Abstract
Background
The optimal treatment of cervical spondylomyelopathy (CSM) is controversial, with the owner’s and clinician’s perception of gait improvement often being used as outcome measures. These methods are subjective and suffer from observer bias.
Objectives
To establish kinematic gait parameters utilizing digital motion capture in normal Doberman Pinschers and compare them with CSM-affected Dobermans.
Animals
Nineteen Doberman Pinschers; 10 clinically normal and 9 with CSM.
Methods
All dogs were enrolled prospectively and fitted with a Lycra® body suit, and motion capture was performed and used to reconstruct a 3-D stick diagram representation of each dog based on 32 reflective markers, from which several parameters were measured. These included stride duration, length, and height; maximal and minimal spinal angles; elbow and stifle flexion and extension; and maximum and minimum distances between the thoracic and pelvic limbs. A random-effects linear regression model was used to compare parameters between groups.
Results
Significant differences between groups included smaller minimum (mean = 116 mm; P = .024) and maximum (mean = 184 mm; P = .001) distance between the thoracic limbs in CSM-affected dogs. Additionally, thoracic limb stride duration was also smaller (P = .009) in CSM-affected dogs (mean = 0.7 seconds) when compared with normal dogs (mean = 0.8 seconds). In the pelvic limbs, the average stifle flexion (mean = 100°; P = .048) and extension (mean = 136°; P = .009), as well as number of strides (mean = 2.7 strides; P = .033) were different between groups.
Conclusions and Clinical Importance
Our findings suggest that computerized gait analysis reveals more consistent kinematic differences in the thoracic limbs, which can be used as future objective outcome measures.
Keywords: Cervical instability, Digital motion capture, Dog, Wobbler
Kinematic gait analysis quantifies the positions, velocities, accelerations, and angles of anatomic landmarks, segments, and joints in space.1 This technique has been used to recognize and characterize clinically normal gait patterns and identify abnormal gait patterns related to pathologic conditions in humans, dogs, and horses.2-4 Kinematic gait analysis is also a valuable, objective outcome measure in models of spinal cord injury in rodents,5,6 and has recently been reported as a method for determining forelimb– hindlimb coordination in spinal cord injured dogs.7,8 These studies in dogs and rodents have utilized digital motion capture to evaluate the gait after thoracolumbar spinal cord injury.
Cervical spondylomyelopathy (CSM), also known as wobbler syndrome, is one of the most common diseases of the cervical spine in large and giant breed dogs, particularly in Doberman Pinschers.9 The disease is secondary to a collection of disorders affecting the caudal cervical vertebrae and intervertebral disks resulting in clinical signs of spinal cord compression, nerve root compression, or both, including neurologic deficits, cervical hyperesthesia, or both.10,11 Gait abnormalities are often one of the first noted signs in dogs with CSM and are observed as a slowly progressive hind limb ataxia or “wobbling” of the pelvic limbs.9,12 Additionally, thoracic limb abnormalities may manifest themselves as varying degrees of ataxia in combination with a short, stilted gait.9,13 Treatment options for CSM include conservative management or surgical decompression; however, the best option for treatment is still highly controversial.9,14 Herein lies the need to develop better methods for assessing a patient’s response to treatment as outcome measures for CSM reported thus far are highly inconsistent.9 Currently, treatment outcomes for dogs with CSM are based on subjective parameters such as the owner’s and clinician’s perception of improvement based on gait and neurologic examination findings. In addition to being subjective, these parameters are dependent on observers’ experience and can be biased. Thus, the need to develop a more reliable and objective means of assessing a patients’ response to treatment.
The purpose of this study was to prospectively utilize digital video motion capture to compare multiple kinematic parameters of the trunk, thoracic, and pelvic limbs in Doberman Pinscher dogs with and without CSM. We hypothesized that a subset of the parameters, including thoracic and pelvic limb stride length, elbow joint flexion and extension, and truncal sway (ie, spinal angles), would be significantly different between normal and CSM-affected Doberman Pinscher dogs. The identification of consistent differences in kinematic parameters between normal and CSM-affected dogs could serve as the initial step in the development of objective outcome measures for dogs with CSM.
Materials and Methods
Animals
Nineteen client-owned mature Doberman Pinscher dogs were prospectively enrolled in this investigation from August 2010 through September 2011. The study was conducted in accordance with the guidelines and with approval of the Clinical Research Advisory Committee and the Institutional Animal Care and Use Committee of The Ohio State University. Written owner consent was obtained before study enrollment.
Normal Dogs
Dogs were considered clinically normal and eligible for study enrollment if they were greater than or equal to 1 year of age, in addition to no abnormalities identified on physical, orthopedic, and neurologic examination. Additionally, their history could not reveal any previous previous orthopedic or neurologic disease.
Affected Dogs
Dogs were considered affected and eligible for study enrollment if they had neurologic examination findings consistent with a cervical myelopathy and were skeletally mature (≥1 year of age). All affected dogs underwent physical and neurologic examinations performed by two of the authors (KF, RdC), complete blood count, biochemistry profile, cervical spinal radiographs, and magnetic resonance imaging (MRI) examination of the cervical spine. Neurologic status at the time of initial examination was graded on a scale from 1 to 5 on the basis of a previously published grading scale.10,15 Dogs classified as Grade 1 were those with cervical hyperesthesia only. These dogs were excluded from participation. Grade 2 dogs were those with mild pelvic limb ataxia or paresis with mild thoracic limb involvement. Thoracic limb involvement was defined as either a short-strided or spastic gait with a floating appearance. Grade 3 dogs were defined as having moderate pelvic limb ataxia or paresis with thoracic limb involvement as described in Grade 2. Grade 4 was defined as marked pelvic limb ataxia or paresis with thoracic limb involvement, and Grade 5 was defined as nonambulatory tetraparesis. Any dog with Grade 5 neurologic status was also excluded from the study. The diagnosis of CSM was confirmed in all dogs with MRI, utilizing a modified standard protocol,10 to support evidence of spinal cord compression with or without spinal cord signal change. Before general anesthesia for the MRI, a minimum database consisting of a CBC and chemistry profile was performed in all dogs. Additionally, thoracic radiographs were performed in all affected dogs >7 years of age (n = 5).
Equipment Setup
Fifteen red LED motion capture cameras with a recording frequency of 120 frames per second were positioned around a designated capture space (24′ × 24′), using the Vicon8i motion capture system, and were calibrated to permit recording from all positions (Figs 1, 2). We defined the sagittal plane (viewing the dog from the side) as the x-plane, the vertical plane as the y-plane, and movement toward/forward and away/backward, as the z-plane in all dogs except for three of the normal dogs. Thirty-two reflective markers were attached to a Lycra® body suit and wrist bands overlying specific anatomical landmarks depicted on Figure 1. Each marker was placed at least 4 cm apart to avoid interference with the LED camera recording.
Fig 1.
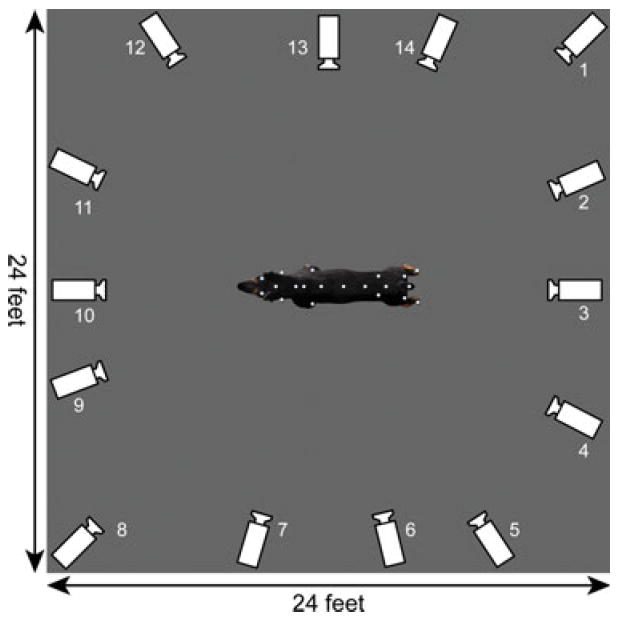
Representation of the motion capture studio showing the dimensions and position of the recording cameras. To facilitate visualization, the image of the dog was magnified in 2.5 times in relation to its actual size.
Fig 2.
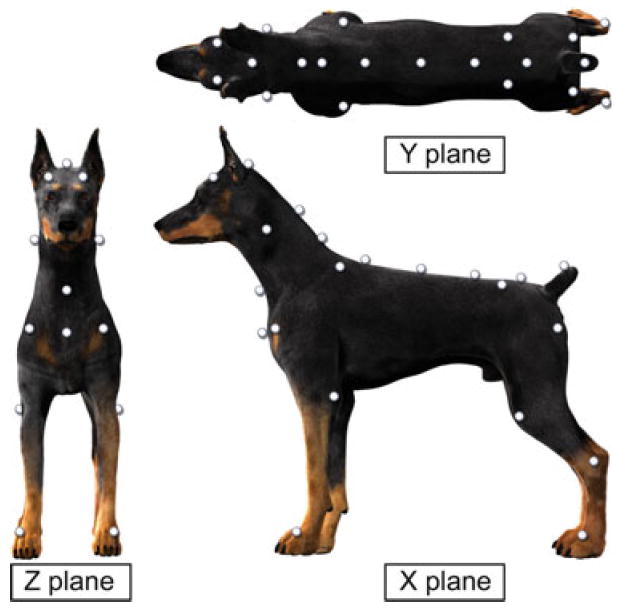
Digital reconstruction showing anatomic marker placement in the doral, lateral, and frontal plane. Anatomic landmarks represented included the parietal bone, the occipital crest, the spine of the scapula, the head of the humerus, the elbow, the fore paws, the iliac crest, the greater trochanter of the femur, the stifle joint, the tarsal joint, the hind paws, the manubrium, and approximately at the level of C1, C7, T3, T12, L3, S1. Each marker was placed at least 4 cm apart to avoid interference with LED camera recording. The recording planes (X, Y, and Z) are also demonstrated.
Recording
Dogs were acclimated to the Lycra® suit, the handler, and the capture space for approximately 10–15 minutes before marker attachment. All dogs were walked at a slow pace (approximately 0.7–0.8 m/s), maintaining a consistent gait and pace along a horizontal line in the capture space. Anytime a dog shook, stopped walking, or was not walking as straight as possible, this pass was excluded from the data. Each horizontal pass providing approximately 10 seconds of valid motion capture. All valid passes were then added together until a total of 45–90 seconds of motion capture data were acquired.
Data Analysis
Processing of the recorded images was carried out with Vicon iQ 2.0 software.a The 32 individual markers were identified and labeled to reconstruct a 3D stick diagram representation of each dog (Fig 2). Visual examination of lateral and forward movement was displayed in the Vicon iQ 2.0 graphical plots of the z-plane and x-plane and was used to exclude any sections of data in which a dog was not walking consistently (either abrupt stopping, shaking, or drifting to one side or the other). Before beginning data collection, a standing pose was performed in the x-plane allowing visualization of all 32 markers. Analysis of coordination was focused on data obtained from the paws and from the spine. Positional data, such as stride height, for all paws were determined in the x- and z- plane, limb angles determined in the x-plane, spine angle from the y-plane, limb distance in the z-plane, and step cycle duration and stride length in the x-plane (Fig 1).
Parameters recorded in all dogs were step cycle duration, stride length, stride height, number of strides from each limb, truncal sway, elbow and stifle flexion, elbow and stifle extension and maximum and minimum distance between contralateral limbs (thoracic and pelvic limbs). Step cycle duration was calculated using each individual paw by measuring the time elapsed between one maximal x-plane position and the next. Stride length was calculated for each individual limb using each foot in the x-plane by measuring the distance in mm from one maximal position to the next. Stride height was determined by the estimated maximum distance between each individual foot and the floor in the x-plane.6 The number of strides was recorded from each limb of every dog and counted as successive maximal x-plane positions. Truncal sway was calculated based on the maximal and minimal lateral deviation of the spine at any given time during the walking phase. Two vectors were established, Vector v1 from cneck2 to spine1, and Vector v2 from cneck2 to spine4. The maximum and minimal angles were the angles made between these two vectors in the y-plane (Fig 3—top). Lastly, the joint angle of the elbow and stifle joints was determined by the maximum joint angle positions in both flexion and extension and measuring the maximum and minimum values for each angle during the step cycle.6 Joint angle for the elbow joint was based on the angle made between the markers in the x-plane located at the humeral head, the elbow, and fore paw (Fig 3— bottom). Stifle angles were defined as the maximum and minimum angle formed by the greater trochanter, the stifle joint, and the tarsal joint (Fig 3—bottom). Maximum and minimum distance between the thoracic and pelvic limbs was the maximum and minimum distance recorded between the contralateral limbs in the z-plane.
Fig 3.
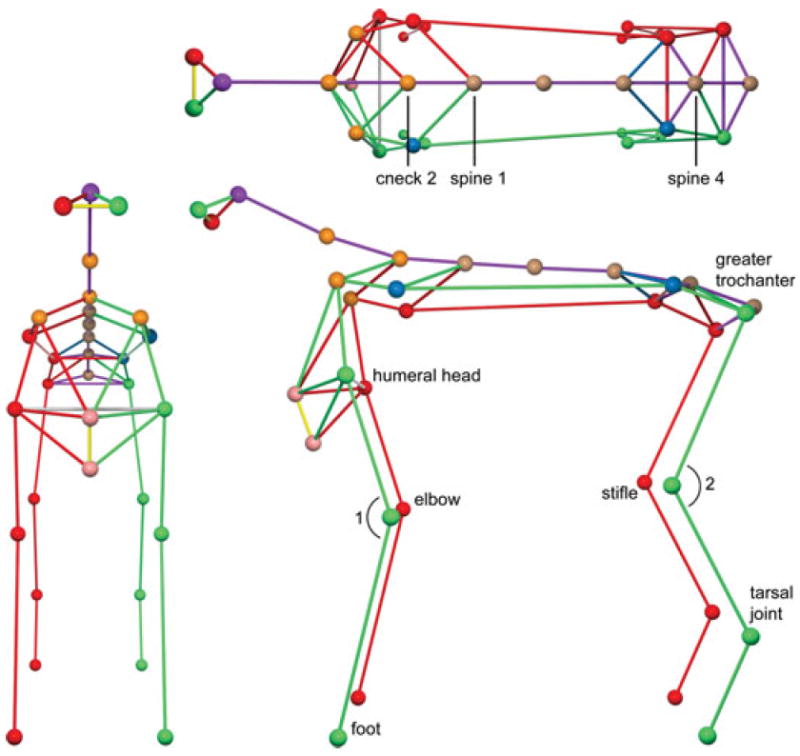
3-D reconstruction showing “ball and stick figures”. Top—Truncal sway was calculated utilizing 2 vectors. Vector v1 from cneck2 to spine1, Vector v2 from cneck2 to spine4. The maximum and minimal angles were the angles made between these two vectors in the y-plane. Bottom—Elbow flexion and extension was defined by the minimum and maximum angle created by the markers located at the level of the humeral head, elbow, and front foot (angle 1). Stifle flexion was determined based on the minimum and maximum angle created by the markers located at the greater trochanter, stifle joint, and tarsal joint (angle 2).
Statistical Analysis
All data were cleaned and exported using Microsoft® Visual Studio 2009.b All data acquired in Vicon iQ 2.0 were then transferred as numerical data into Matlab.c A custom-written script was used to extract the data points of interest. The resulting data were then analyzed by Stata 12.0.d Repeated observations within a dog were considered a technical replicate and were averaged over the number of observations. This average was the estimate of each measurement within a particular dog. Differences in means were tested by a random-effects linear regression model as both the left and right side observations were considered to be nested within a dog. For observations that were not associated with left or right side (maximum thoracic limb distance or maximum spine angle), the values were not repeated within a dog, and the difference in the means between groups was analyzed by a two-sample t-test. Additionally, the coefficient of variation was also calculated for those parameters found to be different between the two groups. Significance level for tests was set at P < .05.
Results
A total of 19 dogs were enrolled (10 clinically normal dogs and 9 CSM-affected dogs)The group of normal dogs was comprised of 9 males and 1 female between 1 and 7 years old (mean 4.2 years, SD 1.87, median 4 years) with body weights ranging from 31.3 to 43.6 kg (mean 37.94, SD 4.53, median 39.25 kg). The CSM-affected group included 6 males and 3 females between the ages of 3 and 12 years (mean 7.7 years, SD 3.35, median 9 years). Clinical signs included mild pelvic limb ataxia or paresis with thoracic limbs involvement (Grade 2; n = 4), moderate pelvic limb ataxia or paresis with thoracic limb involvement (Grade 3; n = 2), and marked pelvic limb ataxia or paresis with thoracic limb involvement (Grade 4; n = 3). The body weight of the dogs ranged from 27.2 to 55.8 kg (mean 37.4 kg, SD 6.67, median 35.6 kg). All CSM-affected dogs had spinal cord compression located in the caudal cervical spine as identified by MRI. The main compression was located at C5-6 in 3 dogs, and at C6-7 in 6 dogs. The main cause of spinal cord compression was disk-associated in 8 dogs, with or without ligamentous compression. One dog had bilateral dorsolateral osseous compression.
Kinematic gait analysis was successfully performed in all nineteen dogs. The entire process for each dog ranged from 45 minutes to 3 hours (average time was approximately 1.5 hours), including set-up (application of the Lycra® suits and marker placement), and data collection to obtain a total of 45–90 seconds of adequate motion capture. The normal dogs took approximately 60 minutes on average, with the CSM dogs taking approximately 65 minutes. The difference between groups was minimal and we observed no association between collection time and neurological status. The number of steps from all limbs ranged from 188 to 340 (mean 236.9) in the normal dogs, and from 190 to 343 (mean 256) in the affected dogs. The range and the mean number of steps were not different between groups. Observations for each dog ranged from 8 to 24 (mean 15.3 ± 1.54 SE) in the normal Dobermans and from 13 to 38 (mean 23.4 ± 2.56) in the affected dogs. The variation in number of observations and amount of motion capture did not appear to be related to the neurologic status of the dog, but seemed to more specifically related to the dog’s behavior (ie, willingness to walk in a straight line wearing a suit with markers). The mean value for each kinematic parameter assessed and its associated 95% confidence interval are presented for both the normal and affected dogs in Table 1.
Table 1.
Kinematic parameters (mean, 95% CI) of clinically normal and CSM-affected Doberman Pinschers
| Parameter | Normal Dogs
|
Affected Dogs
|
P-Valueb | ||||
|---|---|---|---|---|---|---|---|
| Mean and CVa | 95% CI | Mean and CV | 95% CI | ||||
| Number of strides (per 10 seconds)—thoracic limbs | 4.3 (0.37) | 3.4 | 5.2 | 3.5 (0.42) | 2.6 | 4.4 | .235 |
| Number of strides (per 10 seconds)—pelvic limbs | 4.1 (0.41) | 3.2 | 5 | 2.7 (0.55) | 1.7 | 3.6 | .033* |
| Stifle flexion (°) | 109 (0.06) | 103 | 114 | 100 (0.14) | 94 | 106 | .048* |
| Stifle extension (°) | 146 | 141 | 151 | 136 | 130 | 141 | .009* |
| Elbow flexion (°) | 106 | 102 | 109 | 102 | 98 | 106 | .130 |
| Elbow extension (°) | 146 | 141 | 152 | 139 | 133 | 145 | .077 |
| Average flexion (°) | 107 (0.05) | 103 | 111 | 101 (0.08) | 97 | 105 | .023* |
| Average extension (°) | 146 (0.03) | 142 | 150 | 137 (0.06) | 133 | 142 | .005* |
| Pelvic limb stride height (mm) | 83 | 73 | 93 | 84 | 73 | 95 | .856 |
| Thoracic limb stride height (mm) | 75 | 63 | 87 | 90 | 78 | 103 | .088 |
| Average limb stride height (mm) | 79.0 | 68.8 | 89.2 | 87.4 | 76.6 | 98.1 | .268 |
| Stride length—thoracic limbs (mm) | 798 | 740 | 856 | 726 | 665 | 788 | .099 |
| Stride length—pelvic limbs (mm) | 822 | 752 | 892 | 885 | 811 | 959 | .224 |
| Average stride length (mm) | 811 | 756 | 865 | 808 | 751 | 865 | .946 |
| Stride duration—thoracic limbs (sec) | 0.8 (0.08) | 0.8 | 0.9 | 0.7 (0.19) | 0.6 | 0.8 | .009* |
| Stride duration—pelvic limbs (seconds) | 0.8 (0.08) | 0.8 | 0.9 | 0.8 (0.12) | 0.8 | 0.9 | .660 |
| Average stride duration (seconds) | 0.8 | 0.8 | 0.9 | 0.8 | 0.7 | 0.8 | .143 |
| Max thoracic limb distance (mm) | 222 (0.11) | 206 | 237 | 184 (0.13) | 168 | 200 | .001* |
| Min thoracic limb distance (mm) | 146 (0.17) | 128 | 165 | 116 (0.29) | 97 | 135 | .024* |
| Max pelvic limb distance (mm) | 233 | 211 | 255 | 229 | 206 | 252 | .824 |
| Min pelvic limb distance (mm) | 194 | 167 | 221 | 159 | 130 | 187 | .079 |
| Max spine angle (°) | 10.5 | 9.3 | 11.7 | 11.2 | 9.9 | 12.4 | .459 |
| Min spine angle (°) | 2.9 | 1.3 | 4.5 | 3.6 | 1.9 | 5.3 | .567 |
CI, confidence interval; LF, left front; RF, right front; LH, left hind; RH, right hind; CSM, cervical spondylomyelopathy.
P < .05.
CV, coefficient of variation. CV was only established for selected parameters.
P-value tests if the means are different between normal and affected dogs using a random-effects linear regression.
Minimum thoracic limb distance was significantly smaller (P = .024) in CSM-affected dogs (mean = 116 mm) versus normal dogs (mean = 146 mm) (Fig 4), as was maximum thoracic limb distance (P = .001) with a mean of 184 mm in CSM-affected dogs and 222 mm in normal dogs (Fig 5). Additionally, stride duration in the thoracic limbs was also significantly smaller (P = .009) in CSM-affected dogs (mean = 0.7 seconds) when compared with normal dogs (mean = 0.8 seconds), with an effect size using a random-effects linear regression being 0.12 (95% CI: −0.21 to −0.03) lower in the CSM-affected dogs compared with the normal dogs. (Fig 6; also see supplementary videos in the online version). Other values found to be of difference included significantly fewer strides in the pelvic limbs (P = .033) of CSM-affected dogs (mean = 2.7 strides) versus normal dogs (mean = 4.7 strides), as well as CSM-affected dogs having smaller average stifle flexion and extension (100 and 136°, respectively) compared with normal dogs (109 and 146°, respectively) (P = .048) and significantly smaller average flexion (P = .023) and average extension (P = .005) from all 4 limbs of CSM-affected (mean = 101 and 137°, respectively) dogs when compared with that of normal dogs (mean = 107 and 146°, respectively).
Figure 4.
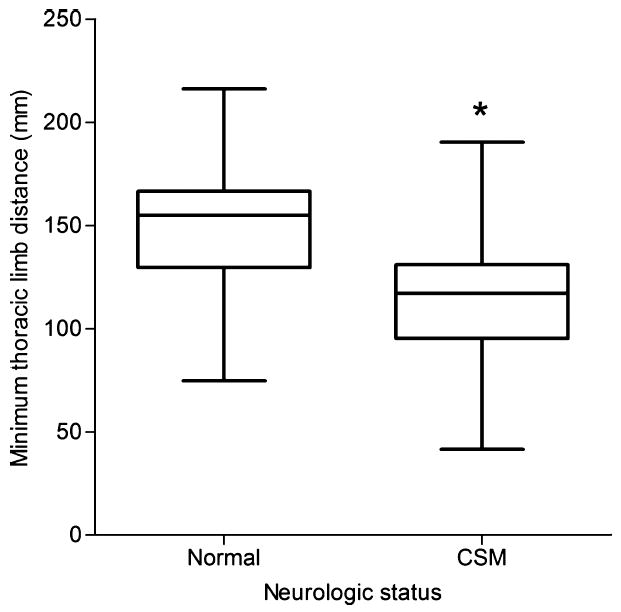
Boxplot data of minimum thoracic limb distance in clinically normal and cervical spondylomyelopathy-affected Doberman Pinschers. *P = .024. The box represents the interquartile range (25–75%), the line in each box delineates the median value.
Figure 5.
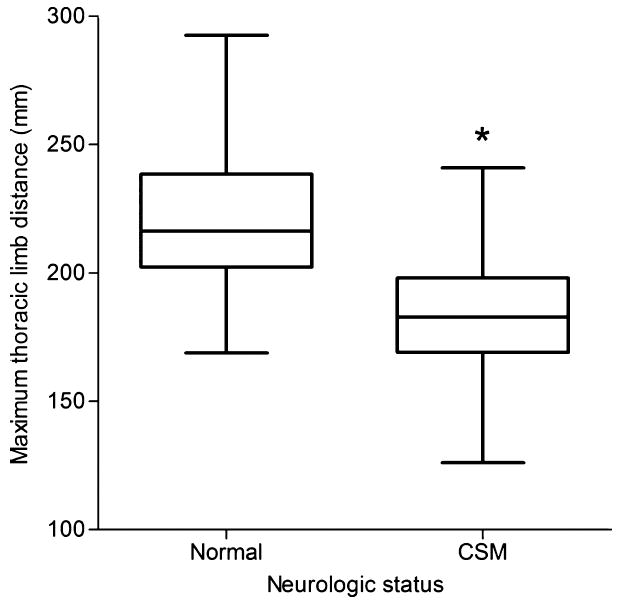
Boxplot data of maximum thoracic limb distance in clinically normal and cervical spondylomyelopathy-affected Doberman Pinschers (n = 19). *P = .001. See Figure 4 for references.
Figure 6.
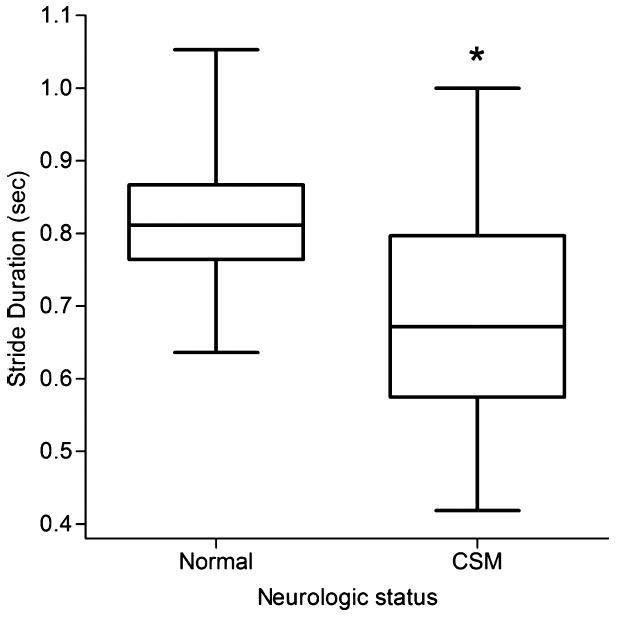
Boxplot of thoracic limb stride duration in clinically normal and cervical spondylomyelopathy-affected Doberman Pinschers (n = 19). *P = .009
Discussion
This study uses digital motion capture to identify gait parameters that are altered in CSM-affected Doberman Pinschers as a means of providing more objective outcome measures when evaluating a treatment. Dogs with CSM have a tendency to have shorter thoracic limb stride duration than normal dogs, as well as a smaller distance between the thoracic limbs during the stance phase of the gait. Additionally, the average number of strides in the pelvic limbs of CSM-affected dogs was not only much smaller than that of the pelvic limbs in normal dogs but also smaller when compared with the thoracic limbs within affected dogs as well as normal dogs. Clinically, Doberman Pinschers with CSM can present with what is a characteristic “two-engine” gait. This is observed as a short-strided thoracic limb gait with a wide-based, long-strided pelvic limb gait often giving the appearance that the gait is “disconnected”9,16 and could explain why CSM-affected dogs tended to have fewer strides in the pelvic limbs versus their thoracic limbs when compared with normal dogs. Normal quadrupedal coordination is defined as for every forelimb step, a hind limb step is taken and the hind limbs alternate with each step.17,18 Thus, based on this definition, our findings indicate that CSM-affected dogs are incoordinated when compared with healthy Dobermans.
Of interest is the fact that despite the CSM-affected dogs clinically showing various degrees of pelvic limb incoordination, there were no differences detected in stride duration, stride length, or distance between the pelvic limbs. Even though we were not expecting this finding, we are not the first to document it. A study examining spatiotemporal gait characteristics in normal dogs and those with thoracolumbar spinal cord disease also found that the thoracic limbs in the neurologic dogs had significantly shorter stride lengths, shorter stride durations, and decreased stance times compared with normal dogs,19 which could also be occurring in dogs with CSM. Additionally, despite the neurologic dogs being ataxic in the pelvic limbs, the study found no differences in stride length, stride duration, or stance time in the pelvic limbs.19 It is possible that ours and this other study suffer from a common issue with clinical research, which is the variability in the patient population. We tried to minimize this by investigating a single disease process in 1 breed only, yet it is possible that a much larger sample size would be required to demonstrate differences in the pelvic limb parameters. As for the finding of there being no differences in the pelvic limbs, despite some dogs having profound ataxia, our study only compared the mean values from each parameter, not evaluating individual limb differences, in accordance with a previous study.19 Ataxic patients can have large variability in stride length and foot placement from stride to stride. Therefore, some strides could have been long and others short, but the mean of these values could have eliminated differences.
We anticipated that the pelvic limbs of CSM-affected dogs would also show marked differences in pelvic limbs kinematic parameters compared with normal dogs. In general, the common clinical presentation of CSM is characterized mostly by pelvic limb ataxia, a long-strided pelvic limb gait, and paresis.9 However, based on the findings of the current study, it would appear that there are also dramatic changes within the thoracic limbs. Most locomotor scoring systems used for assessment of spinal cord injury in dogs assess only the pelvic limbs.20,21 The gait score used in this study was adapted from a previous study on Doberman Pinschers with CSM10 with the goal of also taking into account the thoracic limbs. However, this scale and previous scales based on gait evaluation of canines with cervical spine disorders only state whether or not the thoracic limbs are affected, but do not go into any further detail on the degree of thoracic limb abnormalities. 10,22,23 The clinical changes in the thoracic limbs of CSM-affected dogs need therefore to be better characterized. CSM-affected dogs also had decreased range of motion in the stifle joints, as noted by much smaller angles of stifle flexion and extension in this group, which most likely lead to the overall average smaller flexion and extension. These smaller angles could be attributed to the pelvic limb paresis that is often seen with CSM.9 Additionally, based on the varying severity of pelvic limb ataxia that can be observed, we predicted that CSM-affected dogs would have more truncal sway (larger spinal angles) in comparison with normal dogs, which was not the case in this study. It is unclear why differences were not detected.
Limitations of this study include the fact that while we attempted to keep the dogs moving at a consistent pace, there were no means of monitoring velocity/speed, which may lead to a larger variability in some of the parameters, in particular, stride duration. Other studies in dogs with thoracolumbar lesions chose to maintain constant speed using a treadmill.7 We chose to walk dogs in a large area without specifically controlling the speed as in a treadmill, because this is typically how the gait examination is performed, and we felt that possibly this would allow us detect more gait differences. It is also unknown what role the Lycra® body suit played in potentially altering the gait of the dogs. While all dogs were given time to acclimate to the suit itself, it could have led to minor gait changes. However, all dogs underwent the analysis wearing the suit so any interference was consistent among all dogs. We chose to use the suit to minimize soft tissue artifact, a concern with kinematic gait analysis. This artifact occurs when markers are placed on the skin to monitor the movement of underlying skeletal structures noninvasively and has been shown to affect kinematic measurements in horses, in humans, and most recently, in dogs.24-26 Also, our sample size was small and may not been enough to detect more differences. For example, to detect differences in stride length of approximately 20 mm in the pelvic limbs with an 80% power, we would need 116 dogs (58 per group). Yet for maximum thoracic limb distances, it was found that only 7 dogs were needed per group to detect a difference of 20 mm, thus giving this study adequate power for this parameter. Although large sample sizes are ideal, it is difficult to achieve larger samples sizes in specific funding periods (1–2 years) when dealing with a disease process in a single breed. Additionally, we included 1 dog with dorsolateral osseous compression in the study. We chose to include the dog with dorsolateral osseous compression as there is no indication that the direction of compression affects the spinal cord in different ways. In a recent study, we investigated whether the direction of compression had an effect on spinal cord signal changes and found no relationship. 27 This dog also had mild pelvic limb ataxia (Grade 2), which, we felt, was not any different from the other 3 dogs that were Grade 2 on the gait score with ventral compression.
In summary, the use of 3-D motion capture to evaluate various gait parameters in normal and CSM-affected Doberman Pinschers revealed that the thoracic limb distances and thoracic limb stride duration were significantly smaller in CSM-affected Doberman Pinschers. Additionally, computerized kinematic gait analysis also revealed that CSM-affected Doberman Pinschers have fewer number of strides in the pelvic limbs versus their thoracic limbs, but also fewer pelvic limb strides compared with normal dogs. This finding is suggestive that CSM-affected Doberman Pinschers, by definition, are truly incoordinated in their pelvic limbs. The consistent kinematic differences found on this study will be used to objectively evaluate the treatment response of dogs with CSM.
Supplementary Material
X-plane 3-D reconstruction of a normal Doberman.
X-plane 3-D reconstruction of a CSMaffected Doberman. Note the short-strided, choppy pattern of the thoracic limbs and the long stride of the pelvic limbs.
Acknowledgments
The authors thank Amanda Waln for her assistance with the data collection, as well as Vita Berezine-Blackburn, Tom Heban, Susana del Rio, and Neelima Karanam for their assistance with the digital motion capture and data processing. We also thank TimVojt and Marc Hardman for their work on the illustrations and Gary Phillips for his help with the statistical analysis.
Funding: This study was supported by the Canine Funds of the College of Veterinary Medicine, The Ohio State University.
Abbreviations
- CSM
cervical spondylomyelopathy
- MRI
magnetic resonance imaging
- CV
coefficient of variation
Footnotes
Conflict of Interest: Authors disclose no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Vicon, Los Angeles, CA
Microsoft, Redmond, WA
Matlab, Natick, MA
Stata Corporation, College Station, TX
This study was a collaboration between the Department of Veterinary Clinical Sciences, College of Veterinary Medicine, The Ohio State University and The Ohio State University’s Advanced Computing Center for the Arts and Design. Parts of this study were presented at the 2011 American College of Veterinary Internal Medicine Forum, New Orleans, LA.
References
- 1.DeCamp CE. Kinetic and kinematic gait analysis and the assessment of lameness in the dog. Vet Clin North Am Small Anim Pract. 1997;27:825–840. doi: 10.1016/s0195-5616(97)50082-9. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Bustinduy M, de Medeiros MA, Radke H, et al. Comparison of kinematic variables in defining lameness caused by naturally occurring rupture of the cranial cruciate ligament in dogs. Vet Surg. 2010;39:523–530. doi: 10.1111/j.1532-950X.2010.00672.x. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki E, Nakamura H, Konishi S, Yamano Y. Analysis of the spastic gait caused by cervical compression myelopathy. J Spine Disord Tech. 2002;15:519–522. doi: 10.1097/00024720-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Kelmer G, Keegan KG, Kramer J, et al. Computer-assisted kinematic evaluation of induced compensatory movements resembling lameness in horses trotting on a treadmill. Am J Vet Res. 2005;66:646–655. doi: 10.2460/ajvr.2005.66.646. [DOI] [PubMed] [Google Scholar]
- 5.Leblond H, L’Esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J Neuroscience. 2003;23:11411–11419. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtine G, Gerasimenko Y, van den Brand R, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton L, Franklin RJM, Jeffery ND. Quantification of deficits in lateral paw positioning after spinal cord injury in dogs. BMC Vet Res. 2009;4:47. doi: 10.1186/1746-6148-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton L, Franklin RJM, Jeffery ND. Development of a universal measure of quadrupedal forelimb–hindlimb coordination using digital motion capture and computerised analysis. BMC Neurosci. 2007;8:77. doi: 10.1186/1471-2202-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Costa RC. Cervical spondylomyelopathy (wobbler syndrome) in dogs. Vet Clin North Am Small Anim Pract. 2010;40:881–913. doi: 10.1016/j.cvsm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 10.da Costa RC, Parent JM, Partlow G, et al. Morphologic and morphometric magnetic resonance imaging features of Doberman Pinschers with and without clinical signs of cervical spondylomyelopathy. Am J Vet Res. 2006;67:1601–1612. doi: 10.2460/ajvr.67.9.1601. [DOI] [PubMed] [Google Scholar]
- 11.De Decker S, Van Soens I, Duchateau L, et al. Transcranial magnetic stimulation in Doberman Pinschers with clinically relevant and clinically irrelevant spinal cord compression on magnetic resonance imaging. J Am Vet Med Assoc. 2011;238:81–88. doi: 10.2460/javma.238.1.81. [DOI] [PubMed] [Google Scholar]
- 12.VanGundy TE. Disc-associated wobbler syndrome in the Doberman Pinscher. Vet Clin North Am Small Anim Pract. 1988;18:667–696. doi: 10.1016/s0195-5616(88)50061-x. [DOI] [PubMed] [Google Scholar]
- 13.De Decker S, Gielen IM, Duchateau L, et al. Intraobserver and interobserver agreement for results of low-field magnetic resonance imaging in dogs with and without clinical signs of disk-associated wobbler syndrome. J Am Vet Med Assoc. 2011;238:74–80. doi: 10.2460/javma.238.1.74. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery ND, McKee WM. Surgery for disc-associated wobbler syndrome in the dog – an examination of the controversy. J Small Anim Pract. 2001;42:574–581. doi: 10.1111/j.1748-5827.2001.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 15.da Costa RC, Parent JM. One-year clinical and magnetic resonance imaging follow-up of Doberman Pinschers with cervical spondylomyelopathy treated medically or surgically. J Am Vet Med Assoc. 2007;231:243–250. doi: 10.2460/javma.231.2.243. [DOI] [PubMed] [Google Scholar]
- 16.De Decker S, Bhatti SFM, Duchateau L, et al. Clinical evaluation of 51 dogs treated conservatively for disc-associated wobbler syndrome. J Small Anim Pract. 2009;50:136–142. doi: 10.1111/j.1748-5827.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 17.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating-scale for open-field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 18.Koopmans GC, Deumens R, Honig WMM, et al. The assessment of locomotor function in spinal cord injured rats: The importance of objective analysis of coordination. J Neurotrauma. 2005;22:214–225. doi: 10.1089/neu.2005.22.214. [DOI] [PubMed] [Google Scholar]
- 19.Gordon-Evans WJ, Evans RB, Knap KE, et al. Characterization of spatiotemporal gait characteristics in clinically normal dogs and dogs with spinal cord disease. Am J Vet Res. 2009;70:1444–1449. doi: 10.2460/ajvr.70.12.1444. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox KR. Conservative treatment of thoracolumbar intervertebral disc disease in the dog. J Am Vet Med Assoc. 1965;147:1458–1460. [PubMed] [Google Scholar]
- 21.McMichael MA, Ruaux CG, Baltzer WI, et al. Concentrations of 15F2t isoprostane in urine of dogs with intervertebral disk disease. Am J Vet Res. 2006;67:1226–1231. doi: 10.2460/ajvr.67.7.1226. [DOI] [PubMed] [Google Scholar]
- 22.Rusbridge C, Wheeler SJ, Torrington AM, et al. Comparison of two surgical techniques for the management of cervical spondylomyelopathy in Dobermans. J Small Anim Pract. 1998;39:425–431. doi: 10.1111/j.1748-5827.1998.tb03749.x. [DOI] [PubMed] [Google Scholar]
- 23.McKee WM, Butterworth SJ, Scottt HW. Management of cervical spondylopathy-associated intervertebral disc protrusions using metal washers in 78 dogs. J Small Anim Pract. 1999;40:465–472. doi: 10.1111/j.1748-5827.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- 24.Alexander EJ, Andriacchi TP. Correcting for deformation in skin-based marker systems. J Biomech. 2001;34:355–361. doi: 10.1016/s0021-9290(00)00192-5. [DOI] [PubMed] [Google Scholar]
- 25.Kim SY, Kim JY, Hayashi K, Kapatkin AS. Skin movement during the kinematic analysis of the canine pelvic limb. Vet Comp Orthop Traumatol. 2011;24:326–332. doi: 10.3415/VCOT-10-08-0123. [DOI] [PubMed] [Google Scholar]
- 26.Schwencke M, Smolders LA, Bergknut N, et al. Soft tissue artifact in canine kinematic gait analysis. A pilot study; Proceedings Voorjaarsdagen European Veterinary Conference; April 23–25, 2009; Amsterdam, The Netherlands. p. 283. [Google Scholar]
- 27.da Costa RC. Relationship between spinal cord signal changes and clinical and MRI findings in dogs with cervical spondylomyelopathy. J Vet Int Med. 2012;26:807–808. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
X-plane 3-D reconstruction of a normal Doberman.
X-plane 3-D reconstruction of a CSMaffected Doberman. Note the short-strided, choppy pattern of the thoracic limbs and the long stride of the pelvic limbs.


