Abstract
A novel reactivity mode of a diazo-group, the 1,3-addition of a nucleophile and an electrophile to the diazo-group, has been realized in the intramolecular aminoalkylation of β-amino-α-diazoesters to form tetrasubstituted 1,2,3-triazolines. The reaction proved to have a broad scope, good functional group tolerance, and excellent diastereoselectivity. In addition, a new Au-catalyzed intramolecular transannulation reaction of the obtained propargyl triazolines into pyrroles has been discovered.
Keywords: Diazocompound, Triazoline, Heterocycle, Diastereoselective Reaction, Aminoalkylation
Diazocompounds are important building blocks that have been extensively studied over the years.[1,2] They are widely used in organic chemistry due to the high energy and diverse reactivity of the diazo-group. Primarily, diazocompounds are utilized in denitrogenative generation of metal carbene species.[3] On the other hand, reactions with preservation of the diazo-group are also known. Thus, [3+2] cycloaddition reactions of diazocompounds are commonly used for synthesis of N-heterocycles.[4] There are also scattered reports on the reaction of nucleophiles and electrophiles with diazo-group in a 1,1-fashion when both, nucleophile and electrophile are added to the terminal nitrogen atom of the diazo-group. Reactions of this type are limited to the addition of nucleophiles (RLi or hydride ion), followed by protonation, to produce hydrazones (Scheme 1, eq. 1).[5,6] Herein we disclose a novel reactivity mode of diazocompounds: a 1,3-addition of nucleophile and electrophile at the nitrogen and carbon atoms of the diazo-group (eq. 2). This reactivity mode was accomplished in highly diastereoselective aminoalkylation reaction of the β-amino-α-diazoesters 1 with alkyl halides furnishing 1,2,3-triazolines 2 (eq. 3).
Scheme 1.
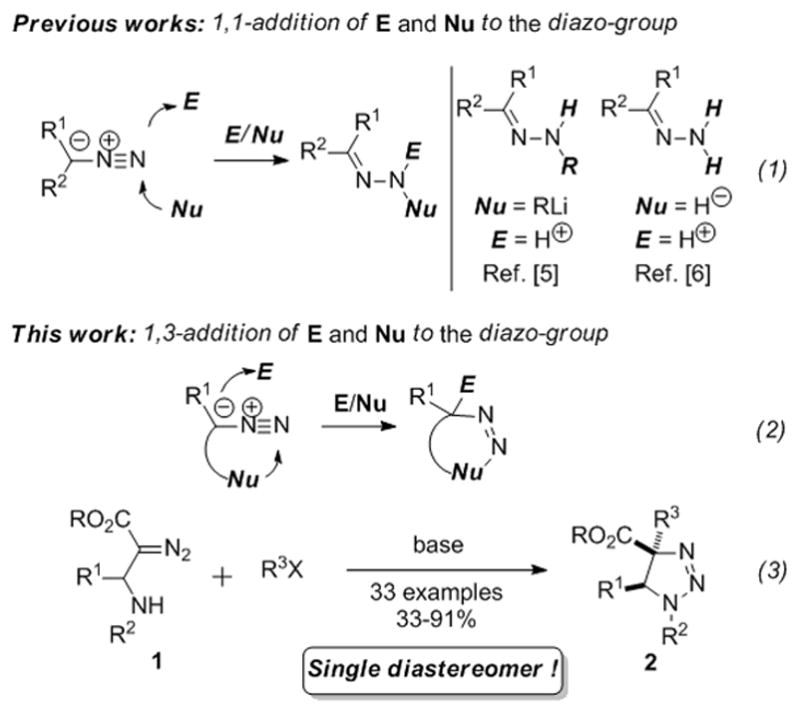
Reaction of diazocompounds with nucleophiles and electrophiles: 1,1- and 1,3 addition.
Recently, we have developed a method for synthesis of β-pyridylamino-α-diazoesters via a three-component coupling reaction of 2-aminoazines, aldehydes, and diazocompounds (Scheme 2, eq. a).[7] Upon investigation of synthetic utility of the obtained products, we found an unexpected reactivity of the diazo-group. Thus, an alkylation reaction of N-pyridyl diazocompound 1aa with benzyl bromide in the presence of NaH, instead of expected N-alkylation product 1′, produced 1,2,3-triazoline 2a, the product of C-alkylation of diazo-group (Scheme 2, eq. b).[8] This reaction proceeds exclusively in a trans-manner with respect to the aryl substituent at the β-position of the diazoester.[9]
Scheme 2.
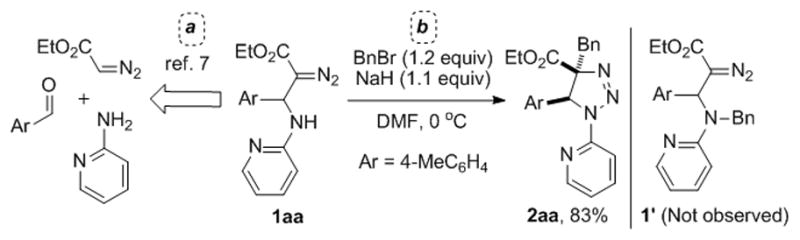
Diastereoselective aminoalkylation of β-pyridylamino-α-diazoester 1aa.
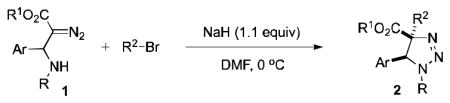
Not only the observed aminoalkylation reaction is interesting conceptually as it represents the first example of intramolecular 1,3-addition of nucleophile and electrophile to the diazo-group, but it also holds a synthetic promise to become a modular approach to valuable 1,2,3-triazoline molecules.[10] Accordingly, we turned our attention to the investigation of the scope of this new transformation (Table 1). Gratifyingly, we found that this aminoalkylation reaction is quite general with respect to the substituent at the β-N-atom of diazoester 1.
Table 1.
The scope of the aminoalkylation reaction of β-amino-α-diazoesters 1.[a]
| ||
|---|---|---|
| entry | product | yield (%) |
| 1 |
 2ab |
71 |
| 2 |
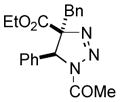 2ac |
81 |
| 3 |
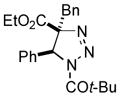 2ad |
49 |
| 4 |
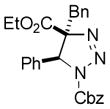 2ae |
58 |
| 5 |
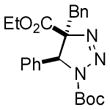 2af |
85 |
| 6 |
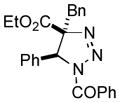 2ag |
36[b] |
| 7 |
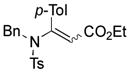 1ah′ |
65 |
| 8 |
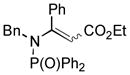 1ai′ |
61[c] |
| 9 |
 2aj |
87 64[d] |
| 10 |
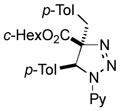 2ak |
83 |
| 11 |
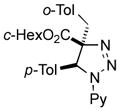 2al |
91 |
| 12 |
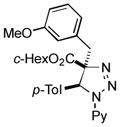 2am |
89 |
| 13 |
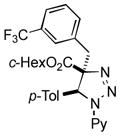 2an |
86 |
| 14 |
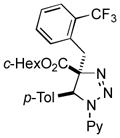 2ao |
69 |
| 15 |
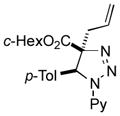 2ap |
78 |
| 16 |
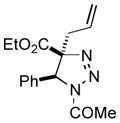 2aq |
71 |
| 17 |
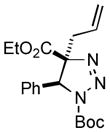 2ar |
75 |
| 18 |
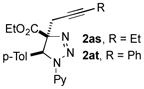
|
69 |
| 19 | 59 | |
| 20 |
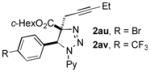
|
64 |
| 21 | 58 | |
| 22 |
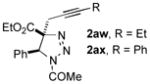
|
62 |
| 23 | 76 | |
| 24 |
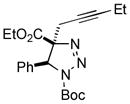 2ay |
70 |
| 25 |
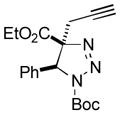 2az |
59 |
| 26 |
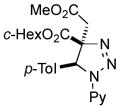 2ba |
41 |
| 27 |
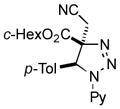 2bb |
45 |
| 28 |
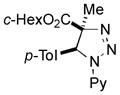 2bc |
71[e] |
| 29 |
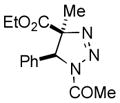 2bd |
79[e] |
| 30 |
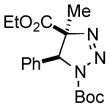 2be |
56[e] |
| 31 |
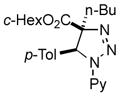 2bf |
47 66[f] |
| 32 |
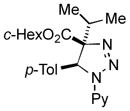 2bg |
49[g] |
| 33 |
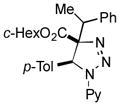 2bh |
67 |
| 34 |
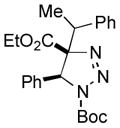 2bi |
59 |
| 35 |
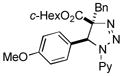 2bj |
70 |
| 36 |
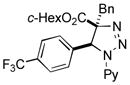 2bk |
67 |
| 37 |
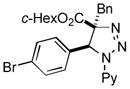 2bl |
54 |
| 38 |
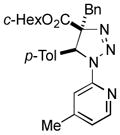 2bm |
62 |
| 39 |
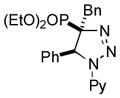
|
-[h] |
Isolated yields, d.r. >99:1 in all cases unless otherwise noted.
d.r. 97:3.
NMR yield.
BnOTf was used.
MeI was used.
n-BuI was used.
i-PrI was used.
Trace of product, the starting diazocompound was recovered.
Py = 2-pyridyl, p-Tol = 4-CH3C6H4, o-Tol = 2-CH3C6H4, Cbz = carboxybenzyl, Boc = tert-butoxycarbonyl.
Thus, upon reaction of 1 with benzyl bromide, triazolines bearing pyrimidyl (2ab), acetyl (2ac), pivaloyl (2ad), carboxybenzyl (2ae), and tert-butoxycarbonyl (2af) groups at N-atom were formed in high yields and diastereoselectivity (Table 2, entries 1–5). In the case of β-N-benzoyl diazoester, the product 2ag was formed in lower yield and diastereoselectivity (entry 6), whereas β-N-tosyl amino and β-N-diphenylphosphonyl diazoesters produced the corresponding enamies 1ah′ and 1ai′ as major products (entries 7,8). Next, we examined the scope of alkylating agents in this transformation. It was found that benzyl triflate is also a competitive reaction partner, however the prodict 2aj was obtained in a slightly lower yield than in the case of reaction with benzyl bromide (Table 1, entry 9). Benzyl bromides having electron-donating (entries 10–12) or electron-withdrawing groups (entries 13,14), as well as allyl bromide (entries 15–17), also underwent aminoalkylation reaction with different diazocompounds producing the desired products in high yields. This reaction also works with internal (entries 18–24) and terminal (entry 25) propargyl bromides, as well as with methyl bromoacetate (entry 26) and bromoacetonitrile (entry 27) as alkylating agents. Notably, simple aliphatic alkylating agents, such as methyl iodide (entries 28–30), n-butyl iodide, as well as n-butyl bromide (entry 31), efficiently participated in the aminoalkylatiown reaction. Notably, secondary alkyl halides (entries 31–33) produced the corresponding triazolines in good yields. The reaction showed good tolerance with respect to the electronic properties of the aryl substituent and N-pyridyl group of β-amino-α-diazoesters (entries 35–38 and 20–21). However, it was found that this aminoalkylation was not efficient with diazomethylenephosphonate derivative (entry 39).
The proposed mechanism for the aminoalkylation reaction of β-amino-α-diazoesters implies initial deprotonation of the amino-group of 1 with sodium hydride to produce anion A, which undergoes cyclization to form enolate B/C (Scheme 3). The following nucleophilic attack of the enolate at the electrophile which approaches from the less sterically hindered site leads to the formation of the corresponding 1,2,3-triazoline 2 in a highly diastereoselective fashion.
Scheme 3.
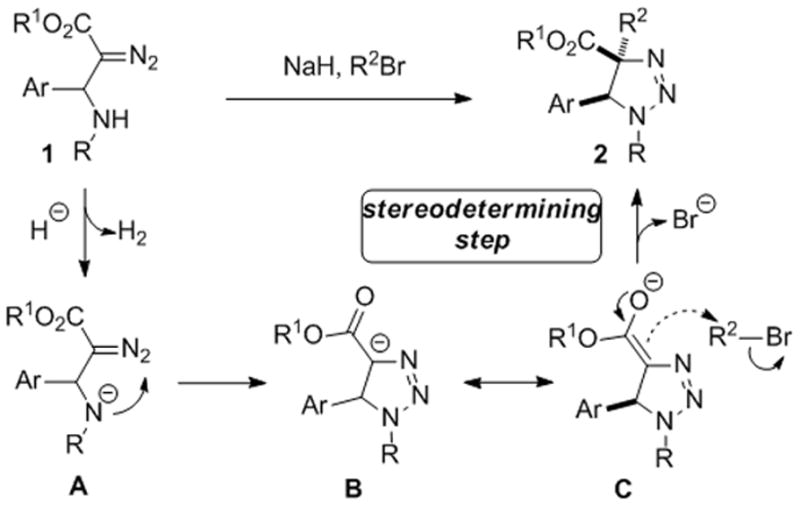
The proposed mechanism for the aminoalkylation reaction of β-amino-α-diazoesters.
Noteworthy, the β-amino-α-diazoesters 1 are easily available via the previously reported methods proceeding via base-[11] or acid-mediated[12] addition of diazoacetates to imines. Furthermore, β-pyridylamino-α-diazoesters can be obtained through a three-component coupling reaction previously developed in our group (Scheme 1).[7] Accordingly, the newly discovered aminoalkylation reaction opens a direct access to triazolines starting from commercially available aldehydes, amines, diazocompounds, and alkyl halides. To this end, we demonstrated the feasibility of this approach by an efficient gram-scale synthesis of 1,2,3-triazoline 2aa via a formal four-component coupling reaction of 2-aminopyridine, aldehyde, diazocompound, and benzyl bromide (Scheme 4).
Scheme 4.
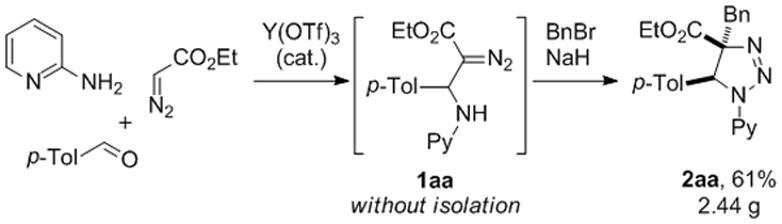
Four-component semi-one-pot synthesis of triazoline 2aa from 2-aminopyridine, aldehyde, ethyl diazoacetate and benzyl bromide. p-Tol = 4- CH3C6H4, Py = 2-pyridyl.
In continuation of our studies on the synthesis of heterocycles via transition metal-catalyzed cycloisomerization reactions of alkynes[13] and transannulation reactions of triazoles,[14] a potential heterocyclization reaction of propargyl triazolines 2as-av was investigated (Scheme 5, eq. 1). Upon screening different transition metal catalysts, it was found that under the Au-catalyzed conditions, propargyl triazolines 2as-av undergo denitrogenative cycloisomerization reaction with the formation of tetrasubstituted pyrroles 5 (Scheme 5, eq. 1). It is believed that first, triazoline 2 produces enamine E through a metal-catalyzed denitrogenative rearrangement of the open triazoline form D.[15] A subsequent aminoauration of the triple bond of E results in the formation of a vinyl-gold intermediate F.[16] The latter upon protiodemetalation and aromatization gives pyrrole 3.[17] The proposed mechanism was supported by observation of trace amounts of intermediate E in the reaction mixtures. Moreover, by treatment of triazoline 2aw with AgBF4 we were able to isolate enamine 4 in good yield. The latter under the standard Au-catalyzed reaction conditions was efficiently transformed into pyrrole 3c (Scheme 5, eq. 2).
Scheme 5.
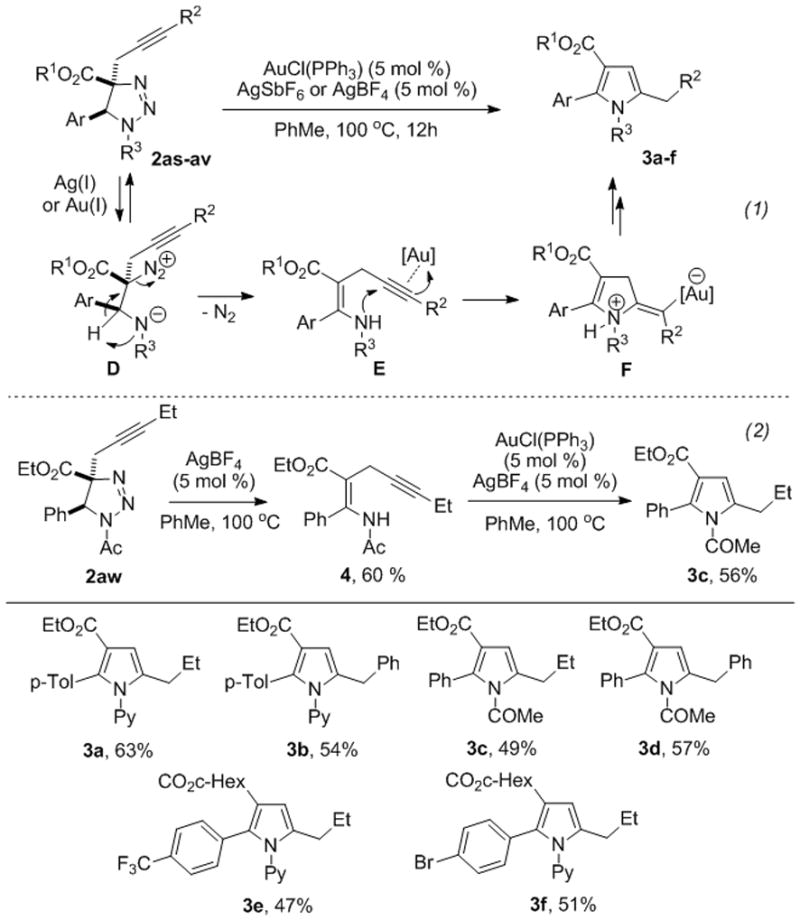
The Au-catalyzed cycloisomerization of triazolines into pyrroles.
The reaction works with N-pyridyl and N-acyl triazolines possessing ethyl- and phenyl substituted propargyl moieties producing pyrroles 3a-d in good yields. The substrates possessing -Br and -CF3 substituents at the aryl ring were also capable reactants producing the corresponding pyrroles 3e, f in moderate yields. This cycloisomerization process represents the first example of denitrogenative transannulation reaction of 1,2,3-triazolines with alkynes, that is complementary to the denitrogenative transannulation of 1,2,3-triazoles.[14,18]
In conclusion, we developed a highly diastereoselective intramolecular aminoalkylation reaction of β-amino-α-diazoesters leading to the tetrasubstituted 1,2,3-triazolines. The reaction features a novel 1,3-addition of nucleophile and electrophile to the diazo-group of a diazocompound. It proceeds with a variety of C-electrophiles and demonstrates a broad scope with respect to the substituent at the amino- and aryl group of β-amino-α-diazoesters, as well as good functional group tolerance. We also discovered the first gold-catalyzed intramolecular denitrogenative transannulation reaction of propargyl triazolines into pyrroles.
Supplementary Material
Footnotes
The support of the NIH (GM-64444) is gratefully acknowledged.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
References
- 1.For general reviews on reactivity of diazocompounds, see: Zhao X, Zhang Y, Wang J. Chem Commun. 2012;48:10162. doi: 10.1039/c2cc34406h.Zhang Y, Wang J. Eur J Org Chem. 2011:1015.Davies HML, Manning JR. Nature. 2008;451:417. doi: 10.1038/nature06485.Timmons DJ, Doyle MP. J Organomet Chem. 2001;98:617–618.
- 2.For reviews on the application of diazocompounds, see: Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. Wiley; New York: 1998. Davies HWL, Morton D. Chem Soc Rev. 2011;40:1857–1869. doi: 10.1039/c0cs00217h.
- 3.For recent review on carbene chemistry, see: Moss RA, Doyle MP, editors. Contemporary Carbene Chemistry. Wiley; New Jersey: 2013.
- 4.For review on cycloaddition reaction of diazocompounds, see: Maas G. In: The Chemistry of Heterocyclic Compounds, Vol. 59: Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products. Padwa A, Pearson W, editors. Wiley; New York: 2002. pp. 539–621.Suga H, Itoh K. In: Methods and Applications of Cycloaddition Reactions in Organic Syntheses. Nishiwaki N, editor. Wiley; New Jersey: 2014. pp. 175–204.
- 5.For selected examples of the reaction of diazocompounds with organometallic reagents, see: Yasui E, Wada M, Takamura N. Tetrahedron Lett. 2006;47:743–746.Yasui E, Wada M, Takamura N. Tetrahedron. 2009;65:461–468.For reaction of diazocompounds with potassium malonate, see: Regitz M, Liedhegener A, Stadler D. Liebigs Ann Chem. 1968;713:101–112.
- 6.For reduction of diazocompounds using hydride reagents, see: Yasui E, Wada M, Takamura N. Chem Pharm Bull. 2007;55:1652–1654. doi: 10.1248/cpb.55.1652.
- 7.Gulevich AV, Helan V, Wink DJ, Gevorgyan V. Org Lett. 2013;15:956–959. doi: 10.1021/ol400148r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The stereoselectivity was determined by GC-MS and NMR analysis of crude reaction mixtures.
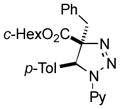
- 9.X-ray analysis of the product 2ay confirmed the trans-orientation of the Ar group and the alkyl residue (see SI for details). CCDC-995385 contains the supplementary crystallographic data for this compound.
- 10.For examples of biologically active 1,2,3-triazolines, see: Kadaba PK, Dixit T. Curr Med Chem. 2003;10:2109–2121. doi: 10.2174/0929867033456819.Dürüst Y, Karakuş H, Kaiser M, Tasdemir D. Eur J Med Chem. 2012;48:296–304. doi: 10.1016/j.ejmech.2011.12.028.For the use of 1,2,3-triazolines as synthetic intermediates, see: Pohlhaus PD, Bowman RK, Johnson JS. J Am Chem Soc. 2004;126:2294–2295. doi: 10.1021/ja0397963.Zeghada S, Bentabed-Ababsa G, Derdour A, Abdelmounim S, Domingo LR, Sáez JA, Roisnel T, Nassar E, Mongin F. Org Biomol Chem. 2011;9:4295–4305. doi: 10.1039/c1ob05176h.Margetić D, Warrener RN, Butler DN, Jin CM. Tetrahedron. 2012;68:3306–3318.Baskar B, Wittstein K, Sankar MG, Khedkar V, Schürmann M, Kumar K. Org Lett. 2012;14:5924–5927. doi: 10.1021/ol3028412.Hayashi K, Tanimoto H, Zhang H, Morimoto T, Nishiyama Y, Kakiuchi K. Org Lett. 2012;14:5728–5731. doi: 10.1021/ol302608q.de Loera D, Stopin A, Garcia-Garibay MA. J Am Chem Soc. 2013;135:6626–6632. doi: 10.1021/ja401577p.For the use of 1,2,3-triazolines in total synthesis, see: Gebhardt B, König CM, Schleth C, Dauber M, Koert U. Chem–Eur J. 2010;16:5934–5941. doi: 10.1002/chem.201000104.
- 11.For base-mediated addition of diazocompounds to imines, see: Jiang N, Qu Z, Wang J. Org Lett. 2001;3:2989–2992. doi: 10.1021/ol016324p.Jiang N, Wang J. Tetrahedron Lett. 2002;43:1285–1287.Zhao Y, Jiang N, Wang J. Tetrahedron Lett. 2003;44:8339–8342.Zhao Y, Ma Z, Zhang X, Zou Y, Jin X, Wang J. Angew Chem. 2004;116:6103–6106.Angew Chem Int Ed. 2004;43:5977–5980. doi: 10.1002/anie.200460730.Chen S, Zhao Y, Wang J. Synthesis. 2006:1705–1710.Goodman CG, Do DT, Johnson JS. Org Lett. 2013;15:2446–2449. doi: 10.1021/ol4009206.For review, see: Zhang Y, Wang J. Chem Commun. 2009:5350–5361. doi: 10.1039/b908378b.
- 12.For the acid-mediated addition of diazocompounds to imines, see: Uraguchi D, Sorimachi K, Terada M. J Am Chem Soc. 2005;127:9360–9361. doi: 10.1021/ja051922a.Hashimoto T, Maruoka K. J Am Chem Soc. 2007;129:10054–1055. doi: 10.1021/ja0713375.Maruoka K, Hashimoto T. Synthesis. 2008:3703–3706.Hashimoto T, Kimura H, Nakatsu H, Maruoka K. J Org Chem. 2011;76:6030–6037. doi: 10.1021/jo2005999.Hashimoto T, Kimura H, Kawamata Y, Maruoka K. Nat Chem. 2011;3:642–646. doi: 10.1038/nchem.1096.Zhang H, Wen X, Gan L, Peng Y. Org Lett. 2012;14:2126–2129. doi: 10.1021/ol300664d.Kantam ML, Balasubrahmanyam V, Kumar KBS, Venkanna GT, Figueras F. Adv Synth Catal. 2007;349:1887–1890.
- 13.For recent examples on cyclosiomerization reactions, see: Kazem Shiroodi R, Dudnik AS, Gevorgyan V. J Am Chem Soc. 2012;134:6928–6931. doi: 10.1021/ja301243t.Li Z, Chernyak D, Gevorgyan V. Org Lett. 2012;14:6056–6059. doi: 10.1021/ol302947r.Dudnik AS, Xia Y, Li Y, Gevorgyan V. J Am Chem Soc. 2010;132:7645–7655. doi: 10.1021/ja910290c.For reviews, see: Kazem Shiroodi R, Gevorgyan V. Chem Soc Rev. 2013;42:4991–5001. doi: 10.1039/c3cs35514d.Gulevich AV, Dudnik AS, Chernyak N, Gevorgyan V. Chem Rev. 2013;113:3084–3213. doi: 10.1021/cr300333u.
- 14.For reviews on transannulation reactions, see: Chattopadhyay B, Gevorgyan V. Angew Chem. 2012;124:886–896. doi: 10.1002/anie.201104807.Angew Chem Int Ed. 2012;51:862–872. doi: 10.1002/anie.201104807.Gulevich AV, Gevorgyan V. Angew Chem. 2013;125:1411–1413. doi: 10.1002/anie.201209338.Angew Chem Int Ed. 2013;52:1371–1373. doi: 10.1002/anie.201209338.
- 15.The test thermal reaction of triazoline 2as under transition metal-free conditions did not produce any product.
- 16.For cycloisomerization of skipped propargyl enamines into pyrroles, see: Barluenga J, Tomás M, Kouznetsov V, Suárez-Sobrino A, Rubio E. J Org Chem. 1996;61:2185–2190.Robinson RS, Dovey MC, Gravestock D. Tetrahedron Lett. 2004;45:6787–6789.
- 17.For selected cyclosiomerization reactions proceeding via formation of vinyl-gold intermediates, see: Hashmi ASK, Schuster AM, Rominger F. Angew Chem. 2009;121:8396–8398.Angew Chem Int Ed. 2009;48:8247–8249. doi: 10.1002/anie.200903134.Hashmi ASK, Schuster AM, Gaillard S, Cavallo L, Poater A, Nolan SP. Organometallics. 2011;30:6328–6337.Hashmi ASK. Gold Bull. 2009;42:275–279.
- 18.For the synthesis of pyrroles via denitrogenative transannulation reaction of triazoles with alkynes, see: Miura T, Yamauchi M, Murakami M. Chem Commun. 2009:1470–1471. doi: 10.1039/b819162j.Chattopadhyay B, Gevorgyan V. Org Lett. 2011;13:3746–3749. doi: 10.1021/ol2014347.Shi Y, Gevorgyan V. Org Lett. 2013;15:5394–5396. doi: 10.1021/ol4027655.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


