Abstract
Background
The contemporary need for repeat revascularization in older patients after percutaneous coronary intervention (PCI) has not been well studied. Understanding repeat revascularization risk in this population may inform treatment decisions.
Methods
We analyzed patients ≥65 years old undergoing native-vessel PCI of de novo lesions from 2005 to 2009 discharged alive using linked CathPCI Registry and Medicare data. Repeat PCIs within 1 year of index procedure were identified by claims data and linked back to CathPCI Registry to identify target vessel revascularization (TVR). Surgical revascularization and PCIs not back linked to CathPCI Registry were excluded from main analyses but included in sensitivity analyses. Independent predictors of TVR after drug-eluting stent (DES) or bare-metal stent (BMS) implantation were identified by multivariable logistic regression.
Results
Among 343,173 PCI procedures, DES was used in 76.5% (n = 262,496). One-year TVR ranged from 3.3% (overall) to 7.1% (sensitivity analysis). Precatheterization and additional procedure-related TVR risk models were developed in BMS (c-indices 0.54, 0.60) and DES (c-indices 0.57, 0.60) populations. Models were well calibrated and performed similarly in important patient subgroups (female, diabetic, and older [≥75 years]). The use of DES reduced predicted TVR rates in high-risk older patients by 35.5% relative to BMS (from 6.2% to 4.0%). Among low-risk patients, the number needed to treat with DES to prevent 1 TVR was 63–112; among high-risk patients, this dropped to 28–46.
Conclusions
In contemporary clinical practice, native-vessel TVR among older patients occurs infrequently. Our prediction model identifies patients at low versus high TVR risk and may inform clinical decision making.
Despite several decades of technological innovation, restenosis after percutaneous coronary intervention (PCI) remains a challenge. Compared with bare-metal stents (BMS), drug-eluting stents (DES) are associated with significantly reduced rates of restenosis.1 The uptake of DES was rapid after its introduction in 2003, with DES use peaking at 90% of PCI procedures in 2005.2 Current rates of DES use, however, are lower, having been tempered by concerns over (1) the need for prolonged dual antiplatelet therapy, which increases the risk for bleeding, medication nonadherence, and stent thrombosis; (2) the complication of very late stent thrombosis associated with DES3,4; and (3) higher technological cost.5,6 Consequently, there is growing interest in identifying patients for whom the risk of selective DES use may be acceptable.
Stent choice is especially important among the growing older US patient population. The past decade has witnessed a marked expansion in the use of PCI in older persons,7 with patients ≥65 years old now representing almost 40% of PCI procedures in the United States.8 However, restenosis or the need for target vessel revascularization (TVR) after PCI has not been well studied in older patients. Although some studies using Centers for Medicare & Medicaid (CMS) data have looked at overall revascularization rates, these studies did not have access to detailed clinical data, nor could they accurately determine TVR.6,9,10 Importantly, the use of DES in older patients is challenged by the significantly higher risk for post-PCI bleeding, particularly among those on prolonged dual antiplatelet therapies.11,12 From a financial perspective, the added costs of DES result in an overall net addition to national health care expenditures in Medicare beneficiaries.5 To date, studies have examined the financial impact of DES for the “average” patient but have not looked at the potential benefits and costs in low- or high-risk patient subgroups.13
Using clinical and procedural data from the National Cardiovascular Data Registry CathPCI Registry linked with longitudinal data from CMS, we sought to (1) examine the overall rate of TVR after PCI, (2) identify predictors of TVR, and (3) examine the number needed to treat (NNT) for DES use in low- versus high-predicted TVR risk subgroups among patients ≥65 years old.
Methods
Data sources
Clinical and procedural data for our study were from the CathPCI Registry, which has been previously described.8,14 The CathPCI Registry is an initiative of the American College of Cardiology Foundation and The Society for Cardiovascular Angiography and Interventions and is the largest PCI registry in the United States, capturing ~85% of PCI procedures performed at >1,400 hospitals.8 Longitudinal revascularization outcomes were identified from administrative inpatient Medicare data.
Study population
We identified CathPCI Registry patients ≥65 years old undergoing PCI from January 2005 to June 2009, linked to CMS data based on indirect identifiers—a process that has been previously described.15 We excluded patients who did not receive a stent, who received both BMS and DES, and who either died or underwent coronary artery bypass graft (CABG) surgery during their index hospitalization. Because of heterogeneity in operator descriptions of bypass graft targets, we did not include index PCI procedures performed in graft lesions. Patients presenting with ST-segment elevation myocardial infarction (STEMI) or undergoing PCI of previously treated lesions were also excluded due to potential differences in restenosis risk and predictors of TVR.
Outcomes and definitions
Our primary outcome was TVR within 1 year of the index procedure. Repeat revascularization procedures were identified from CMS claims and linked back to CathPCI Registry data to examine procedural details. We defined TVR as repeat PCI performed in a vessel that was treated during the index admission. Our primary definition of TVR did not include CABG, nor did it include repeat revascularizations that could not be linked back to the CathPCI Registry. Patients who died within 1 year after the index PCI were included in our analysis. If a TVR procedure was performed before death, then the patient was included in the TVR group; otherwise, the patient was counted in the “no TVR” group. Standardized definitions for CathPCI Registry version 3.04 data variables included in our analyses can be found online at www.ncdr.com/webncdr/cathpci/home/datacollection.
Statistical analysis
We explored bivariate associations of TVR with clinically relevant pre-PCI clinical and procedural variables. Baseline clinical and angiographic characteristics were described according to TVR status and summarized as counts and percentages for categorical variables and means with SDs and median with interquartile ranges for continuous variables. Differences between groups were compared using χ2 and Wilcoxon rank tests for categorical and continuous variables, respectively.
We identified predictors of TVR using multivariable logistic regression in a random selection of two-thirds of the sample population (development cohort). Primary model development was performed separately among patients receiving BMS or DES. Within each population (BMS or DES), 2 models were developed based on (1) precatheterization-only variables (“pre-catheterization model”) and (2) precatheterization plus coronary anatomy and procedure-related variables (“procedural model”). The following demographic and clinical variables were included in both models: age, female sex, race/ethnicity, body mass index, smoking status, hypertension, dyslipidemia, cerebrovascular disease, peripheral artery disease, insulin-treated diabetes, non–insulin-treated diabetes, prior myocardial infarction (MI), prior PCI, prior CABG, prior congestive heart failure (CHF), current CHF, New York Heart Association class, hemodialysis, glomerular filtration rate, presentation symptoms (nonacute coronary syndrome: no angina, atypical chest pain, stable angina; non–ST-segment elevation acute coronary syndrome [NSTE ACS]: unstable angina or non-STEMI [NSTEMI]), and PCI indication (elective, urgent, emergent/salvage). Additional variables in the procedural model included bifurcation lesion, target vessel, number of intervened vessels, minimum stent diameter (per mm increase), and total stent length (per mm increase). Although several of these variables are technically post-PCI, they serve as proxies for information that can be estimated from the diagnostic angiogram and may not otherwise be available in the CathPCI Registry. In each model, we used cubic spline plots to explore the linearity of continuous variables and identified independent predictors of TVR via backward variable selection, with a cutoff of P < .05 to remain in the model.
Models were validated by applying model coefficients to the remaining one-third sample population (validation cohort). Model discrimination was assessed with the c-index, and we evaluated model calibration by plotting predicted versus observed rates of TVR within deciles of predicted TVR risk and testing the difference with the Hosmer-Lemeshow goodness-of-fit test. We next examined the performance of our models developed in the overall BMS or DES populations among respective subgroups of women, diabetic patients, and patients ≥75 years old. In addition, patients receiving BMS were classified by tertile of predicted TVR risk (low, moderate, high) using the procedural BMS model. Predicted TVR risk in these patients was also determined using the procedural DES model. From these data, we calculated the NNT with DES versus BMS to prevent 1 TVR event among patients within each tertile of TVR risk.
Finally, we performed additional sensitivity analyses. Analyses were repeated after revising our definition of TVR to include the following groups in addition to patients already included in our main analysis: (1) patients undergoing CABG within 1 year of the index procedure and (2) patients for whom repeat revascularizations within 1 year of the index procedure could not be linked back to CathPCI Registry data. We also developed precatheterization and procedural models in the combined BMS and DES populations including patients presenting STEMI and undergoing PCI of previously treated lesions. Statistical significance was defined for all analyses as P < .05, and all analyses were performed at the Duke Clinical Research Institute using SAS version 9.2 (SAS Institute, Cary, NC).
Results
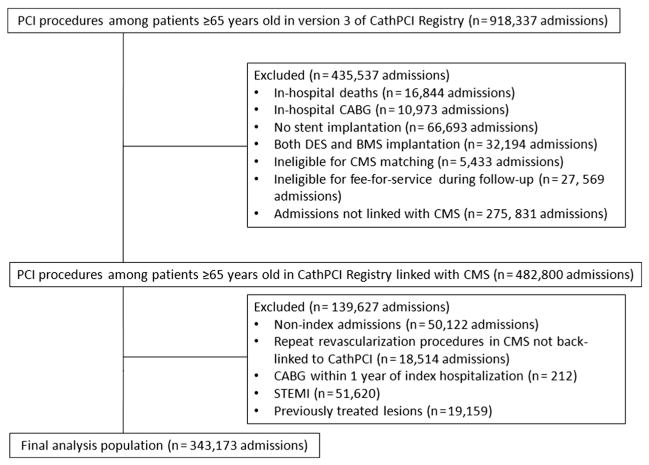
From 918,337 admissions among patients ≥65 years old, we excluded inhospital deaths (n = 16,844), CABG performed during the index admission (n = 10,973), procedures without stent implantation (n = 66,693), and PCIs using both DES and BMS (n = 32,194) (Figure 1). We excluded patients from hospitals without any CMS-matched admissions (n = 5,433), patients ineligible for Medicare fee-for-service during the follow-up year (n = 27,569), admissions that could not be linked to CMS (n = 275,831), nonindex admissions (n = 50,122), repeat 1-year revascularizations identified in CMS that could not be back linked to CathPCI Registry (n = 18,514), and CABG procedures within 1 year of index hospitalization (n = 212). Patients presenting with STEMI (n = 51,620) or undergoing PCI of previously treated lesions (n = 19,159) were also excluded.
Figure 1.
Selection of analysis population. This figure displays the study population; inclusions and exclusions are defined.
Among the 343,173 PCI admissions included in our final analysis population, 3.3% (n = 11,217) of patients had TVR within 1 year of the index PCI, and DES was used in 76.5% (n = 252,494) of overall procedures. Table I shows baseline patient and procedural characteristics stratified according to TVR status. Compared with patients who had no TVR, patients with TVR had more comorbidities (including hypertension, diabetes, and prior MI), had more prior coronary revascularization, and more often presented with NSTE ACS. Patients with TVR also had more multivessel disease, were treated more frequently with stents of smaller diameter and longer length, and more often underwent BMS implantation.
Table I.
Baseline patient and procedural characteristics
| TVR 1 y (n = 11,217) | No TVR 1 y (n = 331,956) | P | |
|---|---|---|---|
| Patient characteristics | |||
| Age (mean ± SD), y | 74.4 ± 6.5 | 74.8 ± 6.6 | <.001 |
| Female, % | 44.9 | 43.8 | .03 |
| Race/ethnicity, % | .64 | ||
| White | 87.9 | 87.8 | |
| African American | 5.0 | 4.8 | |
| Hispanic | 2.1 | 2.2 | |
| Other | 5.0 | 5.2 | |
| Weight (mean ± SD), kg | 80.9 (69.0,93.0) | 80.9 (69.2,93.1) | .60 |
| BMI (mean ± SD), kg/m2 | 28.7 ± 5.7 | 28.7 ± 5.9 | .10 |
| Smoker, % | <.001 | ||
| Current | 10.2 | 11.7 | |
| Former | 42.6 | 41.2 | |
| Hypertension, % | 85.3 | 83.2 | <.001 |
| Dyslipidemia, % | 77.2 | 75.8 | .001 |
| Cerebrovascular disease, % | 18.8 | 15.9 | <.001 |
| Peripheral artery disease, % | 16.6 | 14.9 | <.001 |
| Chronic lung disease, % | 19.0 | 19.7 | .08 |
| Diabetes mellitus, % | 38.4 | 33.6 | <.001 |
| Insulin therapy | 13.1 | 9.8 | |
| Noninsulin therapy | 25.2 | 23.8 | |
| Prior MI, % | 26.1 | 23.9 | <.001 |
| Prior PCI, % | 32.7 | 26.9 | <.001 |
| Prior CABG, % | 21.3 | 17.8 | <.001 |
| Prior CHF, % | 14.1 | 14.0 | .99 |
| Current CHF, % | 11.8 | 12.6 | .01 |
| NYHA class, % | <.001 | ||
| I | 29.8 | 32.5 | |
| II | 24.7 | 25.2 | |
| III | 31.1 | 28.9 | |
| IV | 14.4 | 13.5 | |
| GFR (mean ± SD) | 65.5 ± 25.1 | 65.9 ± 26.2 | .47 |
| Dialysis, % | 34.1 | 26.7 | <.001 |
| Presentation symptoms | <.001 | ||
| Non-ACS | 39.0 | 43.2 | |
| NSTE ACS | 61.0 | 56.8 | |
| PCI indication | .11 | ||
| Elective | 54.6 | 54.6 | |
| Urgent | 41.7 | 42.0 | |
| Emergent/salvage | 3.7 | 3.4 | |
| Procedural characteristics | |||
| Bifurcation lesion, % | 16.5 | 12.8 | <.001 |
| Minimum stent diameter (mean ± SD), mm | 2.5 ± 0.5 | 2.6 ± 0.5 | <.001 |
| Total stent length (mean ± SD), mm | 41.6 ± 23.3 | 37.7 ± 21.2 | <.001 |
| Vessels treated | |||
| RCA | 37.7 | 37.9 | .60 |
| LM | 4.1 | 2.9 | <.001 |
| LAD | 51.6 | 44.8 | <.001 |
| LCx | 35.7 | 31.7 | <.001 |
| No. of intervened vessels, % | <.001 | ||
| 1 | 74.4 | 83.2 | |
| 2 | 23.8 | 15.8 | |
| 3 | 1.8 | 0.9 | |
| DES, % | 70.3 | 76.7 | <.001 |
Abbreviations: ACS, Acute coronary syndrome; BMI, body mass index; GFR, glomerular filtration rate; LAD, left anterior descending artery; LCx, left circumflex artery; LM, left main artery; NSTE, non–ST-segment elevation; NYHA, New York Heart Association; RCA, right coronary artery.
Predictors of TVR
Significant predictors of TVR from each model are listed in Tables II and III. Using only precatheterization variables in the BMS model, we determined that the 3 strongest predictors of TVR based on model χ2 values were insulin-treated diabetes, non–insulin-treated diabetes, and history of prior PCI. After adding procedural characteristics into the BMS model, smaller stent diameter, multivessel PCI, and longer stent length were the greatest predictors of TVR. Among the DES population (Table III), the 3 precatheterization variables with the highest χ2 values were history of prior PCI, older age, and insulin-treated diabetes. Yet, in the procedural DES model, multivessel PCI, smaller stent diameter, and history of prior PCI were the strongest TVR predictors.
Table II.
Significant predictors of 1-year TVR among patients receiving BMS
| Parameters | Precatheterization model
|
Procedural model
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% lower CI | 95% upper CI | χ2 | P | OR | 95% lower CI | 95% upper CI | χ2 | P | |
| Insulin-treated diabetes | 1.521 | 1.332 | 1.737 | 38.46 | <.001 | 1.441 | 1.261 | 1.647 | 28.80 | <.001 |
| Non–insulin-treated diabetes | 1.276 | 1.154 | 1.411 | 22.54 | <.001 | 1.240 | 1.120 | 1.373 | 17.22 | <.001 |
| Prior PCI | 1.250 | 1.136 | 1.375 | 21.02 | <.001 | 1.294 | 1.176 | 1.423 | 27.98 | <.001 |
| Presentation with NSTE ACS | 1.201 | 1.100 | 1.311 | 16.74 | <.001 | 1.181 | 1.081 | 1.290 | 13.69 | <.001 |
| Cerebrovascular disease | 1.210 | 1.089 | 1.344 | 12.57 | <.001 | 1.190 | 1.071 | 1.322 | 10.41 | .001 |
| Age (per 5 y) | 0.948 | 0.918 | 0.978 | 10.88 | <.001 | 0.935 | 0.905 | 0.965 | 16.68 | <.001 |
| Current CHF | 0.828 | 0.732 | 0.936 | 9.09 | .003 | 0.801 | 0.707 | 0.906 | 12.40 | <.001 |
| Prior CABG | 1.161 | 1.043 | 1.292 | 7.43 | .006 | |||||
| BMI per 5 units (when ≥30) | 0.919 | 0.863 | 0.979 | 6.90 | .009 | 0.908 | 0.847 | 0.972 | 7.59 | .006 |
| BMI per 5 units (when <30) | 1.097 | 1.020 | 1.179 | 6.26 | .012 | |||||
| 2-vessel PCI (vs single) | 1.511 | 1.345 | 1.698 | 62.03 | <.001 | |||||
| 3-vessel PCI (vs single) | 2.175 | 1.533 | 3.088 | |||||||
| LCx PCI (vs LAD) | 0.828 | 0.741 | 0.924 | 16.63 | <.001 | |||||
| LM PCI (vs LAD) | 0.959 | 0.719 | 1.279 | |||||||
| RCA PCI (vs LAD) | 0.834 | 0.753 | 0.924 | |||||||
| Minimum stent diameter (per mm) | 0.696 | 0.645 | 0.752 | 85.63 | <.001 | |||||
| Total stent length (per mm) | 1.005 | 1.003 | 1.007 | 29.95 | <.001 | |||||
Table III.
Significant predictors of 1-year TVR among patients receiving DES
| Parameters | Precatheterization model
|
Procedural model
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% lower CI | 95% upper CI | χ2 | P | OR | 95% lower CI | 95% upper CI | χ2 | P | |
| Prior PCI | 1.316 | 1.242 | 1.395 | 85.54 | <.001 | 1.352 | 1.274 | 1.436 | 98.61 | <.001 |
| Age (per 5 y) | 0.935 | 0.914 | 0.956 | 34.44 | <.001 | 0.929 | 0.908 | 0.950 | 42.73 | <.001 |
| Insulin-treated diabetes | 1.304 | 1.193 | 1.425 | 34.31 | <.001 | 1.252 | 1.147 | 1.366 | 25.39 | <.001 |
| Prior CABG | 1.183 | 1.106 | 1.265 | 23.77 | <.001 | 1.190 | 1.109 | 1.277 | 23.31 | <.001 |
| Cerebrovascular disease | 1.176 | 1.094 | 1.265 | 19.35 | <.001 | 1.173 | 1.091 | 1.261 | 18.61 | <.001 |
| Non–insulin-treated diabetes | 1.137 | 1.065 | 1.214 | 14.88 | <.001 | 1.116 | 1.046 | 1.191 | 10.97 | <.001 |
| Presentation with NSTE ACS | 1.121 | 1.053 | 1.194 | 12.81 | <.001 | 1.142 | 1.080 | 1.208 | 21.56 | <.001 |
| Current CHF | 0.852 | 0.777 | 0.933 | 11.78 | <.001 | 0.840 | 0.767 | 0.920 | 14.18 | <.001 |
| Dialysis | 1.342 | 1.119 | 1.609 | 10.09 | .001 | 1.334 | 1.112 | 1.600 | 9.62 | .002 |
| Hypertension | 1.131 | 1.045 | 1.224 | 9.25 | .002 | 1.124 | 1.039 | 1.217 | 8.41 | .004 |
| NYHA Class II (vs I) | 1.076 | 0.999 | 1.158 | 8.42 | .038 | |||||
| NYHA Class III (vs I) | 1.111 | 1.030 | 1.198 | |||||||
| NYHA Class IV (vs I) | 1.102 | 1.000 | 1.213 | |||||||
| Current/recent smoker | 0.928 | 0.878 | 0.981 | 6.91 | .009 | 0.940 | 0.889 | 0.993 | 4.81 | .028 |
| BMI per 5 units (when ≥30) | 0.955 | 0.917 | 0.994 | 5.17 | .023 | |||||
| 2-vessel PCI (vs single) | 1.577 | 1.474 | 1.686 | 190.98 | <.001 | |||||
| 3-vessel PCI (vs single) | 1.751 | 1.416 | 2.165 | |||||||
| LCx PCI (vs LAD) | 0.825 | 0.769 | 0.886 | 65.68 | <.001 | |||||
| LM PCI (vs LAD) | 0.562 | 0.460 | 0.687 | |||||||
| RCA PCI (vs LAD) | 0.818 | 0.765 | 0.874 | |||||||
| Bifurcation lesion | 1.218 | 1.132 | 1.311 | 27.70 | <.001 | |||||
| Minimum stent diameter (per mm) | 0.712 | 0.668 | 0.759 | 108.59 | <.001 | |||||
| Total stent length (per mm) | 1.004 | 1.003 | 1.006 | 45.79 | <.001 | |||||
Model development and validation
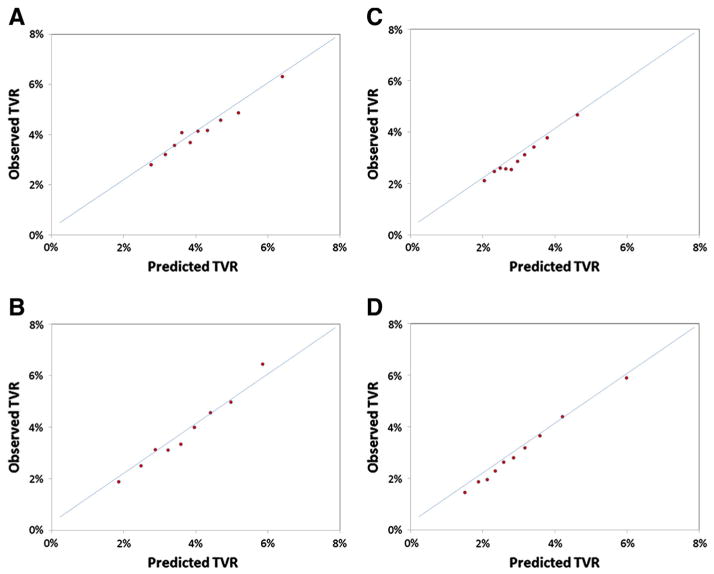
Model performance was assessed in the main BMS and DES validation cohorts as well as several prespecified subgroups. The overall discriminatory capability of the models was modest, whereas model calibration was excellent (Figure 2A and B). The discrimination of the precatheterization models (BMS and DES c-indices 0.54 and 0.57, respectively) improved incrementally with the addition of catheterization and procedural variables (BMS and DES c-indices 0.60 and 0.62, respectively). Precatheterization and procedural models performed similarly among female, diabetic, and older (≥75 years old) patients receiving BMS or DES (Table IV).
Figure 2.
Calibration plots for TVR prediction models. This figure displays calibration plots for TVR prediction models: BMS precatheterization model (A), BMS procedural model (B), DES precatheterization model (C), DES procedural model (D).
Table IV.
Model performance (c-indices) among validation cohort
| Group | BMS model
|
DES model
|
||||
|---|---|---|---|---|---|---|
| n | Precatheterization model | Procedural model | n | Precatheterization model | Procedural model | |
| Overall validation | 26866 | 0.54 | 0.60 | 87410 | 0.57 | 0.62 |
| Female | 11703 | 0.54 | 0.60 | 38470 | 0.57 | 0.63 |
| Diabetic | 8837 | 0.56 | 0.64 | 29616 | 0.57 | 0.63 |
| Age ≥75 y | 14984 | 0.53 | 0.60 | 40881 | 0.57 | 0.62 |
Estimated TVR benefit according to patient risk and stent type
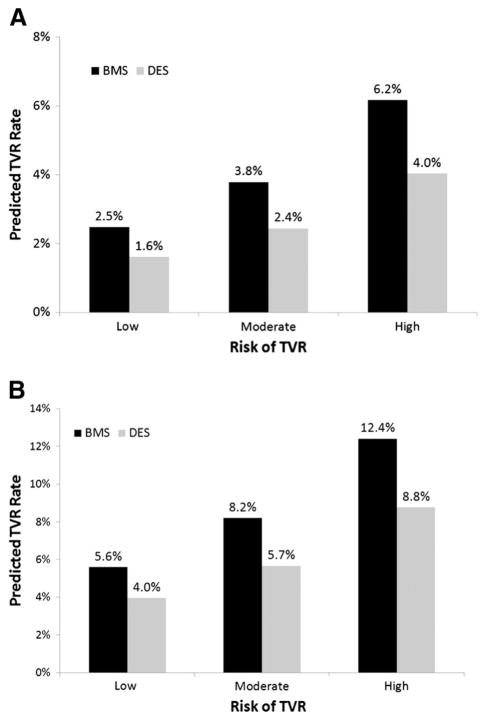
Patients receiving BMS were grouped according to tertile of predicted TVR risk using the BMS procedural model as follows: low (mean 2.5%, range 0.4%–3.2%), moderate (mean 3.8%, range 3.2%–4.5%), and high (mean 6.2%, range 4.5%–29.8%). Compared with patients in the low-risk group, the likelihood of TVR was higher for patients at moderate (odds ratio [OR] 1.5, 95% CI 1.4–1.7) or high (OR 2.6, 95% CI 2.3–2.8) risk. Tertiles of predicted TVR risk according to the DES procedural model were defined as low (mean 1.6%, range 0.3%–2.0%), moderate (mean 2.4%, range 2.0%–2.9%), and high (mean 4.0%, range 2.9%–18.2%). Patients in the moderate- and high-risk categories again had a higher likelihood of TVR compared with those in the low-risk group (OR 1.5, 95% CI 1.3–1.6, and OR 2.4, 95% CI 2.2–2.7, respectively). In high-risk older patients, the use of DES was predicted to reduce TVR by 35.5% relative to BMS (from 6.2% to 4.0%). Comparisons of estimated TVR rates for patients within each tertile based on stent type resulted in absolute risk reductions (NNT for TVR with DES vs BMS) of 0.9% (112 patients), 1.4% (72 patients), and 2.2% (46 patients) for low-, moderate-, and high-risk tertiles, respectively (Figure 3A).
Figure 3.
Predicted 1-year TVR rates restenosis risk and stent type. This figure depicts predicted rates of TVR based on the BMS procedural model or DES procedural model for patients at low-, moderate-, or high-predicted restenosis risk using: the primary definition of TVR (A) and the broader TVR definition (including CABG within 1 year of index PCI and PCIs that could not be back linked to CathPCI to verify TVR status) (B).
Sensitivity analyses
We performed sensitivity analyses using a broader definition of TVR. The overall estimated TVR rate increased to 7.1% when we included CABG within 1 year of the index PCI (n = 212) and repeat PCIs within 1 year identified in Medicare data that could not be back linked to the CathPCI Registry (n = 18,514) as TVR procedures. Nevertheless, this broader TVR definition did not significantly change our primary results (c-indices: precatheterization model 0.57, procedural model 0.61 for both BMS and DES models). Using the broader TVR definition, BMS patients were again classified according to tertiles of predicted TVR risk using the BMS procedural model (low: mean 5.6%, range 1.2%–7.0%, moderate: mean 8.2%, range 7.0%–9.5%, high: mean 12.4%, range 9.5%–39.9%) or DES procedural model (low: mean 4.0%, range 1.0%–4.9%, moderate: mean 5.7%, range 4.9%–6.6%, high: mean 8.8%, range 6.6%–33.4%). Compared with patients in the low-risk group, those in the moderate-(BMS: OR 1.5, 95% CI 1.4–1.6 and DES: OR 1.5, 95% CI 1.4–1.6) and high-risk (BMS: OR 2.4, 95% CI 2.2–2.5 and DES: OR 2.4, 95% CI 2.2–2.5) groups had higher risk of 1-year TVR. Under these assumptions, the use of DES versus BMS would reduce TVR risk (NNT) by 1.6% (63 patients), 2.5% (40 patients), and 3.6% (28 patients) in low-, moderate-, and high-risk patients, respectively (Figure 3B).
Finally, we developed precatheterization and procedural TVR models in a combined BMS and DES population and also included patients presenting with STEMI and undergoing PCI of previously treated lesions (total combined n = 413,952) (online Appendix Supplementary Table and online Appendix Supplementary Figure). Although there were slight changes in which factors were most strongly predictive of TVR (online Appendix Supplementary Table), overall model performance remained unchanged (online Appendix Supplementary Figure) (c-indices: precatheterization 0.57, procedural 0.63).
Discussion
As the population ages, PCI is increasingly being performed in older patients. In this older population, the use of DES can reduce the risk of restenosis but is costly and typically requires prolonged dual antiplatelet therapy, which puts patients at increased risk for bleeding. Therefore, there has been debate as to whether the risk of repeat intervention outweighs the safety concerns and associated cost in older patients. In this large national registry of PCI patients ≥65 years old linked with Medicare claims data, we found that the overall contemporary rate of TVR at 1 year was low (ranging from 3.3% in the overall population to 7.1% in a sensitivity analysis). We identified specific demographic, clinical, angiographic, and procedural variables associated with TVR and developed and validated clinical prediction models for TVR. Despite low overall risk for TVR in older individuals, we found that most patients received DES, and DES use varied only modestly among those with low-versus high-predicted risk for TVR.
Although TVR has represented a clinical interest for decades, our analysis adds to the body of knowledge accumulated from prior studies. A limited number of previous studies using Medicare data have examined revascularization after PCI in older patients,6,9,10 yet these studies were based on administrative data and assumed TVR rates. In contrast, we had access to index procedure clinical and angiographic data and were able to verify TVR status for repeat procedures through back-linking from Medicare to the CathPCI Registry. In addition, our results were based on national community-based findings as opposed to more selected single-center or state-wide registries or clinical trials in which follow-up angiography was protocol driven.
Several explanations may account for the low risk of 1-year TVR in our study. We excluded CABG as an outcome from our primary analysis, as we were unable to verify whether such procedures truly represented TVR events (ie, if the bypass graft targeted a vessel that was stented during the index admission). We also excluded CMS PCI claims that could not be verified as involving the target vessel in the CathPCI Registry. In secondary analyses, the overall TVR rate increased to only 7.1% (assuming the extreme case that 100% of these CABG and unconfirmed repeat PCI procedures represented events involving the incident vessel), which remains lower than the results in the younger patient population. In our older population, more conservative use of repeat intervention due to provider concerns about concomitant comorbidities (eg, renal impairment) and more difficult detection of atypical restenosis symptoms may partly explain lower TVR rates. Even when symptomatic, older patients might be less willing to undergo repeat procedures. Finally, advancements in stent design, improvements in secondary prevention, and better operator stent choice in contemporary practice may have also contributed to our findings.
Our study also found that relative to BMS, DES is associated with reduced TVR rates at 1 year among older patients undergoing PCI. This is in contrast to a recent analysis among Medicare beneficiaries in which DES patients experienced minimal difference in revascularization compared with BMS patients.10 The discrepant findings may be due to a limitation of the prior study to distinguish TVR from any repeat revascularization and inclusion of CABG in their revascularization definition. In the absence of contraindications to dual antiplatelet therapy, the TVR benefit observed in this analysis might support widespread use of DES over BMS in the older population, especially in high-risk patients for whom the greatest benefit of DES versus BMS was observed. However, despite a relative risk reduction of TVR with DES versus BMS, the absolute benefit is small and does not support indiscriminate DES use, especially among low-and moderate-risk patients.
Therefore, we developed well-calibrated models to predict the need for TVR among older patients undergoing PCI. Our models were developed in BMS and DES populations separately to reduce the effect of operator bias when selecting candidates for specific stent types. Our models were also based on extensive and detailed clinical data from the CathPCI Registry using standardized data definitions and could easily be implemented at approximately 1,400 hospitals currently participating in the CathPCI Registry as well as at nonparticipating institutions. Despite the robust clinical and procedural data used in our analysis, the discriminatory ability of our models was modest and similar to that seen in prior work in other populations (c-indices 0.60–0.68).16–20 Taken together, this reflects the challenge of predicting a multifactorial disease process such as restenosis and our current inability to account for all relevant factors, for example, patient factors such as individual genetic predisposition or ability to take prolonged dual antiplate-let therapy. Such continued difficulty predicting TVR and restenosis highlights the need for improved data collection strategies and for further investigation to identify and quantify TVR-related factors.
The ability to accurately identify patients with low-versus high-risk for TVR may have important clinical and financial implications. In particular, the decision to use DES, whose benefits are proportional to the underlying TVR risk, must be weighed against the risk for bleeding, especially in the vulnerable older population. In our study, we observed that most of the older PCI patients receive DES, and DES use increased slightly in those at high versus low risk for TVR. Given that DES use among Medicare beneficiaries has added an estimated $1.57 billion to national health care expenditures from 2002 to 2006,5 a more selective and targeted use of DES in older patients truly at highest risk for TVR could potentially lead to significant reductions in health care costs.
Study limitations
Our study has several important limitations. First, although we examined a large national dataset and included many candidate variables in our study, our data were observational. Second, variables that affect restenosis risk, such as insufficient stent deployment or lesion coverage and patient compliance with medications, may not have been available. Third, we did not have complete data regarding rationale for provider stent choice, although we developed models in separate BMS and DES populations to help address this issue of selection bias. Fourth, procedural variables that might impact restenosis, such as postintervention stenosis and coronary dissection, were available to us but were purposely excluded, as our intent was to develop a predictive model that allows for prospective assessment of restenosis risk to guide PCI therapy. Fifth, despite data that suggest differences in outcomes according to specific DES type,21,22 we classified stents broadly as DES versus BMS to help inform decisions regarding required use of prolonged dual antiplatelet therapy associated with DES in this higher bleeding risk older population. Sixth, we used a narrow definition of TVR to conservatively estimate outcome rates and develop the most accurate TVR prediction model. By excluding index graft interventions and CABG revascularization, we likely excluded true TVR events, but inclusion of the latter population in the definition of TVR in sensitivity analyses produced similar results. Finally, our model included variables related to CHF, which may be more predictive of mortality than TVR, and we counted deaths within 1 year post-PCI as no TVR, which may partially explain why CHF was mildly protective for TVR. However, this strategy was chosen based on its use in previous work.16
Conclusions
Risk-benefit assessment of DES is especially important in the growing older patient population in whom more PCIs are currently being performed and who are at risk for bleeding. Using a large, contemporary national database, we found a low rate of TVR among patients ≥65 years old, suggesting that native-vessel TVR among older patients is uncommon in contemporary practice. We also developed and validated models to predict TVR based on clinical and procedural variables. Based on our model, we can identify a subgroup of patients who are at higher risk for restenosis. These models can inform patient and provider decision making when considering DES versus BMS implantation among older individuals.
Acknowledgments
Sources of funding
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry. The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the National Cardiovascular Data Registry or its associated professional societies identified at www.ncdr.com. Connie N. Hess received support from the National Institutes of Health (grant no. 5T32HL069749-09).
We thank Erin Hanley for her editorial contributions to this manuscript. Ms Hanley did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
Footnotes
Disclosures
C.N. Hess: Dr Hess has no relevant disclosures to report.
S.V. Rao: TBD.
D. Dai: Dr Dai has no relevant disclosures to report.
M.L. Neely: Dr Neely has no relevant disclosures to report.
R.N. Piana: TBD.
J.C. Messenger: TBD.
E.D. Peterson: Dr Peterson reports research funding for the American College of Cardiology, American Heart Association, Eli Lilly & Company, Janssen Pharmaceuticals, and Society of Thoracic Surgeons (all significant); consulting (including CME) for Merck & Co (modest), Boehringer Ingelheim, Genentech, Janssen Pharmaceuticals, and Sanofi-Aventis (all significant).
References
- 1.Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198–206. doi: 10.1161/CIRCULATIONAHA.108.826479. [DOI] [PubMed] [Google Scholar]
- 2.Epstein AJ, Polsky D, Yang F, et al. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305:1769–76. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 4.Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–68. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 5.Groeneveld PW, Polsky D, Yang F, et al. The impact of new cardiovascular device technology on health care costs. Arch Intern Med. 2011;171:1289–91. doi: 10.1001/archinternmed.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan J, Linde-Zwirble W, Engelhart L, et al. Temporal changes in coronary revascularization procedures, outcomes, and costs in the bare-metal stent and drug-eluting stent eras: results from the US Medicare program. Circulation. 2009;119:952–61. doi: 10.1161/CIRCULATIONAHA.108.781138. [DOI] [PubMed] [Google Scholar]
- 7.Wang TY, Masoudi FA, Messenger JC, et al. Percutaneous coronary intervention and drug-eluting stent use among patients >/=85 years of age in the United States. J Am Coll Cardiol. 2012;59:105–12. doi: 10.1016/j.jacc.2011.10.853. [DOI] [PubMed] [Google Scholar]
- 8.Dehmer GJ, Weaver D, Roe MT, et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol. 2012;60:2017–31. doi: 10.1016/j.jacc.2012.08.966. [DOI] [PubMed] [Google Scholar]
- 9.Clark MA, Bakhai A, Lacey MJ, et al. Clinical and economic outcomes of percutaneous coronary interventions in the elderly: an analysis of Medicare claims data. Circulation. 2004;110:259–64. doi: 10.1161/01.CIR.0000135589.85501.DB. [DOI] [PubMed] [Google Scholar]
- 10.Douglas PS, Brennan JM, Anstrom KJ, et al. Clinical effectiveness of coronary stents in elderly persons: results from 262,700 Medicare patients in the American College of Cardiology-National Cardiovascular Data Registry. J Am Coll Cardiol. 2009;53:1629–41. doi: 10.1016/j.jacc.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–5. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- 12.Aronow HD, Steinhubl SR, Brennan DM, et al. CREDO Investigators. Bleeding risk associated with 1 year of dual antiplatelet therapy after percutaneous coronary intervention: insights from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2009;157:369–74. doi: 10.1016/j.ahj.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Amin AP, Spertus JA, Cohen DJ, et al. Use of drug-eluting stents as a function of predicted benefit: clinical and economic implications of current practice. Arch Intern Med. 2012;172:1145–52. doi: 10.1001/archinternmed.2012.3093. [DOI] [PubMed] [Google Scholar]
- 14.Brindis RG, Fitzgerald S, Anderson HV, et al. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–5. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 15.Brennan JM, Peterson ED, Messenger JC, et al. Linking the National Cardiovascular Data Registry CathPCI Registry with Medicare claims data: validation of a longitudinal cohort of elderly patients undergoing cardiac catheterization. Circ Cardiovasc Qual Outcomes. 2012;5:134–40. doi: 10.1161/CIRCOUTCOMES.111.963280. [DOI] [PubMed] [Google Scholar]
- 16.Yeh RW, Normand SL, Wolf RE, et al. Predicting the restenosis benefit of drug-eluting versus bare metal stents in percutaneous coronary intervention. Circulation. 2011;124:1557–64. doi: 10.1161/CIRCULATIONAHA.111.045229. [DOI] [PubMed] [Google Scholar]
- 17.Singh M, Gersh BJ, McClelland RL, et al. Predictive factors for ischemic target vessel revascularization in the Prevention of Restenosis with Tranilast and its Outcomes (PRESTO) trial. J Am Coll Cardiol. 2005;45:198–203. doi: 10.1016/j.jacc.2004.05.089. [DOI] [PubMed] [Google Scholar]
- 18.Stolker JM, Kennedy KF, Lindsey JB, et al. Predicting restenosis of drug-eluting stents placed in real-world clinical practice: derivation and validation of a risk model from the event registry. Circ Cardiovasc Interv. 2010;3:327–34. doi: 10.1161/CIRCINTERVENTIONS.110.946939. [DOI] [PubMed] [Google Scholar]
- 19.Kastrati A, Dibra A, Mehilli J, et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006;113:2293–300. doi: 10.1161/CIRCULATIONAHA.105.601823. [DOI] [PubMed] [Google Scholar]
- 20.Singh M, Gersh BJ, McClelland RL, et al. Clinical and angiographic predictors of restenosis after percutaneous coronary intervention: insights from the Prevention of Restenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2004;109:2727–31. doi: 10.1161/01.CIR.0000131898.18849.65. [DOI] [PubMed] [Google Scholar]
- 21.Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362:1663–74. doi: 10.1056/NEJMoa0910496. [DOI] [PubMed] [Google Scholar]
- 22.Elezi S, Dibra A, Mehilli J, et al. Vessel size and outcome after coronary drug-eluting stent placement: results from a large cohort of patients treated with sirolimus- or paclitaxel-eluting stents. J Am Coll Cardiol. 2006;48:1304–9. doi: 10.1016/j.jacc.2006.05.068. [DOI] [PubMed] [Google Scholar]