Abstract
We have studied the basis for intrinsic resistance to low levels of vancomycin in Clostridium innocuum NCIB 10674 (MIC = 8 μg/ml). Analysis by high-pressure liquid chromatography (HPLC) and mass spectrometry of peptidoglycan nucleotide precursors pools revealed the presence of two types of UDP-MurNac-pentapeptide precursors constitutively produced, an UDP-MurNAc-pentapeptide with a serine at the C terminus which represented 93% of the pool and an UDP-MurNAc-pentapeptide with an alanine at the C terminus which represented the rest of the pool. C. innocuum cell wall muropeptides containing pentapeptide[Ser], either dialanine substituted on the epsilon amino group of lysine or not, were identified and represented about 10% of the monomers while only 1% of pentapeptide[d-Ala] monomers were found. The sequence of a 2,465-bp chromosomal fragment from C. innocuum was determined and revealed the presence of ddlc. innocuum and C. innocuum racemase genes putatively encoding homologues of d-Ala:d-X ligases and amino acid racemases, respectively. Analysis of the pool of precursors of Enterococcus faecalis JH2-2, containing cloned ddlc. innocuum and C. innocuum racemase genes showed in addition to the UDP-MurNAc-pentapeptide[d-Ala], the presence of an UDP-MurNAc-pentapeptide[d-Ser] precursor. However, the expression of low-level resistance to vancomycin was observed only when both genes were cloned in E. faecalis JH2-2 together with the vanXYc gene from Enterococcus gallinarum BM4174 which encodes a d,d-peptidase which eliminates preferentially the high affinity vancomycin UDP-MurNAc-pentapeptide [d-Ala] precursors produced by the host. We conclude that resistance to vancomycin in C. innocuum NCIB 10674 was related to the presence of the two chromosomal ddlc. innocuum and C. innocuum racemase genes allowing the synthesis of a peptidoglycan precursor terminating in serine with low affinity for vancomycin.
Members of the genus Clostridium are a major part of the anaerobic microflora of humans and are a potential cause of human infections. Clostridium innocuum belongs to the normal intestinal flora of human infants and adults and is one of the species which have been reported to cause human infections such as intra-abdominal sepsis, bacteremia, and endocarditis (11, 25). Clostridium spp. are considered susceptible to glycopeptides, vancomycin, and teicoplanin. However, a recent report has shown that MICs of vancomycin were equal to 8 or 16 μg/ml (intermediate resistance) for 28 clinical isolates of C. innocuum and C. innocuum NCIB 10674 while teicoplanin remained active (MICs = 0.25 to 1 μg/ml), suggesting that low-level vancomycin resistance is intrinsic in this species (23). Resistance to glycopeptide antibiotics among gram-positive organisms may be either acquired or naturally expressed (5). Acquired resistance to glycopeptides is generally observed in enterococci and has recently spread to Staphylococcus aureus (10). The VanA, VanB, and VanD types of resistance result from the synthesis of a new pentadepsipeptide peptidoglycan precursors ending in d-lactate [d-Lac] and the elimination of the high-affinity vancomycin pentapeptide[d-Ala] precursor ending in d-alanine and synthesized by the host (27). Low-level resistance to vancomycin is acquired in enterococci with the VanE or VanG phenotypes and is intrinsic in the VanC types Enterococcus gallinarum and Enterococcus casseliflavus-Enterococcus flavescens (5). The basis for this resistance is the synthesis of d-Ala-d-serine (d-Ser) which is substituted for d-Ala-d-Ala in the pentapeptide precursor (6, 7) and in the muropeptides (18). Again, the pentapeptide[d-Ala] precursor with high affinity for vancomycin is completely eliminated by d,d-peptidases and/or d,d-carboxypeptidases, a condition necessary for full expression of resistance (28). In Enterococcus gallinarum, synthesis of d-Ser is carried out by a pyridoxal phosphate-dependent and membrane-bound serine racemase (VanT) (3).
In this report, we analyzed the pool of precursors and the peptidoglycan structure of C. innocuum NCIB 10674. We identified a ddlc. innocuum gene and a C. innocuum racemase gene with homology to genes encoding d-Ala-d-X ligases and amino acid racemases, respectively, and responsible for the synthesis of a precursor and different muropeptides ending in d-Ala-d-Ser.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
C. innocuum NCIB 10674 (MICs of vancomycin and teicoplanin equal to 8 and 0.5 μg/ml, respectively) was grown in Wilkins-Chalgren broth or agar (Difco Laboratories, Detroit, Mich.) at 37°C under anaerobic conditions. For testing the inducible or constitutive mode of expression of vancomycin resistance, strain NCIB 10674 was grown anaerobically overnight in broth without or with subinhibitory concentrations of vancomycin (2 or 4 μg/ml). Induced and noninduced cells were diluted 1/50 in 10 ml of fresh broth in the presence of vancomycin at 0, 2, or 4 μg/ml. Bacterial growth was then measured for 12 h by spectrophotometry at 650 nm (Sequioa-Turner photometer [model 340]) and growth curves were plotted. Enterococcus faecalis JH2-2 was grown in brain heart infusion broth or agar (Difco Laboratories) at 37°C. Amplified genes were cloned in the shuttle multicopy vector pJIM2246 (which confers chloramphenicol resistance) (26). The MICs of vancomycin for enterococci containing various constructs were determined on Mueller-Hinton agar by the E-test method as recommended by the manufacturer (AB Biodisk, Uppsala, Sweden) and read after 48 h of incubation at 37°C. E. coli DH10B was used in transformation experiments. The vanXYc gene was amplified from total DNA of E. gallinarum BM4174 (28).
DNA manipulations.
C. innocuum NCIB 10674 total DNA was extracted as previously described (23). Amplification of fragments internal to genes encoding related ligases with degenerate V1 and V2 primers was performed as previously described (15). Digestion with restriction endonucleases (New England Biolabs Inc., Beverly, Mass.), isolation of plasmid DNA, ligation, and transformation were carried out by standard methods (29). Sequencing was carried out with an ABI 377 automatic sequencer (Applied Biosystems). The entire sequence of the ddlc. innocuum and C. innocuum racemase genes was obtained by inverse PCR (24). Briefly, a digoxigenin-labeled probe (Roche Applied Science, Mannheim, Germany) from the amplified product was obtained with oligonucleotides V1 and V2. This probe hybridized in Southern experiments to a 5-kb SacII fragment, a 6.7-kb DraI fragment, and a 3-kb EcoRI fragment from C. innocuum NCIB 10674 chromosomal DNA. Clostridium DNA was digested with these enzymes and self-ligated at 15°C for 18 h. DNA was also digested with both DraI and SacII and treated with T4 DNA polymerase to generate blunt ends before ligation. The inverse PCR was performed with primers A, B, C, and D (Table 1). The ddlc. innocuum, C. innocuum racemase, and vanXYC genes were cloned in plasmid pJIM2246 using primers shown in Table 1. Nucleotide and amino acid sequences were analyzed by using the BLAST and FASTA softwares available over the Internet at the National Center for Biotechnology Information Web site (http://www.ncbi.nlm.nih.gov/). Multiple sequence alignment and phylogenetic tree were performed with the ClustalX and PHYLIP programs.
TABLE 1.
Primers used in this study
| Primer | Sequencea | Positionb | Primer use |
|---|---|---|---|
| A | −5′ GTATGGGATGCCACTCAGCTCAAAC 3′ | 378-354 | Inverse PCR |
| B | +5′ TGCCGGAAGCAGCTTTGGAATTCAC 3′ | 567-591 | Inverse PCR |
| C | +5′ AAGGCATATCGAGCCATGAACTGCA 3′ | 867-892 | Inverse PCR |
| D | −5′ AGCCGTCAAAGGACTCCATCCTGTG 3′ | 613-591 | Inverse PCR |
| LigBamHI | +5′ CTGGATCCAGTGGTGAATGAGCTGG 3′ | −55-−31 | ddlc. innocuum cloning |
| LigSalI | −5′ GCTTGTCGACGGAATCAGCTTCTG 3′ | 1178-1154 | ddlc. innocuum cloning |
| RacBamHI | +5′ CCGTTTCCGGATCCGATTGACAAG 3′ | 1018-1041 | C. innocuum racemase cloning |
| RacSall | −5′ ATTGTCGACCTTCTCTTGAAAAATAG 3′ | 2265-2240 | C. innocuum racemase cloning |
| XYSacII | +5′ TTGAGAGCTCTGGCAGAGGAG 3′ | −28-−9 | vanXYc cloning |
| XYBamHI | −5′ GTTCGCATAATAAATAAAGGATCCGA 3′ | 589-564 | vanXYc cloning |
Restriction sites introduced in the primer sequence are underlined; +, direct primer; −, reverse primer.
Position relative to the ATG start codon in the ddlc. innocuum or in the vanXYc genes.
Preparation and analysis of the peptidoglycan nucleotide precursor pools.
Enterococcal cells grown to an optical density at 650 nm of 0.7 were treated with vancomycin at 50 times the MIC for 90 min. Peptidoglycan precursors were extracted with formic acid as previously described (6) and analyzed by reverse-phase high-pressure liquid chromatography (RP-HPLC) with a μBondapack C18 column (3.9 by 300 nm; Waters) at a flow rate of 0.5 ml min−1 with 50 mM ammonium acetate, pH 5.0. Products were detected by absorbance at 262 nm. The UDP-MurNAc structures were deduced from their molecular mass determined by liquid chromatography-mass spectrometry and mass spectrometry-mass spectroscopy (MS/MS) as previously described (9).
Peptidoglycan structure analysis.
Muropeptides were prepared from cell walls as described previously (8) except that hydrofluoric acid was used during the peptidoglycan purification (12, 18) and cellosyl (generous gift from Hoechst) was added to mutanolysin (Sigma, Saint-Quentin Fallavier, France) and lysozyme (Sigma) at 250 μg/ml each in phosphate buffer (25 mM, pH: 6.5) containing MgCl2 (10 mM) during the hydrolysis step. The resulting muropeptides were reduced with sodium borohydride and separated by RP-HPLC coupled to mass spectrometry as previously described (8, 20). The structure of the muropeptides were deduced either from their molecular masses or after coelution with other structures previously identified in E. faecalis (13, 19). Some muropeptides were further purified by RP-HPLC and analyzed by MS/MS using the nanoelectrospray source kit for the Finnigam TSQ 7000 Protona A/S (San Jose, Calif.) as previously described (20).
Nucleotide accession number.
The DNA sequences of the ddlc. innocuum and C. innocuum racemase genes have been deposited with GenBank accession number AY479979.
RESULTS
Pool of UDP-linked cytoplasmic precursors in C. innocuum.
Precursors were studied in C. innocuum NCIB 10674 grown in the presence (2 μg/ml) or absence of vancomycin. Chromatograms of precursor pools of C. innocuum NCIB 10674 that were induced or noninduced were similar and showed that two types of UDP-MurNAc-pentapeptide precursors were present. One had a molecular mass of 1,165.8 Da, corresponding to a UDP-MurNAc-pentapeptide with a serine at the C terminus (pentapeptide[d-Ser]) and represented 93% of the pool. The other had a molecular mass of 1,149.4 Da, corresponding to a UDP-MurNAc-pentapeptide with an alanine at the C terminus (pentapetide [d-Ala]) and represented only 7% of the pool. The presence of the serine or an alanine at position 5 as well as the presence of a lysine residue at position 3 was demonstrated by MS/MS (data not shown). Neither UDP-MurNAc-tetrapeptide nor UDP-MurNAc-tripeptide precursors were found. Lack of tetrapeptide precursors suggested the absence of d,d-carboxypeptidase activity in C. innocuum. The quantitative similarity of the precursor pool for induced and noninduced cells suggested that expression of resistance to vancomycin was constitutive in C. innocuum NCIB 10674. This observation was consistent with the finding that growth curves for cells induced or not induced with vancomycin and challenged with subinhibitory concentrations of vancomycin were similar (data not shown).
Muropeptide composition of C. innocuum NCIB 10674.
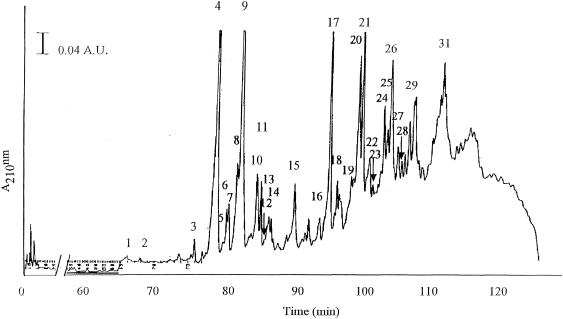
The structure of 29 muropeptides of C. innocuum was identified (Fig. 1) and their deduced structures are shown in Table 2. Among the monomers two major muropeptides (peak 4 and 9) representing about 60% of the monomers were identified by their molecular mass and MS/MS as disaccharide tripeptides with two alanines branched on the ɛ-amino group of the l-lysine3. Peak 9 differed from peak 4 by a mass difference of −42, corresponding to the loss of the N-acetyl residue from the N-acetylglucosaminyl moiety of the disaccharide. This suggested that two alanines could be part of the interpeptide bridge in different oligomers. The presence of such dialanine interpeptide bridges was confirmed by MS/MS in two tetra-tridimers present in peak 20 and 26.
FIG. 1.
Separation of C. innocuum cell wall muropeptides by RP-HPLC.
TABLE 2.
Molecular mass and structure of muropeptides from C. innocuum NCIB 10476
| Peak | Muropeptide type | Alanine no.a | Proposed structureb |
m/zc
|
|
|---|---|---|---|---|---|
| Observed | Calculated | ||||
| 1 | Monomer | DS-mono | 570.2 | 570.2 | |
| 2 | Monomer | DS-di | 698.3 | 698.4 | |
| 3 | Monomer | DS-tetra | 897.4 | 897.6 | |
| 4 | Monomer | DS-A2-trid | 968.5 | 968.7 | |
| 5 | Monomer | DS-tetra(−42) | 855.4 | 855.7 | |
| 6 | Monomer | DS-A-tetra | 968.5 | 968.5 | |
| 7 | Monomer | DS-A-tetra(OH)d | 969.5 | 969.7 | |
| 8 | Monomer | DS-penta[A](−42) | 926.6 | 926.6 | |
| Monomer | DS-A2-penta[S](OH)d | 1,127.5 | 1,128.0 | ||
| 9 | Monomer | DS-A2-tri(−42) | 926.5 | 926.7 | |
| Monomer | DS-A2-penta[S] | 1,126.5 | 1,127.2 | ||
| 10 | Monomer | DS-A-tetra(OH)(−42) | 927.4 | 927.6 | |
| 11 | Monomer | DS-A2-tetra | 1,039.8 | 1,039.8 | |
| 12 | Monomer | DS-A2-penta[S](OH)(−42) | 1,085.5 | 1,085.4 | |
| 13 | Monomer | DS-A-tetra(−42) | 926.5 | 926.8 | |
| 14 | Monomer | DS-A2-penta[S](−42)d | 1,084.5 | 1,084.8 | |
| Monomer | DS-A2-penta[A](OH)d | 1,111.5 | 1,111.5 | ||
| 15 | Monomer | DS-A2-tetra(−42) | 997.7 | 998.4 | |
| 16 | Dimer | [3] | BisDSe | 1,917.9 | 1,918.2 |
| 17 | Dimer | [4] | BisDSe | 1,990.0 | 1,990.1 |
| Dimer | [3] | DS-A2-tetra-A-penta[S](OH)(−42)f | 2,035.0 | 2,034.7 | |
| 18 | Dimer | [4] | BisDS-A2-tetra-A2-penta[S] | 2,148.0 | 2,148.3 |
| 19 | Dimer | [3] | BisDS—(OH)(−42) | 1,876.9 | 1,877.0 |
| 20 | Dimer | [4] | BisDS-A2-tetra-A2-tri(−42)d | 1,947.9 | 1,947.8 |
| 21 | Dimer | [4] | BisDS—(−42)e | 1,947.9 | 1,947.0 |
| 22 | Dimer | [4] | BisDS-A2-tetra-A2-penta[S](OH)(−42) | 2,105.0 | 2,105.0 |
| 23 | Dimer | [4] | BisDS-A2-tetra-A2-penta[S](OH × 2)(−42) | 2,106.0 | 2,105.7 |
| 24 | Trimer | [6] | TerDS—(OH)e | 3,010.5 | 3,010.9 |
| 25 | Trimer | [4] | TerDS-A2-tetra-A-tetra-A-penta[S](OH × 3)f | 3,028.5 | 3,028.3 |
| 26 | Dimer | [4] | BisDS-A2-tetra-A2-tri(OH)(−42 × 2)d | 1,905.9 | 1,905.9 |
| Dimer | [3] | BisDS—(−42 × 2)e | 1,833.9 | 1,833.2 | |
| 27 | Dimer | [4] | BisDS—(OH)(−42 × 2) | 1,905.9 | 1,906.2 |
| 28 | Dimer | [4] | BisDS-A2-tetra-A2-penta[S](OH × 2)(−42 × 2) | 2,064.9 | 2,065.3 |
| 29 | Trimer | [6] | TerDS-tetra-tetra-tri (OH) (−42)e | 2,969.4 | 2,968.9 |
Data in brackets are total number of alanines present in the cross-bridge, in the free N- terminal and C-terminal ends of oligomers.
Proposed structure deduced from the molecular mass or MS/MS. DS, disaccharide (GlcNAC-MurNAC); BisDS, dimeric form; TerDS, trimeric form; mono, monopeptide (l-Ala); di, dipeptide (l-Ala-d-iGln); tri, tripeptide (l-Ala-d-iGln-l-Lys); tetra, tetrapeptide (l-Ala-d-iGln-l-Lys-d-Ala); penta [A], pentapeptide (l-Ala-d-iGln-l-Lys-d-Ala-d-Ala); penta [S], pentapeptide (l-Ala-d-iGln-l-Lys-d-Ala-d-Ser); A, one alanine branched on l-Lys; A2, two alanines branched on L-Lys; OH indicates the presence of Glu instead of iGln; (−42), monomer without acetyl group on GlcNAC, (−42 × 2), dimer without acetyl group on both GlcNAC of the dimer. For other trimers, tetramers, and pentamers (peaks >29), no structure is proposed.
(M + H)+ ion of the reduced muropeptide.
Structure determined after MS/MS.
No structure is proposed, due to the unknown distribution of the alanine(s) (see footnote a) in the interpeptide bridge or at the C terminus: for a dimer it can be either a BisDS-tetra-tetra or a BisDS-tetra-tri, and for a trimer it can be either a TerDS-tetra-tetra-tetra or a TerDS-tetra-tetra-tri.
Assignment of the number of alanine in the cross bridge is arbitrary.
As expected from the presence or cytoplasmic pentapeptide[d-Ser] precursors, different monomers containing pentapeptide[d-Ser]) either dialanine substituted or not were also identified in peaks 8, 9, 12, and 14, and represented about 10% of the monomers. Only small quantities (1%) of monomers pentapeptide[d-Ala] were found in agreement with the low amount of pentapeptide[d-Ala] precursor present in the pool. Structures containing pentapeptide[d-Ser] were also identified among the dimers (peaks 17, 18, 22, 23, 25, and 28). Since the detailed structure of different dimers and trimers could not be exactly determined due to the unknown precise number of alanine present in the interpeptide-bridge or at the C terminus (tetra or tri) only some structures are proposed (Table 2).
Identification of the ddlc. innocuum and C. innocuum racemase genes homologous to ddl and alr genes.
No amplification product was observed with DNA of the strain, using a PCR assay with primers specific for resistance genes vanA, vanB, vanC1, vanC2, vanD, vanE, and vanG (14). The degenerate primers V1 and V2 which allow amplification of fragments internal to genes that encode related ligases (15), were used in a PCR with total DNA of C. innocuum NCIB 10674 as a template. A ca. 600-bp fragment was amplified and cloned into E. coli. Nucleotide sequences of the fragment, determined on both strands, were identical in 10 clones. The deduced amino acid sequence was compared with those encoded by various ddl genes, the d-Ala:d-Ala ligases from E. coli, the VanA and VanB d-Ala:d-Lac ligases, and the VanC1 and VanE d-Ala:d-Ser ligases. The sequence displayed between 28% and 39% of identity with the corresponding portion of those proteins. The motifs conserved in the related ligases were present, suggesting that the amplified fragment was internal to a ligase gene possibly involved in vancomycin resistance. Fragments similar in size were also amplified with oligonucleotides V1 and V2 from two clinical isolates of C. innocuum. The deduced amino acid sequence was found identical to that for C. innocuum NCIB 10674 except for one amino acid substitution (V235A).
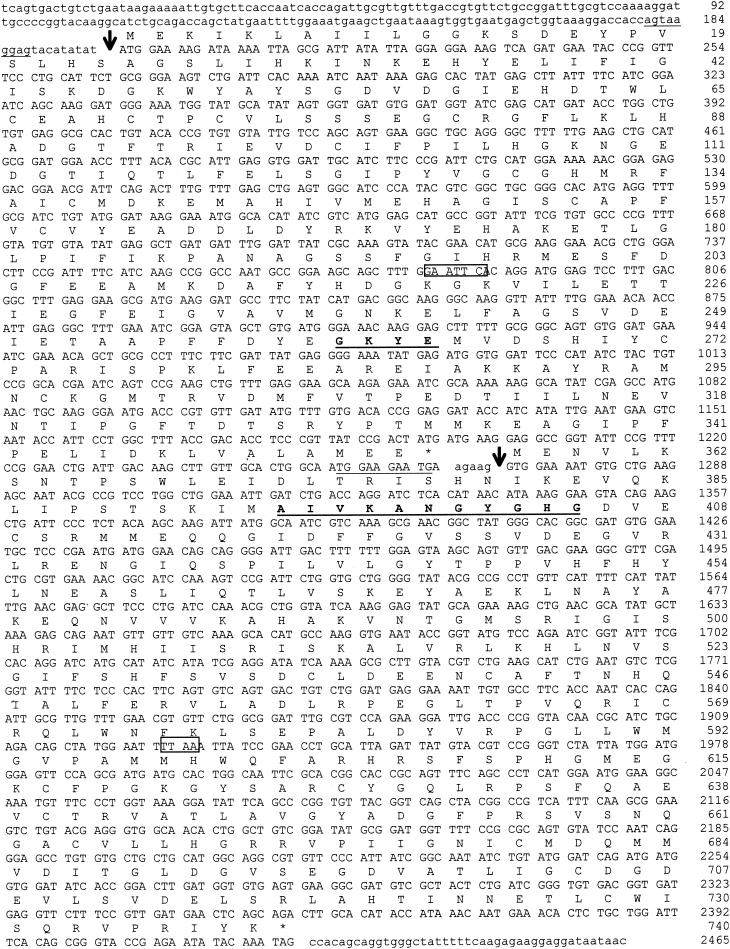
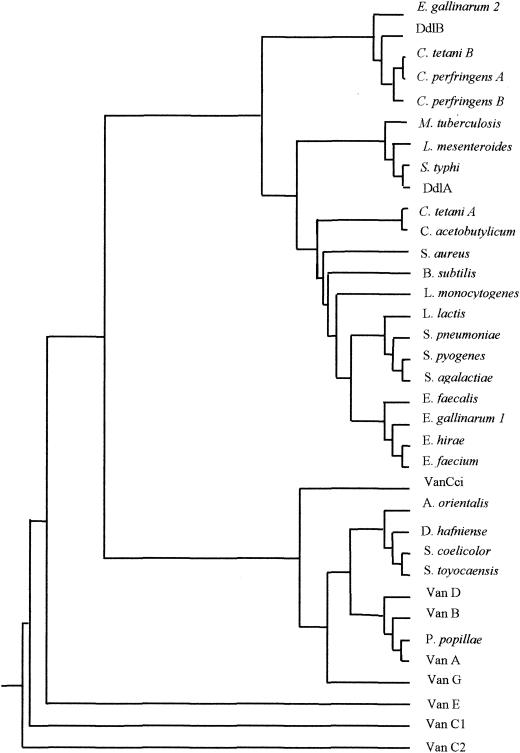
The sequence of the regions upstream and downstream from the V1-V2 PCR product was obtained by inverse PCR as follows. The upstream sequence was obtained from a DraI-SacII fragment and the downstream sequence from an EcoRI fragment. In the 2,465-bp sequenced fragment, two open reading frames (ORF) were identified (Fig. 2). The 1,068-bp upstream ORF (nucleotides [nt] 198 to 1265) was preceded by a putative ribosome binding site (RBS) (5′-AGTAAGGAGTN8ATG) that displayed complementarity (underlined) to the Bacillus subtilis RBS consensus sequence (3′-OH UCUUUCCUCC) (22). The percentages of identity of the putative product, called Ddlc. innocuum, with various d-Ala:d-Lac, d-Ala:d-Ser, and d-Ala:d-Ala ligases were calculated from the sequence alignment. Percentages of identity ranged from 39 to 45% with the d-Ala:d-Ser ligases (VanE, Van C1, VanC2, and VanG), from 36 to 41% with d-Ala:d-lactate (d-Lac) ligases (VanA, Van B, VanD, and ligases from Paenibacillus popillae, Streptomyces toyocaensis, Amycolatopsis orientalis), and from 36 to 38% with the putative d-Ala:d-Ala ligases from clostridia (Clostridium acetobutylicum, Clostridium perfringens, Clostridium tetani, and Desulfitobacterium hafniense). The highest degree of identity (45%) was with the VanG (d-Ala:d-Ser) ligase. The motifs conserved in the related amino acid ligases were found in the deduced 355-amino acid sequence. Of four amino acids that are present in the d-Ala:d-Ser ligases (EKYQ), two (KY) at positions 262 to 263 were conserved (16). Alignment of ligases was used to construct a phylogenetic tree, confirming that Ddlc. innocuum was related to d-Ala:d-X ligases (Fig. 3) (17).
FIG. 2.
Sequence of the ddlc. innocuum and C. innocuum racemase genes. The putative ribosome binding sites are underlined. The deduced amino acid sequence of Ddlc. innocuum and C. innocuum racemase are shown above the nucleotide sequence. Start of the proteins is indicated by an arrow. The EcoRI (nt 781 to 786) and the DraI (nt 1923 to 1926) sites used for inverse PCR are boxed. The conserved motif in d-Ala:d-Ser ligases and the putative pyridoxal attachment site in alanine racemases are in boldface type and are underlined.
FIG. 3.
Phylogenetic tree derived from the alignment of d-Ala:d-Lac, d-Ala:d-Ser, and selected d-Ala:d-Ala ligases. The tree was constructed by the neighbor-joining method, taking into account the results of maximum-parsimony and bootstrapping analysis. Sequences of the ligases are from Amycolatopsis orientalis (AAD19835), Bacillus subtilis 168 (CAB12263), C. acetobutylicum [Ddl] (AAK80837), C. innocuum [Ddl c. innocuum] (), C. perfringens [DdlA] (BAB81021), C. perfringens [DdlB] (BAB80525), C. tetani [DdlA] (AAO34934), C. tetani [DdlB] (AAO35288), D. hafniense [Ddl] (ZP_00099215), E. coli K12 [DdlA] (NP_414915), E. coli K12 [DdlB] (NP_414634), E. casseliflavus [VanC2] (AAA60990), E. faecalis V583 [VanB] (2007289A), E. gallinarum BM4174 [VanC1] (AAA24786), E. faecalis [VanD] (AAM09849), E. faecalis BM4405 [VanE] (AAL27442), E. faecalis WCH9 [VanG] (AAF71281), E. faecalis [Ddl] (AAC43218), E. faecium BM4147 [VanA] (AAA65956), E. faecium [Ddl] (ZP_00036460), E. gallinarum BM4174 [Ddl1] (AAN62561), E. gallinarum BM4174 [Ddl2] (AAK97387), E. hirae [Ddl] (Q47827), Lactococcus lactis (AAK04439), Leuconostoc mesenteroides [Ddl] (Q48745), Listeria monocytogenes (CAC98933), M. tuberculosis [Ddl] (CAB05431), P. popillae (AAF36803), Salmonella enterica serovar Typhi [DdlA] (AA070072), S. aureus [Ddl] (BAB43170), S. agalactiae [Ddl] (AAM99654), S. pneumoniae [Ddl] (CAB64467), Streptomyces coelicolor [Ddl] (NP_627790), and Streptomyces toyocaensis [Ddl] (AAC23582).
Immediately downstream ddlc. innocuum, another ORF (nt 1271 to 2422) was identified that was preceded by an RBS (5′-TGGAAGAATGN6GTG) that displayed complementarity to the 3′ extremity (underlined) to the 3-OH terminus of B. subtilis 16S rRNA (22) and began by an unusual GTG initiation codon. This ORF could possibly code for a 383-amino-acid protein that displayed homology with alanine racemases of different microorganisms encoded by alr genes and was therefore a hypothetical C. innocuum racemase gene. No potential transmembrane domains was detected using a hydrophobicity plot of the predicted amino acid sequence, suggesting that it was a soluble protein. Percentages of identity with serine racemases VanT of E. gallinarum BM4174, VanTE of E. faecalis, VanTC2 from E. casseliflavus and the putative serine racemase VanTG ranged from 30 to 34%. Percentages of identity with putative alanine racemases of clostridia (C. acetobutylicum, C. perfringens, C. tetani, Clostridium thermocellum, and D. hafniense) ranged from 28 to 33%. Analysis revealed the presence of motifs conserved in racemases, in particular the putative pyridoxal attachment site which is highly conserved in alanine racemases (Fig. 2).
Expression of glycopeptide resistance and pool of UDP-linked cytoplasmic precursors in E. faecalis harboring plasmid encoded genes from C. innocuum.
We tested if the putative ddlc. innocuum ligase and C. innocuum racemase genes could confer vancomycin resistance in an heterologous host. They were first amplified from C. innocuum, then cloned either alone or combined on the shuttle plasmid pJIM2246 where they were expressed under the control of the promoter of the chloramphenicol acetyltransferase gene and finally introduced into E. faecalis JH2-2 (Table 3). E. faecalis JH2-2 harboring pJIM2246 containing the cloned ddlc. innocuum ligase or C. innocuum racemase genes showed only the presence of pentapeptide[d-Ala] precursor and no change in the vancomycin MICs. Combination of C. innocuum racemase gene and ddlc. innocuum resulted in the production of pentapeptide[d-Ala] and pentapeptide[d-Ser] whereas resistance to vancomycin was still not expressed. This result was, however, not surprising since synthesis of modified precursors by the cloned genes could result in vancomycin resistance only if the high-affinity vancomycin pentapeptide[d-Ala]precursor produced by the host was eliminated (5, 27, 28). Partial elimination of this latter was achieved, by cloning the vanXYc gene from E. gallinarum BM4174 downstream from the ddlc. innocuum and/or C. innocuum racemase genes (Table 3). VanXYc has a d,d-peptidase activity which degrades UDP-MurNAc-pentapeptide[d-Ala] to UDP-MurNAc-tetrapeptide and can hydrolyze d-Ala:d-Ala, although at a lesser efficiency (28). By contrast, this enzyme has a with very low dipeptidase activity against d-Ala:d-Ser and no activity against UDP-MurNAc-pentapeptide[d-Ser] (28). Introduction of this construct in E. faecalis JH2-2 resulted in the production of pentapeptide[d-Ser], pentapeptide[d-Ala], and tetrapeptide precursors, together with a reproducible threefold increase in the MIC of vancomycin. In the presence of the cloned ddlc. innocuum and vanXYc genes and in the absence of C. innocuum racemase gene similar increased MIC of vancomycin was observed for E. faecalis when d-serine (10 mM) was added to Mueller-Hinton agar. In contrast, the addition of L-serine (10 mM) did not affect susceptibility to vancomycin.
TABLE 3.
Peptidoglycan precursors in extracts of E. faecalis JH2-2 harboring various plasmids and for which MICs of vancomycin differ
| Plasmid | MIC of vancomycin (μg/ml) | Type of peptidoglycan precursors (%)a
|
||
|---|---|---|---|---|
| Tetra | Penta[Ser] | Penta[Ala] | ||
| pJIM2246 | 2 | —b | — | 100 |
| pJIM2246Ω ddlc. innocuum | 2 | — | — | 100 |
| pJIM2246Ω C. innocuum racemase gene | 2 | — | — | 100 |
| pJIM2246Ω ddlc. innocuum, C. innocuum racemase gene | 2 | — | 9 | 91 |
| pJIM2246Ω vanXYc | 2 | 90.8 | — | 9.2 |
| pJIM2246Ω ddlc.innocuum, vanXYc | 2 | NDc | ND | ND |
| pJIM2246Ω C. innocuum racemase gene, vanXYc | 2 | 92.5 | — | 7.5 |
| pJIM2246Ω ddlc. innocuum, C. innocuum racemase gene, vanXYc | 6 | 28.5 | 11.7 | 59.8 |
Tetra, UDP-MurNac-l-Ala-γ-d-Glu-l-Lys-d-Ala; Penta[Ser], UDP-MurNac-l-Ala-γ-d-Glu-l- Lys-d-Ala-d-Ser; Penta[Ala], UDP-MurNac-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala.
—, not detected.
ND, not done.
DISCUSSION
In this work, we have shown that intrinsic low-level resistance to vancomycin in C. innocuum is related to the synthesis of a high proportion of low-affinity precursors ending in d-Ala-d-Ser (4, 18). This is the first report of such a mechanism of resistance in an anaerobic bacteria.
The two genes encoding a putative Ddlc. innocuum ligase and C. innocuum racemase were found to be adjacent on the chromosome. The ligases from other Clostridium spp. form a group distinct from Ddlc. innocuum which was closely related to the VanG d-Ala:d-Ser ligase and to d-Ala:d-Lac ligases, although placed on a separate branch (Fig. 3). C. innocuum racemase was predicted to be a soluble protein, similar to “classical” racemases and therefore differs from the other serine racemases reported previously in enterococci—VanTC (1), VanTE (1), and VanTG (21—which contain 10 transmembrane domains and are probably membrane-bound. The reason for this difference is unknown.
As our results showed that resistance was related to the synthesis of precursors terminating in d-Ser, the presence of a small quantity of precursors ending in d-Ala and of pentapeptide[Ala] monomers in C. innocuum was surprising. It is possible that the Ddlc. innocuum ligase has also some activity of a d-Ala:d-Ala ligase. Alternatively, another d-Ala:d-Ala ligase could be encoded by the chromosome of C. innocuum. However a single gene encoding a d-Ala:d-Ser ligase was amplified by oligodeoxynucleotides V1 and V2 which does not exclude the presence of a second, more structurally remote, ddl gene. There is circumstantial evidence that a single ligase is present as no gene encoding a VanXY-type enzyme is present in the operon from C. innocuum between the ligase and racemase genes while when the two genes are cloned in E. faecalis (which has a d-Ala-d-Ala ligase), VanXYc has also to be added for the organism to become low-level resistant to vancomycin. In general, bacterial chromosomes encode a single enzyme, although there are exceptions such as E. gallinarum with one d-Ala:d-Ser and two d-Ala:d-Ala ligases (2), and enteric bacteria (E. coli and Salmonella enterica serovar Typhimurium) with two d-Ala:d-Ala ligases. The in silico analysis of the sequenced genome of clostridia showed that C. acetobutylicum and D. hafniense contained only one putative d-Ala:d-Ala ligase (GenBank access numbers AAK80837 and ZP_00099215, respectively), C. perfringens two (BAB81021 and BAB80525), and C. tetani two, as well (AAO34934 and AAO35288).
Cloning of the ddlc. innocuum and C. innocuum racemase genes in E. faecalis showed that cooperation of the two genes was necessary for the synthesis of the low vancomycin affinity pentapeptide[d-Ser] precursor in this host and that they confer resistance to vancomycin provided that, in the presence of the cloned vanXYc gene, the high affinity vancomycin pentapeptide[d-Ala] precursor synthesized by the heterologous host was partially eliminated. Homology of C. innocuum racemase with amino acid racemases and expression of vancomycin resistance without addition of d-Ser suggested that the protein catalyses synthesis of d-Ser in vivo from L-Serine available either from the culture medium or synthesized de novo. In confirmation of this hypothesis, the cloned ddlc. innocuum gene alone in presence of vanXYc was sufficient for expression of resistance if bypass of the absent C. innocuum racemase was obtained after addition of d-serine to the culture medium.
Thus, it can be concluded that in C. innocuum, cooperation of ddlc. innocuum and C. innocuum racemase lead to the expression of glycopeptide resistance since they allow the predominant production of cytoplasmic pentapeptide [d-Ser] precursor which is then processed by the cell wall machinery to be integrated in the peptidoglycan.
Acknowledgments
V. David was the recipient of a grant from the Fondation pour la Recherche Médicale. This work was supported in part by a grant from INSERM.
REFERENCES
- 1.Abadia Patiño, L., P. Courvalin, and B. Perichon. 2002. vanE gene cluster of vancomycin-resistant Enterococcus faecalis BM4405. J. Bacteriol. 184:6457-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambur, O. H., P. E. Reynolds, and C. A. Arias. 2002. d-Ala:d-Ala ligase gene flanking the vanC cluster: evidence for presence of three ligase genes in vancomycin-resistant Enterococcus gallinarum BM4174. Antimicrob. Agents Chemother. 46:95-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias, C. A., M. Martin-Martinez, T. L. Blundell, M. Arthur, P. Courvalin, and P. E. Reynolds. 1999. Characterization and modelling of VanT: a novel, membrane-bound, serine racemase from vancomycin-resistant Enterococcus gallinarum BM4174. Mol. Microbiol. 31:1653-1664. [DOI] [PubMed] [Google Scholar]
- 4.Arias, C. A., P. Courvalin, and P. E. Reynolds. 2000. vanC cluster of vancomycin-resistant Enterococcus gallinarum BM4174. Antimicrob. Agents Chemother. 44:1660-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur, M., P. Reynolds, and P. Courvalin. 1996. Glycopeptide resistance in enterococci. Trends Microbiol. 4:401-407. [DOI] [PubMed] [Google Scholar]
- 6.Billot-Klein, D., L. Gutmann, E. Collatz, and J. van Heijenoort. 1992. Analysis of peptidoglycan precursors in vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 36:1487-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billot-Klein, D., L. Gutmann, S. Sable, E. Guittet, and J. van Heijenoort. 1994. Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type Enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J. Bacteriol. 176:2398-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billot-Klein, D., R. Legrand, B. Schoot, J. van Heijenoort, and L. Gutmann. 1997. Peptidoglycan structure of Lactobacillus casei, a species highly resistant to glycopeptide antibiotics. J. Bacteriol. 179:6208-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billot-Klein, D., D. Shlaes, D. Bryant, D. Bell, R. Legrand, L. Gutmann, and J. van Heijenoort. 1997. Presence of UDP-N-acetylmuramyl-hexapeptides and -heptapeptides in enterococci and staphylococci after treatment with ramoplanin, tunicamycin, or vancomycin. J. Bacteriol. 179:4684-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 11.Cutrona, A. F., C. Watanakunakorn, C. R. Schaub, and A. Jagetia. 1995. Clostridium innocuum endocarditis. Clin. Infect. Dis. 21:1306-1307. [DOI] [PubMed] [Google Scholar]
- 12.De Jonge, B. L., Y. S. Chang, D. Gage, and A. Tomasz. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J. Biol. Chem. 267:11248-11254. [PubMed] [Google Scholar]
- 13.De Jonge, B. L., S. Handwerger, and D. Gage. 1996. Altered peptidoglycan composition in vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 40:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutka-Malen, S., S. Evers, and P. Courvalin. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:24-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutka-Malen, S., C. Molinas, M. Arthur, and P. Courvalin. 1992. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a D-alanine:D-alanine ligase-related protein necessary for vancomycin resistance. Gene 112:53-58. [DOI] [PubMed] [Google Scholar]
- 16.Evers, S., B. Casadewall, M. Charles, S. Dutka-Malen, M. Galimand, and P. Courvalin. 1996. Evolution of structure and substrate specificity in D-alanine:D-alanine ligases and related enzymes. J. Mol. Evol. 42:706-712. [DOI] [PubMed] [Google Scholar]
- 17.Felsenstein, J. 1988. Phylogenies from molecular sequences: inference and reliability. Annu. Rev. Genet. 22:521-565. [DOI] [PubMed] [Google Scholar]
- 18.Grohs, P., L. Gutmann, R. Legrand, B. Schoot, and J. L. Mainardi. 2000. Vancomycin resistance is associated with serine-containing peptidoglycan in Enterococcus gallinarum. J. Bacteriol. 182:6228-6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mainardi, J. L., D. Billot-Klein, A. Coutrot, R. Legrand, B. Schoot, and L. Gutmann. 1998. Resistance to cefotaxime and peptidoglycan composition in Enterococcus faecalis are influenced by exogenous sodium chloride. Microbiology 144:2679-2685. [DOI] [PubMed] [Google Scholar]
- 20.Mainardi, J. L., R. Legrand, M. Arthur, B. Schoot, J. van Heijenoort, and L. Gutmann. 2000. Novel mechanism of beta-lactam resistance due to bypass of DD-transpeptidation in Enterococcus faecium. J. Biol. Chem. 275:16490-16496. [DOI] [PubMed] [Google Scholar]
- 21.McKessar, S. J., A. M. Berry, J. M. Bell, J. D. Turnidge, and J. C. Paton. 2000. Genetic characterization of vanG, a novel vancomycin resistance locus of Enterococcus faecalis. Antimicrob. Agents Chemother. 44:3224-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran, C. P., Jr., N. Lang, S. F. LeGrice, G. Lee, M. Stephens, A. L. Sonenshein, J. Pero, and R. Losick. 1982. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol. Gen. Genet. 186:339-346. [DOI] [PubMed] [Google Scholar]
- 23.Mory, F., A. Lozniewski, V. David, J. P. Carlier, L. Dubreuil, and R. Leclercq. 1998. Low-level vancomycin resistance in Clostridium innocuum. J. Clin. Microbiol. 36:1767-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochman, H., A. S. Gerber, and D. L. Hartl. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120:621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onderdonk, A. B., and S. D. Allen. 1995. Clostridium, p. 574-586. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 26.Renault, P., G. Corthier, N. Goupil, C. Delorme, and S. D. Ehrlich. 1996. Plasmid vectors for Gram-positive bacteria switching from high and to low copy number. Gene 183:175-182. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds, P. E., F. Depardieu, S. Dutka-Malen, M. Arthur, and P. Courvalin. 1994. Glycopeptide resistance mediated by enterococcal transposon Tn1546 requires production of VanX for hydrolysis of D-alanyl-D-alanine. Mol. Microbiol. 13:1065-1070. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds, P. E., C. A. Arias, and P. Courvalin. 1999. Gene vanXYC encodes D,D-dipeptidase (VanX) and D,D-carboxypeptidase (VanY) activities in vancomycin-resistant Enterococcus gallinarum BM4174. Mol. Microbiol. 34:341-349. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.