Abstract
Background
Vascular endothelial growth factor-121 (VEGF121), an angiogenic protein secreted in response to hypoxic stress, binds to VEGF receptors (VEGFRs) overexpressed on vessels of ischemic tissue. The purpose of this study was to evaluate 64Cu-VEGF121 positron emission tomography for noninvasive spatial, temporal, and quantitative monitoring of VEGFR2 expression in a murine model of hindlimb ischemia with and without treadmill exercise training.
Methods and Results
64Cu-labeled VEGF121 and a VEGF mutant were tested for VEGFR2 binding specificity in cell culture. Mice (n=58) underwent unilateral ligation of the femoral artery, and postoperative tissue ischemia was assessed with laser Doppler imaging. Longitudinal VEGFR2 expression in exercised and nonexercised mice was quantified with 64Cu-VEGF121 positron emission tomography at postoperative day 8, 15, 22, and 29 and correlated with postmortem γ-counting. Hindlimbs were excised for immunohistochemistry, Western blotting, and microvessel density measurements. Compared with the VEGF mutant, VEGF121 showed specific binding to VEGFR2. Perfusion in ischemic hindlimbs fell to 9% of contralateral hindlimb on postoperative day 1 and recovered to 82% on day 29. 64Cu-VEGF121 uptake in ischemic hindlimbs increased significantly (P<0.001) from a control level of 0.61 ±0.17% ID/g (percentage of injected dose per gram) to 1.62±0.35% ID/g at postoperative day 8, gradually decreased over the following 3 weeks (0.59±0.14% ID/g at day 29), and correlated with γ-counting (R2=0.99). Compared with nonexercised mice, 64Cu-VEGF121 uptake was increased significantly (P≤0.0001) in exercised mice (at day 15, 22, and 29) and correlated with VEGFR2 levels as obtained by Western blotting (R2=0.76). Ischemic hindlimb tissue stained positively for VEGFR2. In exercised mice, microvessel density was increased significantly (P<0.001) compared with nonexercised mice.
Conclusions
64Cu-VEGF121 positron emission tomography allows longitudinal spatial and quantitative monitoring of VEGFR2 expression in murine hindlimb ischemia and indirectly visualizes enhanced angiogenesis stimulated by treadmill exercise training.
Keywords: imaging, arteriosclerosis, exercise, angiogenesis, tomography, peripheral vascular disease, growth substances
As the Western world population continues to age, the prevalence of peripheral arterial disease (PAD), which affects ≈12% of adults, is increasing.1 PAD, a manifestation of systemic atherosclerosis, is caused by atherosclerotic stenosis and/or occlusion of lower-extremity arteries, with consecutive muscle tissue ischemia at stress or rest, and it results in 2 major clinical symptoms: intermittent claudication and critical limb ischemia.2 The natural response to muscle tissue ischemia includes the mobilization of circulating cellular elements and the upregulation of angiogenic growth factors with the corresponding binding ligands that together enable development of collateral vasculature.3–5 Novel therapeutic strategies for patients refractory to conventional treatments of PAD (such as exercise training, drug therapy, bypass grafting, or percutaneous interventions) aim to stimulate or augment these physiological adaptive processes by application of angiogenic cytokines and/or gene and cell therapy protocols.2,4 Although such therapeutic strategies have shown encouraging results, with increased vascular collateral growth and improved clinical symptoms in preclinical studies and early nonrandomized clinical trials, they have failed or have provided unsatisfactory responses in randomized placebo–controlled clinical trials.6,7 These conflicting results may be due to the absence of objective and quantitative measures of the biological response to ischemia to select for patients who would benefit most from a given specific therapeutic approach.8
Molecular imaging is a rapidly expanding field that attempts to noninvasively visualize, characterize, and quantify biological processes at the cellular and subcellular level in living subjects.9 Direct targeted molecular imaging of specific molecular markers of angiogenesis, such as vascular endothelial growth factor (VEGF) and its receptors (VEGFRs), could provide an objective and quantitative measure for individualized monitoring of PAD therapy. VEGFR2 is one of the major regulators of angiogenesis, and activation of the VEGF/VEGFR2 axis triggers multiple downstream signaling networks that result in increased angiogenesis.10
In the present study, we hypothesized that positron emission tomography (PET) imaging would enable noninvasive in vivo spatial, temporal, and quantitative monitoring of angiogenesis with the use of a radiolabeled protein targeted at VEGFR2. To test this hypothesis, we longitudinally monitored VEGFR2 expression levels by 64Cu-VEGF121 PET in murine ischemic hindlimbs over a 4-week period and quantified the effects of proangiogenic treadmill exercise training on both radiotracer uptake and microvessel density (MVD).
Methods
In Vitro Assays and Cell Culture Experiments
Synthesis of VEGF121 and VEGFMutant
The gene for VEGF121 was amplified from mRNA extracted from human umbilical vascular endothelial cells via polymerase chain reaction and inserted into a pRSF-Duet1 prokaryotic expression vector (Novagen, San Diego, Calif). A VEGF mutant (D63A/E64A/ E67A/R82N/I83L/K84S) with decreased affinity for VEGFR2 and, to a lesser extent, for VEGFR1 (henceforth referred to as VEGFMutant) was obtained by 2 rounds of polymerase chain reaction with 2 pairs of overlapping primers and 2 flanking primers and was cloned into a pRSF-Duet1 vector. For protein expression and purification, the plasmids were transformed into BL21 (DE3) competent cells.
Radiolabeling of VEGF121 and VEGFMutant
Copper-64 (64Cu; t1/2= 12.7 hours) was obtained from the University of Wisconsin, Madison, and DOTA (1,4,7,10-tetraazadodecane-N,N′ ,N″,N‴-tetraacetic acid) was purchased from Macrocyclics, Inc (Dallas, Tex). VEGF121 and VEGFMutant were radiolabeled as described previously.11 For 64Cu-DOTA-VEGF121, the radiolabeling yield was 69.7±6.3%, the number of DOTA molecules was 2.2±0.1, and the specific activity was 116.2± 10.5 mCi/mg, with a radiochemical purity >98% in the present study. For 64Cu-DOTA-VEGFMutant, the radiolabeling yield was 53.9±9.1%, the number of DOTA molecules was 0.9±0.2, and the specific activity was 89.8±15.2 mCi/mg, with radiochemical purity >98%.
Binding Assay of VEGF121 and VEGFMutant
Porcine aortic endothelial (PAE-KDR) cells stably transfected to express human VEGFR2 (KDR) and porcine aortic endothelial control cells (PAEs) not expressing VEGFR1 and VEGFR2 were cultured in Ham’s F-12 medium containing 10% fetal bovine serum (Sigma-Aldrich, St Louis, Mo). With 125I-VEGF165 as the radioligand, the receptor-binding affinity of VEGF121, DOTA-VEGF121, VEGFMutant, and DOTA-VEGFMutant for VEGFR2 was determined as described elsewhere for both PAE-KDR and PAE cells.12 To analyze the binding affinity of VEGF121, DOTA-VEGF121 VEGFMutant, and DOTA-VEGFMutant to VEGFR1, soluble VEGFR1D1–D6 (sVEGFR1; Research Diagnostics, Inc, Concord, Mass) was diluted with coating buffer (15 mmol/L NaCO3, 35 mmol/L NaHCO3, pH 9.6) and coated onto 96-well plates (NUNC, Rochester, NY). Serially diluted VEGF121, DOTA-VEGF121 VEGFMutant, or DOTA-VEGFMutant was then added to compete with the radioligand 125I-VEGF165. The best-fit 50% inhibitory concentration (IC50) values were calculated by fitting the data by nonlinear regression by use of GraphPad Prism 4.0 software (GraphPad Software, Inc, San Diego, Calif). Experiments were performed in triplicate.
Animals and Treadmill Exercise Protocol
Mouse Model of Hindlimb Ischemia
Animal protocols were approved by the Stanford Institutional Administrative Panel on Laboratory Animal Care. Unilateral hindlimb ischemia in the left leg was introduced in 4-month-old male C57BL/6J mice (n=58, Jackson Laboratories, Bar Harbor, Me) by ligation of the left femoral artery proximal and distal to the caudal femoral artery. The arterial segment between the ligatures was excised. A sham procedure was performed on the contralateral leg in 10 mice.
Laser Doppler Hindlimb Tissue Perfusion Measurements
One day before surgery and on days 1, 8, 15, 22, and 29 after surgery, in vivo hindlimb tissue perfusion imaging of the mice was performed with a laser Doppler imaging system (Periscan PIM 3; Perimed AB, Järfälla, Sweden). Three perfusion images were obtained in each mouse, with the temperature of the mice kept constant at 37±0.5°C. Average hindlimb perfusion was expressed as the ratio of ischemic to nonischemic hindlimb by drawing regions of interests over both hindlimbs.
In Vivo Assessment of Hindlimb Function and Ischemic Damage
One day before surgery and on days 1, 8, 15, 22, and 29 after surgery, hindlimb function of all mice was assessed clinically and graded by use of a 4-point grading scale (ambulatory impairment score) as described elsewhere, where 4 indicates dragging of foot; 3, no dragging of foot but no plantar flexion; 2, plantar flexion but no flexing of toes; and 1, normal function, with flexing of toes to resist gentle traction on the tail.13 In addition, ischemic damage of hindlimbs was evaluated clinically and graded with a 5-point grading scale (tissue damage score) as follows: 5, any amputation; 4, tissue necrosis; 3, severe discoloration; 2, mild discoloration; and 1, no difference compared with nonischemic contralateral hindlimb.13
Treadmill Exercise Training
A subgroup of 20 mice were exercised on a rodent treadmill (Exer-3–6; Columbus Instruments, Columbus, Ohio). The treadmill exercise training began 3 days after surgery and was performed 5 times a week. Each training session started with a speed of 9 m/min for 3 minutes and was then increased by 3 m/min every 3 minutes until a maximum speed of 18 m/min was reached. The training was performed until the mice were unable to keep pace (mean training time per day 35 minutes; range 30 to 40 minutes). Five mice were trained for 1 week, 5 for 2 weeks, 5 for 3 weeks, and 5 for 4 weeks.
Small-Animal Imaging Experiments
PET Imaging
In all animals, imaging was performed on a Concorde R4 microPET system (Siemens AG, Malvern, Pa) with the animals maintained under 2% isoflurane anesthesia. One, 4, and 20 hours after intravenous injection of 64Cu-VEGF121 (mean 238 µCi; range 217 to 277 µCi) via the tail vein, a 5-minute static scan with an approximate resolution of 2 mm in each axial direction was obtained in all animals. Images were reconstructed with the OSEM (ordered-subsets expectation maximization) algorithm.
64C>u-VEGFMutant was administered in an additional 3 nonexercised mice (day 8 after surgery), and PET scanning was performed as described above. In vivo blocking studies were performed in an additional 3 nonexercised mice (day 8 after surgery). First, 250 µg of rat anti-mouse VEGFR2 monoclonal antibody (Avas 12a1; eBio-science, San Diego, Calif) was injected via tail vein in each of these 3 mice. Sixty minutes after injection of the monoclonal antibodies (to allow for distribution of the antibodies in the tissue), 64Cu-VEGF121 was injected through the tail vein, and PET imaging was performed as described above.
Image Analysis of PET Images
PET images were analyzed offline in random order with nonproprietary PET analysis software (AMIDE version 0.8.2; http://amide.sourceforge.net).14 Regions of interest encompassing both the ischemic and nonischemic muscles of both hindlimbs were drawn. Percentage of injected dose per gram (% ID/g) was calculated. No corrections for partial volume or attenuation were performed.
Postmortem Analysis
γ-Well Counting
After PET imaging, the skeletal muscles of the ischemic and nonischemic hindlimbs were excised and weighed in 3 mice. Tissue radioactivity was measured with a γ-counter (Cobra II Auto-Gamma; PerkinElmer, Wellesley, Mass) and was corrected for background, decay time, and tissue weight.
Biodistribution Studies
At day 8 after surgery, an additional 12 mice were injected with 19.2 µCi (range 15.8 to 22.9 µCi) of 64Cu-VEGF121 through the tail vein, euthanized, and dissected at 1 hour (n=4), 4 hours (n=4), and 20 hours (n=4) after injection. Blood, hindlimb muscle tissue, and major organs and tissues were collected and weighed wet, and the radioactivity in the tissues was measured with a γ-counter.
Immunohistochemistry
Frozen tissue slices (5 µm) of the muscle tissue were incubated with rat anti-mouse VEGFR2 antibody (DC101; ImClone Systems, Inc, New York, NY) and visualized with FITC-conjugated donkey anti-rat secondary antibody (1:200; Jackson ImmunoResearch Laboratories, Inc, West Grove, Pa). For VEGFR1 staining, the slices were incubated with rabbit anti-mouse VEGFR1 antibody and visualized with Cy3-conjugated donkey anti-rabbit secondary antibody (1:50, Laboratory Vision, Fremont, Calif). Slices were also stained for CD31 to localize VEGFR2 expression to muscle vessels and for MVD analysis. For this purpose, the slices were incubated with rat anti-mouse CD31 antibody (1:100; BD Biosciences, San Jose, Calif) and visualized with FITC-conjugated donkey anti-rat secondary antibody (1:200; Jackson ImmunoResearch Laboratories). To identify the nuclei, all slices were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich).
On CD31-stained slices, 7 random, nonoverlapping views of the muscle tissue slices of both exercised and nonexercised mice were selected for MVD analysis. The number of vessels counted was divided by the field of view to yield the MVD, expressed as vessels/mm2.
Western Blotting
Muscle tissue protein was extracted with a tissue protein extraction buffer (T-PER; Pierce Biotechnology, Inc, Rockford, Ill). Forty micrograms of protein from each muscle sample was separated by running the sample on a NuPAGE Bis-Tris gel (Invitrogen, Carlsbad, Calif). The protein was transferred to a nitrocellulose membrane (Invitrogen), and the membrane was then incubated with a rabbit anti-mouse VEGFR2 primary antibody (1:1000; Upstate USA Inc, Charlottesville, Va) and with a horseradish peroxidase–conjugated anti-rabbit secondary antibody (1:5000; GE Healthcare, Piscataway, NJ). As an internal loading control, the same membrane was incubated with anti-α-tubulin antibody. Relative VEGFR2 expression levels were quantified on Western blots with ImageJ software (version 1.32; National Institutes of Health, Bethesda, Md) after densitometric scanning of the exposed films.
Statistical Analysis
Data are given as mean±SD. The 2-tailed paired and unpaired Student t tests were used to test differences within animals (ischemic versus nonischemic contralateral or sham-operated contralateral hindlimb of mice) and between animals (VEGFMutant versus wild-type VEGF121; in vivo blocking versus nonblocking), respectively. To test the effect of treadmill exercise training, a 2×4 between-subjects ANOVA was used, with fixed effects of training (present or not) and time (8, 15, 22, or 29 days). Correlations between PET values and both γ-counting and Western blotting results were expressed with the Pearson correlation coefficient. Differences were considered significant at a probability value <0.05.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Binding Assays Demonstrate Specific Binding Affinity of VEGF121 and Reduced Binding of VEGFMutant to VEGFR2
Using VEGFR2-expressing cells (PAE-KDR), the IC50 value for VEGFMutant (10 µmol/L) was 3400-fold higher than the IC50 value for VEGF121 (2.9 nmol/L), which confirms a reduced binding affinity of VEGFMutant to VEGFR2 (Figure 1). Binding affinity of VEGFMutant to VEGFR1 was reduced to a lesser extent (IC50 value of 12 nmol/L for VEGFMutant compared with an IC50 value of 4 nmol/L for VEGF121; Figure 1). After DOTA labeling, the IC50 values for binding of DOTA-VEGF121 to VEGFR2 (3.3 nmol/L) and VEGFR1 (4.2 nmol/ L), respectively, were minimally reduced. IC50 values for binding of DOTA-VEGFMutant to VEGFR2 (21 µmol/L) and VEGFR1 (28 nmol/L), respectively, were slightly reduced compared with free VEGFMutant. No significant binding was noted of VEGF121, DOTA-VEGF121 VEGFMutant, and DOTA-VEGFMutant to negative control cells (PAEs).
Figure 1.
Graphs demonstrate binding assays of VEGF121 and VEGFMutant binding to VEGFR2 (top) and VEGFR1 (bottom). VEGFMutant has decreased affinity to VEGFR2 (IC50=10 /µmol/L) compared with wild-type VEGF121 (IC50=2.9 nmol/L) and has decreased affinity, although to a lesser extent, to VEGFR1 (IC50=12 nmol/L). Measurements were done in triplicate.
Laser Doppler Imaging and Clinical Assessment Confirm Ischemia After Femoral Artery Ligation and Hindlimb Blood Flow Recovery Over 4 Weeks
To confirm successful induction of tissue ischemia after femoral artery ligation, laser Doppler imaging was performed, and mice were evaluated clinically by use of the ambulatory impairment score and the tissue damage score. Average hindlimb tissue perfusion ratio decreased from 1.0±0.04 before surgery to 0.09±0.02 at day 1 after surgery, gradually recovered over the following 4 weeks, and eventually reached 0.82±0.13 at day 29 after surgery. On serial clinical assessment, a reduced functional use of the ischemic hindlimb was noted at day 1 after surgery (mean ambulatory impairment score 1.6±0.5). Functional use of hindlimb improved by day 8 and recovered to normal levels by days 22 and 29, respectively (Table). The mean clinical tissue damage score increased from 1.0 before surgery to 2.6±0.5 at day 1 and decreased to near-normal levels at day 29 after surgery (1.2±0.4; Table). Clinical ambulatory impairment and tissue damage scores were not significantly different (P>0.35) in exercised versus nonexercised mice at any time point after surgery.
Table 1.
Serial Clinical Evaluation of Ischemic Hindlimbs by Use of the Ambulatory Impairment Score and the Tissue Damage Score at Day 0 (1 Day Before Surgery) and at Days 1, 8, 15, 22, and 29 After Femoral Artery Ligation (5 Mice at Each Time Point)
| Mouse | Day 0 |
Day 1 |
Day 8 |
Day 15 |
Day 22 |
Day 29 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Training | Training | No Training | Training | No Training | Training | No Training | Training | No Training | Training | No Training | Training | |
| Ambulatory impairment score |
||||||||||||
| 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Tissue damage score |
||||||||||||
| 1 | 1 | 1 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| 2 | 1 | 1 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 |
| 3 | 1 | 1 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 1 | 1 | 1 |
| 4 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
| 5 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 |
PET Imaging Shows Significantly Higher 64Cu-VEGF121 Uptake in Ischemic Hindlimbs Than in Nonischemic Hindlimbs
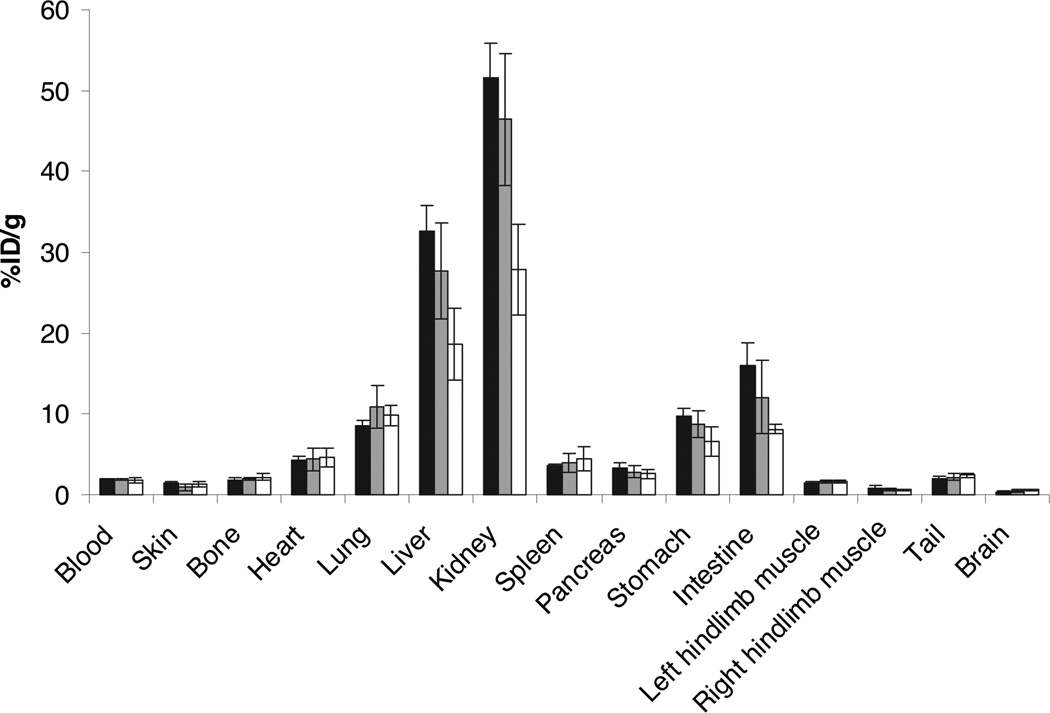
64Cu-VEGF121 uptake in ischemic hindlimbs was significantly higher (P<0.001) than in contralateral nonischemic hindlimbs on PET imaging at days 8, 15, and 22 after surgery (Figure 2). In ischemic hindlimbs, VEGF121 uptake was highest at day 8 after surgery (mean 1.62% ID/g; range, 1.23% ID/g to 2.4% ID/g) and decreased thereafter. Twenty-nine days after femoral artery ligation, VEGF121 uptake in ischemic hindlimbs (mean 0.59% ID/g; range, 0.46% ID/g to 0.76% ID/g) was not significantly different (P=0.1) from radiotracer uptake in the contralateral nonischemic hindlimbs (mean 0.61% ID/g; range 0.40% ID/g to 0.64% ID/g). In vivo 64Cu-VEGF121 uptake correlated well with ex vivo 64Cu γ-well counting of the excised muscle tissues (R2=0.99, P=0.02). No statistically significant difference (P>0.33) existed in radiotracer uptake in ischemic hindlimbs when scans were performed 1 hour, 4 hours, and 20 hours after radiotracer administration. Ex vivo biodistribution of 64Cu-VEGF121 in blood, major organs, and tissues at 1 hour, 4 hours, and 20 hours after tracer injection is summarized in Figure 3.
Figure 2.
Serial 64Cu-VEGF121 uptake in 4 male C57BL/6J mice (supine position) at day 8 (a), day 15 (b), day 22 (c), and day 29 (d) after left femoral arterial ligation. On representative coronal thick-slab maximum-intensity projections of microPET data obtained at 1 hour after intravenous injection of radiotracer, a progressive decrease in 64Cu-VEGF121 uptake (expressed as mean values of % ID/g) in the left ischemic hindlimb (arrow) over successive postoperative time points is demonstrated. Right contralateral nonischemic hindlimbs were used as negative control and show low background 64Cu-VEGF121 uptake. Note radiotracer uptake in liver (L) and left kidney (K; right kidney cannot be differentiated from liver on coronal projections).
Figure 3.
Ex vivo biodistribution of 64Cu-VEGF121 in male C57BL/6J mice (8 days after left femoral arterial ligation) at 1 hour (n=4; black bar), 4 hours (n=4; gray bar), and 20 hours (n=4; white bar) after injection. 64Cu-VEGF121 was highest in liver and kidney. Tracer levels in blood were not significantly different at the 3 different time points after injection (levels before 1 hour [and, thus, the half-life of 64Cu-VEGF121] were not measured). Uptake of 64Cu-VEGF121 was significantly higher in ischemic (left) than in nonischemic (right) hindlimb muscle tissue. Columns indicate means; bars, ±SD.
64Cu-VEGF121 Uptake in Ischemic Hindlimbs Is Specific Through Binding to VEGFR2
To test whether radiotracer uptake was due to specific VEGF121 binding to VEGFR2 in ischemic muscle tissue or nonspecific distribution of radiotracer in the tissue interstitium after surgery, sham-operated hindlimbs in 10 mice were also scanned at day 8 after surgery. In sham-operated hindlimbs, radiotracer uptake (mean 0.67% ID/g; range 0.35% ID/g to 0.71% ID/g) was not significantly different (P=0.52) from nonischemic hindlimbs (mean 0.61% ID/g; range 0.40% ID/g to 0.64% ID/g). To further exclude the possibility of nonspecific retention of VEGF121 in ischemic hindlimbs, PET scanning with 64Cu-VEGFMutant was performed at day 8 after surgery. After administration of 64Cu-VEGFMutant, mean radiotracer uptake was significantly lower (mean 0.88% ID/g; range 0.75% ID/g to 1.07% ID/g) than after administration of 64Cu-VEGF121 (P=0.01). Radiotracer uptake was also significantly lower (P=0.03) after preadministration of anti-VEGF2 antibodies (mean 0.79% ID/g; range 0.72% ID/g to 0.86% ID/g).
Treadmill Exercise Training Increases 64Cu-VEGF121 Uptake in Ischemic Hindlimbs
At all time points after surgery, average 64Cu-VEGF121 uptake in ischemic hindlimbs was higher in exercised versus nonexercised ischemic hindlimbs. The ANOVA found significant main effects of treadmill exercise training (exercised mice had higher uptake values than nonexercised mice, P<0.0001) and time point after surgery (later time points had lower uptake values than earlier time points after surgery, P<0.001; Figure 4).
Figure 4.
Uptake values of serial 64Cu-VEGF121 PET imaging of mice with and without treadmill exercise training at days 8, 15, 22, and 29 after surgery. 64Cu-VEGF121 uptake (expressed as % ID/g) was highest at day 8 after surgery and gradually decreased over the following 3 weeks. 64Cu-VEGF121 uptake was higher in ischemic hindlimbs of exercised mice than in nonexercised mice. 64Cu-VEGF121 uptake was not increased significantly in contralateral nonischemic hindlimbs of exercised mice. Columns indicate means; bars, ±SD.
Immunohistochemistry Shows Higher Amounts of VEGFR2 in Ischemic Hindlimbs Than in Nonischemic Hindlimbs
Immunohistochemical staining for VEGFR2 in ischemic muscle tissue was strong, and staining in nonischemic control muscle tissue was almost nonexistent at day 8 after surgery (Figure 5A). At day 29 after surgery, VEGFR2 expression levels were reduced but still higher than in control muscle tissue. Costaining of VEGFR2 and CD31 was unsuccessful; however, visual examination of CD31 and VEGFR2 staining of slices of the same hindlimb muscle tissue suggested colocalization of VEGFR2 and CD31 on muscle vessels (Figure 5B). Low VEGFR1 expression levels were present in both ischemic and nonischemic control muscle tissues (Figure 5A).
Figure 5.
A, Micrographs (magnification ×200) show representative immunohistochemical staining (VEGFR1, red; VEGFR2, green) of frozen hindlimb muscle tissue slices at day 8 and day 29 after surgery. VEGFR2 was overexpressed in ischemic hind-limb muscle tissue compared with contralateral control nonischemic hindlimb muscle tissue, whereas VEGFR1 staining of ischemic hindlimb muscle tissue was not different from contralateral control hindlimb muscle tissue. B, CD31 staining (green) of ischemic hindlimb muscle tissue (at day 8 after surgery) suggests colocalization of CD31 and VEGFR2 on endothelial cells of muscle vessels. C, Representative CD31 staining of hindlimb muscle tissue at day 8 and day 29 for MVD analysis. CD31 staining was higher in hindlimb muscle tissue of exercised (+Training) than nonexercised mice at both time points. All slices were counterstained with DAPI to identify the nuclei.
MVD Is Increased in Ischemic Hindlimb Muscle Tissue of Exercised Compared With Nonexercised Mice
In ischemic muscle tissue, MVD increased after treadmill training (Figure 5C). At days 8 and 29, respectively, MVD was 179.4±26.2 and 127.8±12.0 vessels/mm2 in nonexercised mice and increased significantly (P<0.001) to 245.7±25.4 and 178.1±18.3 vessels/mm2 in exercised mice. In nonischemic control muscle tissue, MVD was 131.3±21.1 vessels/mm2 in nonexercised mice and did not increase significantly (P = 0.7) in exercised mice (136.4±19.1 vessels/mm2).
Western Blotting Shows Higher VEGFR2 Protein Expression in Exercised Than in Nonexercised Mice
The amount of VEGFR2 protein was higher in ischemic than in nonischemic muscle tissue and was increased after treadmill exercise training at all time points after surgery (Figure 6). A positive correlation existed (R2=0.76, P<0.001) between relative VEGFR2 expression levels as measured by Western blotting and 64Cu-VEGF121 uptake as assessed by PET imaging at different time points after surgery.
Figure 6.
Representative Western blot analysis of VEGFR2 protein expression in ischemic and nonischemic (control) hindlimb muscle tissue of exercised (+Training) and nonexercised mice at days 8, 15, 22, and 29 after femoral artery ligation. Expression of α-tubulin was used as a loading control.
Discussion
In this study, we demonstrated that expression levels of VEGFR2 can be monitored in murine hindlimb ischemia by PET imaging with 64Cu-labeled VEGF121. In addition, we showed that the effects of treadmill exercise training on spatial and temporal VEGFR2 expression levels can be quantified longitudinally by 64Cu-VEGF121 PET imaging. Furthermore, increased VEGFR2 expression levels indicate the presence of angiogenesis, which can be monitored by 64Cu-VEGF121 PET after treadmill exercise training.
We first created a mutant VEGF protein (VEGFMutant) and showed in cell culture that the mean affinity of VEGFMutant for cells expressing VEGFR2 (PAE-KDR) was 3400-fold lower than with wild-type VEGF121. Cells that lack expression of VEGFR2 (PAE cells) served as control for cell culture experiments and showed no binding affinity to either VEGF121 or VEGFMutant. After DOTA labeling, only a minimal difference was noted in VEGFR2 binding affinity compared with free VEGF121, which indicates that the lysine residues or N-terminal amine groups used for DOTA conjugation may not be located at the VEGFR2 binding domain of VEGF121. Taken together, these in vitro results suggest binding specificity between recombinant human VEGF121 and its ligand, VEGFR2 (KDR). Murine VEGFR2 (flk-1) shows ≈85% homology with human VEGFR2 (KDR) and plays a role in mouse physiology analogous to the role of KDR in humans.15,16 In fact, VEGFR2 is very often referred to as KDR/flk-1, which reflects the close analogy between the 2 VEGFR2 homologs.3,10 Therefore, we reasoned that human 64Cu-VEGF121 could be used to noninvasively and quantitatively image VEGFR2 expression levels in vivo in a murine hindlimb model of ischemia in the present study.
Among several growth factor receptor pathways, VEGF and its different isoforms and receptors have been identified as the major regulators of angiogenesis. VEGF is secreted in response to ischemic or hypoxic conditions, and levels of VEGF protein are significantly elevated in skeletal muscles that are ischemic as a result of arterial occlusion.10,17 VEGF121 is a non–heparin-binding VEGF isoform that contains the full biological and receptor-binding activity of VEGF.18 VEGFR2 is an endothelium-specific receptor tyrosine kinase that is known to play a pivotal role in angiogenesis. Activation of the VEGFR2 tyrosine cascade mediates the majority of downstream effects of VEGF in angiogenesis, including microvascular permeability, endothelial cell proliferation, migration, and survival.19 After exercise training or electrical stimulation of muscle tissue, the unique role of VEGFR2 in enhancing capillary growth has been further underlined in several studies. After stimulation of muscle activity in rats, increased protein levels of both VEGF and VEGFR2 were seen, whereas expression levels of VEGFR1 protein were decreased substantially.20,21 In addition, Milkiewicz et al21 showed that increased MVD, a measure of angiogenesis, was only present at increased protein levels of VEGFR2 after stimulated muscle activity. The results of the present study confirm these findings. In the present study, VEGFR2 expression was substantially upregulated in ischemic hindlimb tissue and further increased after treadmill training, as assessed by both immunohistochemistry and Western blotting. In contrast, VEGFR1 expression was not increased in ischemic hindlimb tissue of either nonexercised or exercised mice in the present study. In addition, MVD was substantially elevated in exercised compared with nonexercised mice. Therefore, 64Cu-VEGF121 uptake in ischemic hindlimb tissue in the present study primarily reflects binding of VEGF121 to VEGFR2 and not to VEGFR1; 64Cu-VEGF121 uptake also indicates the presence of angiogenesis in hindlimb ischemia. The specificity of 64Cu-VEGF121 PET to monitor VEGFR2 but not VEGFR1 expression levels is also corroborated by the observation that VEGFMutant, which had substantial binding affinity to VEGFR1 in cell culture experiments, did not accumulate substantially in ischemic hindlimb muscle tissue in vivo.
Several studies using hindlimb ischemia models have addressed the potential of radionuclide imaging approaches for visualization and quantification of molecular markers of angiogenesis in ischemia.22–24 Upregulation of αvβ3-integrin 3 days after creation of hindlimb ischemia in mice has been demonstrated by scintigraphic imaging of radioiodine-labeled RGD [123I-c(RGD(I)yV)].23 In another study, expression levels of αvβ3-integrin were obtained over a 2-week period after induction of hindlimb ischemia in mice by use of a γ-camera and a 99mTc-labeled chelate-peptide conjugate containing an RGD motif.22 Experience with radionuclide imaging of VEGFR2 expression, however, remains very limited. Lu et al24 used 111In-labeled recombinant human VEGF121 and a γ-camera for visualization of VEGFR2 expression over a 2-day period in a rabbit model of hindlimb ischemia. A subtle increase in scintigraphic image counts in the ischemic hindlimb (mean 370 cpm) could be detected compared with nonischemic control (mean 280 cpm) and sham-operated (mean 310 cpm) hindlimbs in that study.24 We significantly add to that study,24 first, by tracking expression levels of VEGFR2 over a 4-week period rather than a 2-day period only, and second, by investigating angiogenesis by PET rather than γ-camera imaging. PET is increasingly being used for cancer and cardiovascular imaging clinically and holds significant advantages over γ-camera imaging in that the sensitivity for detecting molecular probes is up to 2 orders of magnitude higher than that for γ-camera imaging.9 This may explain the much higher relative radiotracer uptake values in ischemic versus nonischemic hindlimbs in the present study compared with the values obtained with γ-camera imaging in the study by Lu et al.24 The present study is unique in that we addressed for the first time the effects of stimulated angiogenesis on PET tracer uptake in a murine hindlimb ischemia model using the clinically relevant and widely used therapeutic approach of treadmill exercise training. In exercised mice, 64Cu-VEGF121 uptake was increased substantially compared with nonexercised mice and reached statistically significant differences 2 to 4 weeks after initiation of exercise training. This increase in radiotracer uptake on PET imaging correlated well with VEGFR2 expression levels in mice after treadmill exercise training as measured semiquantitatively by Western blotting and was paralleled by an increase of MVD. In contrast, we did not observe a significant difference between exercised and nonexercised mice by the 2 clinical assessment scores used in the present study. These findings suggest that 64Cu-VEGF121 PET imaging may provide a more sensitive means to objectively quantify the kinetics of VEGFR2 expression and the presence of angiogenesis than is possible with clinical evaluation scores. Therefore, this novel approach may be used as an imaging surrogate end point for studying various therapeutic approaches for PAD in preclinical studies and ultimately perhaps also in clinical studies. However, this hypothesis needs to be tested in further studies both in large animals and in humans. Further studies are also warranted to address whether the use of αvβ3-integrin, VEGFR2, or the combination of those 2 or other angiogenesis molecular markers may represent the most optimal imaging targets for monitoring angiogenesis in ischemia, both in animals and in humans.
The following limitations of the study need to be addressed. Hindlimb ischemia was created in healthy mice in the present study. Collateral vessel creation and thus expression of molecular markers of angiogenesis depend on the mouse strain and may vary in older, atherosclerotic, and hypercholesterolemic mice.25,26 Therefore, future studies are warranted to test the utility of 64Cu-VEGF121 PET imaging of VEGFR2 expression in animal models that better reflect the vascular status of older, atherosclerotic, and probably hypercholesterolemic patients with PAD. Furthermore, it has been shown that hindlimb perfusion in C57BL/6J mice, which were used in the present study, recovers relatively quickly (within 4 weeks) after femoral artery ligation compared with other mouse strains.25 Therefore, our mouse model may reflect chronic PAD only during a limited time interval after surgical creation of ischemia. Thus, the kinetics of VEGFR2 expression levels as determined in the present study cannot be extrapolated directly to other animal models of hindlimb ischemia or to patients with PAD. Finally, although a recent study has shown only small estimated radiation-absorbed doses of 64Cu-VEGF121 in both rats and humans,11 a radiotracer with a shorter half-life than 64Cu for labeling VEGF121 would be desirable in future translatable studies in humans. This is further supported by our finding that radiotracer uptake in ischemic hindlimbs was not significantly different at 1 hour, 4 hours, and 20 hours after intravenous administration, corroborating the use of a radiotracer with a short half-life such as 18F (t1/2=109.7 minutes) or 68Ga (t1/2=68 minutes).
In conclusion, the results of the present study suggest that PET imaging with 64Cu-labeled VEGF121 allows noninvasive spatial visualization and quantification of VEGFR2 expression in a murine model of hindlimb ischemia. Modulation of VEGFR2 expression levels by treatment with treadmill exercise training in mice can be measured and correlated to increased MVD in angiogenesis by 64Cu-VEGF121 PET imaging.
CLINICAL PERSPECTIVE.
Angiogenesis, the recruitment of new vessels, is part of the adaptive processes in ischemia and involves the interaction of various angiogenic factors. One of the major regulators of angiogenesis is vascular endothelial growth factor (VEGF) receptor type 2 (VEGFR2). VEGFR2 is an endothelium-specific receptor tyrosine kinase that is overexpressed in ischemic tissues and that triggers multiple signaling networks that result in endothelial cell survival, mitogenesis, migration, differentiation, and vascular permeability. By radiolabeling the natural ligand of VEGFR2, the isoform VEGF121, using copper−64 (64Cu), we found that temporal and spatial expression levels of VEGFR2 can be tracked by positron emission tomography (PET) in a chronic hindlimb ischemia model in mice. In mice that underwent treadmill exercise training over several weeks, VEGFR2 expression levels were increased compared with nonexercised mice and could be monitored noninvasively by 64Cu-labeled VEGF121 PET imaging in vivo. With PET imaging being increasingly used for cardiovascular applications in clinics, our proof-of-concept study suggests that molecular imaging with PET-based approaches may be valuable for noninvasive monitoring of angiogenesis in patients with peripheral arterial disease. In particular, in patients with peripheral arterial disease and refractory symptoms who are undergoing novel therapeutic approaches such as gene- and cell-based therapies, quantitative PET imaging may be helpful in patient stratification and decision making in future clinical studies.
Acknowledgments
Sources of Funding
This work has been supported by the Swiss Foundation of Medical-Biological Grants, Novartis Research Foundation, and Swiss Society of Radiology (all to Dr Willmann) and by National Cancer Institute grants SAIRP and ICMIC CA114747 P50 and National Heart, Lung, and Blood Institute grant R01 HL078632 (Dr Gambhir).
Footnotes
Disclosures
None.
References
- 1.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D. The Cardiovascular Health Study Group Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 2.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med. 2002;347:1941–1951. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 4.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease, part I: angiogenic cytokines. Circulation. 2004;109:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 5.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease, part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 6.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 7.Rajagopalan S, Mohler ER, III, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 8.Choksy SA, Chan P. Therapeutic angiogenesis. Br J Surg. 2006;93:261–263. doi: 10.1002/bjs.5254. [DOI] [PubMed] [Google Scholar]
- 9.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005:209–231. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 11.Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, Chen X. PET of vascular endothelial growth factor receptor expression. J Nucl Med. 2006;47:2048–2056. [PubMed] [Google Scholar]
- 12.Chen X, Plasencia C, Hou Y, Neamati N. Synthesis and biological evaluation of dimeric RGD peptide-paclitaxel conjugate as a model for integrin-targeted drug delivery. J Med Chem. 2005;48:1098–1106. doi: 10.1021/jm049165z. [DOI] [PubMed] [Google Scholar]
- 13.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–210. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 14.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–137. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
- 15.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 16.Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 19.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 20.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97:1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 21.Milkiewicz M, Hudlicka O, Verhaeg J, Egginton S, Brown MD. Differential expression of Flk-1 and Flt-1 in rat skeletal muscle in response to chronic ischaemia: favourable effect of muscle activity. Clin Sci (Lond) 2003;105:473–482. doi: 10.1042/CS20030035. [DOI] [PubMed] [Google Scholar]
- 22.Hua J, Dobrucki LW, Sadeghi MM, Zhang J, Bourke BN, Cavaliere P, Song J, Chow C, Jahanshad N, van Royen N, Buschmann I, Madri JA, Mendizabal M, Sinusas AJ. Noninvasive imaging of angiogenesis with a 99mTc-labeled peptide targeted at αvβ3 integrin after murine hindlimb ischemia. Circulation. 2005;111:3255–3260. doi: 10.1161/CIRCULATIONAHA.104.485029. [DOI] [PubMed] [Google Scholar]
- 23.Lee KH, Jung KH, Song SH, Kim DH, Lee BC, Sung HJ, Han YM, Choe YS, Chi DY, Kim BT. Radiolabeled RGD uptake and alphav integrin expression is enhanced in ischemic murine hindlimbs. J Nucl Med. 2005;46:472–478. [PubMed] [Google Scholar]
- 24.Lu E, Wagner WR, Schellenberger U, Abraham JA, Klibanov AL, Woulfe SR, Csikari MM, Fischer D, Schreiner GF, Brandenburger GH, Villanueva FS. Targeted in vivo labeling of receptors for vascular endothelial growth factor: approach to identification of ischemic tissue. Circulation. 2003;108:97–103. doi: 10.1161/01.CIR.0000079100.38176.83. [DOI] [PubMed] [Google Scholar]
- 25.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 26.Tirziu D, Moodie KL, Zhuang ZW, Singer K, Helisch A, Dunn JF, Li W, Singh J, Simons M. Delayed arteriogenesis in hypercholesterolemic mice. Circulation. 2005;112:2501–2509. doi: 10.1161/CIRCULATIONAHA.105.542829. [DOI] [PubMed] [Google Scholar]