Abstract
It is now well accepted that a subgroup of patients with myelodysplastic syndromes (MDS) can recover from pancytopenia following immunosuppressive treatment (IST). For many years immunosuppression with antilymphocyte antibodies has been a standard treatment approach for patients with severe aplastic anemia. The initial concept of using immunosuppression to treat pancytopenic patients with MDS was based on the premise that MDS might share with severe aplastic anemia (SAA) an autoimmune basis for the bone marrow failure common to both conditions. The idea was supported by reports of favorable outcomes in occasional cases of MDS treated with antithymocyte globulin (ATG). Today, various forms of IST have been successfully used to restore hematopoiesis in MDS in many centers worldwide. In this review we outline the rationale for use of IST in MDS, and describe studies which help to define the patients with MDS likely to respond to IST. We summarize 18 published clinical trials using IST for MDS and discuss how these studies have helped to define the MDS subgroups likely to respond to treatment, the nature and durability of the response, the impact of IST on long-term outcome and the best treatment approach.
Autoimmune features of MDS
Bone marrow failure is a common feature of both SAA and MDS. More than 40 years ago the observation that some patients given immunosuppressive conditioning with ATG followed by bone marrow transplantation sometimes achieved full recovery of their autologous marrow prompted Gluckman et al to use ATG immunosuppression to successfully restore marrow function in patients with SAA1. While the autoimmune basis of pancytopenia in the majority of individuals with acquired aplastic anemia is now well accepted, the evidence that there is an autoimmune component to the pancytopenia in some patients with MDS has gained only slow acceptance. This is in part due to the real diagnostic challenge of identifying hypoplastic MDS as distinct from SAA in cases of severe marrow hypoplasia, leading to the perception that hematological responses to IST occur only in cases of MDS/SAA overlap syndromes2. Nevertheless, while there are clearly cases of pancytopenia which are difficult to characterize definitely as either SAA or MDS there is ample clinical data demonstrating that responses to IST are in no way confined to hypoplastic MDS. Evidence for an autoimmune process underlying the pancytopenia of MDS comes from clinical and immunological studies and has been extensively reviewed3, 4.
Association with autoimmune diseases
As well as its overlap with SAA, MDS is found more commonly in patients with rheumatoid disorders including connective tissue disorders such as rheumatoid arthritis and polymyalgia, thyroid disease, chronic vasculitis, glomerulonephritis, polyneuropathy, and the associated large granular lymphocytosis (LGL)5, 6, 7. In addition, patients with MDS frequently exhibit antibody-mediated autoimmune syndromes including ITP and autoimmune neutropenia8.
Immune abnormalities in MDS
A compelling indication that MDS is associated with autoimmune process is the finding in numerous reports of inflammatory cytokine profiles with increase in tumor necrosis factor-α and interferon-γ in the bone marrow9, 10 and skewing of the T cell repertoire affecting both CD8 and CD4 T cells11, 12, 13. As in other autoimmune states, regulatory T cells are decreased and diminish further as the disease progresses14. There are also significant abnormalities of natural killer (NK) cell numbers and function15. However, most data support a central role for T lymphocytes suppressing hematopoietic progenitors and causing marrow failure and cytopenias. Molldrem described the inhibition of granulocyte and erythrocyte progenitors by autologous T cells and the normalization of the T cell repertoire after ATG treatment16, 17. These studies identified CD8 T cells as the main inhibitors of erythroid and granulocytic colony growth. Subsequent studies by Sloand et al found that the myelosuppressive component of the CD8 T cell repertoire was often restricted to expanded V beta 3.1 T cell receptor families suggesting the clonal expansion of myelosuppressive T cells. Further investigation in patients with trisomy 8 MDS showed that colony inhibition was almost entirely restricted to colonies which were positive by fluorescent in situ hybridization for trisomy of chromosome 818. This provided evidence for an autoimmune T cell attack specific for the dysplastic clone. In trisomy 8 and some other forms of MDS the Wilms tumor antigen WT1 was found to be over expressed, and in elegant studies Sloand showed that the clonally expanded T cells of MDS were specific for WT1 and that they recognized WT1 antigens expressed on MDS cells.19 We can conclude that WT1 and probably other overexpressed leukemia associated antigens are the initiators of an autoimmune attack on the marrow in MDS, with the possibility that residual normal marrow cells are also damaged as bystanders contributing to marrow failure and pancytopenia. Lastly, the alteration towards normal of the T cell repertoire in patients with MDS responding to IST strongly suggests a causal relationship between abnormal immune function and pancytopenia in MDS.
Immunosuppresive treatment for MDS
Approaches to IST
Since the first successful application of horse ATG to treat MDS, other immunosuppressive agents have been used either alone (eg: rabbit ATG, and the antilymphocyte monoclonal antibody alemtuzumab) or in combination with ATG (eg: cyclosporine20, and the TNF inhibitor etanacerpt21) (see “clinical trials” below). Steroids and immunosuppressive chemotherapy have not been used because of the risk of causing bacterial infection. Currently there is no clear indication which agents or schedule offer the best chance of achieving a hematological response and how likely it is that the response will be durable.
Hematological recovery and outcome after IST
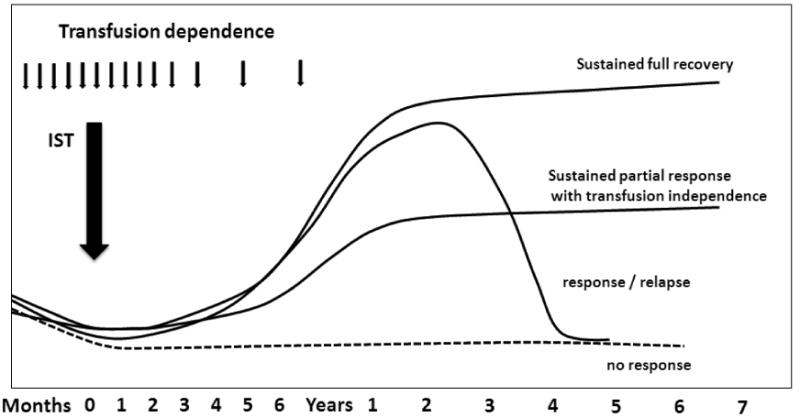
The characteristic pattern of hematological response to IST is a slow but progressive improvement in blood counts and a diminution or cessation of the need for red cell and platelet transfusion support beginning around 6 weeks after treatment and reaching a maximum between 3-6 months (Figure 1).22 Blood counts may return to normal or to levels associated with an improved quality of life with loss of transfusion dependence and no bacterial or fungal infection. Marrow cellularity improves and abnormal marrow karyotypes may diminish in frequency or disappear. However normalization of the marrow is not an absolute requirement for hematological response - some patients with trisomy-8 disease can recover normal marrow function without losing the MDS karyotype.23 Many patients have a sustained normalization of blood counts but about a third relapse. Some of these patients can be rescued by further IST. Only one study reports long-term follow-up of ATG treated patients. Responders survived significantly longer than comparable non-IST treated controls matched for MDS subtype, age and disease stage, whereas non responders progressed at the same pace as controls. Notably leukemic transformation was uncommon among responders.24
Figure 1. Patterns of hematological response to IST in MDS.
Factors affecting response of cytopenia to IST
MDS is best considered as a group of distinct disorders, each with a tendency to progressive deterioration in marrow function, but retaining unique pathophysiologies. The relevance of IST in the treatment of cytopenia in MDS depends on whether there is an autoimmune component to the marrow failure in a particular MDS subtype. Determining which subtype of MDS is susceptible to IST is hampered by lack of a reliable immune marker of hematological response. Furthermore, as MDS progresses, bone marrow function deteriorates as the potential for normal hematopoiesis diminishes with de-differentiation of the marrow and the extinction of residual normal stem cells. Thus even MDS subtypes where an autoimmune process contributes to cytopenia may fail to respond to effective immunosuppression because hematopoietic stem cells are defective or lost. In the absence of a clear understanding of immune pathophysiology, investigators have sought to define predictive factors for response to IST. Table 1 lists the variables that have been explored as predictive factors for hematological response to IST. Very early it emerged that hematological responses were more likely in early stages of the disease (refractory anemia or RA with an excess of blasts) and that secondary MDS and advanced MDS did not respond.22 Among MDS subtypes, secondary MDS and RARS are non-responsive while trisomy-8 disease appears favorable.23 Bone marrow cellularity, degree of cytopenia, and paroxysmal nocturnal hemoglobinuria (PNH) abnormality were not found to be prognostic in analysis of large patient numbers. In a series of analyses at NIH several other factors emerged as favorable for response in MDS RA: younger age, female sex, and HLA DR15+.25, 26 Many of these younger patients had the trisomy-8 karyotype but this did not emerge as an independent predictive factor. These factors were combined in a positive or negative score which identified responders with an 88% probability and non responders with a 7% probability of response.24 In this multivariate analysis of MDS patients treated with IST at NIH, three factors emerged as the only independent predictive factors for response: age <60 years, HLA DR15+ and treatment with ATG and cyclosporine in combination.
Table 1. MDS characteristics and response to IST3, 26.
| Variable | Impact | ||
|---|---|---|---|
| Favorable | No effect | Unfavorable | |
| Disease status | RA, RAEB, IPSS low/int-1 | RARS, Secondary MDS Advanced MDS | |
| HLA type | DR15 | ||
| Age | <60 years | ||
| Sex | female | ||
| Duration of transfusion dependence | short | ||
| Karyotype | trisomy 8 | other karyotype | |
| IST regimen | ATG+CSA | ||
| Other | PNH Hypoplastic marrow | ||
Trials of Immunosuppression in MDS
Since the first reports over 15 years ago of responses of MDS to immunosuppression, investigators worldwide have explored a variety of forms of IST. Currently 18 prospective clinical trials exploring IST in patients with myelodysplastic syndromes have been published. Although smaller studies helped pave the way for larger trials, these larger trials will be discussed below. The most common therapies used were cyclosporine alone, antithymocyte globulin with or without cyclosporine and alemtuzumab. These studies are summarized in Table 2. Below is a detailed description of the outcomes of some of the larger studies.
Table 2. Summary of individual IST trials.
| Authors | Type of MDS | Type of IST | Response | Comments |
|---|---|---|---|---|
| Asano et al.45 | RA | Cyclosporine | 4/8 (50%) | PNH did not correlate to response |
| Atoyebi et al.31 | RA, RARS | Cyclosporine | 0/6 (0%) | Drug withdrawn in 3 patients due to adverse side-effects |
| Catalano et al.29 | RA | Cyclosporine | 5/9 (56%) | All patients had hypocellular marrows |
| Chen et al.27 | RA, RARS, RAEB | Cyclosporine | 20/32(62.5%) | Survival time longer in responders |
| Dixit et al.46 | RA, RAEB, RARS | Cyclosporine | 13/19 (68%) | RA patients had higher response |
| Ishikawa et al.28 | RA, RAEB, RARS | Cyclosporine | 10/19 (53%) | HI seen within 24 weeks |
| Okamoto et al.30 | RA, RAEB | Cyclosporine | 6/10 (60%) | HLA-DR15 favorable predictive factor |
| Selleri et al.47 | Hypocellular MDS | Cyclosporine | 8/11(73%) | All patients were hypocellular |
| Killick et al.32 | RA, RAEB, RARS | Horse ATG | 10/20 (50%) | 8/13 RA patients responded |
| Molldrem et al.22 | RA, RAEB, RARS | Horse ATG | 11/25 (44%) | 9/14 RA patients responded |
| Steensma et al.33 | RA, RAEB-1 | Horse ATG | 0/8 (0%) | Study stopped early due to lack of efficacy, 6 RAEB-1 patients |
| Passweg et al.35 | RA, RARS, RAEB-1, RAEB-2, hypocellular | Horse ATG + cyclosporine | 13/45(29%) | Trial compared to best supportive care |
| Yazji et al.48 | RA, RARS, RAEB-t, CMML | Horse ATG + cyclosporine | 5/31 (16%) | 3/18 RA/RARS patients achieved durable CR |
| Scott et al.21 | RA, RARS, RCMD, RCMD-RS, RAEB-1 | Horse ATG + etanercept | 14/25(56%) | All responders responded by 8 weeks |
| Stadler et al.34 | RA, RAEB, CMML | Horse ATG vs. Rabbit ATG | 12/35 (34%) | Horse or Rabbit ATG efficacious in RA patients with short duration of disease |
| Broliden et al.36 | RA, RAEB | Rabbit ATG + cyclosporine | 6/20 (30%) | Poorly tolerated in patients older than 70 |
| Sloand et al.37 | Low-risk, Intermediate-1, Intermediate-2 | Alemtuzumab | 17/22 (77%) Int-1 4/7 (57%) Int-2 | Patients selected based on likely to respond criteria |
| Platzbecker et al.40 | RA, RARS, RCMD, RCMD-RS, RAEB-1, RAEB-2 | Sirolimus | 3/19 (16%) | 2 responders were RAEB-1 and RAEB-2 |
Cyclosporine alone
The first prospective studies using cyclosporine in patients with MDS was reported by Jonasova et al. Fourteen of 17 (82%) cytopenic patients with MDS RA subtype with variable marrow cellularity were treated with cyclosporin A. Substantial hematological responses with complete transfusion independence sustained for up to 30 months were observed.20 Chen et al. reported a 62.5% response rate in 32 patients with RA, RARS and RAEB treated with cyclosporine, with the vast majority being hematological improvements.27 Patients were enrolled from 4 different centers with a median age of 6 to 71 years old. Patients could have any type of MDS but had to be off any treatment for their MDS prior to enrollment. Cyclosporine was administered twice daily at a dose of 3 to 6 mg/kg and was adjusted by the cyclosporine blood concentration and patient's responses with mean concentrations of 110 to 241 ng/mL. Patients must have been treated for at least 2 months to be included in the analysis and for at least 3 months to be regarded as having completed therapy. The median time to elicit responses was 5 weeks. Eighteen patients showed a hematologic improvement with 5 patients demonstrating trilineage improvements. Most patients tolerated cyclosporine, although one patient discontinued treatment due to renal dysfunction. This study demonstrated that responders had a longer average survival than nonresponders. Ishikawa et al. demonstrated that 10 of 19 evaluable patients had a hematological improvement after cyclosporine treatment.28 Most smaller studies have also demonstrated some hematological improvement with cyclosporine as monotherapy. Catalano et al. reported a study of nine patients in which four benefited from cyclosporine.29 Okamoto et. al observed a positive response in lower risk MDS patients and especially those who were HLA-DR15 positive.30 While these results were promising, Atoyebi et al. in the UK found no responses in 6 patients treated with cyclosporine alone.31
Antithymocyte globulin
There have been 7 prospective trials using rabbit or horse antithymocyte globulin (ATG) with or without cyclosporine. The first large prospective study was reported by Molldrem et al. at NIH.22 They demonstrated a 44% response rate with horse ATG alone at a median of 14 months in unselected MDS patients. Patients were treated with horse ATG at a dose of 40 mg/kg/d for four doses. 44% of patients became transfusion-independent, three had complete trilineage hematological responses, six had partial responses, and two achieved minimal responses. Responses were observed mostly in refractory anemia patients although two patients with refractory anemia with excess blasts also responded. Median duration of response was 10 months. The overall survival was 84% at 38 months and the main side-effect was mild serum sickness in all patients. Alteration of the natural progression of disease was not noted. Another study by Killick et al. observed a 50% response rate in 20 evaluable patients. These patients, however, were lower risk defined by less than 10% blast count.32 However, Steensma et al. treated 8 patients but stopped their study due to lack of efficacy. Notably, 6 of their 8 patients were higher risk with RAEB-1 MDS.33 Stadler et al. from Germany compared horse ATG to rabbit ATG in a phase II multicenter trial in low-risk MDS patients. Thirty-five MDS patients were randomized to either horse ATG at a dose of 15 mg/kg/day or rabbit ATG at 3.75 mg/kg/day each for 5 days. Patients with RARS were excluded. Median age was 63 years with a mean follow up of 15 months. The mean time to response was 3 months with a median duration of response of 9 months. They found similar responses (34%) in patients treated with horse ATG and rabbit ATG and concluded that either form of ATG was efficacious34. Predictors for response were associated with FAB or WHO criteria and interval from diagnosis to study therapy. Age, bone marrow cellularity and HLA-DR15 status were not statistically significant, although HLA typing was only done in a minority of patients and their median age was higher than in other studies.
Antithymocyte globulin with cyclosporine
The largest trial to date studying the combination of ATG and cyclosporine is the recent phase III SAKK 33/99 trial carried out by 17 centers comparing horse ATG with cyclosporine to best supportive care. All subtypes of MDS were treated. The median age was 62 years. Forty-five patients received horse ATG at a dose of 15mg/kg for 5 days and oral cyclosporine for 180 days compared to 43 patients who received best supportive care. There was a 29% hematologic response to IST compared to a 9% response with best supportive care35. By 6 months, 13 of 45 patients on the treatment arm had a hematologic response compared to 4 of 43 patients receiving supportive care. Hypocellular MDS patients were noted to respond better but HLA-DR status was not assessed, as information that HLA-DR15 was a prognostic factor was not available when the trial was started. Patients were allowed to cross over to the treatment arm if disease progression was noted before 6 months or no response was seen after 6 months. Patients were censored at the time of cross over. The authors noted, however, no impact on transformation free survival or overall survival. Broliden et al. conducted a multicenter phase II study in which they treated 20 patients with low-risk MDS with rabbit ATG and cyclosporine and had an overall response rate of 30%36. The median age of the responders was 56 years compared to 64 years of age for the nonresponders. The initial ATG dose was 10 mg/kg/day for 4 days, but with response rates lower than expected in the first ten patients, the dose was increased to 20 mg/kg/day for 3 days. Oral cyclosporine was initiated on day 4 and continued for 32 weeks with a target level of 200 ng/mL. Five patients with RA and one with RAEB responded to this combination. The median time to response was 2 months.
Alemtuzumab
Alemtuzumab has only been recently explored as an immunosuppressive agent for MDS. In a prospective, non-randomized phase II study done by Sloand et al. at the NIH, responses occurred in 77% of intermediate-1 patients and 57% of intermediate-2 patients. Patients were selected based on their likelihood of responding to IST. Patients likely to respond to IST were defined as HLA-DR15-negative individuals whose age plus the number of months of red blood cell transfusion dependence (RCTD) was less than 58 (e.g., 45 years old and 12 months of TD); and those who were HLA-DR15-positive and whose age plus RCTD was less than 72.37 Patients were treated with a test dose of 1 mg of intravenous alemtuzumab on the first day, followed by 10 mg per day for ten consecutive days. The median age was 57 years and most patients were intermediate-1 risk, with seven patients intermediate-2 risk and two patients low-risk. No significant toxicities were noted and there was no clinically significant reactivation of EBV or CMV disease. Hypocellularity was not a predictor of response, although most responders were HLA-DR15 positive. Cytogenetic abnormalities disappeared by 12 months in a subset of patients.
Other treatments
An accumulating body of data suggests that sirolimus may interfere with the dysfunctional pathways of angiogenesis, apoptosis, proliferation and differentiation in MDS38,39. Platzbecker et al. studied sirolimus as a sole agent for treatment of patients with MDS. Nineteen patients with a median age of 72 years were enrolled on the trial and received sirolimus orally, starting with a 6 mg loading dose followed by 2 mg once daily. The dose was further adjusted to maintain a blood concentration of 3-12 ng/mL. Three of 19 patients achieved a major or minor hematological response according to the International Working Group criteria at a median follow-up of 13 months40. Six of the 19 patients did not receive the drug for more than 3 months due to side effects such as grade IV thrombocytopenia in one patient. Interestingly, all responders were higher risk MDS (1 RAEB-2, 1 RAEB-1, and 1 RCMD). Scott et al. examined the combination of horse ATG with etanercept in a phase II trial. Twenty-five patients with all types of MDS with IPSS risk scores of low and intermediate-1 were treated with horse ATG at 40 mg/kg/day for 4 days, followed by etanercept, 25 mg subcutaneous twice a week for 2 weeks, every month for 4 months. The median age was 65 years and responses were assessed using the modified guidelines proposed by the IWG41. The overall response by intent to treat analysis among 25 patients treated was 56%.21 The authors suggest that etanercept may have additional effects outside of T-cell suppression leading to enhanced responses. Previous experiments have documented higher levels of TNF-α levels in bone marrow samples of MDS patients and TNF-α induced apoptosis may lead to ineffective hematopoiesis, particularly in the early stages of MDS. The authors conclude that ATG/etanercept combinations may be superior to ATG/cyclosporine 42, 43, 44.
Overview of IST for MDS
From these studies several broad conclusions can be made about the potential of IST in the treatment of MDS.
IST is most effective in the early phase of MDS described variously as MDS RA, MDS IPSS-low-risk.
Comparisons between trials is limited by variable inclusion criteria. Studies that selected for a certain patient population, notably lower-risk MDS and HLA-DR15 positivity, demonstrated more favorable responses. Young age has also been demonstrated to be a marker of response, while the importance of marrow cellularity in predicting response is equivocal.
Because of these variables, it is not entirely clear which treatment approach emerges as the most favorable. However, with the proviso that future studies are still needed to fully validate optimum treatment approaches, it reasonable to conclude that sirolimus is least efficacious, and alemtuzumab may be superior to other single agents (Table 3). However, the alemtuzumab study did select for patients felt to have a high chance of responding to immunosuppression. Whether combinations of a particular ATG with cyclosporine or etanercept is comparable with alemtuzumab would require a prospective comparative study.
Table 3.
Cumulative overall response rates from 18 separate trials of IST for MDS.
| Regimen | response rate % |
|---|---|
| Sirolimus | 16 |
| Cyclosporine | 56 |
| Horse ATG | 35 |
| Rabbit ATG | 27 |
| Horse ATG + cyclosporine | 25 |
| Rabbit ATG + cyclosporine | 30 |
| Alemtuzumab | 68 |
Unresolved issues
Does IST induce leukemic progression?
The administration of IST in MDS where the immune response is directed against preleukemic MDS clones could theoretically risk leukemic progression by removal of an effective immune control of the malignant process. Curiously, in the analysis of long term outcome of IST treated patients, responders not only sustained hematopoiesis over many years but also had a lower risk of leukemic progression than a matched group of patients with similar MDS IPSS risk scores who did not receive IST. Patients who were nonresponders did not show accelerated disease progression but progressed at the same pace as controls not given IST. Thus IST appears to be safe in the longterm.24
Future directions
Treatment of MDS with antilymphocyte antibodies is clearly an effective therapy for selected patients with MDS. There is clearly a need for multicenter prospective studies in large numbers of MDS patients to evaluate and compare promising IST strategies. Unresolved questions are whether IST can be further optimized. Given the variablilty of ATG and its relative ease of administration, alemtuzumab would appear to be the best choice of antibody for further evaluation. Combining an antibody with anti TNF treatment with etanercept also deserves consideration. It is not clear whether MDS patients with more advanced disease might also respond more frequently if the IST were optimized. For this reason it will be important to continue to explore IST in a spectrum of MDS disorders to include RA/IPSS int -1, RARS, RAEB/IPSS int -2. Interpretation and comparison of results between treatment centers would be much improved by standardization of treatment response definitions and clear definition of the MDS subtype.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gluckman E, Devergie A, Faille A, et al. Treatment of severe aplastic anemia with antilymphocyte globulin and androgens. Exp Hematol. 1978 Sep;6(8):679–87. [PubMed] [Google Scholar]
- 2.Barrett J, Saunthararajah Y, Molldrem J. Myelodysplastic syndrome and aplastic anemia: distinct entities or diseases linked by a common pathophysiology? Semin Hematol. 2000 Jan;37(1):15–29. doi: 10.1016/s0037-1963(00)90027-1. [DOI] [PubMed] [Google Scholar]
- 3.Barrett AJ, Sloand E. Autoimmune mechanisms in the pathophysiology of myelodysplastic syndromes and their clinical relevance. Curr Hematol Malig Rep. 2008 Jan;3(1):23–8. doi: 10.1007/s11899-008-0005-y. [DOI] [PubMed] [Google Scholar]
- 4.Olnes MJ, Sloand EM. Targeting immune dysregulation in myelodysplastic syndromes. JAMA. 2011;305:814–819. doi: 10.1001/jama.2011.194. [DOI] [PubMed] [Google Scholar]
- 5.Stern M, Buser AS, Lohri A, Tichelli A, Nissen-Druey C. Autoimmunity and malignancy in hematology-more than an association. Crit Rev Oncol Hematol. 2007 Aug;63(2):100–10. doi: 10.1016/j.critrevonc.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Giannouli S, Kanellopoulou T, Voulgarelis M. Myelodysplasia and autoimmunity. Curr Opin Rheumatol. 2012 Jan;24(1):97–102. doi: 10.1097/BOR.0b013e32834db4ee. [DOI] [PubMed] [Google Scholar]
- 7.Saunthararajah Y, Molldrem JL, Rivera M, et al. Coincident myelodysplastic syndrome and T-cell large granular lymphocytic disease: clinical and pathophysiological features. Br J Haematol. 2001 Jan;112(1):195–200. doi: 10.1046/j.1365-2141.2001.02561.x. [DOI] [PubMed] [Google Scholar]
- 8.Mewawalla P, Dasanu CA. Immune alterations in untreated and treated myelodysplastic syndrome. Expert Opin Drug Saf. 2011 May;10(3):351–61. doi: 10.1517/14740338.2011.534456. [DOI] [PubMed] [Google Scholar]
- 9.Kordasti SY, Afzali B, Lim Z, et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009 Apr;145(1):64–72. doi: 10.1111/j.1365-2141.2009.07593.x. [DOI] [PubMed] [Google Scholar]
- 10.Sugimori C, List AF, Epling-Burnette PK. Immune dysregulation in myelodysplasticsyndrome. Hematol Rep. 2010 Jan 26;2(1):e1. doi: 10.4081/hr.2010.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melenhorst JJ, Eniafe R, Follmann D, Nakamura R, Kirby M, Barrett AJ. Molecular and flow cytometric characterization of the CD4 and CD8 T-cell repertoire in patients with myelodysplastic syndrome. Br J Haematol. 2002 Oct;119(1):97–105. doi: 10.1046/j.1365-2141.2002.03802.x. [DOI] [PubMed] [Google Scholar]
- 12.Campregher PV, Srivastava SK, Deeg HJ, Robins HS, Warren EH. Abnormalities of the alphabeta T-cell receptor repertoire in advanced myelodysplastic syndrome. Exp Hematol. 2010 Mar;38(3):202–12. doi: 10.1016/j.exphem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fozza C, Contini S, Galleu A, et al. Patients with myelodysplastic syndromes display several T-cell expansions, which are mostly polyclonal in the CD4(+) subset and oligoclonal in the CD8(+) subset. Exp Hematol. 2009 Aug;37(8):947–55. doi: 10.1016/j.exphem.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Kordasti SY, Ingram W, Hayden J, et al. CD4+CD25 high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007 Aug 1;110(3):847–50. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- 15.Fozza C, Longinotti M. Are T-cell dysfunctions the other side of the moon in the pathogenesis of myelodysplastic syndromes? Eur J Haematol. 2012 May;88(5):380–7. doi: 10.1111/j.1600-0609.2012.01762.x. [DOI] [PubMed] [Google Scholar]
- 16.Molldrem JJ, Jiang YZ, Stetler-Stevenson M, Mavroudis D, Hensel N, Barrett AJ. Haematological response of patients with myelodysplastic syndrome to antithymocyte globulin is associated with a loss of lymphocyte-mediated inhibition of CFU-GM and alterations in T-cell receptor Vbeta profiles. Br J Haematol. 1998 Sep;102(5):1314–22. doi: 10.1046/j.1365-2141.1998.00920.x. [DOI] [PubMed] [Google Scholar]
- 17.Kochenderfer JN, Kobayashi S, Wieder ED, Su C, Molldrem JJ. Loss of T-lymphocyte clonal dominance in patients with myelodysplastic syndrome responsive to immunosuppression. Blood. 2002;100:3639–45. doi: 10.1182/blood-2002-01-0155. [DOI] [PubMed] [Google Scholar]
- 18.Sloand EM, Mainwaring L, Fuhrer M, et al. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005 Aug 1;106(3):841–51. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sloand EM, Melenhorst JJ, Tucker ZC, et al. T-cell immune responses to Wilms tumor 1 protein in myelodysplasia responsive to immunosuppressive therapy. Blood. 2011 Mar 3;117(9):2691–9. doi: 10.1182/blood-2010-04-277921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonasova A, Neuwirtova R, Cermak J, et al. Cyclosporin A therapy in hypoplastic MDS patients and certain refractory anaemias without hypoplastic bone marrow. Br J Haematol. 1998;200:304–9. doi: 10.1046/j.1365-2141.1998.00551.x. [DOI] [PubMed] [Google Scholar]
- 21.Scott BL, Ramakrishnan A, Fosdal M, et al. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Scott Br J Haematol. 2010 Jun;149(5):706–10. doi: 10.1111/j.1365-2141.2010.08145.x. Epub 2010 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molldrem JJ, Caples M, Mavroudis D, Plante M, Young NS, Barrett AJ. Antithymocyte globulin for patients with myelodysplastic syndrome. Br J Haematol. 1997 Dec;99(3):699–705. doi: 10.1046/j.1365-2141.1997.4423249.x. [DOI] [PubMed] [Google Scholar]
- 23.Sloand EM, Mainwaring L, Fuhrer M, et al. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005 Aug 1;106(3):841–51. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008 May 20;26(15):2505–11. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunthararajah Y, Nakamura R, Nam JM, et al. HLA-DR15 (DR2) is overrepresented in myelodysplastic syndrome and aplastic anemia and predicts a response to immunosuppression in myelodysplastic syndrome. Blood. 2002 Sep 1;100(5):1570–4. [PubMed] [Google Scholar]
- 26.Saunthararajah Y, Nakamura R, Wesley R, Wang QJ, Barrett AJ. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood. 2003 Oct 15;102(8):3025–7. doi: 10.1182/blood-2002-11-3325. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Jiang B, Da W, Gong M, Guan M. Treatment of myelodysplastic syndrome with cyclosporin A. Int J Hematol. 2007;85(1):11–17. doi: 10.1532/IJH97.A10513. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa T, Tohyama K, Nakao Y, et al. A prospective study of cyclosporine A treatment of patients with low-risk myelodysplastic syndrome: presence of CD55(-)CD59(-) blood cells predicts platelet response. Int J Hematol. 2007;86(2):150–157. doi: 10.1532/IJH97.07052. [DOI] [PubMed] [Google Scholar]
- 29.Catalano L, Selleri C, Califano C, et al. Prolonged response to cyclosporin-A in hypoplastic refractory anemia and correlation with in vitro studies. Haematologica. 2000;85(2):133–138. [PubMed] [Google Scholar]
- 30.Okamoto T, Okada M, Yamada S, et al. Good response to cyclosporine therapy in patients with myelodysplastic syndromes having the HLA-DRB1*1501 allele. Leukemia. 2000;14(2):344–346. doi: 10.1038/sj.leu.2401665. [DOI] [PubMed] [Google Scholar]
- 31.Atoyebi W, Bywater L, Rawlings L, Brunskill S, Littlewood TJ. Treatment of myelodysplasia with oral cyclosporin. Clin Lab Haematol. 2002;24(4):211–214. doi: 10.1046/j.1365-2257.2002.00446.x. [DOI] [PubMed] [Google Scholar]
- 32.Killick SB, Mufti G, Cavenagh JD, A, et al. A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low-risk’ myelodysplasia. Br J Haematol. 2003;120(4):679–684. doi: 10.1046/j.1365-2141.2003.04136.x. [DOI] [PubMed] [Google Scholar]
- 33.Steensma DP, Dispenzieri A, Moore SB, Schroeder G, Tefferi A. Antithymocyte globulin has limited efficacy and substantial toxicity in unselected anemic patients with myelodysplastic syndrome. Blood. 2003;101(6):2156–2158. doi: 10.1182/blood-2002-09-2867. [DOI] [PubMed] [Google Scholar]
- 34.Stadler M, Germing U, Kliche KO, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia. 2004;18(3):460–465. doi: 10.1038/sj.leu.2403239. [DOI] [PubMed] [Google Scholar]
- 35.Passweg JR, Giagounidis AA, Simcock M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care--SAKK 33/99. J Clin Oncol. 2011;29(3):303–309. doi: 10.1200/JCO.2010.31.2686. [DOI] [PubMed] [Google Scholar]
- 36.Broliden PA, Dahl IM, Hast R, et al. Antithymocyte globulin and cyclosporine A as combination therapy for low-risk non-sideroblastic myelodysplastic syndromes. Haematologica. 2006;91(5):667–670. [PubMed] [Google Scholar]
- 37.Sloand EM, Olnes MJ, Shenoy A, et al. Alemtuzumab treatment of intermediate-1 myelodysplasia patients is associated with sustained improvement in blood counts and cytogenetic remissions. J Clin Oncol. 2010 Dec 10;28(35):5166–73. doi: 10.1200/JCO.2010.29.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Recher C, Beyne-Rauzy O, Demur C, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105(6):2527–34. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- 39.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature Medicine. 2002 Feb;8(2):128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 40.Platzbecker U, Haase M, Herbst R, et al. Activity of sirolimus in patients with myelodysplastic syndrome - Results of a pilot study. Br J Haematol. 2005;128(5):625–630. doi: 10.1111/j.1365-2141.2005.05360.x. [DOI] [PubMed] [Google Scholar]
- 41.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical applications and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 42.Kitagawa M, Saito I, Kuwata T, et al. Overexpression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leukemia. 1997;11:2049–2054. doi: 10.1038/sj.leu.2400844. [DOI] [PubMed] [Google Scholar]
- 43.Gersuk GM, Beckham C, Loken MR, et al. A role for tumor necrosis factor-a, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. British Journal of Haematology. 1998;103:176–188. doi: 10.1046/j.1365-2141.1998.00933.x. [DOI] [PubMed] [Google Scholar]
- 44.Sawanobori M, Yamaguchi S, Hasegawa M, et al. Expression of TNF receptors and related signaling molecules in the bone marrow from patients with myelodysplastic syndromes. Leukemia Research. 2003;27:583–591. doi: 10.1016/s0145-2126(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 45.Asano Y, Maeda M, Uchida N, et al. Immunosuppressive therapy for patients with refractory anemia. Ann Hematol. 2001;80(11):634–638. doi: 10.1007/s002770100360. [DOI] [PubMed] [Google Scholar]
- 46.Dixit A, Chatterjee T, Mishra P, et al. Cyclosporin A in myelodysplastic syndrome: a preliminary report. Ann Hematol. 2005;84(9):565–568. doi: 10.1007/s00277-005-1016-6. [DOI] [PubMed] [Google Scholar]
- 47.Selleri C, Maciejewski JP, Catalano L, et al. Effects of cyclosporine on hematopoietic and immune functions in patients with hypoplastic myelodysplasia: In vitro and in vivo studies. Cancer. 2002;95(9):1911–1922. doi: 10.1002/cncr.10915. [DOI] [PubMed] [Google Scholar]
- 48.Yazji S, Giles FJ, Tsimberidou AM, et al. Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia. 2003;17(11):2101–2106. doi: 10.1038/sj.leu.2403124. [DOI] [PubMed] [Google Scholar]


