Abstract
Introduction
This work reports on the development of a biodegradable dual-drug-eluting stent with sequential-like and sustainable drug-release of anti-platelet acetylsalicylic acid and anti-smooth muscle cell (SMC) proliferative paclitaxel.
Methods
To fabricate the biodegradable stents, poly-L-lactide strips are first cut from a solvent-casted film. They are rolled onto the surface of a metal pin to form spiral stents. The stents are then consecutively covered by acetylsalicylic acid and paclitaxel-loaded polylactide-polyglycolide nanofibers via electrospinning.
Results
Biodegradable stents exhibit mechanical properties that are superior to those of metallic stents. Biodegradable stents sequentially release high concentrations of acetylsalicylic acid and paclitaxel for more than 30 and 60 days, respectively. In vitro, the eluted drugs promote endothelial cell numbers on days 3 and 7, and reduce the proliferation of SMCs in weeks 2, 4, and 8. The stents markedly inhibit the adhesion of platelets on days 3, 7, and 14 relative to a non-drug-eluting stent. In vivo, the implanted stent is intact, and no stent thrombosis is observed in the stent-implanted vessels without the administration of daily oral acetylsalicylic acid. Promotion of endothelial recovery and inhibition of neointimal hyperplasia are also observed on the stented vessels.
Conclusion
The work demonstrates the efficiency and safety of the biodegradable dual-drug-eluting stents with sequential and sustainable drug release to diseased arteries.
Keywords: sequential-like and sustainable release, biodegradable drug-eluting stents, poly-L-lactide, polylactide-polyglycolide, mechanical properties
Introduction
The use of endoluminal metallic stents in percutaneous transluminal coronary angioplasty (PTCA) has become common in the treatment of arterial stenosis.1 Despite the fact that using stents reduces the rate of restenosis below that achieved using PTCA alone, it remains a serious problem in patients with coronary artery disease. Restenosis is characterized by the formation of neointima by the migration of proliferating medial vascular smooth muscle cells (SMCs) to the intima, which cause stenosis and constrictive remodeling.2 Although great improvements have been made in the design of stents and the materials used in them, the development of in-stent restenosis is still an important issue associated with the use of bare-metal stents (BMS).3 Drug-eluting stent (DES) reduces stent restenosis by delivering drugs directly to the site of the vessel injury. The drug, frequently sirolimus or paclitaxel, inhibits neointimal hyperplasia and reduces the incidence of restenosis in high-risk patients.4 However, although in-stent restenosis rates can be greatly reduced by the use of DES, the implantation of DES increases the incidence of late stent thrombosis, which causes non-lethal myocardial infarction and, thereby, a high mortality rate.5,6 Stent thrombosis is caused by hypersensitivity to drug loading and by incomplete re-endothelialization caused by the anti-proliferative coating of the stent struts.7,8 Delayed re-endothelialization after DES deployment necessitates aggressive dual anti-platelet therapy, which is associated with its own set of complications.9 Another potential drawback of metallic stents is the impairment of images obtained by multi-slice computed tomography (CT) and magnet resonance (MR) tomography.10
To overcome the aforementioned potential limitations, stents that are made of biodegradable materials that may dissolve or be absorbed in the body have been proposed as promising substitutes for metallic stents. The potential advantages of biodegradable stents include the reduction or elimination of late stent thrombosis, improved lesion imaging by CT or MR, facilitation of repeat surgical or percutaneous treatments to the same site, restoration of vasomotion, and freedom from side-branch obstruction by struts and from strut fracture-induced restenosis.11 These polymeric stents initially exhibit enough radial strength to stabilize the vessel wall following implantation and to prevent mechanical recoil.12 Furthermore, various drugs, such as a combination of anti-proliferative and anti-inflammatory drugs to accelerate endothelial healing, can be incorporated to improve cellular or functional specificity.13,14
The Igaki-Tamai stent, which was the first absorbable stent to be implanted in humans, is constructed from poly-L-lactide (PLLA).15 In its degradation process, the hydrolysis of bonds between repeating lactide units forms lactic acid, which enters the Krebs cycle and is metabolized to carbon dioxide and water. Acceptable rates of major adverse cardiac events and scaffold thrombosis, which are similar to those associated with the use of BMS, and without stent recoil and vessel remodeling suggest the long-term safety of the Igaki-Tamai stent.16 Recently, the everolimus-eluting bioabsorbable stents have shown clinical and imaging outcomes similar to those for metallic DES implantation but with the potential problem of size-limited polymeric scaffolds and the risk of late stent recoil.17–19 Venkatraman et al20 developed a bi-layered biodegradable spiral stent that comprised PLLA and poly-D-L-lactide-glycolide (PLGA), that could self-expand at 37°C. However, the minimum time for the full expansion of this bi-layered stent in an aqueous environment was approximately 8 minutes, and the significant initial recoil of the stent with the reduced diameter resulted in a risk of migration or movement of the stent. Chen et al21 developed a self-expandable spiral stent that was made of a chitosan-based film that can rapidly self-expand (150 seconds) in an aqueous medium with a shape memory. Despite the fact that these biodegradable stents have so far provided satisfactory results, most require a complex manufacturing process22 or have a complex structure that may increase the long-term risk of stent fracture upon degradation. An ideal stent should 1) possess sufficient strength to perform its mechanical function; 2) have good longitudinal flexibility to facilitate insertion; 3) deliver adequate drug concentrations to the target site to prevent restenosis via acceleration of re-endothelialization and inhibition of SMC proliferation within days and months, respectively; and 4) be biodegradable after serving its purpose and biocompatible so that the material breakdown process does not cause any tissue irritation. Furthermore, to prevent migration during deployment, cardiologists prefer to be able to control the timing of stent expansion against the vessel during the procedure. A balloon-expandable DES with a simple structure is thus urgently sought.
PLLA is one of the most promising biodegradable biomaterials and has a degradation time of over 2 years.23 It is a semi-crystalline polymer with a glass transition temperature of 60°C–66°C. PLGA, on the other hand, is a biodegradable material that has been approved by the US Food and Drug Administration for use in the manufacture of medical devices. It has been extensively studied as a therapeutic delivery vehicle because of its biodegradability and biocompatibility. Both PLLA and PLGA are non-toxic and tissue compatible, and can be eventually reabsorbed in the vital organs.24,25
In this work, a simple and functional balloon-expandable DES made of a spiral PLLA backbone with acetylsalicylic acid- and paclitaxel-loaded dual-layered PLGA nanofibers was developed and fabricated using a solvent-casting and electrospinning method. To fabricate the biodegradable stents, PLLA strips were first cut from a solvent-casted film. They were rolled onto the surface of a metal pin to form spiral stents. These stents were then covered by acetylsalicylic acid- and paclitaxel-loaded PLGA nanofibers by electrospinning. Scanning electron microscopy (SEM) was utilized to observe the surface morphology of the electrospun nanofibers. The sequential-like and sustainable-release characteristics of the drugs were determined via an in vitro elution method. The cell viability, the proliferation of eluted acetylsalicylic acid and paclitaxel, and the adhesion of platelets to the stents were also examined. Finally, the feasibility of deploying the developed stent in the abdominal aorta of a rabbit model without the administration of daily oral acetylsalicylic acid was evaluated.
Materials and methods
Materials
Acetylsalicylic acid (Bayer HealthCare, Berlin, Germany) and paclitaxel (T1912, Mn =853.93 Da; Sigma-Aldrich Co, St Louis, MO, USA) were used in this study. The biodegradable polymers that were used to form the backbones of the stents were commercially available PLLA with an inherent viscosity of 2.6–3.2 dl/g (Resomer L209S). Two forms of PLGA were used to make the nanofibers; one had a lactide:glycolide ratio of 50:50 (Resomer RG 503) with an inherent viscosity of 0.32–0.44 dl/g and the other had a lactide:glycolide ratio of 75:25 (Resomer RG 756S) and an inherent viscosity of 0.71–1.00 dl/g. All polymers were obtained commercially from Sigma-Aldrich Co. The solvent was 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), which was also purchased from Sigma-Aldrich Co.
Fabrication of biodegradable stents
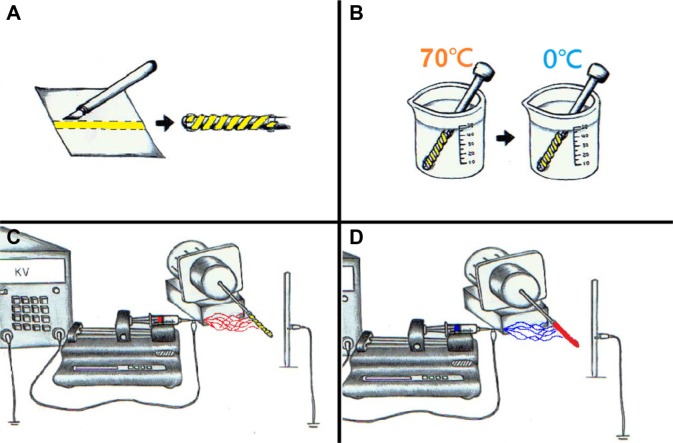
Figure 1 schematically depicts the manufacturing process for biodegradable DES. To fabricate a biodegradable stent, a PLLA strip with an area of 100×3 mm and a thickness of 0.25 mm was first cut from a solvent-cast film. It was rolled onto the surface of a metal pin with a diameter of 3.5 mm and fixed at the two ends with tape (Figure 1A). The metal pin, wrapped by the strip, was placed in an isothermal water bath at 70°C for 10 minutes, and then in a water bath at 0°C for quenching (Figure 1B). When the polymeric material cooled to a state of sufficient rigidity, which occurred when all of it had cooled to below the glass transition temperature of the polymer, a spiral PLLA stent with an internal diameter of 3.5 mm was formed.
Figure 1.
Steps for manufacturing newly developed stents.
Notes: (A) A poly-L-lactide strip was first cut from a solvent-cast film and rolled onto the surface of a metal pin that was fixed at the two ends with tape. (B) The metal pin, wrapped with the strip, was placed in an isothermal water bath at 70°C, and then in a water bath at 0°C for quenching. (C) The electrospinning of acetylsalicylic acid-loaded poly-D-L-lactide-glycolide nanofibers onto the biodegradable stent. (D) The spinning of paclitaxel-loaded nanofibers onto the stent.
The spiral stents were tightly rolled onto another steel pin with a diameter of 0.95 mm, which was connected to a motor. They were first covered by acetylsalicylic acid-loaded PLGA nanofibers as the inner layer so as to favor an early acetylsalicylic acid release after stent implantation, followed by paclitaxel-loaded PLGA nanofibers as the outer layer via electrospinning (Figure 1). The electrospinning set-up in this work involved a syringe and needle with an internal diameter of 0.42 mm, a ground electrode, a metallic pin/PLLA stent that was mounted on a motor, a collection plate, and a high voltage supply. The needle was connected to the high voltage supply, which generated positive direct current voltages and currents of up to 35 kV and 4.16 mA/125 W, respectively. The rotational speed of the motor was 300 rpm. PLGA with a lactide:glycolide ratio of 50:50 (30 mg) and acetylsalicylic acid (5 mg) were dissolved into 1 mL of HFIP that contained 0.1 mL of contrast dye (Omnipaque, Iohexol 350 mg/mL; GE Healthcare). Separately, PLGA with a lactide:glycolide ratio of 75:25 (30 mg) and paclitaxel (5 mg) were dissolved into the same solution. The PLGA 50:50/acetylsalicylic acid solution was first delivered and electrospun by a syringe pump with a volumetric flow rate of 3.6 mL/h, to yield nanofibers on the spiral stent. The distance between the needle tip and the ground electrode was 10 cm, and the positive voltage that was applied to the polymer solution was 17 kV. All electrospinning experiments were conducted at room temperature. Then, another layer of PLGA 75:25/paclitaxel nanofibers were spun. After electrospinning, the spiral PLLA stents were covered by both the PLGA 50:50/acetylsalicylic acid as the inner layer (acetylsalicylic acid 25 μg/mm2, 3,000 μg) and PLGA 75:25/paclitaxel nanofibers as the outer (paclitaxel, 20 μg/mm2, 3,000 μg). The stents were then taken off the pin. Constrained by the nanofibers, the PLLA stent could not regain its shape after it was removed from the pin. All nanofiber-mounted stents were placed in a vacuum oven at 40°C for 72 hours to evaporate off the solvent.
To test the expandability of fabricated stents, the biodegradable stent was passed over a commercial balloon (Maverick 3.5×20 mm; Boston Scientific, Natick, MA, USA) to enable it to be expanded. The stent and the balloon were placed inside a flexible plastic tube with an internal diameter of 3.5 mm to be inflated by a commercially available manual balloon catheter pump (Perouse Medical, France).
Compression test
The compression test was carried out on a LLOYD tensiometer. A 2.5 kN load cell was used, and the cross-head speed was 1 mm/min. The stent to be tested was compressed in the radial direction. Deformations under various loads were recorded. Three specimens of each material were tested.
Stability of stent in phosphate buffer
Weight variation of the biodegradable stent was monitored by immersing the stents in a phosphate buffer, 0.15 mol/L (pH 7.4), at 37°C. The samples were removed from the solutions, dried inside an oven for 24 hours, and weighed at 60 days. The weight retention (WR, %) of the stents was calculated as follows:
| (1) |
where W0 and W were the initial weight of the stents and the weight of the stents at different weeks, respectively.
SEM observations
After the electrospun nanofibers were coated with gold, the morphology was observed using an SEM (Hitachi S-3000N; Tokyo, Japan). The average diameter of ten randomly selected nanofibers and the distribution of their diameters were determined.
The densities of the electrospun nanofibers were calculated by dividing their mass by their volume. The apparent porosity of the nanofibrous membranes was calculated using the following equation:
| (2) |
where ρmembrane and ρpolymer are the densities of the nanofibrous membrane and the polymer, respectively.
In vitro elution of acetylsalicylic acid and paclitaxel from stents
An in vitro elution approach was utilized to characterize the release of acetylsalicylic acid and paclitaxel from the biodegradable stents. A 0.15 mol/L phosphate buffer (pH 7.4) was used as the dissolution medium. The stents were placed in glass test tubes with 1 mL of phosphate buffer. All tubes were incubated at 37°C. The dissolution medium was collected at 24-hour intervals. The extracted medium was subsequently analyzed using high-performance liquid chromatography (HPLC). Fresh phosphate buffer (1 mL) was then added. This procedure was repeated every 24 hours for 60 days.
HPLC analysis
The concentrations of acetylsalicylic acid and paclitaxel in the buffer in the elution studies were determined from the standard curves of HPLC assay for the drugs. The HPLC analyses were conducted on a Hitachi D-2000 Elite Delivery System. The column that was used for separation of the acetylsalicylic acid and paclitaxel was a ZORBAX ODS, C18, 5 μm, 4.6×250 mm HPLC column. The mobile phase contained distilled water and acetonitrile (Mallinckrodt, St. Louis, MO, USA) (50/50, v/v). The absorbencies at 254 nm and 227 nm for acetylsalicylic acid and paclitaxel, respectively, were monitored at a flow rate of 1.0 mL/min. All samples were assayed in triplicate, and sample dilutions were conducted to bring the unknown concentrations into the range of the assay standard curve. A calibration curve was plotted for each set of measurements (correlation coefficient >0.99). The HPLC calibration of the standard curves for acetylsalicylic acid and paclitaxel with six standard concentrations was performed as follows:
| (3) |
| (4) |
The elution products were identified and quantified with high sensitivity using the HPLC system.
Effect of drug loading on platelet adhesion and cell cultures
The DES for eluents at three time points (3, 7, and 14 days) were tested for platelet adhesion. Blood was drawn from a healthy rabbit before the in vivo DES implantation and mixed with 3.2% sodium citrate in 1/9 volume ratio. Platelet-rich plasma (PRP) was obtained by centrifugation at 150 g for 10 minutes. The blood was maintained at 22°C before the PRP was separated. The number of platelets in the PRP was 2×105 cells/μL, determined using a semi-automated hematology analyzer (SYSMEX F820). Fifty microliters (107 platelets) of the platelet suspension was placed on the surfaces of the nanofibers at the three time points, and incubated at 37°C for 3 hours. Following incubation with PRP, the plates were washed three times in phosphate-buffered solution (PBS). The platelets that adhered to the fiber surface were mixed with 1% glutaraldehyde and immersed in PBS before being stood in the fixative for 60 minutes at 4°C. The adhesion of platelets to the stents was determined by counting the adherent cells in the SEM images. Cells on the surface of the stent were counted in 20 rectangular fields that were selected at random. Adherent cells in these photographs (enlargement ×1,000, area 11,136 μm2) were counted manually. From these data, the mean densities of the adherent platelets per mm2 were calculated.
The natural history of endothelial regrowth begins within the first 24 hours after arterial denudation.26 Meanwhile, paclitaxel exhibits anti-proliferative properties against endothelial cells. Since the biodegradable stents include controlled-release minimum paclitaxel concentrations for the first 3 weeks, the influence of eluted paclitaxel on the proliferation of endothelial cells was examined. Endothelial cells were isolated from human umbilical cord veins (HUVACs) via a modified collagenase method.27 Cells were grown to confluence in medium 199 that was supplemented with 20% fetal bovine serum (FBS) at 37°C in a humidified 95% air/5% CO2 incubator. Confluent cultures of endothelial cells at passage 3 were used in the experiments.
The vascular SMCs multiply three to five times, accounting for 90% of the ultimate intimal proliferation over the first 2 weeks after injury.28 Inhibition of SMC proliferation by paclitaxel was confirmed after 2 weeks. Rat vascular SMCs29 were prepared by the enzymatic digestion of the thoracic aortic medium from adult Sprague-Dawley rats and cultured in Dulbecco’s Modified Eagle’s Medium and supplemented with 10% FBS.30 The cells used in all of the experiments were taken from the fourth passage and cultured on conventional uncoated dishes.
The proliferative activities of cells were measured by 5-bromo-2-deoxyuridine (BrdU) incorporation using an enzyme-linked immunosorbent assay (ELISA) detecting kit (Roche, Mannheim, Germany), following the manufacturer’s instructions. The cytotoxicity test was performed on elution products that had been treated for 24 hours and washed once with PBS, before 1 mL M199 that contained 0.05 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to them. Following incubation at 37°C for 1 hour, the media were removed and formazan crystals in the cells were solubilized in 1 mL dimethyl sulfoxide (DMSO) to obtain an optical density (OD) reading at 570 nm using a spectrophotometer.
Animal study
A total of 20 adult male New Zealand white rabbits with an average weight of 3.2±0.4 kg were used in the animal study. They were housed in individual cages in a temperature- and light-controlled room and given standard rabbit chow ad libitum with free access to sterilized drinking water. All animal procedures were institutionally approved, and all of the studied animals were cared for under the supervision of a licensed veterinarian in a manner consistent with the regulations of the National Institute of Health of Taiwan.
Rabbits were divided into two groups: group A (n=12) received a balloon injury followed by the deployment of a biodegradable DES; group B (n=8) received only a balloon injury. To perform the balloon injury, the rabbits were sedated and anesthetized with a muscular injection of xylazine (9.3 mg/kg), and with the administration of Zoletil 50™ (tiletamine-zolazepam 10 mg/kg) and oxygen (2 L/min) through a face mask. To mimic the coronary artery, rabbit aorta was selected for implantation in terms of its diameter and the patency of small arterial branches after stent deployment.31,32 A 6 French sheath was inserted into the femoral artery using the puncture technique. Briefly, the technique for femoral artery cannulation is the Seldinger technique, also known as ‘catheter over wire’. The location of the vessel was first determined by palpation of the arterial pulse. The overlying skin was prepared with chlorhexidine, and a small nick was made on the skin at the needle entry site. The femoral artery was punctured and the needle was advanced slowly until pulsating blood flow was achieved. After the guidewire was deployed, the needle over the wire was removed and the catheter was advanced over the guidewire into the vessel. The guidewire was then removed, leaving the catheter in place. Finally, the successful artery cannulation was confirmed by pulsatile blood flow from the catheter.
During the procedure, the rabbits first underwent endothelial denudation of the descending abdominal aorta using a 3.5×20 mm Maverick™ balloon to cause an angioplasty balloon injury. The balloon was passed three times over a 0.014 inches guidewire to the aorta, inflated to nominal pressure (8 atm with 50% (v/v) contrast/saline) and withdrawn in a retrograde manner to the low descending abdominal aorta.
The biodegradable DES was deployed in 12 rabbits in the stented group via the guidewire and balloon. When the stents reached the target sites, they were expanded for 30 seconds (8 atm) to a diameter of 3.5 mm, yielding a stent-to-artery diameter ratio of 1.2:1. Acetylsalicylic acid (40 mg/day) was administered orally as an anticoagulant to all rabbits 24 hours before catheterization. The drug was continuously administered to the rabbits in the balloon injury only group throughout the in-life phase of the study, whereas no drug was administered to the stented group. A single dose of intra-arterial heparin (150 IU/kg) was administered to each rabbit upon catheterization.
The hemocompatibility of the implanted stent and the patency of the artery were evaluated using an iE33 ultrasound device (Philips Medical Systems, Best, the Netherlands) and by angiography at 3 days (from group A, n=4) and at 4 weeks (group A, n=8; group B, n=8). Endothelial function of the abdominal aorta was performed via ultrasound evaluation at 4 weeks.33 On day 28, marginal ear veins were cannulated for endothelium-dependent vasomotor function at 5–10 mm distal from non-stented reference segments and evaluated following the infusion of two incremental doses of acetylcholine (Ach) 0.05 and 0.5 μg/mL/min. Ach was delivered via an infusion pump (Harvard Apparatus, Holliston, MA, USA) at 1 mL/min for 3 minutes, with intervals of precisely 5 minutes between each injection. Endothelium-independent function was evaluated using nitroglycerine (NTG) 5 μg/mL/min. Two-dimensional and color Doppler ultrasound were performed before and 30 seconds after each drug administration.
The anesthetized animals were then exsanguinated by a lethal-dose injection of lidocaine (100 mg/kg). Explanted vessels were flushed with lactated Ringer’s solution before fixation.
The vessels that were and were not treated with a stent were collected for microscopic observation and histological examination of both inflammatory and injury responses following standard guidelines.34,35 Briefly, the inflammation scale was 0= none; 1= mild, including minimal infiltrated inflammatory cells; 2= moderate; and 3= severe, with large clusters of inflammatory cells with granulomatous morphology. Vessel injuries were scored on the following scale: 0= strut not in contact with internal elastic lamina (IEL); 1= strut in contact with IEL and profile in neointima; 2= strut penetrates IEL with profile in media; 3= strut penetrates media and is in contact with external elastic lamina; and 4= strut is in adventitia. The scores of all struts were averaged to obtain the mean score for each of the 72 histological sections of eight stented arteries and eight balloon-injury arteries in 16 animals on day 28 (n=16), respectively.
Immunofluorescence
The chemicals that were used in the assay were obtained from Sigma-Aldrich Co. The fluorescent dyes were purchased from Molecular Probes Inc. (Eugene, OR, USA). The tissue samples on day 28 (n=16) were embedded in optimal cutting temperature compound before they underwent frozen sectioning on a microtome-cryostat. To perform immunostaining, frozen sections were washed in PBS and blocked with 2% bovine serum albumin for 30 minutes at room temperature. The sections were then incubated for 1 hour at room temperature with primary antibodies against calponin and diluted in blocking solution. Nuclei were visualized by diamidine-2-phenylindole (DAPI) staining. The experiments were performed in triplicate.
Western blot analysis
For Western blotting, immunoblotting was carried out using anticalponin (Dako, CA, USA), and antitubulin (Santa Cruz, CA, USA) antibodies as primary antibodies. Signals were detected using the enhanced chemiluminescence-detection method (Amersham, Netherlands) and quantified by densitometry. The amount of the protein of interest was expressed relative to the amount of tubulin.
Statistical analysis
All data are presented as mean ± standard deviation. Differences between two groups were identified by the unpaired t-test. One-way analysis of variance (ANOVA) was used to find statistically significant differences. Within ANOVA, a post hoc Bonferroni method for multiple comparisons was applied to detect significant differences between pairs. Differences were regarded as statistically significant at P<0.05. SPSS software (version 17.0 for Windows; SPSS Inc., Chicago, IL, USA) was used to analyze the data.
Results and discussion
In this work, biodegradable dual-drug-eluting spiral stents were successfully fabricated by the solvent-casting and electrospinning technique (Figure 1). Figure 2 presents the SEM of the electrospun drug-loaded nanofibers at a magnification ×6,000. The diameters of the electrospun PLGA/acetylsalicylic acid nanofibers ranged from 530 nm to 710 nm, and the porosity of the nanofibrous membrane was calculated to be 75.3%±0.8%. The densities of the nanofibers and polymer were 0.33 and 1.34 g/cm3, respectively. The diameters of the spun PLGA/paclitaxel nanofibers ranged from 690 nm to 1,210 nm, while the porosity of the nanofibrous membrane was found to be 70.5%±1.4%. The densities of the nanofibers and polymer were 0.38 and 1.3 g/cm3, respectively (porosity, P<0.01).
Figure 2.
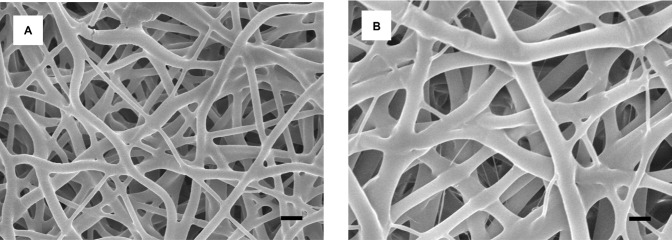
Scanning electron microscopy images of electrospun nanofibers.
Notes: (A) Acetylsalicylic acid nanofibers with a diameter of 530–710 nm and 75.3% porosity. (B) Paclitaxel-loaded nanofibers with a diameter of 690–1,210 nm and 70.5% porosity (scale bar: 1 μm).
PLGA is a biodegradable and non-toxic material that has been extensively investigated as a therapeutic delivery vehicle. The presence of drugs in the PLGA nanofibers could increase the biocompatibility of the electrospun matrices and act as an excellent scaffold, promoting cell proliferation and growth.36 Furthermore, nanofibers with high porosities advantageously allowed the red blood cells to pass to the branch vessels, minimizing the compromising of those branch vessels following the implantation of the biodegradable stents.
Inflation of balloon-expandable biodegradable stents and mechanical strengths of stents
Figure 3 displays the expansion of the stent inside a plastic tube with an internal diameter of 3.5 mm. The fabricated biodegradable stent was passed over a commercial balloon (Figure 3A) to enable it to be expanded. During inflation of the balloon, the stents with the nanofibers expanded. When the balloon pressure reached 8 atm, the nanofibers on the surface of the PLLA spiral stent broke and a crevice in the membrane was observed in the longitudinal direction. The nanofibers no longer constrained the PLLA stents, which therefore expanded freely with the elastic recovery of the polymeric materials. The force exerted by the balloon also promoted the expansion. A spiral stent with internal and external diameters of 3.5 mm and 3.75 mm, respectively, and two layers of drug-loaded nanofibers were thus obtained. With the release of the balloon pressure, the stent retracted somewhat but remained attached to the plastic tube owing to the elastic recovery force of the polymers. Finally, the balloon was removed, leaving the stent and the nanofibers in situ (Figure 3B).
Figure 3.
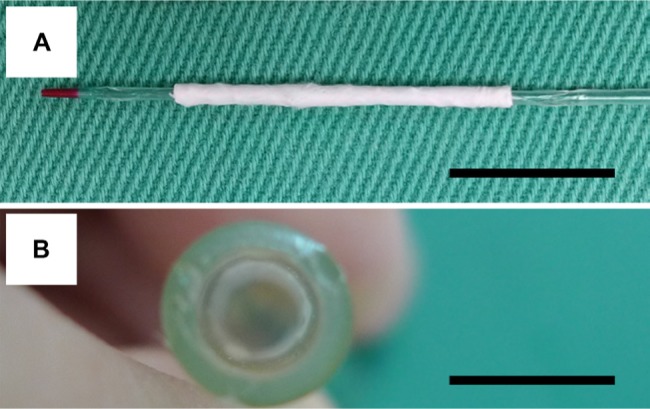
Balloon expansion of a biodegradable stent inside a plastic tube.
Notes: (A) The electrospun nanofibers mounted on a spiral poly-L-lactide stent (3.5×20 mm). (B) During inflation of the balloon, the stents with the nanofibers expanded inside a plastic tube. The balloon was removed, leaving the stent and the nanofibers in situ (scale bar: 10 mm).
The compression strengths of the stents fabricated as described above were measured using a tensile tester and compared with those of commercially available metallic stents (Driver 3.5 mm ×20 mm; Medtronic, Minneapolis, MN, USA). The measurements in Figure 4 suggest that biodegradable stents had higher mechanical strengths than metallic stents. Moreover, after being eluted in PBS for 60 days, the stents did not exhibit any obvious change in geometry or weight, perhaps because the total degradation time of PLLA exceeds 2 years. The degradation of PLLA after 60 days of elution in PBS was minimal, and no obvious difference in diameter compared with day 0 (3.5±0.1 vs 3.4±0.1 mm, P=0.96) was observed between the eluted specimens.
Figure 4.
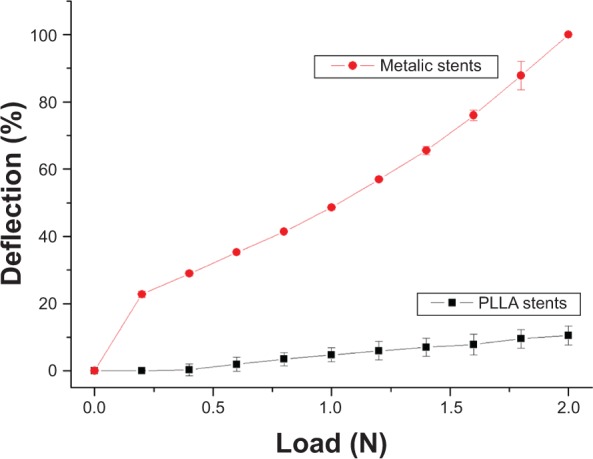
Comparison of biodegradable drug-eluting stents and metallic stents under compression. Biodegradable spiral stents had higher mechanical strengths than metallic stents.
Abbreviation: PLLA, poly-L-lactide.
An earlier study reported a drop in the collapse pressure of PLLA stents that was greater when more of the drug was incorporated more than five percentage of weight.37 To retain the mechanical properties of the PLLA material, the drug concentration should not exceed 1 wt%. The use of a solvent-cast PLLA backbone and electrospun nanofibers can increase the drug loading of biodegradable stents without detectably reducing their mechanical strength.
Self-expandable stents have been developed by other researchers, but the expansion of these stents mainly depends on the elastic or shape memory of the materials from which they are made.12,13,16 Nevertheless, the shape memory of the stents may vary among materials, and may be degraded by environmental conditions. Moreover, the self-expansion process may require heating to a high temperature (such as 70°C) and take many minutes or even days. Unlike these previous bioabsorbable stents, the spiral PLLA stent that was developed in this work is balloon expandable and has excellent mechanical strength that enables it to resist the external pressure of the blood vessels after it is expanded using a balloon. The developed stent was fully expanded at relatively low pressure (8 atm) within a short duration (30 seconds). This pressure is similar to the deployment pressure that is used for metallic stents.15 The recoil and change in the longitudinal dimension of the stents following expansion are minimal. Additionally, the stent delivery and deployment system uses conventional angioplasty balloon catheters. The use of similar deployment techniques as currently used in clinical practice shortens the learning curve, depends on familiar clinical principles and experience, and thereby fosters clinical acceptance. This would provide advantages in terms of endoluminal deployment relative to the aforementioned stents.
Release of acetylsalicylic acid and paclitaxel from PLLA stents
The release curve of acetylsalicylic acid from drug-loaded stents/nanofibers, plotted in Figure 5 from the results of the HPLC analysis, revealed two stages of drug release: an initial burst and a more gradual and sustained drug release. The release characteristic of paclitaxel was steady and slow for the first 2 weeks, with a minor peak on day 10, a major peak on day 21, and two other peaks on days 35 and 45. Whereas the nanofiber provided sustained release of acetylsalicylic acid for 30 days, it had released 50% of the loaded paclitaxel after 60 days of elution in the PBS. The combined release curves of the DES/nanofibers exhibited ‘sequential-like’ release behavior: a high concentration of acetylsalicylic acid was released in the first few days, and then a high concentration of paclitaxel was released from day 10.
Figure 5.
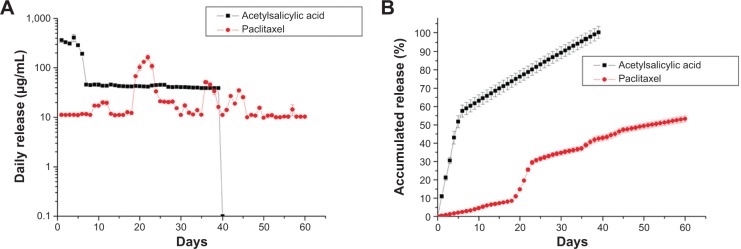
Effect of drug loading on rate of release by biodegradable stents.
Notes: The combined release curves of the drug-eluting stent/nanofibers exhibited ‘sequential-like’ release behavior: a high concentration of acetylsalicylic acid was released in the first few days, and then a high concentration of paclitaxel was released from day 10.
In this study, the in vitro release curves of biodegradable stents exhibited an initially high release of acetylsalicylic acid during the first few days. After the electrospinning process, most pharmaceutics were dispersed in the bulk of the PLGA matrix; however, some drugs might be located on the surface of the nanofibers. This led to the initial burst release of acetylsalicylic acid. Meanwhile, the surface PLGA/acetylsalicylic acid nanofibrous membrane acted as a barrier to the drugs at the outer nanofibrous membrane; the initial burst release of paclitaxel was thus reduced. Moreover, the time of peak drug release can be varied by adjusting the weight percentage of the drug in the nanofibers.22 Following the initial burst, drug release was controlled by osmotic pressure and polymer degradation. In a hydrophobic PLGA matrix, water will be taken up by the poorly water-soluble paclitaxel,38 with high osmotic pressure through the polymer matrix, causing swelling of the drug. Then, the polymer matrix may break under this swelling to form openings for drug release. Furthermore, when the polymer molecular weight decreases sufficiently, loss of polymer begins. The drug will then be released along with this polymer loss. The combined effects of these two factors (osmotic pressure and polymer degradation) may thus lead to the daily peaks of release of paclitaxel from the nanofibers. Overall, this work demonstrates the ‘sequential-like’ release behavior of dual-layered nanofibers, which release a high concentration of acetylsalicylic acid in the first few days, and then sustain the release of a high concentration of paclitaxel after 10 days.
Despite the fact that electrospinning may produce a non-uniform distribution of nanofibers on the stents, the experimental results demonstrated that the biodegradable PLLA stents herein released high concentrations of acetylsalicylic acid and paclitaxel for more than 30 and 60 days, respectively.
Test of adhesion of platelets to DES and responses of cells to these eluents
The effectiveness of the acetylsalicylic acid that was released from the DES was determined by SEM observation in platelet adhesion tests on days 3, 7, and 14. Biodegradable stents/nanofibers without drug loading were prepared as controls. The results in Figure 6 suggest that the released drug effectively inhibited the adhesion of platelets. Significantly fewer platelets adhered to the nanofibers with drug-loaded stents than to those without (day 3: 451±34 vs 4,339±463/mm2; day 7: 448±38 vs 6,544±680/mm2; day 14: 455±41 vs 10,998±727/mm2, all P<0.01) (Figure 7).
Figure 6.
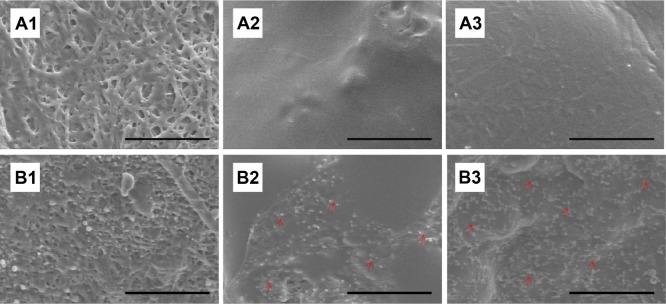
Platelet adhesion test in vitro.
Notes: Following immersion of the drug-loaded and non-loaded nanofibrous membranes in platelet-rich plasma, significantly fewer platelets adhered to the drug-loaded nanofibers at 3 (A1), 7 (A2), and 14 (A3) days than to the non-loaded nanofibers (B1–3) on days 3, 7, and 14, respectively. Red arrows indicate platelet adhesion. B2 exhibits larger platelet aggregates and more extensive platelet pseudopod formation than B3 (scale bar: 50 μm).
Figure 7.
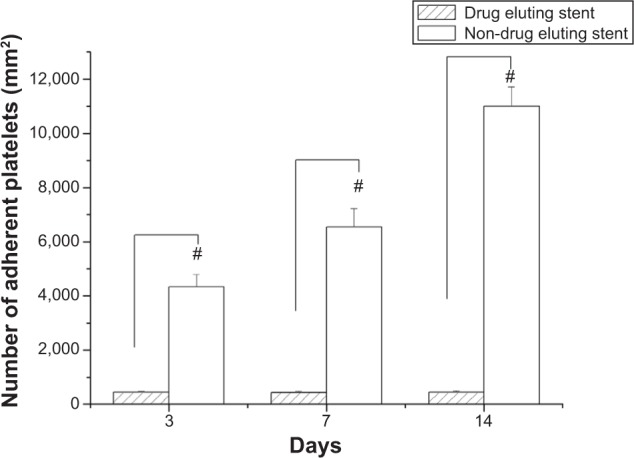
Number of adherent platelets.
Notes: Following immersion of the two groups of stents in platelet-rich plasma for 3 hours, significantly fewer platelets adhered to the drug-loaded stents than to the non-drug-loaded stents on days 3, 7, and 14 (#P<0.01).
Following vascular injury, platelets are implicated in the pathogenesis of atherothrombosis and inflammation. The extent of activation and adherence of platelets is higher in patients who have clinical events.39–42 Biodegradable stents with combined acetylsalicylic acid and paclitaxel can prevent the activation of platelets and reduce the formation of platelet thrombus. This characteristic may underlie the protective effect of stenting treatment followed by subsequent local platelet recruitment.43 Figures 6 and 7 reveal that significantly fewer platelets adhered to the drug-loaded stents, and the interaction of the two drugs in the stent system may have additively improved the adhesion of platelets.14
BrdU assays revealed that the eluent promoted the proliferation (P<0.05) of HUVACs over that in the control group on day 3. The rate of cell growth declined with time after day 14 (Figure 8). The reduction of cell proliferation on days 14 and 21 to below that of the control group and day 7 was significant (P<0.05). The BrdU assays from vascular SMCs indicated that the eluents significantly reduced the proliferation of cells below that in the control group (P<0.05) on days 14, 28, and 56 (Figure 9). The MTT results also demonstrated comparable cell viability on days 0, 7, 14, 21, 28, 35, 42, 49, and 56 (ANOVA P=0.169).
Figure 8.
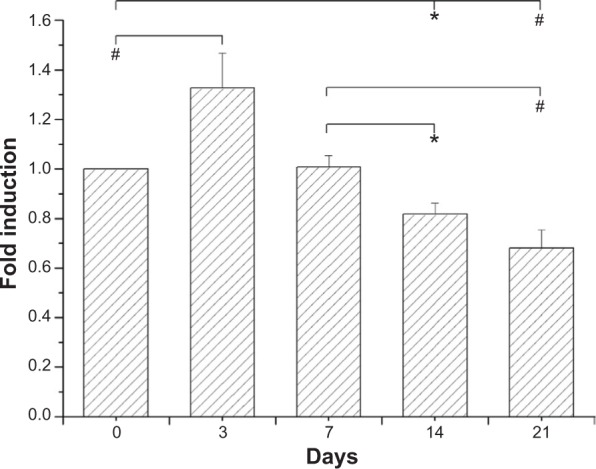
Incorporation of BrdU into HUVACs was assayed by BrdU cell proliferation ELISA, as described in the Methods section.
Notes: Data from BrdU assays revealed that elution on day 3 promoted the proliferation of HUVACs over that of the control group. The reduction of cell proliferation on days 14 and 21 relative to the control group and day 7 was also significant. Each value (mean ± standard error [n=4]) is expressed as a multiplier of the amount of BrdU incorporated into control cells (*P<0.05; #P<0.01).
Abbreviations: BrdU, 5-bromo-2-deoxyuridine; ELISA, enzyme-linked immunosorbent assay; HUVACs, human umbilical cord veins.
Figure 9.
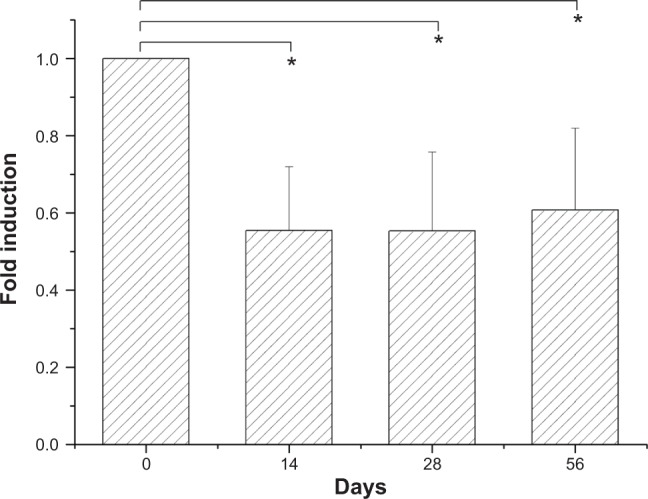
Incorporation of BrdU into vascular SMCs was assayed by BrdU cell proliferation ELISA, as described in the Methods section.
Notes: These BrdU assays revealed that elution reduced the proliferation of cells below that of the control group as detected on different days. Each value (mean ± standard error [n=4]) is expressed as a multiplier of the amount of BrdU incorporated into control cells (*P<0.05).
Abbreviations: BrdU, 5-bromo-2-deoxyuridine; ELISA, enzyme-linked immunosorbent assay; SMCs, smooth muscle cells.
The results of the BrdU and MTT assays in this work verify that the biodegradable stents significantly reduce the proliferation of vascular SMCs with no obvious cytotoxicity against them. Paclitaxel, apart from being able to inhibit the proliferation of SMCs, inhibits the growth of endothelial cells, resulting in incomplete endothelization and potential thrombosis of the arteries. Unlike commercially available DES, which release high concentrations of paclitaxel immediately after their deployment, the biodegradable stents that were developed in this work released only low concentrations of paclitaxel for the first few days. Their inhibition of the growth of endothelial cells is minimal. This fact may explain the effective increase in proliferation of HUVACs in the first few days, and the minimization of the delayed endothelialization that causes acute stent thrombosis.
In vivo animal study
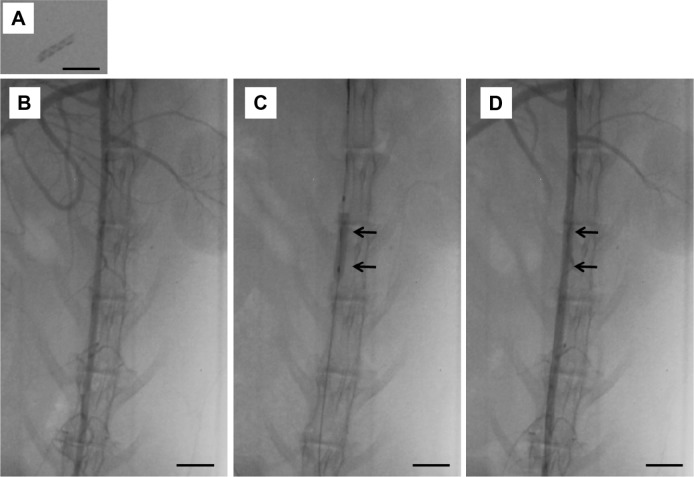
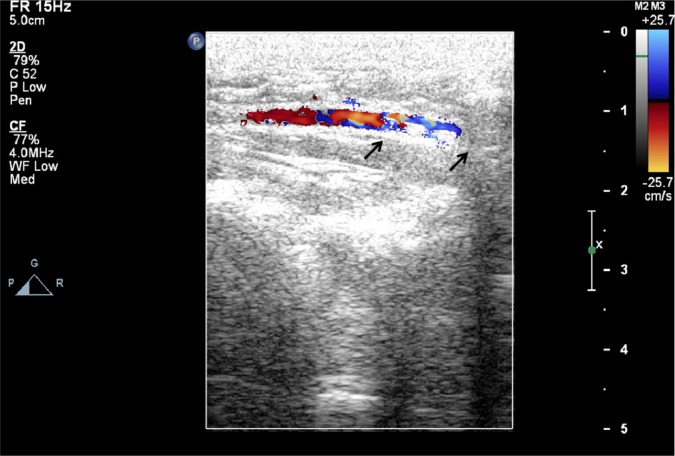
Figure 10 shows photographs of the delivery of the deployed biodegradable stent/nanofibers in the abdominal aorta of the rabbit. No migration of the implanted stent was observed 3 days and 4 weeks following the procedure. Peripheral vascular ultrasound and angiography suggested that the stented abdominal aorta was patent in all of the test animals (Figure 11 and supplemental video).
Figure 10.
The delivery of the developed stent in the descending abdominal aorta of the rabbit.
Notes: (A) Photographs of stent on X-ray film. (B and C) A balloon was used to deliver biodegradable stents into the rabbit abdominal aorta. (D) No migration of the implanted stent was observed after deployment and dye injection (scale bar: 10 mm). Arrows indicate stent site.
Figure 11.
No migration of the implanted stent (arrows) was observed at 3 days and 4 weeks following the procedure, and the stented abdominal aorta was patent for all test animals based on the peripheral vascular ultrasound study (see supplemental video).
Changes in the diameter of the abdominal aorta in response to Ach infusions were examined for endothelial function (Figure 12). Endothelial function was assessed following 4 weeks of stenting treatment. Endothelia-dependent vasodilatory responses to Ach were significantly stronger in group A than in group B. The group A spiral stents with sequential-like and sustained release of acetylsalicylic acid exhibited a more vasodilatory response than did the groups treated with no stents (P<0.01).
Figure 12.
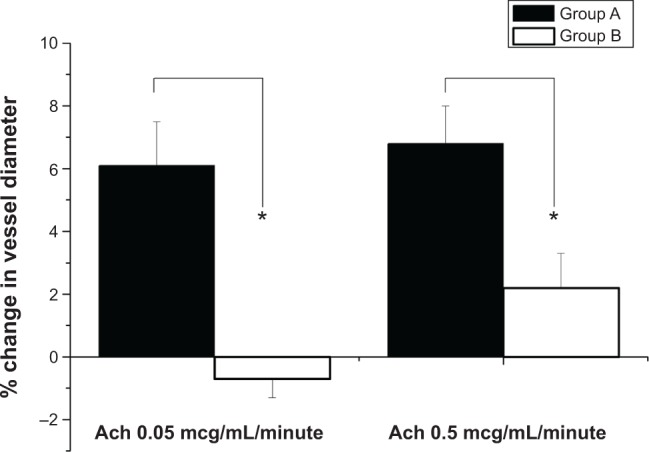
In vivo assessment of endothelial function in rabbits.
Notes: Change in diameter of abdominal aorta in response to acetylcholine infusions. Endothelial function was evaluated after 4 weeks of stenting treatment. Endothelial-dependent vasodilatory response to Ach was maintained in drug-loaded stenting for a significantly greater period than in balloon-injury aorta (*P<0.01).
Abbreviation: Ach, acetylcholine.
Various pharmaceuticals are under investigation in animals and in humans for use in combination with DES to promote endothelial recovery and prevent restenosis. Reduced arterial endothelium-dependent vasodilation is a key role in the pathophysiology of in-stent restenosis. Endothelial dysfunction would progress atherosclerosis in coronary arteries in patients who have undergone percutaneous coronary intervention.44 Moreover, the recovery of endothelial dysfunction provides therapeutic options to prevent in-stent restenosis. The beneficial effects of endothelium-dependent vasodilation have been demonstrated in patients with coronary artery disease.45 In this study, vasodilation was also increased via local controlled drug therapy. Therefore, improved functionality of the endothelium, as the major regulator of vascular homeostasis by sequential-like release of acetylsalicylic acid and paclitaxel, might maintain the above vasoprotective effects.
Paclitaxel has been proven to be clinically effective in inhibiting arterial SMC proliferation and migration and in preventing the formation of neointima following stenting.46,47 However, stent thrombosis remains a serious complication following either BMS or DES treatment, and its occurrence is associated with 40%–50% mortality and a composite death/myocardial infarction rate of 50%–70%.48,49 Stent thrombosis has been shown to be a multileveled process in which the vasculoprotective reserve is overcome by prothrombotic factors.50 The release of a high concentration of acetylsalicylic acid from drug-loaded biodegradable stents after deployment may thus solve the problem of incomplete endothelization by preventing the adhesion of activated platelets and the associated stent thrombosis. However, the sustained release of paclitaxel from the biodegradable drug-eluting PLLA stents should be able to control or reduce the growth and migration of SMCs and also prevent inflammatory responses, which are the dominant causes of neointimal proliferation and in-stent restenosis.51 The sequential-like release of acetylsalicylic acid and paclitaxel from the biodegradable stents may therefore provide an ideal solution to the prevention of arterial thrombus and restenosis.
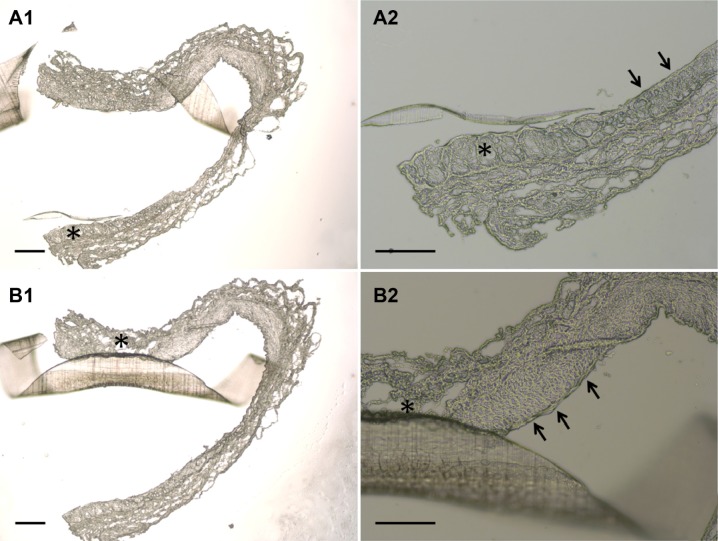
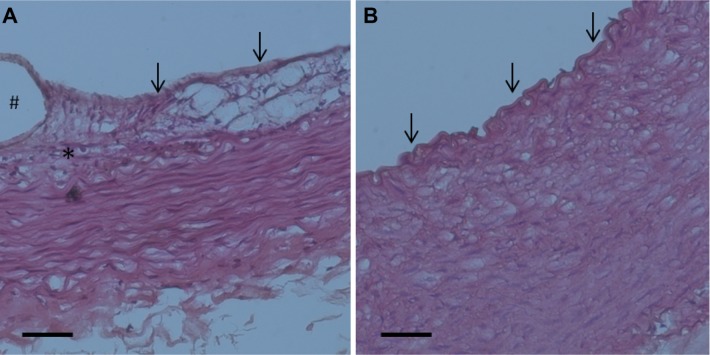
Following incision of the aorta, the implanted stent was found to be intact. Furthermore, the photomicrographs of the cross-sections showed that no thrombus was formed in the stent-implanted vessel (Figure 13). These results demonstrate the feasibility of the deployment of the developed biodegradable stent in an artery using a 6 French sheath. Additionally, the vessel segments from treated animals had endothelial injury comparable with other vessel areas on day 3 (Figure 13). Moreover, by day 28, group A exhibited reconstituted anatomically intact endothelium without significant intimal hyperplasia, but group B exhibited consistent intimal hyperplasia, with a thickness of approximately 75 μm (Figure 14).
Figure 13.
Photomicrographs of cross-sections showing the condition of the stent on the vessel lumen three days following balloon angioplasty and stent deployment.
Notes: Segment of vessel of a treated animal shows a characteristic fragment of a spiral stent, wide vessel lumen without thrombus formation and non-significant stenosis without a lumen reduction of greater than 50% (A1, and B1, 40×) (scale bar: 250 mm). Additionally, segment of vessel of a treated animal has greater endothelial injury (asterisk) comparing with other vessel area (single arrow) (A2, and B2, 100×) (scale bar: 100 mm).
Figure 14.
Photomicrographs of representative rabbit vessel sections 28 days after intervention.
Notes: Four-week high-power photomicrographs (hematoxylin-eosin staining, ×200) of pathology of groups A and B were shown. Inflammation response was low in stent groups (A). By day 28, an anatomically intact endothelium had been re-constituted in group A (almost no intimal hyperplasia), and groups (B) exhibited consistent intimal hyperplasia with a thickness of approximately 100 μm. A single arrow represents the endothelial cells, and asterisk indicates the inflammatory cells surrounding the stent struts. Scale bar: 50 mm. # indicates the stent strut area.
The spiral stent also demonstrated its potential to inhibit thrombus formation following intervention, even without administration of oral acetylsalicylic acid. Otherwise, no difference in the inflammatory and injury responses was observed between animals in the balloon-injury-only group (control) and those in the biodegradable stent group (0.54±0.10 vs 0.58±0.11) (P=0.79). Vascular injury scores were also comparable between the two groups at week 4 (1.69±0.23 vs 1.68±0.24) (P=0.84). No toxic or allergic side effects were noted in any animal at any time in the study.
Local delivery of drugs is preferred to systematic therapy as a treatment of restenosis, mainly because of the systemic side effects that are associated with the anti-restenotic compounds used. In this work, PLGA was used in the electrospinning of acetylsalicylic acid- and paclitaxel-loaded nanofibers onto the surface of spiral PLLA stents to provide local drug delivery. Various polymeric materials have been utilized for this purpose, based on their capacity to provide the sustained release of pharmaceuticals. The PLGA form depends on the lactide:glycolide ratio used in the polymerization. The copolymer with a 50:50 lactide:glycolide ratio degrades faster than that with a 75:25 ratio. The use of PLGA with different lactide:glycolide ratios allows the release rates of various pharmaceuticals to be controlled.
Additionally, the use of dual-layer electrospinning enables the formation of nanofibers that released different drugs sequentially. These nanofibers eliminate the risk of toxicity that is associated with paclitaxel and offer the advantage of sustained drug release for cardiovascular applications. According to Figure 14, an anatomically intact endothelium was reconstituted in the treatment group, and no obvious adverse result was noted during the 28-day period. Hence, the risk of vessel blockage by PLLA degradation may have been minimal in the following months.
Detection of calponin after 4 weeks of response to injury
Immunofluorescent labeling of calponin, a vascular SMC differentiation marker, was observed on stented arteries by confocal fluorescence microscopy. The specimens were also co-stained with DAPI to reveal cell nuclei. Additionally, these changes in the degree of calponin could be quantitatively documented by Western blot analysis (samples of non-injured rabbit aorta were used as controls).
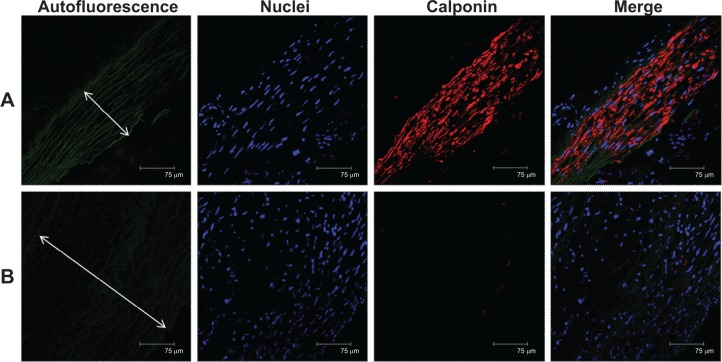
In all samples, less calponin-positive labeling at the tunica media of the aorta was observed on balloon-injury vessels than those on the drug-eluting spiral stented vessels (Figure 15). Furthermore, marked neointima formation was found due to proliferation of SMCs in the media giving rise to the appearance of fused intima and media in the ‘no stenting’ groups (Figure 15B). The level of calponin observed via Western blot analysis in the control and spiral stent groups was higher than in the balloon-injury group (Figure 16) (post hoc P<0.05).
Figure 15.
Immunofluorescence of calponin on stented arteries after 4 weeks.
Notes: Calponin immunostaining (red) of (A) a drug-eluting spiral stent and (B) balloon-injury artery. Autofluorescence on tunica media (green) and DAPI-stained nuclei are also shown. A high degree of positive labeling with calponin was observed on the drug-loaded spiral stent vessels. The double arrow depicts tunica media. Group B showed that marked neointimal hyperplasia resulted from proliferation of smooth muscle cells.
Abbreviation: DAPI, diamidine-2-phenylindole.
Figure 16.

Quantitative analysis of calponin content of arteries on day 28.
Notes: Drug-eluting spiral stent and control groups had significantly higher calponin levels than did the balloon-injury group (*post hoc P<0.05).
Based on functional molecular determinants, vascular SMCs can be divided into contractile or proliferative types.52 The proliferative vascular SMCs, which are negative for calponin, contribute to the progression of atherosclerosis and the formation of neointimal hyperplasia after vascular injury.53 Therefore, the dual-drug-eluting stent may block atherosclerosis and restenosis by modulating the phenotype change of vascular SMCs.
In summary, sequential-like and sustained release of acetylsalicylic acid and paclitaxel from novel biodegradable spiral stents provided vessel patency and inhibited stent thrombosis after balloon injury, even without the administration of daily oral acetylsalicylic acid. The present study provides evidence that the release of drugs from spiral stents promotes endothelial cells and vascular SMC differentiation in vivo as indicated by a high expression of contractile proteins. Histopathological evaluation also demonstrated the biocompatibility and safety of the developed stents, suggesting that the biodegradable dual-drug-eluting stents can be an ideal candidate for various cardiovascular applications. Since investigations of the long-term functionality of the proposed stent are required, further experimental studies in larger animal models will be needed to evaluate the benefits and risks of using biodegradable DES in diseased arteries.
Conclusion
This work developed balloon-expandable biodegradable drug-eluting spiral stents that performed the sustained and sequential-like release of anti-platelet acetylsalicylic acid and anti-proliferative paclitaxel. Biodegradable DES show better mechanical properties than metallic stents. HPLC analysis revealed that biodegradable stents sequentially released high concentrations of acetylsalicylic acid and paclitaxel for more than 30 and 60 days, respectively. The results of the cell culture indicated that the eluted drug promoted endothelial cell proliferation on days 3 and 7, and reduced SMC proliferation in weeks 2, 4, and 8, relative to the control groups. Moreover, the stent significantly inhibited the adhesion of platelets on days 3, 7, and 14. In the animal study, the implanted stent was intact, and no thrombus formation was observed in the stent-implanted vessel. Furthermore, in vivo histopathological assessment also demonstrated the biocompatibility and safety of the stent on diseased arteries. Promotion of endothelial recovery and inhibition of neointimal hyperplasia were demonstrated in the stented rabbits. Solvent-casting and electrospinning techniques will enable the fabrication of biodegradable DES of various sizes that provide drug release for the treatment of various cardiovascular diseases.
Supplementary materials
See the supplemental video. No migration of the implanted stent (arrow) was observed 3 days and 4 weeks after the procedure, and the stented abdominal aorta was patent for all test animals based on the peripheral vascular ultrasound study.
Acknowledgments
The authors would like to thank the National Science Council of Taiwan (Contract no. NSC-102-2314-B-182A-109) and Chang Gung Memorial Hospital (Contract no. CMRPD2A0083) for financially supporting this research. Ted Knoy and Yichia Lin are appreciated for their editorial assistance.
Footnotes
Disclosure
The authors report no conflicts of interest with respect to this work.
References
- 1.Babapulle MN, Eisenberg MJ. Coated stents for the prevention of restenosis: Part I. Circulation. 2002;106(21):2734–2740. doi: 10.1161/01.cir.0000038982.49640.70. [DOI] [PubMed] [Google Scholar]
- 2.Ruygrok PN, Serruys PW. Intracoronary stenting. From concept to custom. Circulation. 1996;94(5):882–890. doi: 10.1161/01.cir.94.5.882. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann R, Mintz GS. Coronary in-stent restenosis – predictors, treatment and prevention. Eur Heart J. 2000;21(21):1739–1749. doi: 10.1053/euhj.2000.2153. [DOI] [PubMed] [Google Scholar]
- 4.James SK, Stenestrand U, Lindbäck J, et al. SCAAR Study Group Long-term safety and efficacy of drug-eluting versus bare-metal stents in Sweden. N Engl J Med. 2009;360(19):1933–1945. doi: 10.1056/NEJMoa0809902. [DOI] [PubMed] [Google Scholar]
- 5.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 6.Pfisterer ME. Late stent thrombosis after drug-eluting stent implantation for acute myocardial infarction: a new red flag is raised. Circulation. 2008;118(11):1117–1119. doi: 10.1161/CIRCULATIONAHA.108.803627. [DOI] [PubMed] [Google Scholar]
- 7.Cook S, Ladich E, Nakazawa G, et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120(5):391–399. doi: 10.1161/CIRCULATIONAHA.109.854398. [DOI] [PubMed] [Google Scholar]
- 8.Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Farb A, Boam AB. Stent thrombosis redux – the FDA perspective. N Engl J Med. 2007;356(10):984–987. doi: 10.1056/NEJMp068304. [DOI] [PubMed] [Google Scholar]
- 10.Mahnken AH, Buecker A, Wildberger JE, et al. Coronary artery stents in multislice computed tomography: in vitro artifact evaluation. Invest Radiol. 2004;39(1):27–33. doi: 10.1097/01.rli.0000095471.91575.18. [DOI] [PubMed] [Google Scholar]
- 11.Ormiston JA, Serruys PW. Bioabsorbable coronary stents. Circ Cardiovasc Interv. 2009;2(3):255–260. doi: 10.1161/CIRCINTERVENTIONS.109.859173. [DOI] [PubMed] [Google Scholar]
- 12.Waksman R. Biodegradable stents: they do their job and disappear. J Invasive Cardiol. 2006;18(2):70–74. [PubMed] [Google Scholar]
- 13.Blindt R, Vogt F, Astafieva I, et al. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006;47(9):1786–1795. doi: 10.1016/j.jacc.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Lin YH, Chang SH, et al. Local sustained delivery of acetylsalicylic acid via hybrid stent with biodegradable nanofibers reduces adhesion of blood cells and promotes reendothelialization of the denuded artery. Int J Nanomedicine. 2014;9:311–326. doi: 10.2147/IJN.S51258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamai H, Igaki K, Kyo E, et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102(4):399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 16.Nishio S, Kosuga K, Igaki K, et al. Long-term (>10 years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai stents. Circulation. 2012;125(19):2343–2353. doi: 10.1161/CIRCULATIONAHA.110.000901. [DOI] [PubMed] [Google Scholar]
- 17.Meredith IT, Verheye S, Dubois CL, et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J Am Coll Cardiol. 2012;59(15):1362–1370. doi: 10.1016/j.jacc.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Serruys PW, Ormiston JA, Onuma Y, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373(9667):897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 19.Tanimoto S, Bruining N, van Domburg RT, et al. Late stent recoil of the bioabsorbable everolimus-eluting coronary stent and its relationship with plaque morphology. J Am Coll Cardiol. 2008;52(20):1616–1620. doi: 10.1016/j.jacc.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Venkatraman SS, Tan LP, Joso JF, Boey YC, Wang X. Biodegradable stents with elastic memory. Biomaterials. 2006;27(8):1573–1578. doi: 10.1016/j.biomaterials.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Chen MC, Tsai HW, Chang Y, et al. Rapidly self-expandable polymeric stents with a shape-memory property. Biomacromolecules. 2007;8(9):2774–2780. doi: 10.1021/bm7004615. [DOI] [PubMed] [Google Scholar]
- 22.Liu SJ, Chiang FJ, Hsiao CY, Kau YC, Liu KS. Fabrication of balloon-expandable self-lock drug-eluting polycaprolactone stents using micro-injection molding and spray coating techniques. Ann Biomed Eng. 2010;38(10):3185–3194. doi: 10.1007/s10439-010-0075-6. [DOI] [PubMed] [Google Scholar]
- 23.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21(23):2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 24.Ji W, Yang F, Seyednejad H, et al. Biocompatibility and degradation characteristics of PLGA-based electrospun nanofibrous scaffolds with nanoapatite incorporation. Biomaterials. 2012;33(28):6604–6614. doi: 10.1016/j.biomaterials.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Baker SC, Rohman G, Southgate J, Cameron NR. The relationship between the mechanical properties and cell behaviour on PLGA and PCL scaffolds for bladder tissue engineering. Biomaterials. 2009;30(7):1321–1328. doi: 10.1016/j.biomaterials.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Reidy MA, Clowes AW, Schwartz SM. Endothelial regeneration. V. Inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983;49(5):569–575. [PubMed] [Google Scholar]
- 27.Nachman RL, Jaffe EA. Endothelial cell culture: beginnings of modern vascular biology. J Clin Invest. 2004;114(8):1037–1040. doi: 10.1172/JCI23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv. 2011;4(1):104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sollott SJ, Cheng L, Pauly RR, et al. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995;95(4):1869–1876. doi: 10.1172/JCI117867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen WJ, Pang JH, Lin KH, Lee DY, Hsu LA, Kuo CT. Propylthiouracil, independent of its antithyroid effect, promotes vascular smooth muscle cells differentiation via PTEN induction. Basic Res Cardiol. 2010;105(1):19–28. doi: 10.1007/s00395-009-0045-z. [DOI] [PubMed] [Google Scholar]
- 31.Phinikaridou A, Hua N, Pham T, Hamilton JA. Regions of low endothelial shear stress colocalize with positive vascular remodeling and atherosclerotic plaque disruption: an in vivo magnetic resonance imaging study. Circ Cardiovasc Imaging. 2013;6(2):302–310. doi: 10.1161/CIRCIMAGING.112.000176. [DOI] [PubMed] [Google Scholar]
- 32.Masuo O, Terada T, Walker G, et al. Study of the patency of small arterial branches after stent placement with an experimental in vivo model. AJNR Am J Neuroradiol. 2002;23(4):706–710. [PMC free article] [PubMed] [Google Scholar]
- 33.Drolet MC, Plante E, Battistini B, Couet J, Arsenault M. Early endothelial dysfunction in cholesterol-fed rabbits: a non-invasive in vivo ultrasound study. Cardiovasc Ultrasound. 2004;2:10. doi: 10.1186/1476-7120-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31(1):224–230. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19(2):267–274. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 36.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3(2):232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 37.Venkatraman S, Poh TL, Vinalia T, Mak KH, Boey F. Collapse pressures of biodegradable stents. Biomaterials. 2003;24(12):2105–2111. doi: 10.1016/s0142-9612(02)00640-3. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Z, Lee KS, Zhang X, et al. Fibromodulin-deficiency alters temporospatial expression patterns of transforming growth factor-β ligands and receptors during adult mouse skin wound healing. PLoS One. 2014;9(3):e90817. doi: 10.1371/journal.pone.0090817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mickelson JK, Lakkis NM, Villarreal-Levy G, Hughes BJ, Smith CW. Leukocyte activation with platelet adhesion after coronary angioplasty: a mechanism for recurrent disease? J Am Coll Cardiol. 1996;28(2):345–353. doi: 10.1016/0735-1097(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 40.Beekhuizen H, van Furth R. Monocyte adherence to human vascular endothelium. J Leukoc Biol. 1993;54(4):363–378. [PubMed] [Google Scholar]
- 41.Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program. 2011;2011:51–61. doi: 10.1182/asheducation-2011.1.51. [DOI] [PubMed] [Google Scholar]
- 42.Jennings LK. Role of platelets in atherothrombosis. Am J Cardiol. 2009;103(3 Suppl):4A–10A. doi: 10.1016/j.amjcard.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Luo J, Li J, Shen X, et al. The effects and mechanisms of high loading dose rosuvastatin therapy before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol. 2013;167(5):2350–2353. doi: 10.1016/j.ijcard.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 44.Celik T, Iyisoy A, Kursaklioglu H, Celik M. The forgotten player of in-stent restenosis: endothelial dysfunction. Int J Cardiol. 2008;126(3):443–444. doi: 10.1016/j.ijcard.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 45.Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA. 1998;279(20):1643–1650. doi: 10.1001/jama.279.20.1643. [DOI] [PubMed] [Google Scholar]
- 46.Axel DI, Kunert W, Göggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96(2):636–645. doi: 10.1161/01.cir.96.2.636. [DOI] [PubMed] [Google Scholar]
- 47.Herdeg C, Oberhoff M, Baumbach A, et al. Local paclitaxel delivery for the prevention of restenosis: biological effects and efficacy in vivo. J Am Coll Cardiol. 2000;35(7):1969–1976. doi: 10.1016/s0735-1097(00)00614-8. [DOI] [PubMed] [Google Scholar]
- 48.Holmes DR, Jr, Kereiakes DJ, Laskey WK, et al. Thrombosis and drug-eluting stents: an objective appraisal. J Am Coll Cardiol. 2007;50(2):109–118. doi: 10.1016/j.jacc.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 49.Lasala JM, Cox DA, Dobies D, et al. ARRIVE 1 and ARRIVE 2 Participating Physicians Drug-eluting stent thrombosis in routine clinical practice: two-year outcomes and predictors from the TAXUS ARRIVE registries. Circ Cardiovasc Interv. 2009;2(4):285–293. doi: 10.1161/CIRCINTERVENTIONS.109.852178.109.852178. [DOI] [PubMed] [Google Scholar]
- 50.Cook S, Wenaweser P. Off-label use and the spectre of drug-eluting stent thrombosis. Circ Cardiovasc Interv. 2009;2(4):273–276. doi: 10.1161/CIRCINTERVENTIONS.109.891218. [DOI] [PubMed] [Google Scholar]
- 51.Regar E, Sianos G, Serruys PW. Stent development and local drug delivery. Br Med Bull. 2001;59:227–248. doi: 10.1093/bmb/59.1.227. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96(3):280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- 53.Yang HM, Kim BK, Kim JY, et al. PPARγ modulates vascular smooth muscle cell phenotype via a protein kinase G-dependent pathway and reduces neointimal hyperplasia after vascular injury. Exp Mol Med. 2013;45:e65. doi: 10.1038/emm.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See the supplemental video. No migration of the implanted stent (arrow) was observed 3 days and 4 weeks after the procedure, and the stented abdominal aorta was patent for all test animals based on the peripheral vascular ultrasound study.