INTRODUCTION
Programmed death 1(PD-1) is a strategic receptor on activated T-cells that mediates immunosuppression in cancer. Lambrolizumab is a humanized monoclonal IgG4–kappa isotype antibody designed to block the negative immune regulatory signaling of the PD-1 receptor expressed by T cells.1 Anti–PD-1 monoclonal antibodies have activity against several solid tumors.2 Recently, the FDA designated lambrolizumab as a breakthrough therapy for patients with advanced melanoma.
We describe a patient with metastatic melanoma placed on a clinical trial with lambrolizumab who developed CNS toxicity.
CASE REPORT
The patient is a 66-year-old man with stage IV, M1c melanoma in the lung, liver, subcutaneous soft tissue, and inguinal lymph nodes without a BRAF (v-raf murine sarcoma viral oncogene homolog B) gene mutation. Three months after diagnosis, he began dacarbazine and ipilimumab for four cycles. Subsequently, he had progressive disease, and was enrolled onto the study NCT01295827, a Phase I study of single-agent lambrolizumab at the 2 mg/kg dose level every 3 weeks. After four cycles of treatment, he had a partial response with improvement in the pulmonary nodules, stable liver metastases, and a decrease in size of the inguinal and subcutaneous masses.
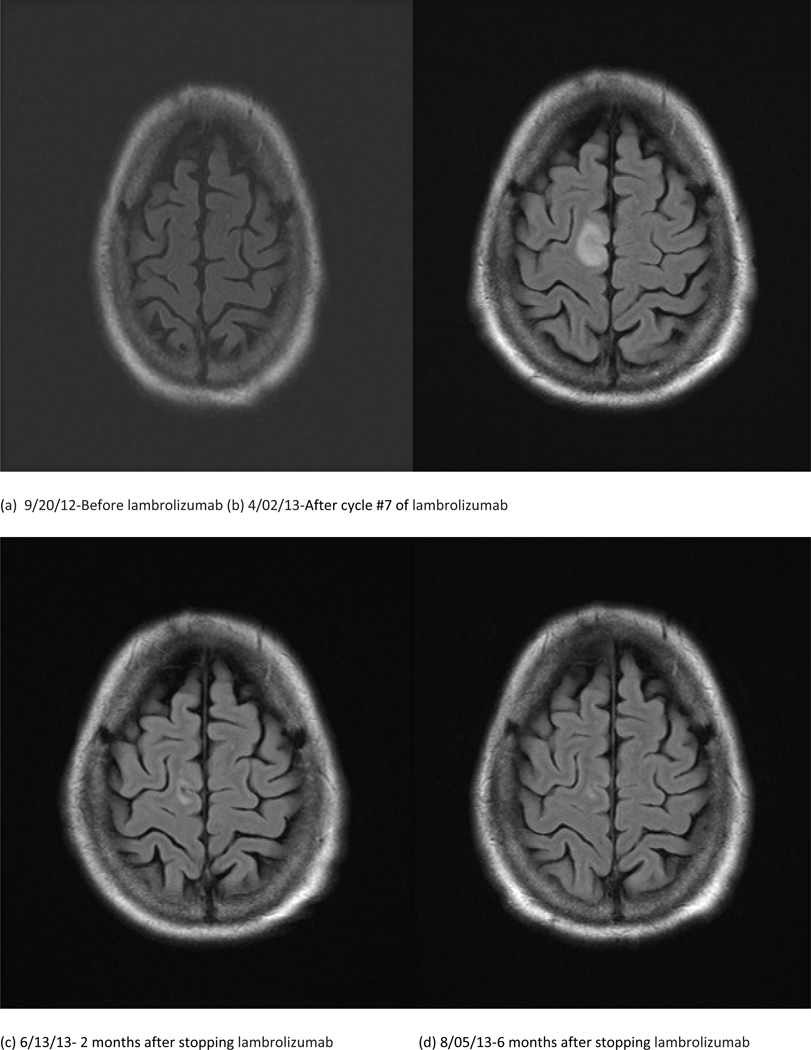
Shortly before cycle 5, he presented with gradual onset ataxia and vertigo for 2 weeks, and intermittent numbness in his left arm for about 4 weeks. The patient was evaluated, and treated symptomatically. A brain magnetic resonance (MR) scan was unremarkable but limited due to motion artifact. A previous, baseline MR scan of the brain prior to starting the trial was also normal (Figure 1a). The patient continued treatment and before cycle #7 his left arm began to twitch. A repeat brain MR scan showed new FLAIR hyperintensities bilaterally in the claustrum, as well as in the right frontal and left occipital lobes (Figure 1b).
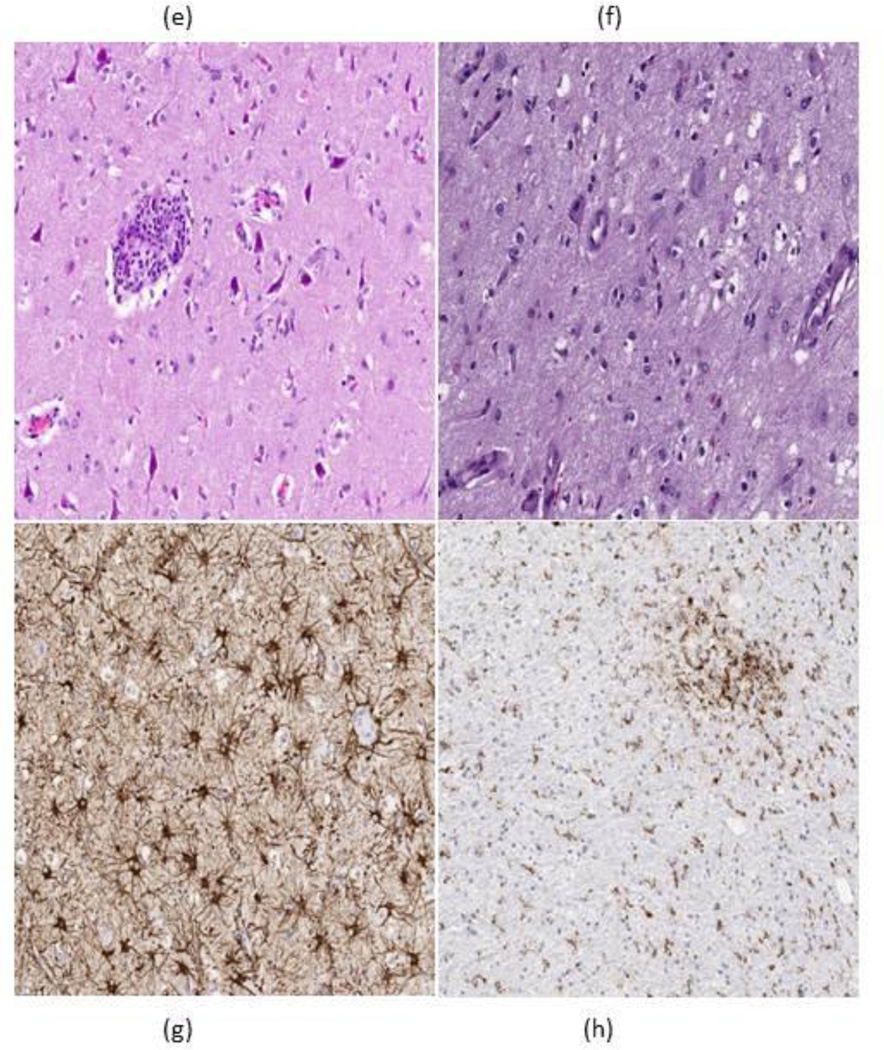
Figure 1.
We clinically assessed this patient after his repeat imaging and found that he was in partial motor convulsive status. He began treatment with oxcarbazepine. The seizures persisted, requiring admission 4 days later. He then underwent a lumbar puncture, with cerebrospinal fluid (CSF) notable for 6 white blood cells (WBC), 2 red blood cells (RBC), protein level of 103 mg/dl, and a glucose of 100mg/dl. Cytology was negative for malignant cells in the CSF. CSF was also negative for Epstein-Barr virus, Herpes, West Nile virus, and cytomegalovirus. A follow up brain MR scan showed stable T2 FLAIR hyperintensities in the right frontal and left occipital lobes with improvement in the signal originating from both external capsules. An electroencephalogram (EEG) showed periodic epileptiform discharges. We increased the oxcarbazepine and added levetiracetam before his discharge. However, his seizures continued, for 3 weeks after his discharge. He then had an open biopsy of the right frontal lesion. The specimen showed neuropil with diffuse microglial activation and focal perivascular inflammation with lymphocytic infiltrates (Figure 1e–f) with no malignancy identified and no immunostaining seen for HSV or JC virus, consistent with nonspecific CNS inflammatory disease. Treatment with lambrolizumab was stopped prior to admission. The partial motor seizures gradually improved after adding phenobarbital to oxcarbazepine and levetiracetam, but over several weeks we tapered all the anticonvulsants without a single seizure. A brain MR scan 2 and 4 months after stopping lambrolizumab (Figure 1c and 1d) showed resolution of the FLAIR changes with the patient’s clinical symptoms completely resolving as well.
DISCUSSION
This patient treated with lambrolizumab developed focal CNS toxicity that clinically presented as partial motor convulsive status (epilepsia partialis continua). This toxicity occurred despite being on the lower dose level of 2 mg/kg every 3 weeks. These seizures and the radiographic abnormalities disappeared gradually once lambrolizumab was stopped. This type of neurotoxicity has not been associated with lambrolizumab to our knowledge. We suspect this toxicity to likely be immune related with other neurological adverse events including CIDP, transverse myelitis, and myasthenia gravis type syndrome having been recently reported with patients on ipilumimab an autoimmune checkpoint inhibitor of CTLA-4.3
A Phase I / II study examining the safety of lambrolizumab in 135 patients found that 79% reported drug-related adverse events of any grade and 13% reported grade 3 or 4 drug-related adverse events.4 The most common toxicities observed included fatigue, rash, diarrhea, nausea, cough, and itching. Other noted side effects are hypothyroidism, abdominal pain, myalgia, headache, asthenia, chills, decreased appetite, pyrexia, elevated transaminases, renal failure, dyspnea, pneumonitis, and vitiligo. Seven lambrolizumab-related Grade 3/4 adverse events were reported as potentially “immune related.”4 None of the adverse events suspected to be of an inflammatory or autoimmune nature were reported to affect the central nervous system.
More studies in patients with advanced melanoma are planned as the FDA recently granted lambrolizumab status as a breakthrough therapy. With the prevalence of brain metastases in advanced melanoma patients and the potential targeting of PD-1pathways in future glioma trials, it is important to be aware of this adverse effect in future studies.
Highlights.
Many immune related neurological adverse events have been reported with ipilimumab
Lambrolizumab can also induce CNS toxicity
CNS toxicity appears reversible with prompt cessation of lambrolizumab
It is important to be aware of this toxicity in future studies of lambrolizumab
Acknowledgements
Funding
No funding was obtained for this project. Dr. Adriana Olar was supported by the National Institutes of Health/National Cancer Institute (Training Grant No. 5T32CA163185)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
No conflict of interests to report.
Contributor Information
Jacob J. Mandel, The University of Texas MD Anderson Cancer Center, Department of Neuro-Oncology, 1515 Holcombe Blvd Unit 431, Houston, Texas 77030-4009
Adriana Olar, The University of Texas MD Anderson Cancer Center, Department of Pathology, 1515 Holcombe Blvd, Houston, Texas 77030
Kenneth D. Aldape, The University of Texas MD Anderson Cancer Center, Department of Pathology, 1515 Holcombe Blvd, Houston, Texas 77030
Ivo W. Tremont-Lukats, The University of Texas MD Anderson Cancer Center, Department of Neuro-Oncology, 1515 Holcombe Blvd Unit 431, Houston, Texas 77030-4009
References
- 1.Patnaik A, Kang SP, et al. Phase I study of MK3475 (anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. J Clin Oncol. 2012;30(suppl) doi: 10.1158/1078-0432.CCR-14-2607. abstr 2512. [DOI] [PubMed] [Google Scholar]
- 2.Topaliam SL, Hodi FS, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Eng J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao B, Shroff S, et al. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014;16(4):589–593. doi: 10.1093/neuonc/nou001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid O, Robert C, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]