Abstract
A phase I trial consisting of panobinostat (a HDAC inhibitor), carboplatin and etoposide was condacted in patients with lung cancer. Patients and Methods: Patients received carboplatin AUC5 on day 1 and etoposide 100 mg/m2 on days 1, 2 and 3, every 21 days. Concurrent oral panobinostat was given 3 times weekly on a 2-weeks-on and 1-week-off schedule during the 4–6 cycles of chemotherapy and then continued as maintenance therapy. Results: Six evaluable patients were treated at the first dose level of panobinostat (10 mg). Dose-limiting toxicity occurred in two patients (33%) during the first cycle. One patient developed grade 4 thrombocytopenia and another grade 4 febrile neutropenia. Therefore, the study was suspended based on the pre-specified study design. No recommended phase II starting dose was established. Conclusion: The addition of panobinostat to carboplatin and etoposide was not tolerable at the lowest dose level tested in this trial. Further research and development into this combination is not recommended.
Keywords: Lung cancer, panobinostat, carboplatin, etoposide
The role of inappropriate gene expression in tumorigenesis and progression of cancer is well-known (1). De-acetylation of histones is associated with decreased expression of tumor suppression genes (2). Histone deacetylases (HDACs) has emerged as a promising therapeutic target. HDACs are divided into four classes: Class I (HDAC1, 2, 3 and 8), class IIa (HDAC4, 5, 7 and 9), class IIb (HDAC6 and 10), class III (SIRT1, 2, 3, 4, 5, 6 and 7) and class IV (HDAC11) (3). Inhibitors of HDACs can induce differentiation, cell-cycle arrest, or apoptosis in tumor cells and may inhibit tumor growth in animal models (4). Tumor growth inhibition and apoptosis in response to HDAC inhibitors may also be mediated by acetylation of non-histone proteins (such as HSP-90, p53, HIF1-α, α-tubulin) (5).
Panobinostat, a hydroxamic acid derivative, is an oral pan-deacetylase inhibitor (6). It affects proteins involved in cell-cycle regulation (p53, p21), angiogenesis (HIF-1α), gene transcription (transcription factors), protein stabilization (Hsp90) and cytoskeleton (α-tubulin), through inhibition of HDACs (7, 8).
Panobinostat exhibits increased histone acetylation and has potent antiproliferative activity against a broad range of tumor cell lines, including lung cancer cell lines (4). Oral panobinostat has been previously studied on a three-times weekly dosing schedule in a phase I, first-in-human study in patients with advanced solid tumors or non-Hodgkin’s lymphoma (7). The most common adverse events were anorexia, nausea, fatigue, diarrhea and transient thrombocytopenia when panobinostat was administered as a single agent. The maximum tolerated dose (MTD), administered, three times per week was found to be 20 mg.
Platinum and etoposide combination regimens are commonly utilized in a variety of aggressive neuroendocrine tumors (small cell lung carcinoma (SCLC), extra-pulmonary small cell carcinoma, large cell neuroendocrine carcinoma, merkel cell carcinoma). Response rates as high as 35–75% with platinum/etoposide are reported for these tumors but with very limited durability and there is a need for more effective regimens that may improve durability (9–11). We have conducted a phase I trial testing for combination of panobinostat with carboplatin and etoposide in patients with lung cancer. Our primary study objective was to determine the dose-limiting toxicities (DLTs) and MTD of panobinostat given on a three times per week schedule when combined with carboplatin and etoposide for treatment of patients with advanced solid tumors.
Patients and Methods
Study design and treatment
Patients with progressive, advanced, or metastatic lung cancer (any histology) were enrolled in an open-label phase I single-arm study of panobinostat in combination with carboplatin and etoposide. The trial was designed as a phase I/II study. The phase I portion focused on estimating the MTD and finding the recommended phase II dose. The phase II component was intended to evaluate the recommended dose by studying the 6-month rate of non-progression. Previously-treated patients were permitted in the phase I portion, however the efficacy study was intended for previously-untreated patients. Panobinostat was administered orally three times per week for two out of three weeks. Panobinostat was administered concurrently with carboplatin at an AUC of 5 given on day 1 and etoposide at a dose of 100 mg/m2 on days 1, 2, and 3 of each cycle. However, for the pharmacokinetic studies, panobinostat was started the week prior to the first carboplatin/etoposide cycle. The majority of DLTs in this study were expected to be hematological (primarily thrombocytopenia and neutropenia) with an expected additive adverse myelosuppressive effect of chemotherapy, and we planned to initiate panobinostat at a dose of 10 mg.
The dose escalation design planned for an initial dose of panobinostat was of 10 mg three times per week, given for 2 weeks, out of a 3-week cycle. If no dose-limiting toxicities were encountered with the first cohort, the dose could be escalated to higher dose levels (level 2: 15 mg; level 3: 20 mg; and level 4: 30 mg). After establishing the MTD of panobinostat at a 2 out of 3 weeks dosing schedule, a 3 out of 3 weeks dosing schedule was planned to be tested at that dose and this schedule was to be adopted if no limiting toxicities occurred. Patients continued on the study regimen until the development of progressive disease or intolerable side effects.
Patient eligibility
Eligibility criteria included patients greater than 18 years of age or older with the ability to give informed consent and Eastern Cooperative Oncology Group (ECOG) performance status of 2 or better. Patients with progressive advanced or metastatic lung cancer (any histology) were allowed. Patients must have had progressed on one or more standard therapies for the disease, or to have had incurable and poorly-responsive disease. Priority was given to patients with small cell lung cancer and neuroendocrine tumors (carcinoid, extrapulmonary small cell carcinoma, peripheral neuroepithelioma, merkel cell tumor, neuroblastoma, large cell neuroendocrine cancer, esthesioneuroblastoma and other neuroendocrine carcinomas of the head and neck) where the combination of carboplatin and etoposide is standard first-line therapy and therefore were allowed to be treated on this trial as first line. Patients could not have previously received panobinostat, any other HDAC inhibitors (including valproic acid), or etoposide. Electrocardiograms were performed on all patients to screen for prolonged QTc (a measure of the time between the start of the Q wave and the end of the T wave in the heart’s electrical cycle) at baseline as required by the study protocol based on prior studies (12). Patients with severe cytopenias, electrolyte abnormalities, cardiac or liver dysfunction, or a history of cardiac arrhythmia were excluded.
Statistical analysis
The primary objective of this phase I trial was to establish a recommended dose for an efficacy (Phase II) trial. Four dose tiers of panobinostat were planned to be investigated (10, 15, 20 and 30 mg); the dosage of carboplatin and etoposide were fixed. A two out of three week dosing schedule was planned to be first-tested. After establishing the MTD of panobinostat at a 2 out of 3 weeks dosing schedule, a 3 out of 3 weeks dosing schedule was planned to be tested, and this schedule was to be adopted in the absence of limiting toxicities. The recommended phase II dose was defined as the maximum dose of panobinostat that was associated with a 25% rate of dose limiting toxicity (DLT) or less. Dose escalation was planned to proceed according to a Narayana k-in-a-row design and the selected dose was estimated by isotonic regression (13). For this trial, k was set to 3, so that three patients were treated without a DLT before the dose could be increased. If no DLTs were observed at the highest dose tier, 3 additional patients were planned to be added. If no DLTs were observed at any dose tier, MTD would be the highest dose utilized. Dose escalation to the next dose level did not occur until the cohort of subjects in the preceding dose level had been observed for a complete cycle. A maximum of 24 patients were planned to be treated in the dose-finding phase.
Toxicity and response assessment
The descriptions and grading scales found in the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0, were utilized for adverse events (AE) grading and reporting. Toxicity assessments were performed weekly during the first cycle and then every 3 weeks with subsequent cycles. ECGs were performed during week 2 of all treatment cycles to monitor the QTc interval. Dose-limiting toxicity was defined as toxicity attributable to the study drug, where during the first cycle of the study regimen, occurrence of one or more of the following constituted a DLT: Grade 3 or higher non-hematological toxicity except nausea and vomiting and elevation of ALT/AST and alkaline phosphatase; nausea or vomiting (≥Grade 3) that lasted longer than 24 h despite maximal medical therapy; elevations of alkaline phosphatase were not considered a DLT; Grade 3 AST/SGOT or ALT/SGPT lasting for >7 days or Grade 4 AST/SGOT or ALT/SGPT; absolute neutrophil count <500/μL lasting longer than 7 days; grade 4 thrombocytopenia (platelet ≤25,000/μL); grade 3 or 4 neutropenia associated with sepsis or fever ≥38.5°C; any other adverse event unrelated to disease progression, intercurrent illness or concomitant medication that did not allow for administration of oral panobinostat for >7 days of the total 21-day cycle doses; delay in starting cycle 2 by more than 2 weeks due to toxicity; abnormal non-hematological laboratory criteria (Grade ≥3), were considered a DLT, if clinically significant and drug-related.
For the purpose of tumor response assessment (RECIST criteria v.1), imaging staging studies were carried out every 6 weeks (2 cycles). Patients were classified as complete response (CR), partial response (PR), stable disease (SD) or disease progression (PD) according to these criteria.
Biospecimen collection and biomarker analysis
Peripheral blood samples were obtained in patients at three time points: (1) At baseline; (2) after three doses of panobinostat alone; and (3) following completion of one cycle of panobinostat/carboplatin/etoposide. Samples promptly underwent ficoll hypaque gradient separation and mononuclear cells were collected from the interface. Mononuclear cells were washed, resuspended and maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) at 0.5×106/ml. Cells were then washed with phosphate buffered-saline (PBS), and re-suspended in Triton X lysis buffer. After brief centrifugation, the insoluble component was removed, and lysates quantified using the Bradford assay and read with a UV-spectrophotometer at a wavelength of 595 nm. Equivalent amounts (50–100 μg) of protein lysates were boiled in Laemmli buffer; size-separated on 12% SDS-polyacrylamide gels, and transferred to nitrocellulose membranes utilizing a submergible transfer cell. The membranes were blocked for 1 h at room temperature with TTBS and NAP-Sure Blocker at a 3:1 ratio, respectively. The membranes were then incubated with the corresponding antibody. Western blotting was performed using high-quality commercially available antibodies for p21, HDAC1, and HDAC6.
Results
Patients’ characteristics
Six evaluable subjects were enrolled in a dose escalation study from October 2009 to March 2011. One additional subject was removed from the protocol prior to receiving any therapy due to development of atrial fibrillation with rapid ventricular response. This patient was not evaluable. Patients’ characteristics are presented on Table I. All subjects enrolled in the study had metastatic lung cancer – adenocarcinoma (3 patients), extensive-stage SCLC (2 patients), large cell neuroendocrine carcinoma (1 patient), squamous cell carcinoma (1 patient). Three subjects enrolled received no prior therapy. Four subjects had failed up to four prior chemotherapy regimens prior to enrollment.
Table I.
Patients’ characteristics.
| Patient no | Age | Histology | Stage | Prior regimen |
|---|---|---|---|---|
| 1 | 66 | SCLC | Extensive | None |
| 2 | 56 | SCLC | Extensive | None |
| 3 | 77 | NSCLC (adenocarcinoma) | AJCC IV | Carboplatin/docetaxel, Carboplatin/pemetrexed/bevacizumab |
| 4 | 45 | NSCLC (adenocarcinoma) | IV | Cisplatin/pemetrexed, docetaxel |
| 5 | 56 | NSCLC (squamous cell carcinoma) | IV | Cisplatin/docetaxel, gemcitabine, paclitaxel |
| 6 | 59 | Large cell neuroendocrine carcinoma | IV | None |
| 7 | 60 | NSCLC (adenocarcinoma) | IV | Cisplatin/paclitaxel, cisplatin/pemextrexed, erlotinib, docetaxel |
SCLC: Small cell lung cancer; NSCLC: non-small cell lung cancer.
Study treatment
Six subjects received the study drug regimen at a first dose level of 10 mg of panobinostat. Three subjects completed 1 cycle of the study regimen, two subjects completed 2 cycles, and one subject completed 4 cycles.
Safety
In the first cohort of three subjects, one DLT occurred at the initial dose level. The first subject developed grade 4 thrombocytopenia after 1 cycle of therapy. The second and third subjects received 2 cycles of therapy without DLT. Subject three required a one-week delay of the second cycle of therapy due to persistent grade 2 neutropenia.
Three additional subjects were enrolled at the same dose level; one DLT occurred during the first cycle of therapy. The fifth subject developed grade 4 febrile neutropenia during the first cycle of therapy and panobinostat was permanently discontinued. In addition, in the absence of limiting toxicities during the first cycle, the fourth subject received four cycles, but subsequently developed grade 3 QTc prolongation, which is a known class effect of HDAC inhibitors, and panobinostat was permanently discontinued. The QTc prolongation resolved after discontinuation of panobinostat. The sixth subject developed grade 4 thrombocytopenia and grade 3 neutropenic fever after 2 cycles of therapy and panobinostat was permanently discontinued.
Dose-limiting toxicity during the first cycle of therapy occurred in 2 out of 6 patients (33%) treated at the initial dose level of panobinostat (10 mg). The observed DLT rate was greater than the target rate of 25% and no provision for de-escalation below 10 mg of panobinostat was permitted as this was not felt to be clinically meaningful. Therefore, the study was suspended for additional enrollment based on the pre-specified dose escalation scheme and study suspension criteria. Study regimen-related adverse events (all cycles) and dose-limiting toxicities (first cycle) are presented in Table II.
Table II.
The number of patients with dose-limiting toxicities (DLT) during the first cycle (N=2 patients with DLT)* and study regimen-related adverse events during any cycle (N=6 patients).
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | DLT Y/N (#, grade) |
|---|---|---|---|---|---|
| Hematological | |||||
| Anemia | 2 | 1 | 1 | 0 | N |
| Neutropenia | 1 | 1 | 1 | 3 | N |
| Febrile neutropenia | 0 | 0 | 1 | 1 | Y (1, Grade 4) |
| Thrombocytopenia | 3 | 0 | 1 | 2 | Y (1, Grade 4) |
| Gastrointestinal | |||||
| Nausea | 3 | 0 | 0 | 0 | N |
| Vomiting | 1 | 0 | 0 | 0 | N |
| Diarrhea | 1 | 1 | 0 | 0 | N |
| Constipation | 1 | 0 | 0 | 0 | N |
| Other | |||||
| Anorexia | 3 | 0 | 0 | 0 | N |
| Cough | 2 | 0 | 0 | 0 | N |
| Dyspnea | 1 | 0 | 0 | 0 | N |
| Fatigue | 2 | 2 | 0 | 0 | N |
| Hyperglycemia | 4 | 0 | 0 | 0 | N |
| Hypoalbuminemia | 2 | 1 | 0 | 0 | N |
| Hypomagnesaemia | 3 | 0 | 0 | 0 | N |
| Hypocalcemia | 1 | 0 | 0 | 0 | N |
| Hyponatremia | 1 | 0 | 0 | 0 | N |
| Insomnia | 1 | 0 | 0 | 0 | N |
| Limb pain | 0 | 2 | 0 | 0 | N |
| QTc prolongation | 0 | 0 | 1 | 0 | N |
| Abnormal liver test | 2 | 0 | 0 | 0 | N |
| Weight loss | 1 | 0 | 0 | 0 | N |
DLT: One patient with grade 4 thrombocytopenia and one with grade 4 febrile neutropenia.
Response
There were no complete or partial responses. Among the second cohort of three subjects, the fourth subject maintained stable disease for 4 cycles when therapy was discontinued for QTc prolongation. For all other evaluable patients progression was the best response. As of June 2013, 5 out of 6 patients treated on the study had died from cancer progression. Response data are summarized in Table III.
Table III.
Treatment response.
| Patient no. | Duration of response (days) | Best response (RECIST) | Off-study reason |
|---|---|---|---|
| 1 | 85 | PD | Grade 4 thrombocytopenia |
| 2 | 45 | PD | Progressive disease |
| 3 | 53 | PD | Progressive disease |
| 4 | 119 | SD | Grade 3 QTc prolongation |
| 5 | 25 | PD | Grade 4 neutropenia |
| 6 | 53 | PD | Grade 4 neutropenia and thrombocytopenia |
Biomarket analysis
Peripheral blood samples were collected from all patients treated with panobinostat. Four subjects had 3 samples drawn at (1) baseline, (2) at one week after 3 doses of LBH589 alone (72 h after last dose of LBH589) and (3) after 1 cycle of LBH589 plus chemotherapy (21 days after chemotherapy). One subject had 2 samples drawn (baseline and one week), and one subject had 1 sample drawn (baseline). For the five subjects with at least 2 available samples, mononuclear cells isolated in each peripheral blood sample underwent cell lysis and protein extraction at the 3 time points. Based on prior pre-clinical data, we hypothesized that there will be increased p21 expression with panobinostat therapy (4). Western blot analysis showed a relative increase in p21 expression after one week of exposure to panobinostat alone in 3/5 of the subjects and after completion of one cycle of panobinostat and carboplatin/etoposide in 3/4 of the subjects tested compared to baseline, as shown in Figure 1. HDAC1 and HDAC6 showed increases in expression after exposure to one cycle of panobinostat and carboplatin/etoposide (3/4 of subjects) compared to baseline as shown in Figure 2.
Figure 1.
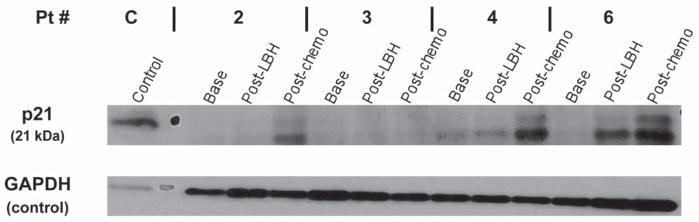
Effects of panobinostat in combination with carboplatin and etoposide on p21 expression. The expression of p21 increased with LBH589 in combination with carboplatin and etoposide in 3 out of 4 subjects.
Figure 2.
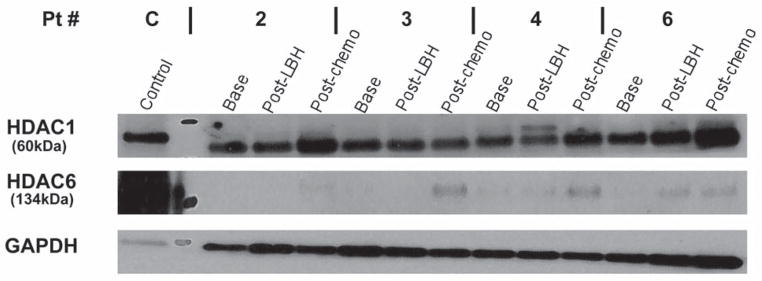
Effects of panobinostat in combination with carboplatin and etoposide on HDAC1 and HDAC6 expression. An increase in the expression of HDAC1 and HDAC6 was seen in 3 out of 4 patients.
Discussion
Panobinostat is a pan-inhibitor of Class I, II and IV histone deacetylases that has been shown to inhibit the de-acetylation of histone and non-histone cellular proteins, targeting lysine groups on chromatin and transcription factors and various non-histone proteins such as p53, tubulin, heat shock protein-90, and retinoblastoma protein (14). Pre-clinical studies have demonstrated antitumor activity for panobinostat, leading to phase I/II studies in advanced solid tumors and hematological malignancies. These studies have tested panobinostat in 2 formulations, as an oral capsule and as a solution for intravenous injection. The clinical development was later focused on the oral formulation in hematological malignancies (7, 14, 15). A phase III study in relapsed multiple myeloma is testing the combination of oral panobinostat with intravenous bortezomib and oral dexamethasone.
The results of our study showed that toxicity limited the use of oral panobinostat in combination with etoposide and carboplatin at the initial dose level. In patients who received panobinostat and carboplatin/etoposide, 33% developed DLTs at the initial dose level of 10 mg administered three times weekly. The majority of toxicities were hematological in nature. As expected, thrombocytopenia was the most common AE on this study. In fact, the panobinostat oral formulation, a review of data from phase I/II panobinostat monotherapy studies showed that thrombocytopenia was the most common laboratory abnormality of any grade. Grade 3–4 thrombocytopenia was also seen in patients treated with the three-times-a-week, every week and every-other-week schedules. The magnitude of platelet count decrease was dose-dependent and was also related to the baseline platelet count (16). Grade 3 QT prolongation was seen in one patient on this study and this appears to be a class effect of HDAC inhibitors. The safety and activity of panobinostat in combination with standard chemotherapy regimens was tested in various solid tumors, including paclitaxel, docetaxel, gemcitabine and capecitabine (7, 17, 18). Myelosuppression (primarily neutropenia, febrile neutropenia, thrombocytopenia) and infections have been reported in panobinostat combination studies with chemotherapy and appear to be dose limiting.
Oral panobinostat used as a single agent has been tolerated at a dose of 20–40 mg given three times-a-week in patients with advanced solid tumors or non-Hodgkins lymphoma (19). Panobinostat in combination with bortezomib for the treatment of relapsed multiple myeloma (20) and in combination with erolotinib for the treatment of non-small cell lung cancer (8) have been relatively well-tolerated.
Western blot analysis in peripheral blood mononuclear cells showed an increase in p21 expression with exposure to panobinostat-alone and panobinostat and carboplatin/etoposide compared to baseline levels. This finding, although in a small number of patients, is consistent with prior data on the effect of panobinostat on deacetylated tumor suppressor genes (4). HDAC1 and HDAC6 expression was also increased after exposure to panobinostat and carboplatin/etoposide. It is unclear how this finding is related to HDAC inhibition. These findings are limited to the small number of samples tested. Also, it cannot be assumed that findings in peripheral blood mononuclear cells will be similar to findings in tumor cells.
Panobinostat has been studied in several hematological malignancies with promising results. A phase IA/II study investigating the escalating doses of oral panobinostat in advanced hematological malignancies showed initial evidence of clinical efficacy in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) patients. Out of 27 evaluable Hodgkin lymphoma (HL) patients, 9 were considered responders (either complete (CR) or partial (PR) with an overall response rate of 28.2%. Seven patients with myelofibrosis (53.8%) showed stable disease. Patients with multiple myeloma (MM), chronic lymphocytic leukemia (CLL) and non-Hodgkin’s lymphoma (NHL) also showed various degrees of responses (21). In the phase I portion of the trial, investigating the combination of oral panobinostat with 5-Azacitidine in high-risk/intermediate-2 MDS and AML, 45% patients with AML showed complete response/complete response with incomplete peripheral blood count recovery (CR/Cri) and 20% patients with MDS/chronic myelomoncytic leukemia showed bone marrow (BM) CR or PR (22). PANORAMA 2 is a phase II study of oral panobinostat plus bortezomib and dexamethasone in patients with bortezomib-refractory MM and preliminary results confirm the efficacy of this combination with an overall response rate (ORR) 35%, a clinical benefit rate of 53% and a median PFS of 5.4 months (23).
Experience of panobinostat in solid tumors has not been encouraging. To improve clinical efficacy, it was tested with explored in combination with various standard treatment regimens (24, 25). Rathkopf et al. studied intravenous (i.v.) panobinostat in castration-resistant prostate cancer (CRPC) who had previously received chemotherapy and were unable to show enough clinical efficacy to further pursue its role in CRPC as a single agent (25). It is unclear whether dose or schedule were suboptimal or the drug itself was inactive against CRPC (25). Also, the clinical activity of panobinostat is monitored through PSA levels and histone acetylation in mononuclear cells which may not be the best surrogates of clinical activity (25). Two trials investigated the role of i.v. and oral panobinostat in combination with trastuzumab or trastuzumab and paclitaxel in patients with HER2-positive metastatic breast cancer and were prematurely terminated due to insufficient level of clinical activity (CLBH589C2204, CLBH589C2114). Three phase I/II studies investigated oral panobinostat as a single agent or in combination with docetaxel and prednisone ([CLBH589B2105]), and as i.v. single-agent or in combination with docetaxel and prednisone (CLBH589C2208 and CLBH589C2205 respectively) and did not show enough clinical efficacy to warrant further investigation.
Given the encouraging results of panobinostat in hematological malignancies, two phase II trials are underway. The study investigating the role of oral panobinostat in refractory cutaneous T-Cell lymphoma is currently ongoing and will assess the response rates in patients with progressive disease (CLBH589B2201). PANORAMA 1 is a multicenter, randomized phase III clinical trial which is investigating two combination therapies, panobinostat with bortezomib and dexamethasone or placebo with bortezomib and dexamethasone, in multiple myeloma patients with recurrent or progressive disease (CLBH589D2308). The study will compare the progression-free survival of the two combination therapies.
In conclusion, this phase I study demonstrated that addition of panobinostat to carboplatin and etoposide, was not tolerable at the lowest dose level of panobinostat tested in this trial. HDAC inhibitors, such as panobinostat, remain a promising class of anti-neoplastic agents. It is worthwhile to pursue rational strategies to study these agents in cancers in which standard treatments need to be improved.
Acknowledgments
This investigator-initiated study was supported by a grant from Novartis and in part by NIH grants P50 CA090440 and UO1CA099168. UPCI shared resources that are supported in part by NIH award P30CA047904 were used for this project. We also thank Ms. Donna Gaspich for her administrative assistance.
Footnotes
Conflicts of Interest
A.A. receives research grant support from Novartis. All other Authors have no conflicts of interest to declare.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 3.Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Cancer Lett. 2008;269:7–17. doi: 10.1016/j.canlet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Crisanti MC, Wallace AF, Kapoor V, Vandermeers F, Dowling ML, Pereira LP, Coleman K, Campling BG, Fridlender ZG, Kao GD, Albelda SM. The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther. 2009;8:2221–2231. doi: 10.1158/1535-7163.MCT-09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro GI, Frank R, Dandamudi UB, Hengelage T, Zhao L, Gazi L, Porro MG, Woo MM, Lewis LD. The effect of food on the bioavailability of panobinostat, an orally active pan-histone deacetylase inhibitor, in patients with advanced cancer. Cancer Chemother Pharmacol. 2012;69:555–562. doi: 10.1007/s00280-011-1758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prince HM, Bishton MJ, Johnstone RW. Panobinostat (LBH589): a potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol. 2009;5:601–612. doi: 10.2217/fon.09.36. [DOI] [PubMed] [Google Scholar]
- 8.Gray JE, Haura EB, Chiappori A, Tanvetyanon T, Williams CC, Pinder MC, Neuger A, Giglia JL, Bepler G, Altiok S. Phase I study of LBH589 in combination with erlotinib for advanced aerodigestive tract cancers. J Clin Oncol. 2010;28(suppl):abstr e13016. [Google Scholar]
- 9.Di Meglio G, Massacesi C, Radice D, Boselli S, Pelosi G, Squadroni M, Spada F, Lorizzo K, De Braud FG, Fazio N. Carboplatin with etoposide in patients with extrapulmonary “aggressive” neuroendocrine carcinoma. J Clin Oncol. 2010;28(suppl):abstr e13072. [Google Scholar]
- 10.Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, Gilchrist J. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18:2493–2499. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- 11.Le Treut JH, Sault M, Chouaid C, Vergnenegre A, Arpin D, Geriniere L, Berard H, Lena H, Paillotin D, Caer HL. Multicentric phase II trial of cisplatin/etoposide chemotherapy in advanced large-cell neuroendocrine carcinoma of the lung (LCNEC): Study GFPC 03-02 from the Groupe Francais de Pneumo-cancerologie. J Clin Oncol. 2010;28(suppl):abstr e18018. [Google Scholar]
- 12.Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, Masson E, Rae P, Laird G, Sharma S, Kantarjian H, Dugan M, Albitar M, Bhalla K. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 13.Ivanova A, Montazer-Haghighi A, Mohanty SG, Durham SD. Improved up-and-down designs for phase I trials. Stat Med. 2003;22:69–82. doi: 10.1002/sim.1336. [DOI] [PubMed] [Google Scholar]
- 14.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280:233–241. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Rasheed W, Bishton M, Johnstone RW, Prince HM. Histone deacetylase inhibitors in lymphoma and solid malignancies. Expert Rev Anticancer Ther. 2008;8:413–432. doi: 10.1586/14737140.8.3.413. [DOI] [PubMed] [Google Scholar]
- 16.Lin R, Hu J, Paul S, Schindler J, Woo MW, Spence S, Hirawat S, Weber HA. Characteristics of thrombocytopenia in pateints treated with oral panobinostat (LBH589) Blood. 2009;114:abstract 2740. [Google Scholar]
- 17.Rathkopf D, Wong BY, Ross RW, Anand A, Tanaka E, Woo MM, Hu J, Dzik-Jurasz A, Yang W, Scher HI. A phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2010;66:181–189. doi: 10.1007/s00280-010-1289-x. [DOI] [PubMed] [Google Scholar]
- 18.Jones SF, Bendell JC, Infante JR, Spigel DR, Thompson DS, Yardley DA, Greco FA, Murphy PB, Burris HA., 3rd A phase I study of panobinostat in combination with gemcitabine in the treatment of solid tumors. Clin Adv Hematol Oncol. 2011;9:225–230. [PubMed] [Google Scholar]
- 19.Prince HM, George D, Patnaik A, Mita M, Dugan M, Butterfoss D, Masson E, Culver KW, Burris HA, 3rd, Beck J. Phase I study of oral LBH589, a novel deacetylase (DAC) inhibitor in advanced solid tumors and non-hodgkin’s lymphoma. J Clin Oncol. 2007;25(suppl):abstract 3500. [Google Scholar]
- 20.Alsina M, Lonial S, Weber DM, Coutre SE, Kang BP, Glynos T, Warsi G, Snodgrass SM, Richardson PG. PANORAMA 2: A phase II study of panobinostat (LBH589) in combination with bortezomib (BTZ) and dexamethasone (DEX) in patients with relapsed and BTZ-refractory multiple myeloma (MM) J Clin Oncol. 2010;28(suppl):TPS308. [Google Scholar]
- 21.Deangelo DJ, Spencer A, Bhalla KN, Prince HM, Fischer T, Kindler T, Giles FJ, Scott JW, Parker K, Liu A, Woo M, Atadja P, Mishra KK, Ottmann OG. Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia. 2013 doi: 10.1038/leu.2013.38. [DOI] [PubMed] [Google Scholar]
- 22.Ottmann OG, DeAngelo DJ, Garcia-Manero G, Lubbert M, Jillella A, Sekeres MA, Zahlten A, Squier M, Acharyya S, Winiger IJ, Fenaux P. Determination of a phase II dose of panobinostat in combination with 5-azacitidine in patients with myelodysplastic syndromes, chronic myelomonocytic leukemia, or acute myeloid leukemia. Blood. 2011;118:abstract 459. [Google Scholar]
- 23.Richardson PG, Alsina M, Weber D, Coutre SE, Lonial S, Gasparetto C, Mukhopadhyay S, Ondovik M, Khan M, Paley C, Schlossman R. PANORAMA 2: Panobinostat combined with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory multiple myeloma. Blood. 2012;120:abstract 1852. doi: 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- 24.Conte P, Campone M, Pronzato P, Amadori D, Frank R, Schuetz F, Rea D, Wardley A, Britten C, Elias A. Phase I trial of panobinostat (LBH589) in combination with trastuzumab in pretreated HER2-positive metastatic breast cancer (mBC): Preliminary safety and tolerability results. J Clin Oncol. 2009;27(suppl):abstr 1081. [Google Scholar]
- 25.Rathkopf DE, Chi KN, Vaishampayan U, Hotte S, Vogelzang N, Alumkal J, Agrawal M, Nydam TM, Fandi A, Scher HI. Phase Ib dose finding trial of intravenous panobinostat with docetaxel in patients with castration-resistant prostate cancer (CRPC) J Clin Oncol. 2009;27(suppl):abstr 5064. [Google Scholar]


