significantly higher body mass index than Controls and smoking ALC (P = 0.01). Smoking ALC had higher average drinks per month over 8 years prior to enrollment and over lifetime than non-smoking ALC (both P < 0.007; see Table 1). Among the 33 non-smoking ALC, 29 reported no history of smoking or smoked less than 20 cigarettes over lifetime. Four non-smoking ALC reported a previous history of chronic smoking, and all had quit more than 8 years prior to enrollment. There were no significant differences in the above demographic variables between the cortical thickness sample and the smaller sample with NAA data.
Co-morbid psychiatric, medical and substance use disorders
Non-smoking and smoking ALC were equivalent on BDI and STAI scores and on the frequency of medical conditions (primarily hypertension and hepatitis C), co-morbid psychiatric conditions (primarily major depression and substance-induced mood disorder with depressive features) and substance use disorders (see Table 1). Approximately 25% of participants diagnosed with a unipolar mood disorder took an antidepressant medication, and approximately 55% of hypertensive participants took antihypertensive medications. There were no differences between smoking and non-smoking ALC in frequency of use of these medications.
Group comparisons on regional thickness measures and ICV
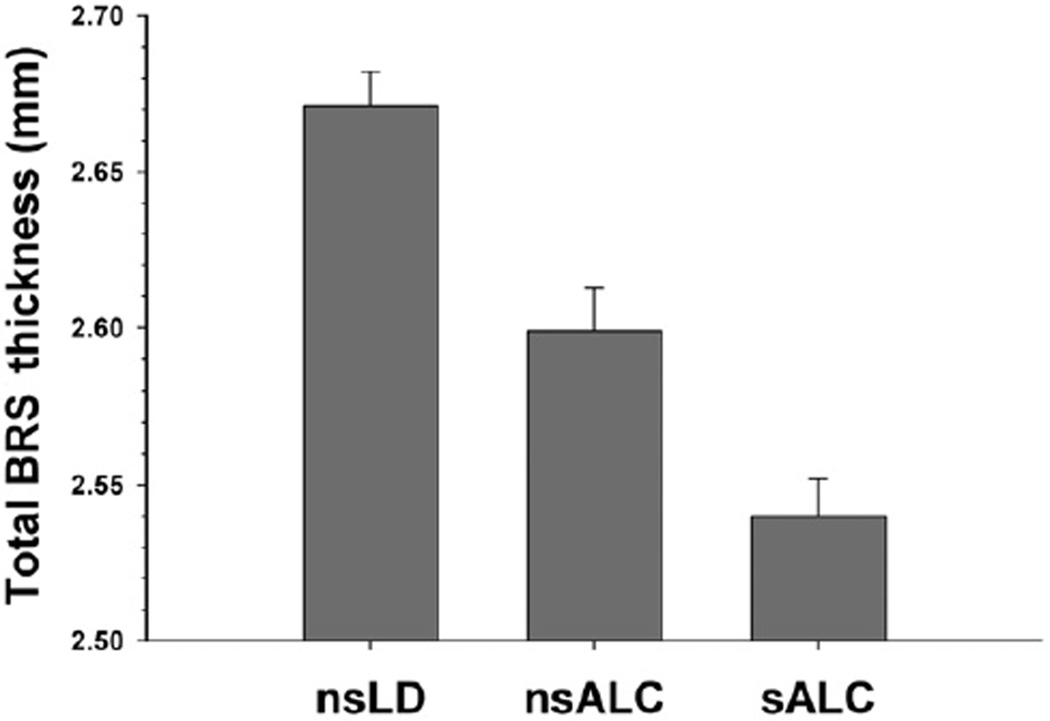
The omnibus MANCOVA indicated significant group differences on thickness measures [F (12, 208) = 3.62, P < 0.001]. Univariate tests were significant for the DLPFC, insula, OFC, total BRS, total frontal cortex and global cortical thickness (all P < 0.001), but not the ACC. There were no group differences on ICV. Pairwise comparisons (see Table 2) indicated that both smoking ALC and non-smoking ALC demonstrated significantly decreased thickness across all measures compared with Controls, except for the ACC, which was thinner only in smoking ALC. Smoking ALC had significantly thinner cortex than non-smoking ALC in the ACC, insula, total BRS and total frontal cortex, with a trend for thinner OFC cortex (P = 0.05). AMNART, education, alcohol consumption variables, medical, psychiatric and substance abuse co-morbidities were not significant predictors of thickness measures in tests specifically comparing smoking and non-smoking ALC, and did not mediate the thickness differences between these groups. To control for different amounts of tissue contained in each of the ROI that comprised the total BRS, total frontal cortex and global cortex, the average thickness for each individual region was scaled to its surface area. Analyses were repeated for the surface area-scaled measures and yielded identical results to those reported above.
Table 2.
Cortical thickness measures (mm) and intracranial volume (mm3). Mean (SD).
| Control |
nsALC |
sALC |
Effect size (Cohen’s d) |
|||
|---|---|---|---|---|---|---|
| Variable | (n = 42) | (n = 33) | (n = 43) | CON versus nsALC |
CON versus sALC |
nsALC versus sALC |
| ACC | 2.77 (0.16) | 2.74 (0.14) | 2.68 (0.14)b,c | 0.20 | 0.64 | 0.43 |
| DLPFC | 2.52 (0.10) | 2.41 (0.11)a | 2.39 (0.10)b | 1.06 | 1.19 | 0.18 |
| OFC | 2.60 (0.11) | 2.52 (0.12)a | 2.47 (0.12)b | 0.70 | 1.12 | 0.43 |
| Insula | 3.07 (0.14) | 2.99 (0.15)a | 2.90 (0.14)b,c | 0.57 | 1.18 | 0.62 |
| Total BRS | 2.67 (0.09) | 2.59 (0.10)a | 2.55 (0.09)b,c | 0.87 | 1.28 | 0.43 |
| Total frontal neocortex | 2.69 (0.09) | 2.58 (0.10)a | 2.54 (0.09)b,c | 1.18 | 1.58 | 0.43 |
| Global neocortex | 2.51 (0.07) | 2.42 (0.09)a | 2.39 (0.09)b | 1.07 | 1.34 | 0.33 |
| Intracranial volume | 1.60 × 106 | 1.59 × 106 | 1.58 × 106 | 0.07 | 0.14 | 0.07 |
| (1.60 × 105) | (1.20 × 105) | (1.50 × 105) | ||||
nsALC < CON;
sALC < CON;
sALC < nsALC; all pairwise tests, P≤ 0.029.
ACC = anterior cingulate cortex; BRS = brain reward system; CON = Control; DLPFC = dorsolateral prefrontal cortex; nsALC = non-smoking alcohol-dependent participant; OFC = orbitofrontal cortex; sALC = smoking alcohol-dependent participant.
Group comparisons on regional NAA measures
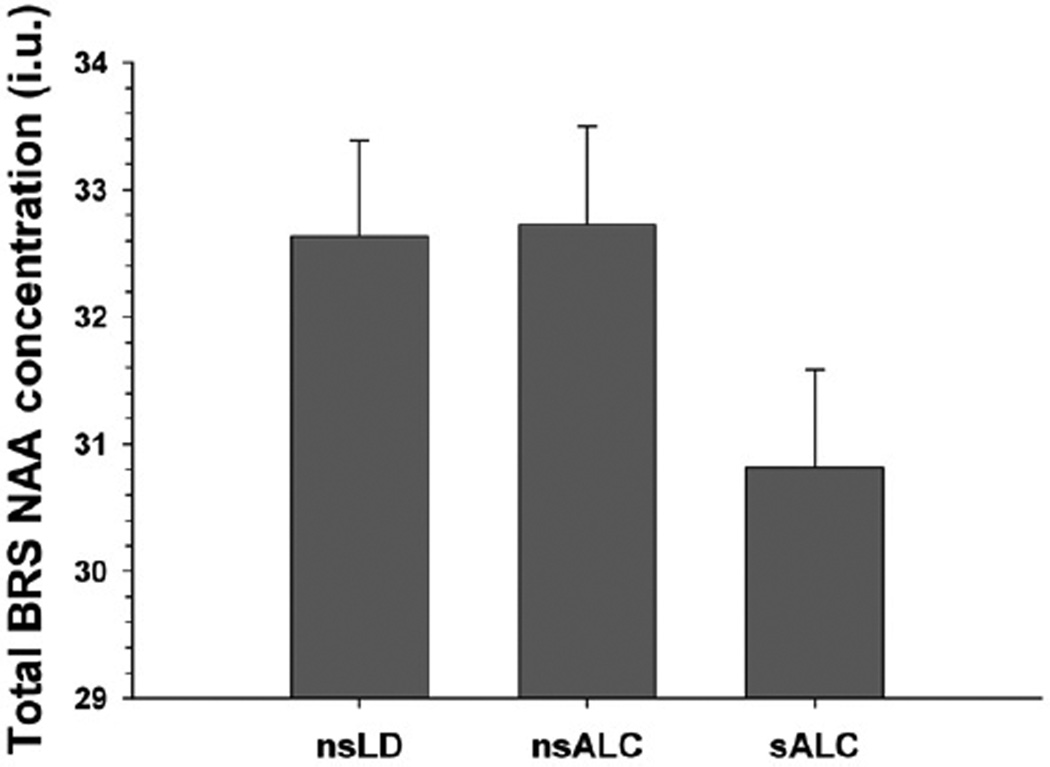
The omnibus MANCOVA indicated groups were significantly different on NAA concentrations [F (10, 160) = 2.55, P < 0.001]. Univariate tests were significant for the DLPFC, insula, vermis, SCR and total BRS, while ACC, total frontal cortex and global cortical NAA levels did not differ significantly between groups. Pairwise comparisons (see Table 3) indicated that smoking ALC had lower NAA concentrations than both non-smoking ALC and Controls in the DLPFC, insula, SCR and total BRS. Smoking ALC showed lower NAA than non-smoking ALC in the cerebellar vermis. Non-smoking ALC and Controls were not significantly different on any regional or global NAA measure. AMNART, education, alcohol consumption variables, medical, psychiatric and substance abuse co-morbidities were not significant predictors of NAA concentrations in pairwise tests for smoking and nonsmoking ALC, and these factors did not mediate the concentration differences between these groups.
Table 3.
N-acetylaspartate measures (institutional units). Mean (SD).
| Control |
nsALC |
sALC |
Effect size (Cohen’s d) |
|||
|---|---|---|---|---|---|---|
| Variable | (n = 33) | (n = 25) | (n = 36) | CON versus nsALC |
CON versus sALC |
nsALC versus sALC |
| ACC | 29.85 (3.85) | 30.23 (3.25) | 29.89 (3.90) | 0.11 | 0.01 | 0.10 |
| DLPFC | 33.39 (3.90) | 33.36 (3.74) | 31.05 (3.79)a,b | 0.01 | 0.62 | 0.61 |
| Insula | 32.23 (3.33) | 32.88 (3.53) | 30.57 (3.15)a,b | 0.19 | 0.49 | 0.68 |
| Vermis | 33.01 (5.97) | 35.77 (5.62) | 32.62 (5.92)b | 0.47 | 0.08 | 0.54 |
| SCR | 32.28 (3.04) | 31.37 (2.95) | 29.49 (3.22)a,b | 0.30 | 0.90 | 0.61 |
| Total BRS | 32.52 (3.88) | 32.72 (3.97) | 30.42 (3.95)a,b | 0.12 | 0.45 | 0.58 |
| Total frontal neocortex | 31.76 (3.91) | 31.40 (3.76) | 31.33 (3.64) | 0.09 | 0.12 | 0.02 |
| Global neocortex | 30.57 (3.51) | 30.54 (3.61) | 30.46 (3.46) | 0.01 | 0.03 | 0.02 |
sALC < CON, P ≤ 0.024;
sALC < nsALC, P ≤ 0.024.
ACC = anterior cingulate cortex; BRS = brain reward system; CON = Control; DLPFC = dorsolateral prefrontal cortex; nsALC = non-smoking alcohol-dependent participant; OFC = orbitofrontal cortex; sALC = smoking alcohol-dependent participant; SCR = superior corona radiata.
Associations of measures of alcohol and cigarette consumption with regional thickness and NAA
After controlling for age, no drinking or smoking measure was significantly related to thickness and NAA measures from any of the BRS regions or from the larger composite regions.
Associations between regional thickness and NAA
In the combined ALC group (i.e. non-smoking + smoking ALC; n = 60), the following relationships were observed between cortical thickness and NAA concentrations (partial correlations controlling for GM contribution to NAA voxels): ACC, r = 0.34, P = 0.049; insula, r = 0.35, P = 0.047; DLPFC, r = 0.15, P = 0.42; total frontal GM, r = 0.22, P = 0.35.There were no significant associations in the corresponding measures for Controls (n = 30).
DISCUSSION
The findings for cortical thickness and NAA concentration measures provide convergent and complementary information on the neurobiological consequences of chronic smoking in this treatment-seeking alcohol-dependent cohort. Both smoking and non-smoking ALC demonstrated significantly thinner cortex than Controls across all regions examined except the ACC. Overall, a stair-step pattern was apparent with smoking ALC demonstrating significantly thinner cortices than non-smoking ALC, who had thinner cortices than Controls (see Fig. 1 for general pattern). Cortical thickness differences between smoking and non-smoking ALC were observed in the ACC, insula, total BRS and total frontal cortex, but not for the global cortex. The magnitude of cortical thickness differences between smoking and nonsmoking ALC in the total BRS and total frontal lobe were identical, suggesting that the effects of smoking on cortical thickness were not specific to the BRS but generally apparent across the entire frontal GM (see Table 2). With respect to NAA concentrations, smoking ALC had lower NAA concentrations than both non-smoking ALC and Controls in the DLPFC, insula, SCR and total BRS. Controls and non-smoking ALC were not significantly different on any regional NAA level. Contrary to the pattern of results for thickness, the lower NAA concentrations in smoking ALC relative to both non-smoking ALC and Controls were significant only in components of the BRS (DLPFC, insula, vermis, SCR), but not in the total frontal or global cerebral cortex (see Fig. 2 for general pattern), suggesting BRS specific neuronal abnormalities in anterior BRS regions. AMNART, education, alcohol consumption measures, medical and psychiatric co-morbidities did not mediate the significant differences between smoking and non-smoking ALC on regional cortical thickness and NAA concentration. ROIs showed no significant left versus right hemisphere differences within groups for thickness or NAA levels.
Figure 1.
Total BRS cortical thickness. BRS =brain reward system; nsALC = non-smoking alcohol-dependent participants; sALC = smoking alcohol-dependent participants; nsLD = nonsmoking, light drinking controls
Figure 2.
Total BRS NAA concentration. BRS=brain reward system; i.u. = institutional units; NAA = N-acetylaspartate; nsALC = non-smoking alcohol-dependent participants; sALC = smoking alcohol-dependent participants; nsLD = non-smoking, light drinking controls
Chronic smoking in this alcohol-dependent cohort was associated with significantly thinner cortex primarily in frontal/BRS brain regions rather than a generalized pattern of thinning across the entire cerebral cortex. The thinner regional and global cortex demonstrated by this ALC cohort (i.e. smoking and non-smoking ALC combined) at approximately 1 week of abstinence is congruent with our recent morphometric study (Durazzo et al. 2011) and suggests cortical thickness may serve as a surrogate marker of increased risk for the development of AUD, particularly since cortical volume, surface area and thickness phenotypes may have independent genetic contributions (see Winkler et al. 2010). The moderately strong associations in the ALC cohort between cortical thickness and NAA concentrations in the ACC and insula suggest that cortical thickness measures may also reflect the integrity of tissue in these regions. In our earlier volumetric work (Gazdzinski et al. 2005b), we found no significant differences between smoking and non-smoking ALC in total frontal cortical volume. This disparity may be related to the smaller sample size in our previous volumetric study or, given that brain volumes are a function of thickness and surface area, cortical thickness measures may be more sensitive to the effects of AUD and chronic smoking. The overall pattern of the results in smoking ALC of this study is consistent with previous neuroimaging results from non-alcohol/substance-dependent cohorts that found chronic smoking-associated structural abnormalities in the anterior frontal GM and WM by voxel-based morphometry methods and standard volumetric measures (Brody et al. 2004; Gallinat et al. 2006; Kuhn, Schubert & Gallinat 2010; Zhang et al. 2011).
The NAA concentration findings for the total frontal cortex were consistent with an earlier study in a smaller cohort (Durazzo et al. 2004), where we also observed no significant differences between smoking ALC, nonsmoking ALC and Controls in cortical NAA from the entire frontal lobe. In this report, however, we extended our earlier results by describing significant NAA reductions in smoking ALC specifically in frontal BRS components (i.e. DLPFC, SCR), which are regions that are involved in inhibitory control/executive functions. The lower SCR NAA concentration in 1-week-abstinent smoking ALC in this report also parallels the findings from an earlier cohort of 1-month-abstinent ALC, in which smoking ALC showed lower NAA levels than non-smoking ALC and Controls in the SCR of the frontal WM (Wang et al. 2009). Nearly all individuals contributing to these earlier NAA studies were part of this larger study.
Although non-smoking ALC demonstrated significant reductions in regional and global cortical thickness compared with Controls, they were not different from Controls on NAA measures in any region. This is surprising, given the protracted history of hazardous alcohol consumption and co-morbid psychiatric and medical conditions, and suggests that the neuronal integrity of the corresponding tissue was not significantly compromised in non-smoking ALC. The potential ‘protective’ factors associated with the normal regional NAA levels demonstrated by non-smoking ALC likely relate to genetic or other pre-morbid and/or co-morbid environmental factors not assessed in this phase of our research. In contrast, smoking ALC showed abnormalities in cortical thickness and NAA levels specific to multiple components of the BRS. Similar to non-smoking ALC, smoking ALC did not show widespread NAA reductions in the frontal GM or across the global cortex. This suggests that the greatest neurobiological abnormalities in chronic smokers of this cohort were localized to the extended BRS regions assessed in this study.
Substance-induced plastic changes (e.g. structural, metabolic, biochemical) in the ‘bottom up’ dopaminergic mesocorticolimbic projections of the BRS originating in the ventral tegmental area are indicated to be strongly involved in acute rewarding effects of alcohol/substances and the transition from social/recreational use to mal-adaptive use/dependence (Baler & Volkow 2006; Kalivas 2008; Kalivas & O’Brien 2008; Volkow et al. 2010). Alternately, ‘late/end-stage addiction’ (Kalivas & Volkow 2005), characterized by the chronic relapse/remit cycle, is suggested to be associated with enduring, and possibly permanent, substance-induced plastic changes in the cortical and subcortical BRS regions that subserve ‘top-down’ inhibitory control/executive functions (Baler & Volkow 2006; Crews & Boettiger 2009; Gazzaley & D’Esposito 2007; Kalivas 2009; Volkow et al. 2010; Potenza et al. 2011). The treatment-seeking ALC participants of this study are clearly in late/end-stage addiction. The neocortical, paralimbic and subcortical regions of the BRS, where smoking ALC exhibited greater abnormalities than non-smoking ALC, are concerned with ‘top-down’ inhibitory control/executive functions. These BRS regions subserve complex functions such as anticipation of future consequences, decision-making, problem-solving, abstraction, set-shifting, working memory, impulse control, regulation of mood and affect, evaluation and anticipation of stimulus salience and hedonic valence (Cummings 1998; Baler& Volkow 2006; Fellows 2007; Paulus 2007; Sinha & Li 2007; Redish, Jensen & Johnson 2008; Rolls & Grabenhorst 2008; Crews & Boettiger 2009). The greater neurobiological abnormalities observed in these regions of smoking ALC may underlie the significantly inferior neurocognitive performance observed in these individuals compared with their non-smoking counterparts in early recovery (Durazzo et al. 2008, 2010a). However, the lack of associations of either cortical thickness or NAA measures with alcohol and cigarette consumption variables and other co-morbid conditions in this study may indicate that the patterns demonstrated by both non-smoking and smoking ALC were apparent prior to the onset of smoking and/or hazardous drinking (Fineberg et al. 2010; Tessner & Hill 2010). If the observed thickness abnormalities in this cohort are indeed pre-morbid, then cortical thickness abnormalities may serve as a risk factor for the development of AUD. It is also possible that the patterns evidenced by the alcohol-dependent cohorts are a function of concurrent environmental factors and other co-morbid conditions not assessed in this study (Meyerhoff& Durazzo 2008; Durazzo et al. 2011). For discussion of potential biological mechanisms associated with the greater abnormalities in regional cortical thickness and neuronal integrity in smoking ALC, see Durazzo & Meyerhoff (2007) and Durazzo et al. (2010b).
This study has limitations that may influence the genseralizability of the findings. The limited number of females in the study cohorts did not permit assessment of potential effects of sex on the outcome measures. There was not a one-to-one correspondence of participants in each of the neuroimaging modalities and of regions comprising the BRS for thickness and NAA concentration measures. Also, given the nominal resolution of our 1H MRSI sequence (approximately 1 ml), the 1H MRSI ROIs likely include a somewhat larger amount of GM tissue than cortical ROIs defined through Freesurfer anatomical parcellation.
Results from this dual modality MR study indicated that, relative to non-smoking ALC, smoking ALC demonstrated greater thinning in the frontal cortex and components of the BRS, as well as compromised neuronal integrity in the BRS. The overall pattern suggests that chronic smoking in this alcohol-dependent cohort is associated with neurobiological abnormalities in anterior brain regions that are implicated in the development and maintenance of all addictive disorders, and that smoking contributes to the significant heterogeneity observed in the scope and magnitude of neurobiological abnormalities in AUD. The current findings reinforce our previous work indicating consideration of smoking status and other prevalent co-morbid conditions in AUD is critical to fully understand how this clinical syndrome impacts brain neurobiology and function. Longitudinal research clearly is necessary to identify pre-morbid and co-morbid factors that may have influenced these findings, as well as to determine if the abnormalities demonstrated by smoking ALC at entry into treatment recover with abstinence from alcohol and smoking cessation. Cigarette smoking is a modifiable health risk that is directly associated with at least 440 000 annual deaths in the United States alone and 10 million deaths worldwide, with greater mortality among those with alcohol and substance use disorders (see Durazzo & Meyerhoff 2007 for review). A growing clinical movement offers smoking cessation programs to all smokers seeking treatment for alcohol/substance used disorders. Data from this report and our other neurocognitive and neuroimaging studies, combined with reports of high mortality associated with cigarette smoking in AUD (Hurt et al. 1996), lend strong support to this pro-wellness clinical practice.
Acknowledgements
This work was supported by the National Institutes of Health [AA10788 to D.J.M.; DA24136 to T.C.D.] and by the use of resources and facilities at the San Francisco Veterans Administration Medical Center. The authors have no disclosures or conflicts of interest to report. We thank Mary Rebecca Young, Bill Clift, and Drs. Peter Banys and Ellen Herbst of the Veterans Administration Substance Abuse Day Hospital, which routinely offers smoking cessation with substance abuse treatment, and Dr. David Pating, Karen Moise and their colleagues at the Kaiser Permanente Chemical Dependency Recovery Program in San Francisco for their valuable assistance in recruiting participants. We also wish to extend our gratitude to the study participants, who made this research possible.
Footnotes
Authors Contribution
TCD was responsible for the study concept, all statistical analyses and drafted the manuscript. TCD conducted or supervised all psychiatric diagnostic interviews. All authors contributed to MR data acquisition, processing and quality assurance under the direction of DJM. TCD and DJM were responsible for interpretation of the data. AM and SG provided critical editing of the manuscript for scientific and intellectual content. All authors thoroughly reviewed the content and approved the final version for publication.
References
- Anderson CM, Maas LC, Frederick B, Bendor JT, Spencer TJ, Livni E, Lukas SE, Fischman AJ, Madras BK, Renshaw PF, Kaufman MJ. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31:1318–1326. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- Aralasmak A, Ulmer JL, Kocak M, Salvan CV, Hillis AE, Yousem DM. Association, commissural, and projection pathways and their functional deficit reported in literature. J Comput Assist Tomogr. 2006;30:695–715. doi: 10.1097/01.rct.0000226397.43235.8b. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Guilfoyle DN. Using proton magnetic resonance imaging and spectroscopy to understand brain ‘activation’. Brain Lang. 2007;102:153–164. doi: 10.1016/j.bandl.2006.06.119. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Dorsolateral prefrontal lesions do not impair tests of scene learning and decision-making that require frontal-temporal interaction. Eur J Neurosci. 2008;28:491–499. doi: 10.1111/j.1460-9568.2008.06353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Depression Inventory. Philadelphia, PA: Center for Cognitive Therapy; 1978. [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, Phillips A, Tanaka K. Dissociable components of rule-guided behavior depend on distinct medial and pre-frontal regions. Science. 2009;325:52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YY, Shamosh NA, Cho SH, DeYoung CG, Lee MJ, Lee JM, Kim SI, Cho ZH, Kim K, Gray JR, Lee KH. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J Neurosci. 2008;28:10323–10329. doi: 10.1523/JNEUROSCI.3259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann N Y Acad Sci. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. J Psychosom Res. 1998;44:627–628. doi: 10.1016/s0022-3999(98)00034-8. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995;34:721–727. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, Blacker D, Albert MS, Killiany RJ, Fischl B. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39:10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Fryer SL, Rothlind JC, Vertinski M, Gazdzinski S, Mon A, Meyerhoff DJ. Measures of learning, memory and processing speed accurately predict smoking status in short-term abstinent treatment-seeking alcohol-dependent individuals. Alcohol Alcohol. 2010a;14:507–513. doi: 10.1093/alcalc/agq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin Exp Res. 2006;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. 2010b;7:3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J Stud Alcohol Drugs. 2010c;71:278–289. doi: 10.15288/jsad.2010.71.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Meyerhoff DJ. The relationships of sociodemographic factors, medical, psychiatric, and substance-misuse co-morbidities to neurocognition in short-term abstinent alcohol-dependent individuals. Alcohol. 2008;42:439–449. doi: 10.1016/j.alcohol.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Grattan LM, Geder L. Impact of frontal lobe lesions on rehabilitation and recovery from acute brain injury. NeuroRehabilitation. 1995;5:161–182. doi: 10.3233/NRE-1995-5206. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1991;69:763–765. [PubMed] [Google Scholar]
- Fellows LK. Advances in understanding ventromedial prefrontal function: the accountant joins the executive. Neurology. 2007;68:991–995. doi: 10.1212/01.wnl.0000257835.46290.57. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P, Version 2.0, 8/98 Revision) New York, NY: Biometrics Research Department; 1998. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatic parcellation of the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005a;78:263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Meyerhoff DJ. Body mass index is associated with brain injury in alcohol dependence - a multimodal magnetic resonance study. Alcohol Clin Exp Res. 2010a;34:2089–2096. doi: 10.1111/j.1530-0277.2010.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005b;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008;162:133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Millin R, Kaiser LG, Durazzo TC, Mueller SG, Weiner MW, Meyerhoff DJ. BMI and neuronal integrity in healthy, cognitively normal elderly: a proton magnetic resonance spectroscopy study. Obesity (Silver Spring) 2010b;18:743–748. doi: 10.1038/oby.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, D’Esposito M. Unifying prefrontal cortex function: executive control, neural networks, and top-down modulation. In: Miller BL, editor. The Human Frontal Lobes: Functions and Disorders. New York: The Guilford Press; 2007. pp. 187–206. [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. 2009;30:353–363. doi: 10.1016/j.neurobiolaging.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hugg JW, Kuzniecky RI, Gilliam FG, Morawetz RB, Faught RE, Hetherington HP. Normalization of contralateral metabolic function following temporal lobectomy demonstrated by h-1 magnetic resonance spectroscopic imaging. Ann Neurol. 1996;40:236–239. doi: 10.1002/ana.410400215. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ3rd. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Vercelli A. Dendritic bundles, minicolumns, columns, and cortical output units. Front Neuroanat. 2010;4:1–7. doi: 10.3389/neuro.05.011.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Yeo RA, Chiulli SJ, Sibbitt WL, Jr., Weers DC, Hart BL, Brooks WM. Biochemical markers of cognition: a proton MR spectroscopy study of normal human brain. Neuroreport. 1999;10:3327–3331. doi: 10.1097/00001756-199911080-00014. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, Bartzokis G, Stanley J, Royall D, Schlosser AE, Null M, Fox PT. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage. 2010;49:1190–1199. doi: 10.1016/j.neuroimage.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow N. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:1–22. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, Franz CE, Lyons MJ, Pacheco J, Perry ME, Stevens A, Schmitt JE, Grant MD, Seidman LJ, Thermenos HW, Tsuang MT, Eisen SA, Dale AM, Fennema-Notestine C. Genetic and environmental influences on the size of specific brain regions in midlife: the VETSA MRI study. Neuroimage. 2010;49:1213–1223. doi: 10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Schubert F, Gallinat J. Reduced thickness of medial orbitofrontal cortex in smokers. Biol Psychiatry. 2010;68:1061–1065. doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, Kim BW, Blood AJ, Evins AE, Seidman LJ, Iosifescu DV, Lee S, Baxter C, Perlis RH, Smoller JW, Fava M, Breiter HC. Cortical thickness abnormalities in cocaine addiction—a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron. 2008a;60:174–188. doi: 10.1016/j.neuron.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008b;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Solch C, Magyar S, Kunig G, Missimer J, Schultz W, Leenders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers. A positron emission tomography study. Exp Brain Res. 2001;139:278–286. doi: 10.1007/s002210100751. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci. 1994;6:358–370. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Lu YW, Parthasarathy S, Moore C, Weisner CM. Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: comparison with matched controls. Arch Intern Med. 2003;163:2511–2517. doi: 10.1001/archinte.163.20.2511. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol Clin Exp Res. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- Meyerhoff D, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner H. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Durazzo TC. Proton magnetic resonance spectroscopy in alcohol use disorders: a potential new endophenotype? Alcohol Clin Exp Res. 2008;32:1146–1158. doi: 10.1111/j.1530-0277.2008.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Paris C, Hagenbuch F, Ebert D, Juengling FD. Brain activation during craving for alcohol measured by positron emission tomography. Aust N Z J Psychiatry. 2006;40:171–178. doi: 10.1080/j.1440-1614.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh RS, Klag MJ. Alcohol: role in the development of hypertension and end-stage renal disease. Curr Opin Nephrol Hypertens. 2001;10:385–390. doi: 10.1097/00041552-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Paul R, Grieve SM, Chaudary B, Gordon N, Lawrence J, Cooper N, Clark CR, Kukla M, Mulligan R, Gordon E. Relative contributions of the cerebellar vermis and prefrontal lobe volumes on cognitive function across the adult lifespan. Neurobiol Aging. 2007;30:457–465. doi: 10.1016/j.neurobiolaging.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Neural basis of reward and craving—a homeostatic point of view. Dialogues Clin Neurosci. 2007;9:379–387. doi: 10.31887/DCNS.2007.9.4/mpaulus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci. 2008;31:415–437. doi: 10.1017/S0140525X0800472X. discussion 437–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Room R. Smoking and drinking as complementary behaviours. Biomed Pharmacother. 2004;58:111–115. doi: 10.1016/j.biopha.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Grant I. The neurobehavior correlates of alcoholism. In: Litten R, Allen J, editors. Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. New York, NY: Oxford University Press; 2009. pp. 398–454. [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Cyr JA. Frontal-striatal circuit functions: context, sequence, and consequence. J Int Neuropsychol Soc. 2003;9:103–127. doi: 10.1017/s1355617703910125. [DOI] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schottenbauer MA, Momenan R, Kerick M, Hommer DW. Relationships among aging, IQ, and intracranial volume in alcoholics and control subjects. Neuropsychology. 2007;21:337–345. doi: 10.1037/0894-4105.21.3.337. [DOI] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Meyerhoff DJ, Mueller S, Chao L, Sacrey DT, Laxer K, Weiner MW. N-acetylaspartate as a marker of neuronal injury in neurodegenerative disease. Adv Exp Med Biol. 2006;576:241–262. doi: 10.1007/0-387-30172-0_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Totwa, NJ: The Humana Press Inc; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40:822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Self-Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologist Press; 1977. [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sullivan E. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. NIAAA Research Monograph No. 34: human brain vulnerability to alcoholism—evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2000. pp. 473–508. [Google Scholar]
- Sullivan EV, Harding AJ, Pentney RJ, Dlugos CA, Martin PR, Parks MH, Desmond JE, Chen SHA, Pryor MR, De Rosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychol Rev. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vion-Dury J, Meyerhoff DJ, Cozzone PJ, Weiner MW. What might be the impact on neurology of the analysis of brain metabolism by in vivo magnetic resonance spectroscopy? [Editorial] J Neurol. 1994;241:354–371. doi: 10.1007/BF02033352. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. PNAS. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, Makris N, Salat D, Reinvang I. Regional cortical thickness matters in recall after months more than minutes. Neuroimage. 2006;31:1343–1351. doi: 10.1016/j.neuroimage.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Durazzo TC, Gazdzinski S, Yeh PH, Mon A, Meyerhoff DJ. MRSI and DTI: a multimodal approach for improved detection of white matter abnormalities in alcohol and nicotine dependence. NMR Biomed. 2009;22:516–522. doi: 10.1002/nbm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedermann D, Schuff N, Matson GB, Soher BJ, Du AT, Maudsley AA, Weiner MW. Short echo time multislice proton magnetic resonance spectroscopic imaging in human brain: metabolite distributions and reliability. Magn Reson Imaging. 2001;19:1073–1080. doi: 10.1016/s0730-725x(01)00441-6. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]