Abstract
Here, we review the angular vestibulocollic reflex (VCR) focusing on its function during unexpected and voluntary head movements. Theoretically, the VCR could (1) stabilize the head in space during body movements and/or (2) dampen head oscillations that could occur as a result of the head’s underdamped mechanics. The reflex appears unaffected when the simplest, trisynaptic VCR pathways are severed. The VCR’s efficacy varies across species; in humans and monkeys, head stabilization is ineffective during low-frequency body movements in the yaw plan. While the appearance of head oscillations after the attenuation of semicircular canal function suggests a role in damping, this interpretation is complicated by defects in the vestibular input to other descending motor pathways such as gaze premotor circuits. Since the VCR should oppose head movements, it has been proposed that the reflex is suppressed during voluntary head motion. Consistent with this idea, vestibular-only (VO) neurons, which are possible vestibulocollic neurons, respond vigorously to passive, but not active, head rotations. Although VO neurons project to the spinal cord, their contribution to the VCR remains to be established. VCR cancelation during active head movements could be accomplished by an efference copy signal negating afferent activity related to active motion. Oscillations occurring during active motion could be eliminated by some combination of reflex actions and voluntary motor commands that take into account the head’s biomechanics. A direct demonstration of the status of the VCR during active head movements is required to clarify the function of the reflex.
Keywords: Vestibulocollic reflex, Head stabilization, Damping, Control systems, Efference copy
Introduction
The vestibular system has traditionally been thought to function as a balance system, stabilizing gaze and posture. We now know that the system and other multisensory inputs combine and contribute to a multitude of functions ranging from automatic reflexes and more complicated motor strategies to spatial perception and path finding (Horak and MacPherson 1996; Melvill Jones 2000; Harada et al. 2001; Taube 2007; Angelaki and Cullen 2008; Moser et al. 2008; Winklhofer and Kirschvink 2010). Nevertheless, reflexes remain a fertile territory in which to test notions of vestibular processing. Two classes of reflexes have played particularly important roles in this regard: (1) the vestibulo-ocular reflex (VOR), which serves to stabilize the visual axis to minimize retinal image motion, and (2) the vestibulocollic (VCR) reflex and the related cervicocollic (CCR) reflex, which stabilize the head in space through the activation of the neck musculature in response, respectively, to vestibular and muscle-stretch receptors.
The VOR has been well characterized and is compensatory over a broad frequency range corresponding to that encountered in everyday life (Huterer and Cullen 2002). The function of the VCR is less clear with two not mutually exclusive ideas dominating thinking about the reflex. The first hypothesis is that the VCR stabilizes the head in space during active body movements, e.g., during locomotion. But were the VCR to remain active during voluntary head movements, it would oppose intended motion. This has led to the hypothesis that the VCR is canceled during such movements (Ezure and Sasaki 1978; Roy and Cullen 2001, 2004; Peterson and Boyle 2004). An alternative hypothesis is that the VCR could serve to dampen head oscillations, which would otherwise occur during active head movements because of the large mass of the head (Peng et al. 1996, 1999). According to this second perspective, VCR cancellation during active movement might prove counterproductive. A goal of the present paper is to review our current understanding of the VCR with a view to considering whether the reflex remains active during voluntary head movements. Unless explicitly stated otherwise, we shall concentrate on the angular VCR based on yaw rotations of the head that activate the horizontal semicircular canals (SCCs). There are also linear reflexes, dependent on otolith inputs, but these have not been as well studied at a functional level (Wilson and Schor 1999; Uchino et al. 2005). Studies of vertical VCRs have involved combined canal and otolith inputs (Pozzo et al. 1990, 1991; Wilson and Schor 1999) and will be only briefly considered. Where instructive, comparisons will be made between the horizontal VCR and the corresponding VOR.
Characteristics of the VCR
The VCR counteracts rotations with an efficacy that varies across species
As elegantly demonstrated in the pioneering experiments of Flourens (1824) and Ewald (1892), the VCR functions to help stabilize the head relative to inertial space by generating a command to move the head in the direction opposite to that of the current head-in-space motion. When a single semicircular canal (SCC) is stimulated, for example by rotation of the head in the plane of that canal, muscles are activated that would produce a compensatory rotation of the head in the same plane. If more than one canal is activated, a reflex response appropriate to the combined stimulus occurs. The spatial properties of the reflex are illustrated by electrical stimulation of individual ampullary nerves (Fig. 1) (Suzuki and Cohen 1964). Activation of the right horizontal ampullary nerve (RLC) occurs during head rotates to the right; upward head rotations activate the posterior ampullary nerves bilaterally (RPC + LPC); roll head movements to the right excite both vertical canals on the same side (RAC + RPC). The evoked movements are compensatory: they are in the same plane, but opposite in direction to the head movements that would activate the canals. As such, the reflexes contribute to stabilization of the head in space.
Fig. 1.
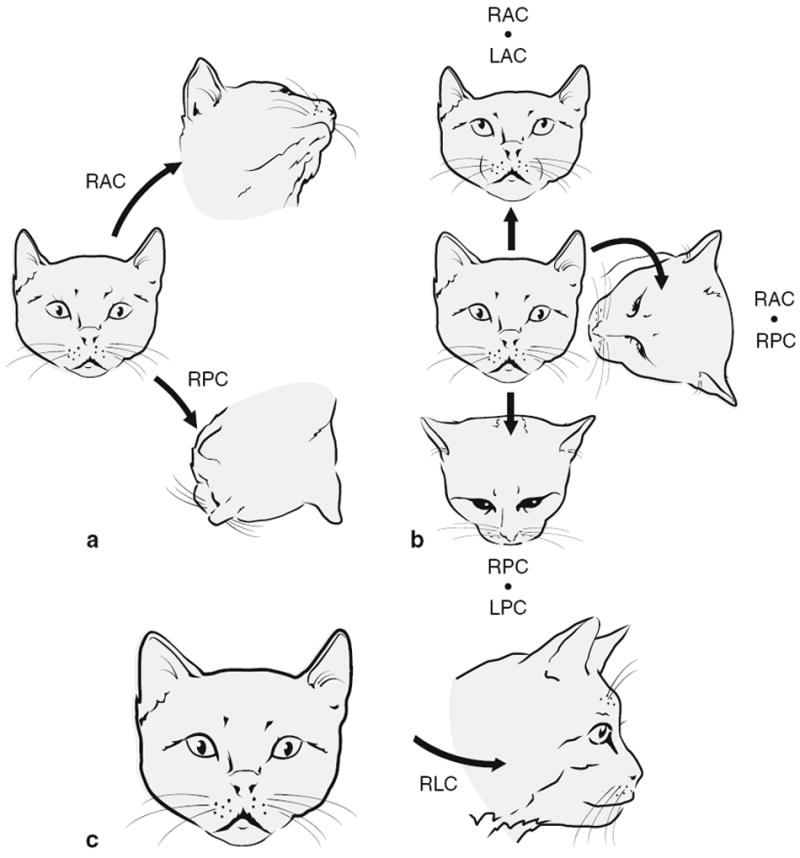
Head movements induced in the cat by electric-shock stimulation of individual ampullary nerves are in the plane of the corresponding canal. Comparison with the directional properties of canals indicates that the evoked head movements are opposite in direction to the head rotations that would excite the individual canals or canal pairs. RAC and LAC right and left anterior canals, RPC and LPC right and left posterior canals, RLC right lateral canal a. Stimulation of individual vertical canals evokes diagonal movements. b Stimulation of pairs of vertical canals produces movements in pitch or roll. c RLC stimulation produces yaw movement to the left. From Suzuki and Cohen 1964
The efficacy of the VCR appears to vary substantially across species. For example, while it is robust in animals that make minimal eye movements such as pigeons (Gioanni 1988) or owls (Money and Correia 1972), its contribution to head stabilization has been described as less significant in macaque monkeys and humans—species that have large (~50°) oculomotor ranges (Guitton et al. 1986; Sadeghi et al. in press). For example, when human subjects are distracted by mental arithmetic, the most substantial part of active yaw-axis stabilization at low frequencies (<1 Hz) is generated by longer-latency voluntary mechanisms, suggesting that the VCR contribution is negligible (Guitton et al. 1986; Keshner and Peterson 1995). The VCR is more active in the range of 1–3 Hz (Peng et al. 1996, 1999; Keshner and Peterson 1995; Keshner et al. 1995), where it would be most effective in counteracting the underdamped mechanics of the head.
Three-neuron reflex arcs interconnect semicircular canals and neck motoneurons
Presumably reflecting the need for speed and security in the VOR, there are well-developed three-neuron pathways interconnecting vestibular afferents, secondary vestibular neurons and ocular motoneurons (Lorente de Nó 1933; Szentagothai 1950; Highstein et al. 1971; Ito et al. 1976a, b). Similarly, the most direct pathway mediating the VCR is comprised of three neurons (Wilson and Yoshida 1969; Wilson and Maeda 1974; Shinoda et al. 1992, 2006). The existence of a direct vestibulocollic pathway was initially demonstrated when electrical stimulation of individual ampullary nerves was found to give rise to short-latency EPSPs and IPSPs in neck motoneurons, consistent with the presence of only two central synapses, one on vestibulocollic neurons in the vestibular nuclei and the second on neck motoneurons (Fig. 2). Counting the peripheral synapse, the pathway is trisynaptic. Multiple peaks whose origin remains to be determined typically followed the short-latency potentials. Most contralateral pathways pass through the medial vestibulospinal tract (MVST) in the medial longitudinal fasciculus (MLF), as do ipsilateral inhibitory pathways (Fig. 3a, b). Ipsilateral excitatory pathways can run in the lateral vestibulospinal tract (LVST). In addition, the most direct pathways mediating some contralateral inhibition in vertical canal-related pathways include a commissural inhibitory neuron located in the cervical spinal cord (Sugiuchi et al. 2004) (Fig. 3b).
Fig. 2.
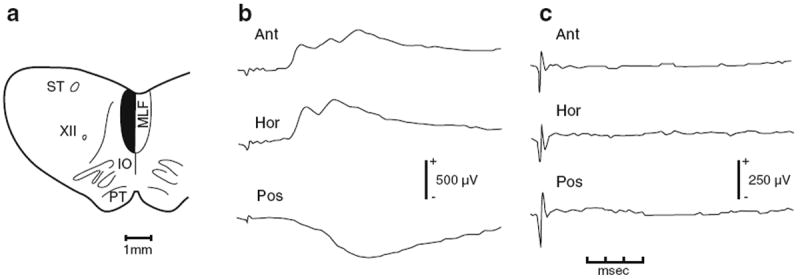
Effects of cutting the MLF ipsilateral to a dorsal neck motoneuron from which synaptic potentials were recorded in response to stimulation of contralateral ampullary nerves. ST solitary tract, XII hypoglossal nerve, IO inferior olive, PT pyramidal tract, MLF medial longitudinal fasciculus. Stimulated contralateral ampullary nerve for each trace: Ant anterior canal, Hor horizontal canal, Pos posterior canal. Lesion is shown by the dark area in a. Responses recorded in a complexus motoneuron before (b) and after the cut (c). Stimulus was 50 μA (Hor) and 75 μA (Ant and Pos) before the cut. All stimuli were 75 μA after the cut. Potentials are averages of 50 sweeps. From Wilson and Maeda (1974)
Fig. 3.
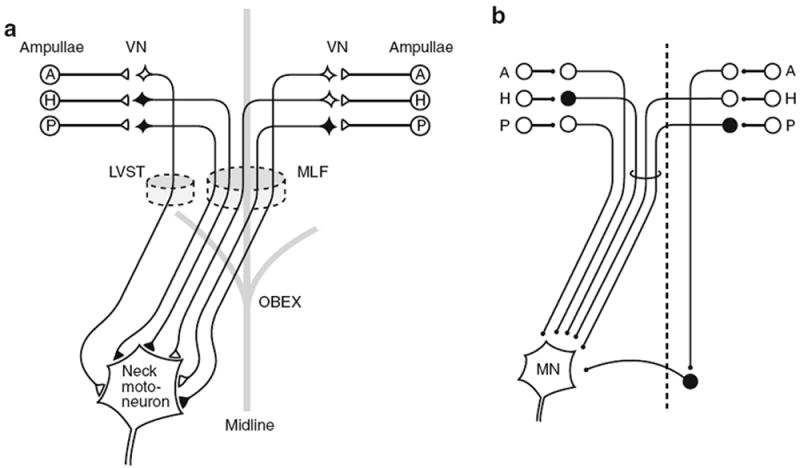
Pathways between ipsilateral and contralateral ampullary nerves and neck motoneurons. Inhibitory vestibulocollic neurons and their terminals are shown in black; excitatory vestibulocollic neurons, in white. a Pattern observed in motoneurons innervating dorsal neck muscles, including biventer and complexus. MVST axons travel in the MLF. From Wilson and Maeda (1974). b Pattern observed in motoneurons of a rotator, obliquus capitis caudalis. Axons in circle are in the MVST; those outside the circle are in the LVST. Note that the inhibition from the contralateral anterior canal acts via an inhibitory commissural neuron at segmental levels. From Sugiuchi et al. (2004). A, H, P, anterior, horizontal, and posterior ampullae, VN vestibular nuclei, MN motoneuron, MLF medial longitudinal fasciculus, LVST and MVST lateral and medial vestibulospinal tracts
The organization of direct reflexes between SCCs and neck motoneurons is consistent with the directional properties of the VCRs summarized in Fig. 1 (reviewed in Wilson and Schor 1999). For example, dorsal extensor neck motoneurons receive bilateral excitatory inputs from the LAC and RAC and bilateral inhibitory inputs from the LPC and RPC. Downward pitches activate both ACs and inhibit both PCs. Hence, the reflex should produce a compensatory, upward head movement acting by the way of AC excitation and PC disinhibition of dorsal extensor motoneurons. These same muscles also result in yaw head rotations that are driven by the reciprocal inputs from the LLC and RLC. In a similar vein, short-latency canal inputs to other classes of neck motoneurons—lateral flexors, rotators, and ventroflexors—should result in head movements in a direction opposite to the head rotations evoking them. Based on the VCRs obtained by stimulation about different rotational axes, specific SCCs should provide inputs to several neck motoneurons pools. Indeed, intra-axonal labeling of individual vestibulocollic neurons has indicated that their axons make such connections (Shinoda et al. 1992; Perlmutter et al. 1998).
In contrast to the VOR, the VCR controls a complex musculature. The VOR involves three, reciprocally related extraocular muscle pairs acting around a single rotation axis (Robinson 1982; Sparks 2002). In contrast, the neck has over 30 muscles controlling pitch, roll and yaw rotations (Peterson et al. 2001). Only some of this complexity can be explained by the multiple rotation axes involved in pitch (C1-skull and C6-7-T1) and roll head movements (C2–C7), as well as a single axis (C1–C2) that dominates horizontal rotations (Graf et al. 1995a, b). From a mathematic perspective, the system is underdetermined with more muscles than rotation axes. One consequence of this property is that the same head motion can be produced by the activation of several different muscle patterns. While electromyographic (EMG) studies have shown that this is the case for voluntary head movements, it is not the case for the VCR where a particular head movement is associated with a stereotyped muscle activation pattern (Peterson et al. 2001).
The VCR involves complex circuitry
Three-neuron arcs have the correct connectivity to mediate the VCR. Yet, there is compelling evidence that more complicated circuitry makes a dominant contribution to the reflex, at least in decerebrate cats (Ezure and Sasaki 1978; Ezure et al. 1978). First, transection of the MLF, which contains all the trisynaptic pathways between the horizontal canal ampullae and dorsal neck motoneurons (Fig. 3), has little or no effect on the gain or phase of neck EMG activity evoked by horizontal rotations. This is so even though MLF lesions, as expected, abolish short-latency EPSPs and IPSPs evoked by stimulation of the LHC and RHC (Fig. 2c). Similarly, MLF lesions have little effect on the vertical VCR (Thomson et al. 1995). Second, intravenous infusion of small doses of the anesthetic, sodium pentobarbital, sharply decreases reflex gain. This is significant, since the anesthetic depresses synaptic transmission, thus affecting multisynaptic pathways sooner and more strongly than trisynaptic ones. Finally, the response dynamics of the VCR are consistent with processing by multisynaptic pathways. Notably, the reflex shows large phase lags relative to vestibular nerve and vestibular-nuclear discharge (Fig. 4b, c). The phase lags, which are most pronounced near 0.1 Hz and disappears at higher frequencies, imply that central pathways perform a partial mathematically equivalent integration of the vestibular signal. Integration is required to convert the angular head-velocity signals carried by vestibular neurons to the head-position signals needed for proper operation of the VCR. Consistent with its presumed action on multisynaptic pathways, barbiturate administration reduces the phase lags as it reduces the gain of the reflex.
Fig. 4.
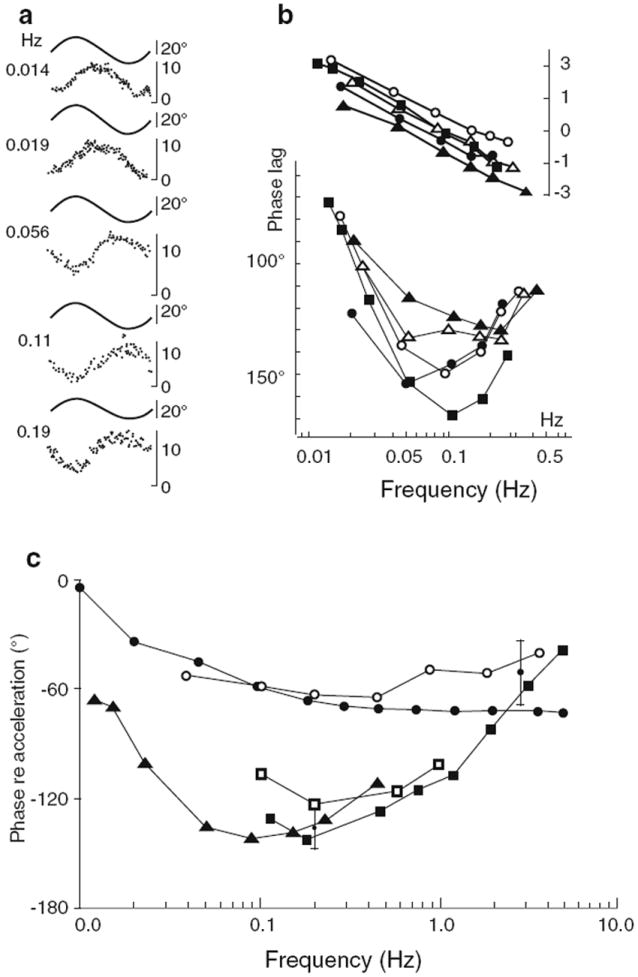
Dynamics of the vestibulocollic reflex (VCR). a EMG responses from a single unit in a dorsal neck muscle. Data are expressed re angular position for responses to different frequencies (Hz) of sinusoidal horizontal rotation. b Bode plot of 5 motor units. From Ezure and Sasaki (1978). c Phase lags of the VCR and of primary vestibular afferents compiled by Bilotto et al. (1982). Multi-unit EMG from dorsal neck muscles. Filled squares VCR phase from one experiment (complexus muscle). Filled triangles average VCR data from Ezure and Sasaki (1978). Open squares data from Berthoz and Anderson (1971). Upper curves: filled circles mean phase of primary afferent population; open circles phase of a single irregular primary afferent from Tomko et al. (1981)
The finding that bilateral inactivation of the interstitial nucleus of Cajal (INC) produces a relative phase advance and gain reduction in vertical and torsional VCRs has led to the proposal that the INC contributes to the indirect pathways controlling these reflexes (Fukushima et al. 1994). In an attempt to identify candidates in horizontal VCR pathways, a search has been made for neurons whose responses to head rotations are muscle-like in paralleling angular position, rather than angular velocity. Such muscle-like neurons have been found in the dorsal LVN (Peterson et al. 1980), the one region of the vestibular nuclei nearly lacking direct vestibular-nerve inputs (Korte 1979), and in the medial reticular formation near the abducens nucleus. Although reticulospinal neurons in this region project to spinal segment C2 (Grantyn and Berthoz 1987, Grantyn et al. 1987; Robinson et al. 1994), their contribution to the VCR remains to be determined (Kitama et al. 1995; Wilson and Schor 1999).
There is at least one situation involving transmission over direct pathways. Loud, air-borne sounds, delivered monaurally through earphones (clicks: 140 dB SPL; 500–1,000 Hz tone bursts, 120 dB SPL), evoke a synchronized EMG response in the ipsilateral sternocleidomastoid (SCM) muscle (Fig. 5) (Colebatch et al. 1994). Such responses, which are called vestibular-evoked myogenic potentials or VEMPs, are probably of vestibular origin as they are abolished on the side of a unilateral vestibular neurectomy, but are present in patients with intact vestibular function but a profound hearing loss. Ipsilateral responses consist of a positive wave (p13) followed by a negative wave (n13). Contralateral responses are of smaller amplitude and opposite polarity (n1, p1). The earliest responses have latencies of 8 ms, consistent with the activation of a three-neuron arc. Ipsilateral responses depend on the SCM being in a heightened state of activation and represent muscle relaxation (motoneuron inhibition), while the much smaller contralateral responses are of opposite polarity and are the result of motoneuron excitation. VEMPs have proved useful in the evaluation of several clinical syndromes (Welgampola and Colebatch 2005).
Fig. 5.
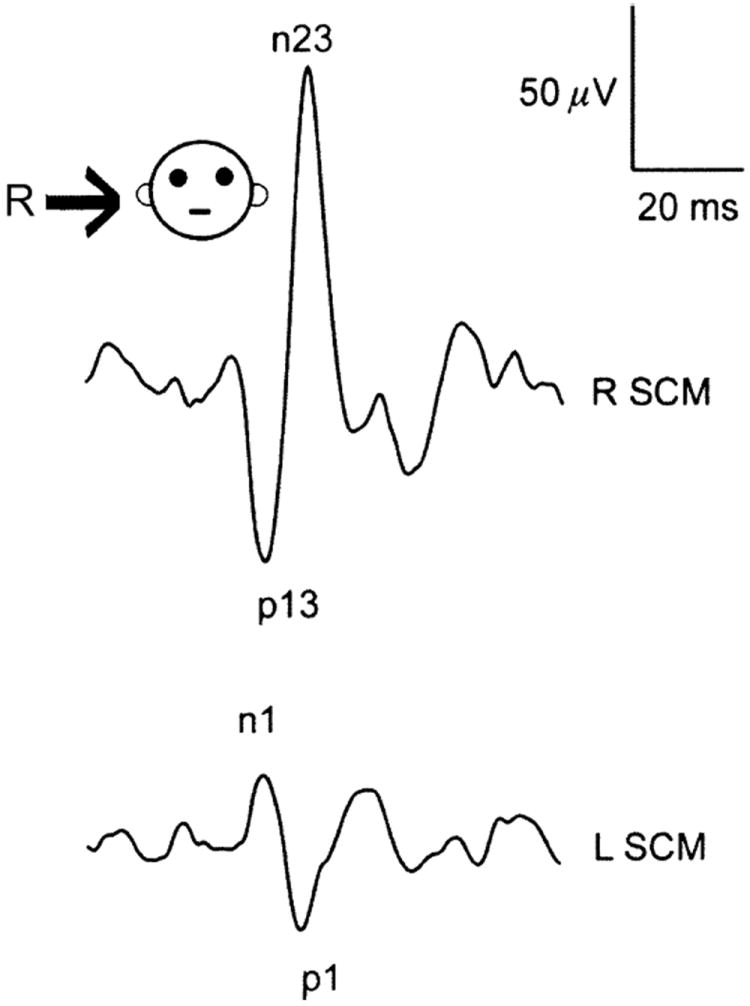
Vestibular-evoked myogenic potentials (VEMPs) evoked by a 100-db normal hearing level click delivered to the right ear via earphones. The traces consist of averaged unrectified electromyograms (EMGs) from the sternocleidomastoid muscles (SCMs) on the two sides. A large biphasic (p13-n23) response is seen ipsilateral to the stimulated ear (R SCM) and a smaller response of opposite polarity (n1-p1) is seen contralaterally (L SCM). From Welgampola and Colebatch (2005)
A control systems model of the VCR and CCR
A model proposed by Goldberg and Peterson (1986) and analyzed in some detail by Peng et al. (1996, 1999) has proved useful in evaluating proposed functions of the VCR. Figure 6 is a summary diagram that includes relevant input and output variables, as well as the interconnections of the various components. The model includes two negative-feedback reflexes, the VCR and CCR; P, a transfer function summarizing the mechanical properties of the neck and head; and a low-pass torque converter that transforms EMG muscle actions into mechanical torques. There are three potential inputs: the angular position of the trunk re space (Ψ), the angular position of the head re the trunk (Θ) and voluntary commands (VOL). Θ functions as the input to the CCR and is also considered the output of the system. The sum, Θ + Ψ, represents the head re space (H) and is the input to the VCR. To obtain a pure CCR, rotate the body under a stationary head, i.e., H = Θ + Ψ = 0 or Θ = −Ψ. A pure VCR occurs during whole-body rotations where the head rotates in tandem with the body, i.e., Θ = 0 so H = Ψ. In Peng et al. (1996, 1999), the transfer functions for the VCR and CCR are written as if the reflex inputs are the second derivatives (accelerations) of Θ and Ψ. To be consistent with the Peng papers, we adopt the same convention (see “Appendix”). At the same time, the convention is arbitrary as the VCR and CCR transfer functions can be expressed for velocity or position inputs by multiplying Peng’s transfer functions by s or s2, respectively, where s is the Laplace variable.
Fig. 6.
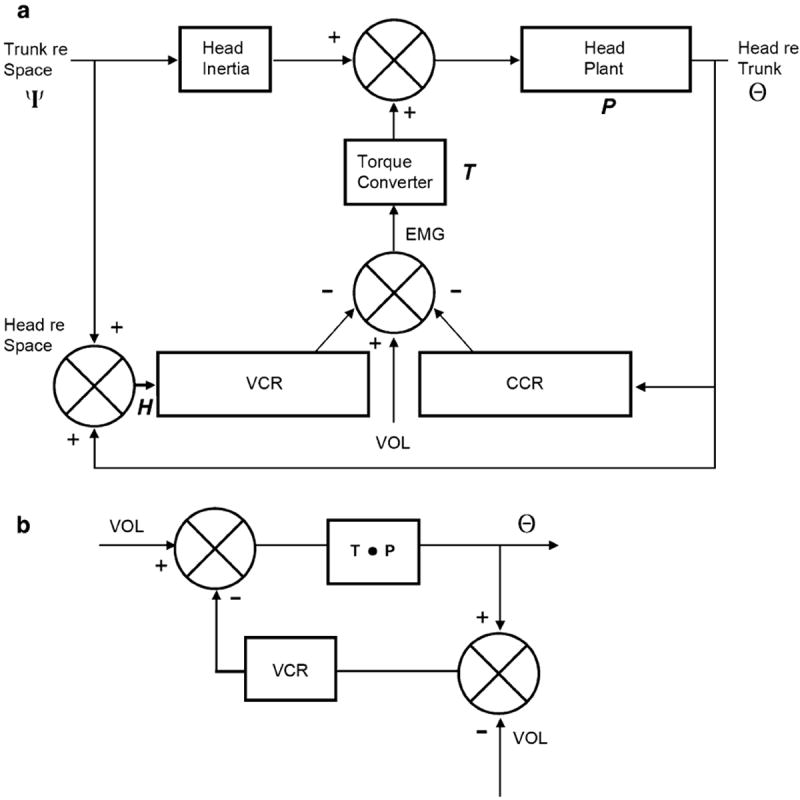
a Block diagram of head–neck plant including input from trunk re space (ψ) and output, head re trunk (neck, Θ). Two reflexes (VCR and CCR) sum with voluntary motor commands (VOL) to result in muscle activation (EMG), which is transformed to torques by a low-pass torque converter (T). Head re space (H) is the sum of ψ and Θ. Head inertia (I) and passive plant mechanics (P). Details in the “Appendix”. From Peng et al. 1996. b In the absence of trunk movements (and ignoring the CCR), the diagram is that of a conventional negative-feedback system with a single input, a voluntary motor command (VOL). The possibility that the VCR is partially canceled during active head movements is depicted by subtracting VOL from the VCR input, leaving deviations from desired head movement as the error signal driving the VCR
We only consider the control of yaw head movements and simplify the situation by assuming that these take place about a single rotation axis passing through the axis–atlantis (C1–C2) joint (Peng et al. 1996, 1999). Figures 8 and 9 are based on calculations done by us in Microsoft Excel worksheets. The model’s transfer functions, gains and time constants are summarized in the “Appendix”.
Fig. 8.
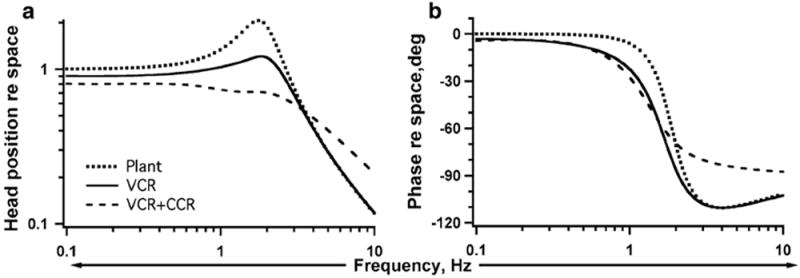
Effects of the VCR and the combined VCR and CCR on H, the head position re space in response to sinusoidal trunk position perturbations with a gain = 1 and a phase = 0°. Curves are based on calculations from the Peng et al. (1996) model. Details as in Fig. 6 and “Appendix”. Bode plots of gain (a) and phase (b) in the absence of reflex control (Plant) and with the VCR alone (CCR = 0) and with CCR present (KCCR = 1.0). In all cases, the VCR gain (KVCR = 30). At low frequencies (<1 Hz), the head stabilization is negligible (i.e., the head and trunk are nearly aligned at gain ≈ 1 and phase ≈ 0°). Near the resonant frequency (≈2 Hz), the VCR increases stiffness and damping, thereby decreasing the resonant peak. At higher frequencies, the head stabilizes as a result of its inertia, rather than reflex actions; the near absence of reflex compensation is indicated by the plant and VCR curves being almost superimposable. The CCR antagonizes stabilization
Fig. 9.
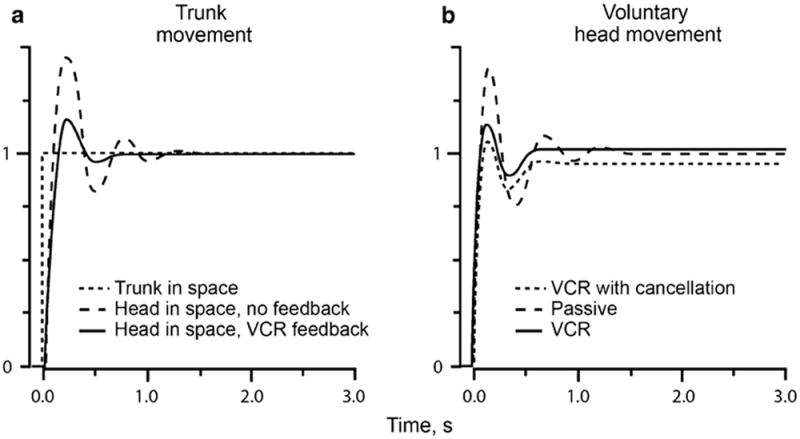
The vestibulocollic reflex (VCR) reduces oscillations during voluntary step displacements of the trunk (a) and of the head on a stationary trunk (b). In b, VCR with cancellation, VCR feedback is obtained by subtracting step motor command from actual head movement (see Fig. 6b). Suppression of head oscillations is almost as effective as happens with unmodified VCR. In these calculations, CCR was ignored. Curves are based on calculations from the Peng et al. (1996) model. Details as in Fig. 6 and “Appendix”
The plant: passive properties of the head and neck
We start with the situation where the trunk is perturbed in the absence of voluntary motor commands or reflex control. The mechanics is termed passive even though it is affected by the postural tone of neck muscles, which depends on activity of the central nervous system (CNS). It is customary in a lumped linear model, such as this one, to characterize its passive behavior by means of its moment of inertia (I), viscosity (B), and elasticity (K). B and K provide restoring torques proportional, respectively, to head velocity and head position. The model summarizes passive behavior by the angular equivalent of Newton’s second law, in which the sum of the restoring torques is equal to the product of I and the angular acceleration of the head in space, i.e.,
| (1) |
or on rearranging terms
| (2) |
The negative signs in Eq. 1 identify the viscous and elastic terms as restoring torques. Equation 2 is a second-order, ordinary differential equation. Depending on the relative values of B2 and 4KI, solutions may be underdamped (B2 < 4KI), critically damped (B2 = 4KI), or overdamped (B2 > 4KI). An underdamped solution to a step input consists of damped oscillations, whereas there are no oscillations in an overdamped step response. Critical damping constitutes a boundary between the other two regimes: solutions are as fast as possible without oscillations. The resonant or natural frequency (in rad/s), , is the frequency of the sinusoidal step response that would occur in the absence of damping (B = 0).
In simple physical systems, it is usually easy to specify I, B, and K. For the head–neck system, the moment of inertia can be measured in cadavers since it only depends on the geometry and density of the head and the distance between its center of gravity and the rotation axis (Peng et al. 1996, 1999). In contrast, there are relatively few reliable measures of viscosity (Jex and Magdaleno 1978; Winters and Stark 1985), and the variability of stiffness measured across subjects is considerable (McGill et al. 1994), in part because the viscosity and stiffness depend on the postural tone set by the CNS. We have adopted the values used by Peng et al. (1996, 1999) (see “Appendix”). They were chosen to match data from human subjects, who were rotated in a pseudorandom fashion with their heads free as they were distracted by doing mental arithmetic (Keshner and Peterson 1995). That the model captures the features of the system is indicated by the qualitative agreement of its open-loop behavior with that of animal experiments, the latter obtained by recording EMG activity with the head fixed to the body (Θ = 0) (Fig. 7).
Fig. 7.
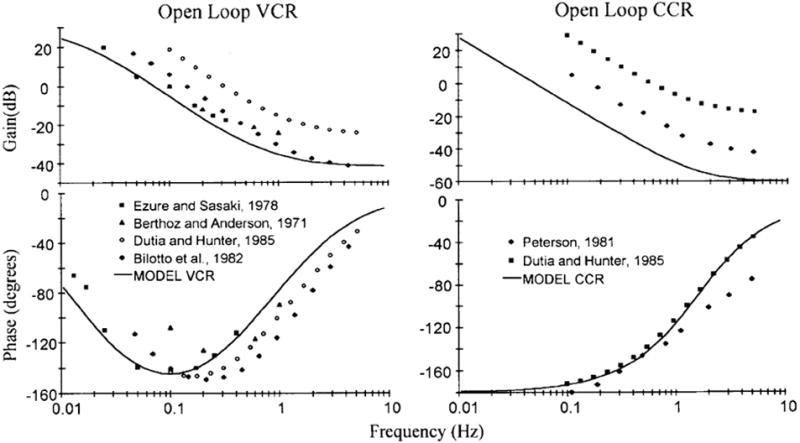
Open-loop transfer functions (curved lines) of the VCR and CCR re trunk angular acceleration based on the parameters listed in Fig. 6. Points are experimental data based on EMG recordings in the decerebrate cat (references in legends). From Peng et al. (1996)
The passive head plant is underdamped, reflecting the large inertia of the head compared to the plant’s viscosity. From the assumed values of the physical constants, viscosity is <0.3 times that needed to prevent oscillations. The natural frequency is 1.89 Hz. Reflecting these features, the calculated passive (plant) transfer function shows a resonant peak slightly above 2 Hz with the gain reaching a maximum value of 1.83 (Fig. 8a). Underdamping is also evident in the oscillations of the head that decay with time during a step displacement of the trunk (Fig. 9a) or rapid, voluntary head saccades (Fig. 9b).
Role of the VCR in the model
Having characterized open-loop behavior, we now close the feedback loops. The VCR is usually thought to stabilize the head in space during trunk movements. To investigate this possibility, it is best to consider the head position in space (H = Θ + Ψ), rather than the head position re the neck, (θ). There is an increase in the damping of head movements as indicated by a reduction in both the resonant peak (Fig. 8) and in the head oscillations during step inputs of the trunk (Fig. 9a) or of the head (Fig. 9b). As illustrated in Fig. 8a, the influence of the VCR on damping is concentrated in a relatively narrow band (1–3 Hz) centered on the resonant frequency. At lower frequencies, stabilization is poor as the head has a gain near unity and a near-zero phase, indicating that it is in register with the trunk rather than stationary in space. Experimental evidence confirms that low-frequency stabilization is poor. This is particularly true for the horizontal VCR (Guitton et al. 1986; Keshner and Peterson 1995). In the frequency range below resonance, stabilization of the head in space can be greatly improved by visual feedback of earth-fixed targets or by voluntary motor commands. As frequency is raised above the resonant point, stabilization progressively improves, but this is the result of head inertia, rather than reflex action. That the VCR has a negligible influence at higher frequencies is indicated by the convergence of the passive (plant) and active (VCR) gain and phase curves. The CCR has a modest effect, slightly diminishing stabilization at high frequencies.
Stabilization may be better for the vertical VCR, possibly because otolith, as well as canal inputs, are involved (Keshner et al. 1995). During various forms of bipedal locomotion, the vertical VCR may contribute to head stabilization (Pozzo et al. 1990, 1991), while both linear (LVOR) and angular (AVOR) vestibulo-ocular reflexes help to stabilize gaze (Moore et al. 1999).
Head stabilization mechanisms
The role of vestibular inputs in head stabilization
Evidence that the vestibular system is involved in head control comes from canal plugging experiments in which the ducts of SCCs are occluded, greatly reducing the SCC response to head rotations except at frequencies approaching 10 Hz (Goldberg and Fernandez 1975; Rabbitt et al. 1999; Sadeghi et al. 2009). The resting activity of the afferents is maintained. A feature of canal plugging is the appearance of oscillations evoked by voluntary head movements or by postural adjustments of the trunk. Similar results have been reported in cats (Money and Scott 1962; Schor 1974; Baker et al. 1982), squirrel monkeys (Paige 1983), and Old World monkeys (B. Cohen and S. Newlands, personal communications). When animals are placed in a normal cage environment, the symptoms disappear within a few days. Compensation can be delayed by preventing visual–vestibular interaction either by placing an unrestrained animal in the dark or by restraining its head in the light (Paige 1983). Oscillations have been reported in humans “with chronic unilateral labyrinthine loss, [where] sudden perturbations of the trunk…cause greater head oscillations and diminished head stability in space when they are rotated toward the lesioned side” (Peng et al. 2004 quoted in Leigh and Zee 2006). While head oscillations might be due to a loss of the VCR, other mechanisms need to be considered. For example, their emergence during voluntary head movements can also be taken as evidence that the gaze control system uses vestibular feedback to estimate an internal gaze motor error signal—equivalent to the distance between the target and the gaze position at that time—to control the final positions of the head and the eyes (Galiana and Guitton 1992; Guitton 1992; Guitton et al. 2003). This point is further considered in under Unsolved Issues (point 4).
Head stabilization in response to unexpected head and body perturbations, as measured by neck-muscle EMGs, has been compared in normal and labyrinthine-defective (LD) subjects (Shupert and Horak 1996). LD subjects showed heightened neck responses during postural adjustments of the entire body, but no responses to isolated head displacements. The orientation of the head in the pitch plane varies considerably in labyrinthine patients, as compared to control subjects (Pozzo et al. 1991). Presumably, the effect reflects the bilateral loss in patients of otolith function. There are also static signs, including the otolith tilt reaction, attributable to the acute loss of otolith function on one side. The reaction consists of a tilt of the head and a counter-rolling of the eyes toward the side of vestibular hypofunction; in addition, there is a vertical misalignment or skew deviation of the eyes (Carey and Della Santina 2005).
Is the VCR abolished during active head movements?
Were the VCR active during voluntary head movements, it could increase damping and reduce oscillations. But because the VCR opposes voluntary movements, its presence necessitates an increase in the voluntary command, referred to in the model as VOL. This has led to the notion that the VCR is turned off during voluntary head movements (Ezure and Sasaki 1978; Cullen 2004; Peterson and Boyle 2004). A possible mechanism comes from recordings of secondary vestibular-only (VO) neurons in the vestibular nuclei, so-called because they do not exhibit eye movement-related signals. VO neurons, including some that project into vestibulospinal pathways, respond appropriately to passive head movements, but their response to active head movements is markedly attenuated, on average by 70% (Boyle et al. 1996; McCrea et al. 1999; Roy and Cullen 2001, 2004). There is evidence that the cancellation signal delivered to VO neurons is based on a comparison of the expected afferent signal, constructed by the brain based on the intended movement, with the actual neck-afferent signal (Roy and Cullen 2004). An example of the behavior of a VO neuron during passive and active head rotations is seen in Fig. 10.
Fig. 10.
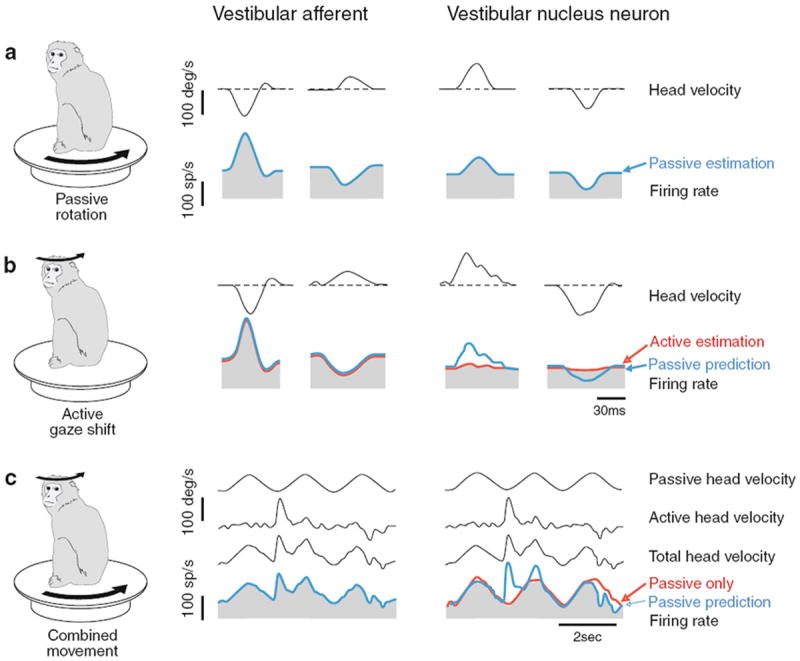
In the vestibular nuclei, vestibular-only (VO) neurons distinguish between sensory inputs that result from the alert monkey’s own actions and those that arise externally. The activity of a horizontal canal afferent (left panel) and VO neuron (right panel) is shown for (a) passive head movements, (b) active head movements, and (c) combined active and passive head movement. Afferents reliably encode head motion in all conditions. In contrast, VO neurons show significantly attenuated responses to the active component of head motion, but remain responsive to passive head movements alone and during combined movements. Afferent responses are based on data from Cullen and Minor (2002); vestibular nucleus responses are based on Roy and Cullen (2001). From Angelaki and Cullen (2008)
A number of important issues remain to be resolved before we can ascertain whether the VCR is turned off during voluntary behavior. For example, an alternative argument is that the strength of the reflex needed to dampen head movements is relatively small, such that only a modest increase in VOL would be required were the reflex to remain active. In the model, for example, the presence of a VCR would necessitate only a 10% increase in the VOL command. In addition, the role and efficacy of the direct VCR pathways has to be clarified. Work in decerebrate cats emphasizes the functional importance of indirect, as yet to be defined pathways. Whether this is true in alert animals has not been studied. In addition, the contribution of VO neurons to direct VCR pathways remains to be ascertained. Studies in alert monkeys show that VO neurons could contribute to the VCR as they can be antidromically activated from cervical segments (Boyle 1993; Boyle et al. 1996). Yet, only about half the antidromically identified neurons in these studies were VO neurons, the remaining neurons having a variety of oculomotor signals. In addition, the antidromic paradigm used could not distinguish vestibulocollic neurons from vestibulospinal neurons only projecting below upper cervical segments.
Perhaps the most direct way of ascertaining the status of the VCR during active head movements would be by applying electrical stimulation to individual ampullae (Suzuki and Cohen 1964) or by evoking VEMPs (Welgampola and Colebatch 2006), while animals are making voluntary eye–head gaze shifts (for more details see section “Difficulties in studying the VCR: comparison with the VOR”).
Cancellation of the VCR and reafference
The possible cancelation of the VCR during voluntary movements can be related to a proposal by von Holst and Mittelstaedt (1950) (Fig. 11a), where a copy of the expected sensory consequences of a motor command (termed efference copy) is subtracted from the actual sensory signal (reafference) to create a sensation of external perturbations (termed exafference). In this way, the nervous system can distinguish sensory inputs arising from external sources and those resulting from self-generated movements. Recent behavioral investigations have provided support for these ideas (Decety 1996; Farrer et al. 2003; Wolpert et al. 1995). Work in several model systems including the electrosensory systems of mormyrid fish and elasmobranchs, the mechanosensory system of the crayfish, and the auditory system of the cricket have provided evidence that sensory information arising from self-generated behaviors can be selectively suppressed at the level of afferent fibers or their central targets (reviewed in Cullen 2004).
Fig. 11.
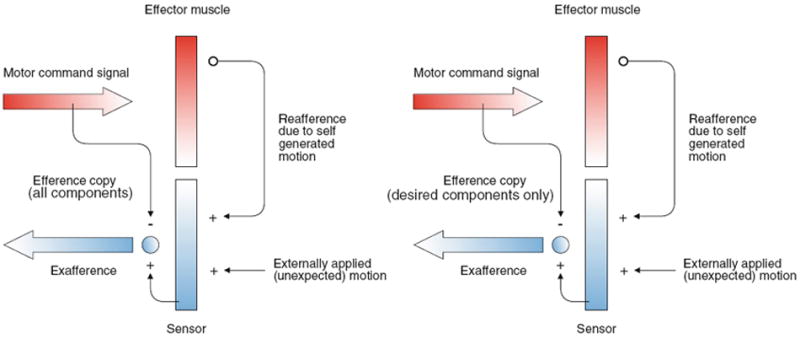
von Holst and Mittelstaedt’s (1950) reafference principle as applied to the vestibular system. a A motor command is sent to the effector muscle and, in turn, there is reafference resulting from the effector’s activation of vestibular sensors. The reafference (+) is compared with an efference copy of the original motor command (−). When the reafference and efference copy signals are equal, they cancel and no sensory information is transmitted to higher levels. In contrast, a difference between afference and efference copy indicates an externally generated event (exafference) that is behaviorally relevant and is further processed by the vestibulocollic reflex (VCR). b. Here, the efference copy signal includes only the desired head movement (minus oscillations). The exafference signal, which includes the oscillations, as well as external perturbations, is processed by the VCR. Modified from Angelaki and Cullen (2008)
The working of the scheme depends on the nature of the efference copy signal. In the present case, that signal might be the desired smooth head-movement trajectory, not including the oscillations of the underdamped plant. In the absence of external sensory inputs, the exafferent signal would only include oscillations and other deviations from the desired movement. The Peng model can be modified to incorporate this idea (Fig. 6b). Not unexpectedly, damping of oscillations is as effective in the modified model (Fig. 9b, VCR with cancellation) as in the original model (Fig. 9b, VCR).
Alternative mechanisms to prevent oscillations
We have suggested that the VCR prevents head oscillations during rapid head saccades. Here, we consider alternative mechanism when the reflex is absent either because canal plugging reduces vestibular input or else because descending vestibulocollic neurons are silenced during active head movements. There are two scenarios that might overcome the absence of a VCR: (1) a learned modification of voluntary commands and/or (2) a change in the strength of remaining reflexes.
The brain could learn to modify an internal model of head mechanics, thereby altering the voluntary motor command to produce the desired movement trajectory (reviews: Kawato 1999; Scott and Norman 2003). If one assumes, that the brain has properly modeled the altered biomechanics of the head and neck, then the VCR would not be needed. The modified motor command, by going through the new biomechanical model, could still produce an efference copy mirroring the desired motor output. To consider a specific example of reprogramming, we note that many rapid movements of the limbs are characterized by a triphasic pattern of EMG muscle activity consisting two bursts of agonist activity separated by an antagonist burst (Terzuolo et al. 1973; Hallett et al. 1975; Ghez and Martin 1982). A similar triphasic pattern is seen as a normal feature of neck muscles during head saccades (Fig. 12) (Zangemeister and Stark 1981; Hannaford et al. 1986). Of particular interest is the antagonistic burst as it is largely responsible for terminating the head movement. Its etiology has not been studied. Conceivably, it could arise as part of a centrally programmed voluntary command and/or the reflex activation of the CCR or VCR. Regardless of its origin, an increase in the antagonistic burst could compensate for the absence of the VCR.
Fig. 12.
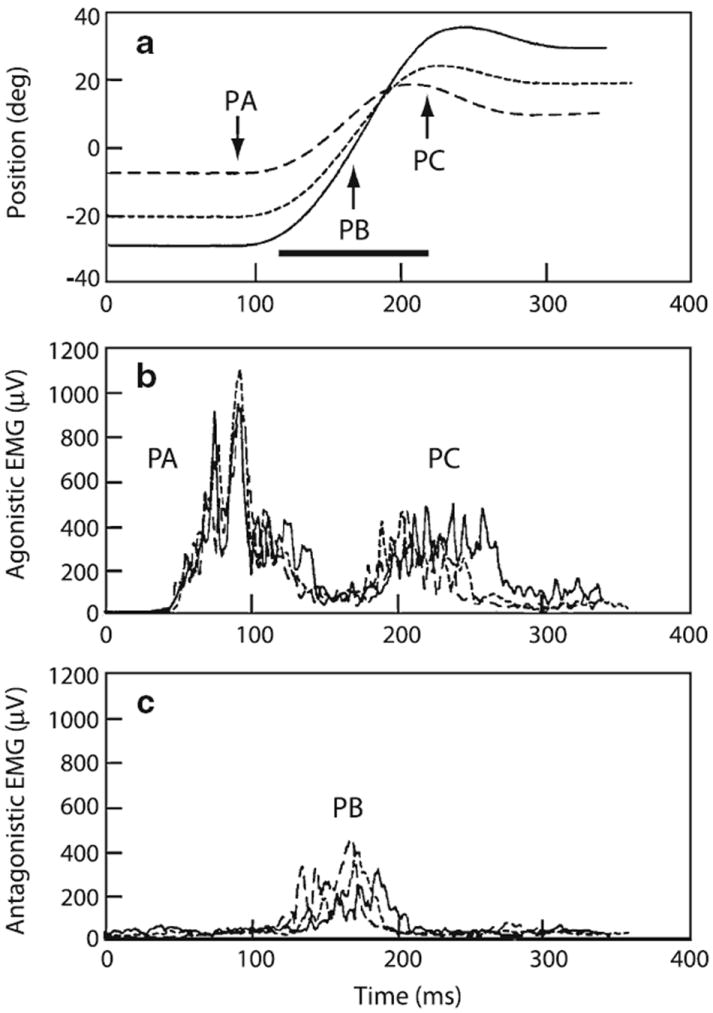
Triphasic electromyographic (EMG) activity during head saccades. a Three head saccades differing in amplitude. Arrows, peak activity in agonist (PA, PC) and antagonist (PB) muscles. Line, duration of activity in PB. b Two burst in agonist muscles (PA, PC) before and after a burst in antagonist muscle (PB) shown in c. Based on Hannaford et al. (1986)
A second possible compensatory mechanism is an adaptive increase in the gain of the CCR. The cervicoocular reflex (COR), which is normally very weak, becomes strengthened in labyrinthine-defective subjects (Baker et al. 1982; Bronstein and Hood 1986). While a parallel increase in the CCR would make functional sense, its strength even following vestibular loss can remain relatively weak (Ito et al. 1997).
Eye–head gaze shifts
In everyday life, we commonly make orienting head turns that are combined with eye movements to redirect our gaze. Bizzi (1981) and his colleagues concluded that the contribution of the head to the gaze shift was removed by the VOR. But numerous studies indicate that the VOR is significantly suppressed during active gaze shifts (Laurutis and Robinson 1986; Tomlinson and Bahra 1986, Tabak et al. 1996; Cullen et al. 2004), which implies that the head and eyes need to be coordinated to ensure accurate gaze shifts.
Two classes of models have emerged to describe the neural control of gaze shifts. In the first model, an integrated controller is used to minimize gaze error rather than enforce a specific eye or head trajectory, making it conceptually analogous to optimal feedback control (Guitton and Volle 1987; Laurutis and Robinson 1986; Cullen et al. 2004). Alternatively, it has been proposed that a gaze displacement command is decomposed early on to control separate eye and head motion. In the scheme described by Freedman (2001, 2008), eye displacement is under internal feedback control, whereas head movements modify eye movements either by reafference or by corollary discharge. It goes beyond the scope of this review to consider the relative merits of the two approaches (see Guitton et al. 2003; Sylvestre and Cullen 2006; Freedman 2008).
Most studies of feedback have recorded eye movements (Tomlinson and Bahra 1986; Freedman and Sparks 2000) and/or omnipause (OMP) or burst (EBN or IBN) neurons during head perturbations (Sylvestre and Cullen 2006). Strictly speaking, then, it is the influence of vestibular or other afferent signals on the eyes, not the head that is being studied. Moreover, such paradigms cannot easily distinguish afferent input from corollary discharge. The role, then, of the vestibular system in the control of voluntary head saccades remains to be studied.
Difficulties in studying the VCR: comparison with the VOR
The VOR is among the best-characterized reflexes, both in terms of its properties and its function. In comparison, our knowledge of the VCR in mammals is fragmentary. It is instructive to compare the two reflexes to understand why the VOR is so much better understood. Both reflexes potentially stabilize their targets, the eyes or the head. In this regard, the VOR is a strong reflex that obviously stabilizes the eyes during head motion. In contrast, the VCR is relatively weak in mammals, showing virtually no stabilization of the head on the body for frequencies below the resonant frequency of the head (Guitton et al. 1986; Gdowski and McCrea 1999; Reynolds and Gdowski 2008). Remarkably, the VCR is stronger in birds, especially birds that have a very limited ocular motility (Money and Correia 1972; Gioanni 1988)
Of equal importance, the limited repertoire of the oculomotor system allows a clear distinction between the VOR and the voluntary behavior of ocular saccades. A similar distinction is not easily made for the neck, which is continually under voluntary control. Consider the stability of the head, which can be enhanced by increasing the strength of the VCR or by voluntary co-contraction of antagonistic muscles (Keshner 2000). It has been suggested that co-contraction strategies are employed by LD subjects, who show heightened muscular activity even in the absence of head movements (Shupert and Horak 1996).
The VCR is most easily demonstrated in decerebrate preparations in the absence of voluntary behavior; open-loop behavior can be studied by EMG recordings during whole-body rotations with the head yoked to the trunk, while closed-loop operation requires whole-body rotations with an unrestrained head. Movement of the body under an earth-fixed head probes the associated stretch reflex or CCR. Perhaps the most serious deficiency in understanding the function of the VCR (and CCR) is our inability to evoke these reflexes in alert animals in the absence of voluntary behavior or without disturbing the trunk. Two methods offer promise for isolated activation of the VCR (1) Loud clicks (>140 dB SPL) delivered to one ear evoke VEMPs, a vestibular-based relaxation of the ipsilateral SCM. Single clicks evoke a short-latency response as a result of the activation of direct (trisynaptic) VCR pathways, which work in decerebrates suggests may not be fully functional. To demonstrate a relaxation requires that the SCM be tonically activated and the recruitment of indirect pathways may require multiple, closely spaced clicks. (2) Trains of electric shocks (40 shocks. 400/s) delivered to individual ampullae produce head movements (Fig. 1). Combining either ampullary or acoustic stimulation with head saccades should test whether the VCR is shut down during voluntary head movements. Comparing EMG activity under various conditions of head and body restraint should help to distinguish the contributions of the VCR and CCR to head stabilization and to isolate the roles of neck and body postural adjustments.
Unresolved issues
We end by listing several issues that need clarification if we are to understand the role of the VCR in alert animals.
As we have seen, movements of the head are determined by the interaction of the mechanical properties of the head and neck, reflexes (the VCR and CCR), and voluntary commands. Assessing the operation and function of the VCR will require experimental approaches that allow systematic dissociation of these factors and are likely to reveal differences in the strength of the reflexes across species.
In decerebrate cats, indirect pathways are functionally more important than direct, trisynaptic pathways. Is the same true in alert animals? If so, we need to determine the structures that make up the indirect pathways and how they convert vestibular-like (angular velocity) signals to reflex-like (angular position) signals.
Is the VCR modified by simultaneous voluntary motor commands? If so, what are the mechanisms involved? In particular, do VO neurons play a critical role and do they exert their influence via direct or indirect pathways?
Do head oscillations and other instabilities that appear immediately after canal plugging reflect the loss of the VCR or are multiple factors involved? Plugging compromises the vestibular input to other motor pathways such as gaze control premotor circuits, as well as ascending pathways that contribute to our sense of spatial orientation. How do these other pathways contribute?
Appendix
The model depicted in Fig. 6 includes the following transfer functions, expressed in terms of the Laplace operator, s (Peng et al. 1996).
| (3) |
| (4) |
| (5) |
| (6) |
The s2 operator in Eq. 5, which is equivalent in the time domain to taking a second derivative, converts the position variables (Ψ, Θ and VOL) to acceleration variables.
Parameters: I = 0.0148 kg m2, B = 0.1 N m s/rad, K = 2.077 N m/rad, KVCR = 30, Ktc = 1, τtc = 0.1 s, τ1A = 0.1 s, τC = 7 s, τCNS1 = 0.4 s, τCNS2 = 20 s, KCCR = 0.1, τMS1 = 0.1 s, τMS2 = 0.1 s. Mechanical properties of the head: I, moment of inertia; B, viscosity; K, elasticity. KVCR, KCCR, and KTC, gains of VCR, CCR, and T.
Calculations in Figs. 8 and 9 were based on the following transfer function:
| (7) |
Contributor Information
Jay M. Goldberg, Email: jgoldber@bsd.uchicago.edu, Department of Neurobiology, Pharmacology and Physiology, University of Chicago, Chicago, IL 60637, USA.
Kathleen E. Cullen, Aerospace Medical Research Unit, Department of Physiology, McGill University, Montreal, QC H3G 1Y6, Canada
References
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- Baker J, Goldberg J, Peterson B, Schor R. Oculomotor reflexes after semicircular canal plugging in cats. Brain Res. 1982;252:151–155. doi: 10.1016/0006-8993(82)90989-1. [DOI] [PubMed] [Google Scholar]
- Berthoz A, Anderson JH. Frequency analysis of vestibular influence on extensor motoneurons. II. Relationship between neck and forelimb extensors. Brain Res. 1971;34:376–380. doi: 10.1016/0006-8993(71)90290-3. [DOI] [PubMed] [Google Scholar]
- Bilotto G, Goldberg J, Peterson BW, Wilson VJ. Dynamic properties of vestibular reflexes in the decerebrate cat. Exp Brain Res. 1982;47:343–352. doi: 10.1007/BF00239353. [DOI] [PubMed] [Google Scholar]
- Bizzi E. Eye-head coordination. In: Brooks VB, editor. Handbook of physiology the nervous system section 1. part 2. Vol. 2. American Physiological Society; Bethesda: 1981. pp. 1321–1336. [Google Scholar]
- Boyle R. Activity of medial vestibulospinal tract cells during rotation and ocular movement in the alert squirrel monkey. J Neurophysiol. 1993;70:2176–2180. doi: 10.1152/jn.1993.70.5.2176. [DOI] [PubMed] [Google Scholar]
- Boyle R, Belton T, McCrea RA. Responses of identified vestibulospinal neurons to voluntary eye and head movements in the squirrel monkey. Ann N Y Acad Sci. 1996;781:244–263. doi: 10.1111/j.1749-6632.1996.tb15704.x. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Hood JD. The cervico-ocular reflex in normal subjects and patients with absent vestibular function. Brain Res. 1986;373:399–408. doi: 10.1016/0006-8993(86)90355-0. [DOI] [PubMed] [Google Scholar]
- Carey JP, Della Santina CC. Principles of applied vestibular physiology. In: Cummings CC, editor. Otolaryngology—head & neck surgery. 4. Vol. 4. Elsevier Mosby; Philadelphia: 2005. pp. 3115–3159. [Google Scholar]
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57:190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci. 2002;22:RC226. doi: 10.1523/JNEUROSCI.22-11-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE. Sensory signals during active versus passive movement. Curr Opin Neurobiol. 2004;14:698–706. doi: 10.1016/j.conb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Huterer M, Braidwood DA, Sylvestre PA. Time course of vestibuloocular reflex suppression during gaze shifts. J Neurophysiol. 2004;92:3408–3422. doi: 10.1152/jn.01156.2003. [DOI] [PubMed] [Google Scholar]
- Decety J. Neural representations for action. Rev Neurosci. 1996;7:285–297. doi: 10.1515/revneuro.1996.7.4.285. [DOI] [PubMed] [Google Scholar]
- Dutia MB, Hunter MJ. The sagittal vestibulocollic reflex and its interaction with neck proprioceptive afferents in the decerebrate cat. J Physiol. 1985;359:17–29. doi: 10.1113/jphysiol.1985.sp015572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald JR. Physiologische Untersuchungen über das Endorgan des Nervus octavus. Wiesbaden: Bergmann; 1892. [Google Scholar]
- Ezure K, Sasaki S. Frequency-response analysis of vestibular-induced neck reflex in cat. I. Characteristics of neural ransmission from horizontal semicircular canal to neck motoneurons. J Neurophysiol. 1978;41:445–458. doi: 10.1152/jn.1978.41.2.445. [DOI] [PubMed] [Google Scholar]
- Ezure K, Sasaki S, Uchino Y, Wilson VJ. Frequency-response analysis of vestibular-induced neck reflex in cat. II. Functional significance of cervical afferents and polysynaptic descending pathways. J Neurophysiol. 1978;41:459–471. doi: 10.1152/jn.1978.41.2.459. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Paillard J, Jeannerod M. The role of proprioception in action recognition. Conscious Cogn. 2003;12:609–619. doi: 10.1016/s1053-8100(03)00047-3. [DOI] [PubMed] [Google Scholar]
- Flourens M. Recherches expérimentales sur les propriétés du système nerveux dans les animaux vertébrés. Paris: 1824. [Google Scholar]
- Freedman EG. Interactions between eye and head control signals can account for movement kinematics. Biol Cybern. 2001;84:453–462. doi: 10.1007/PL00007989. [DOI] [PubMed] [Google Scholar]
- Freedman EG. Coordination of the eyes and head during visual orienting. Exp Brain Res. 2008;190:369–387. doi: 10.1007/s00221-008-1504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Coordination of the eyes and head: movement kinematics. Exp Brain Res. 2000;131:22–32. doi: 10.1007/s002219900296. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Ohashi T, Fukushima J. Effects of chemical deactivation of the interstitial nucleus of Cajal on the vertical vestibulo-collic reflex induced by pitch rotation in alert cats. Neurosci Res. 1994;20:281–286. doi: 10.1016/0168-0102(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Galiana HL, Guitton D. Central organization and modeling of eye-head coordination during orienting gaze shifts. Ann N Y Acad Sci. 1992;656:452–471. doi: 10.1111/j.1749-6632.1992.tb25228.x. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, McCrea RA. Integration of vestibular and head movement signals in the vestibular nuclei during whole-body rotation. J Neurophysiol. 1999;82:436–449. doi: 10.1152/jn.1999.82.1.436. [DOI] [PubMed] [Google Scholar]
- Ghez C, Martin JH. The control of rapid limb movement in the cat. III. Agonist—antagonist coupling. Exp Brain Res. 1982;45:115–125. doi: 10.1007/BF00235770. [DOI] [PubMed] [Google Scholar]
- Gioanni H. Stabilizing gaze reflexes in the pigeon (Columba livia). I. Horizontal and vertical optokinetic eye (OKN) and head (OCR) reflexes. Exp Brain Res. 1988;69:567–582. doi: 10.1007/BF00247310. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C. Responses of peripheral vestibular neurons to angular and linear accelerations in the squirrel monkey. Acta Otolaryngol. 1975;80:101–110. doi: 10.3109/00016487509121307. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Peterson BW. Reflex and mechanical contributions to head stabilization in alert cats. J Neurophysiol. 1986;56:857–875. doi: 10.1152/jn.1986.56.3.857. [DOI] [PubMed] [Google Scholar]
- Graf W, de Waele C, Vidal PP. Functional anatomy of the head-neck movement system of quadrupedal and bipedal mammals. J Anat. 1995a;186(1):55–74. [PMC free article] [PubMed] [Google Scholar]
- Graf W, de Waele C, Vidal PP, Wang DH, Evinger C. The orientation of the cervical vertebral column in unrestrained awake animals. II. Movement strategies. Brain Behav Evol. 1995b;45:209–231. doi: 10.1159/000113551. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. I. Behavioral properties. Exp Brain Res. 1987;66:339–354. doi: 10.1007/BF00243309. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Ong-Meang Jacques V, Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. II. Morphological properties as revealed by intra-axonal injections of horseradish peroxidase. Exp Brain Res. 1987;66:355–377. doi: 10.1007/BF00243310. [DOI] [PubMed] [Google Scholar]
- Guitton D. Control of eye-head coordination during orienting gaze shifts. Trends Neurosci. 1992;15:174–179. doi: 10.1016/0166-2236(92)90169-9. [DOI] [PubMed] [Google Scholar]
- Guitton D, Volle M. Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. J Neurophysiol. 1987;58:427–459. doi: 10.1152/jn.1987.58.3.427. [DOI] [PubMed] [Google Scholar]
- Guitton D, Kearney RE, Wereley N, Peterson BW. Visual, vestibular and voluntary contributions to human head stabilization. Exp Brain Res. 1986;64:59–69. doi: 10.1007/BF00238201. [DOI] [PubMed] [Google Scholar]
- Guitton D, Bergeron A, Choi WY, Matsuo S. On the feedback control of orienting gaze shifts made with eye and head movements. Prog Brain Res. 2003;142:55–68. doi: 10.1016/S0079-6123(03)42006-2. [DOI] [PubMed] [Google Scholar]
- Hallett M, Shahani BT, Young RR. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry. 1975;38:1154–1162. doi: 10.1136/jnnp.38.12.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannaford B, Won SK, Lee SH, Stark L. Neurological control of head movements: inverse modeling and electromyography. Math Biosci. 1986;78:159–178. [Google Scholar]
- Harada Y, Taniguchi M, Namatame H, Iida A. Magnetic materials in otoliths of bird and fish lagena and their function. Acta Otolaryngol. 2001;121:590–595. [PubMed] [Google Scholar]
- Highstein SM, Ito M, Tsuchiya T. Synaptic linkage in the vestibulo-ocular reflex pathway of rabbit. Exp Brain Res. 1971;113:306–326. doi: 10.1007/BF00234952. [DOI] [PubMed] [Google Scholar]
- Horak FB, MacPherson JM. Postural orientation and equilibrium. In: Rowell LB, Shepherd JT, editors. Handbook of physiology Section 12 Exercise, regulation and integration of multiple systems. Oxford University Press; New York: 1996. pp. 255–292. [Google Scholar]
- Huterer M, Cullen KE. Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. J Neurophysiol. 2002;88:13–28. doi: 10.1152/jn.2002.88.1.13. [DOI] [PubMed] [Google Scholar]
- Ito M, Nisimaru N, Yamamoto M. Pathways for the vestibulo-ocular reflex excitation arising from semicircular canals of rabbits. Exp Brain Res. 1976a;24:257–271. doi: 10.1007/BF00235014. [DOI] [PubMed] [Google Scholar]
- Ito M, Nisimaru N, Yamamoto M. Postsynaptic inhibition of oculomotor neurons involved in vestibulo-ocular reflexes arising from semicircular canals of rabbits. Exp Brain Res. 1976b;24:273–283. doi: 10.1007/BF00235015. [DOI] [PubMed] [Google Scholar]
- Ito Y, Corna S, von Brevern M, Bronstein A, Gresty M. The functional effectiveness of neck muscle reflexes for head-righting in response to sudden fall. Exp Brain Res. 1997;117(2):266–272. doi: 10.1007/s002210050221. [DOI] [PubMed] [Google Scholar]
- Jex HR, Magdaleno RE. Biomechanical models for vibration feedthrough to hands and head for a semisupine pilot. Aviat Space Environ Med. 1978;49:304–316. [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Keshner EA. Modulating active stiffness affects head stabilizing strategies in young and elderly adults during trunk rotations in the vertical plane. Gait Posture. 2000;11:1–11. doi: 10.1016/s0966-6362(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Peterson BW. Mechanisms controlling human head stabilization. I. Head-neck characteristics during random rotations in the horizontal plane. J Neurophysiol. 1995;73:2293–2301. doi: 10.1152/jn.1995.73.6.2293. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Cromwell RL, Peterson BW. Mechanisms controlling human head stabilization. II. Head-neck characteristics during random rotations in the vertical plane. J Neurophysiol. 1995;73:2302–2312. doi: 10.1152/jn.1995.73.6.2302. [DOI] [PubMed] [Google Scholar]
- Kitama T, Grantyn A, Berthoz A. Orienting-related eye-neck neurons of the medial ponto-bulbar reticular formation do not participate in horizontal canal-dependent vestibular reflexes of alert cats. Brain Res Bull. 1995;38:337–347. doi: 10.1016/0361-9230(95)00106-o. [DOI] [PubMed] [Google Scholar]
- Korte GE. The brainstem projection of the vestibular nerve in the cat. J Comp Neurol. 1979;184:279–292. doi: 10.1002/cne.901840205. [DOI] [PubMed] [Google Scholar]
- Laurutis VP, Robinson DA. The vestibulo-ocular reflex during human saccadic eye movements. J Physiol. 1986;373:209–233. doi: 10.1113/jphysiol.1986.sp016043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. In the neurology of eye movements. Oxford; New York: 2006. Eye-head movements; pp. 315–342. [Google Scholar]
- Lorente de Nó R. Vestibulo-ocular reflex arc. Arch Neurol Psychiat. 1933;30:245–291. [Google Scholar]
- McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol. 1999;82:416–428. doi: 10.1152/jn.1999.82.1.416. [DOI] [PubMed] [Google Scholar]
- McGill SM, Jones K, Bennett G, Bishop PJ. Passive stiffness of the human neck in flexion, extension, and lateral bending. Clin Biomech. 1994;9:193–198. doi: 10.1016/0268-0033(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Melvill Jones G. Chapter 41: Posture. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 4. McGraw-Hill; New York: 2000. pp. 816–831. [Google Scholar]
- Money KE, Correia KE. The vestibular system of the owl. Comp Biochem Physiol. 1972;42A:353–358. doi: 10.1016/0300-9629(72)90116-8. [DOI] [PubMed] [Google Scholar]
- Money KE, Scott JW. Functions of separate sensory receptors of nonauditory labyrinth of the cat. Am J Physiol. 1962;202:1211–1220. doi: 10.1152/ajplegacy.1962.202.6.1211. [DOI] [PubMed] [Google Scholar]
- Moore ST, Hirasaki E, Cohen B, Raphan T. Effect of viewing distance on the generation of vertical eye movements during locomotion. Exp Brain Res. 1999;129:347–361. doi: 10.1007/s002210050903. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Paige GD. Vestibuloocular reflex and its interactions with visual following mechanisms in the squirrel monkey. II. Response characteristics and plasticity following unilateral inactivation of horizontal canal. J Neurophysiol. 1983;49:152–168. doi: 10.1152/jn.1983.49.1.152. [DOI] [PubMed] [Google Scholar]
- Peng GC, Hain TC, Peterson BW. A dynamical model for reflex activated head movements in the horizontal plane. Biol Cybern. 1996;75:309–319. doi: 10.1007/s004220050297. [DOI] [PubMed] [Google Scholar]
- Peng GC, Hain TC, Peterson BW. Predicting vestibular, proprioceptive, and biomechanical control strategies in normal and pathological head movements. IEEE Trans Biomed Eng. 1999;46:1269–1280. doi: 10.1109/10.797986. [DOI] [PubMed] [Google Scholar]
- Peng GC, Minor LB, Zee DS. Coupled asymmetries of the vestibulo-ocular (VOR) and vestibulocollic (VCR) reflexes in patients with unilateral vestibular loss (UVL) Soc Neurosci Abstr. 2004;23:1294. [Google Scholar]
- Perlmutter SI, Iwamoto Y, Barke LF, Baker JF, Peterson BW. Relation between axon morphology in C1 spinal cord and spatial properties of medial vestibulospinal tract neurons in the cat. J Neurophysiol. 1998;79:285–303. doi: 10.1152/jn.1998.79.1.285. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Boyle R. Vestibulocollic reflexes. In: Highstein SM, Fay RR, Popper AN, editors. The vestibular system. Springer; New York: 2004. pp. 343–374. [Google Scholar]
- Peterson BW, Fukushima K, Hirai N, Schor RH, Wilson VJ. Responses of vestibulospinal and reticulospinal neurons to sinusoidal vestibular stimulation. J Neurophysiol. 1980;43:1236–1250. doi: 10.1152/jn.1980.43.5.1236. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Choi H, Hain T, Keshner E, Peng GC. Dynamic and kinematic strategies for head movement control. Ann N Y Acad Sci. 2001;942:381–393. doi: 10.1111/j.1749-6632.2001.tb03761.x. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Berthoz A, Lefort L. Head stabilization during various locomotor tasks in humans. I. Normal subjects. Exp Brain Res. 1990;82:97–106. doi: 10.1007/BF00230842. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Berthoz A, Lefort L, Vitte E. Head stabilization during various locomotor tasks in humans. II. Patients with bilateral peripheral vestibular deficits. Exp Brain Res. 1991;85:208–217. doi: 10.1007/BF00230002. [DOI] [PubMed] [Google Scholar]
- Rabbitt RD, Boyle R, Highstein SM. Influence of surgical plugging on horizontal semicircular canal mechanics and afferent response dynamics. J Neurophysiol. 1999;82:1033–1053. doi: 10.1152/jn.1999.82.2.1033. [DOI] [PubMed] [Google Scholar]
- Reynolds JS, Gdowski GT. Head movements produced during whole body rotations and their sensitivity to changes in head inertia in squirrel monkeys. J Neurophysiol. 2008;95:2369–2382. doi: 10.1152/jn.00320.2007. [DOI] [PubMed] [Google Scholar]
- Robinson DA. The use of matrices in analyzing the three-dimensional behavior of the vestibulo-ocular reflex. Biol Cybern. 1982;46:53–66. doi: 10.1007/BF00335351. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Phillips JO, Fuchs AF. Coordination of gaze shifts in primates: brainstem inputs to neck and extraocular motoneuron pools. J Comp Neurol. 1994;346:43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci. 2001;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Goldberg JM, Minor LB, Cullen KE. Effects of canal plugging on the vestibuloocular reflex and vestibular nerve discharge during passive and active head rotations. J Neurophysiol. 2009;102:2693–2703. doi: 10.1152/jn.00710.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Multimodal integration after unilateral labyrinthine lesion: single vestibular nuclei neuron responses and implications for postural compensation. J Neurophysiol. doi: 10.1152/jn.00788.2010. (in press), (Epub 2010 Dec 8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor RH. Responses of cat vestibular neurons to sinusoidal roll tilt. Exp Brain Res. 1974;20:347–362. doi: 10.1007/BF00237380. [DOI] [PubMed] [Google Scholar]
- Scott SH, Norman KE. Computational approaches to motor control and their potential role for interpreting motor dysfunction. Curr Opin Neurol. 2003;16:693–698. doi: 10.1097/01.wco.0000102631.16692.71. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Ohgaki T, Sugiuchi Y, Futami T. Morphology of single medial vestibulospinal tract axons in the upper cervical spinal cord of the cat. J Comp Neurol. 1992;316:151–172. doi: 10.1002/cne.903160203. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sugiuchi Y, Izawa Y, Hata Y. Long descending motor tract axons and their control of neck and axial muscles. Prog Brain Res. 2006;151:527–563. doi: 10.1016/S0079-6123(05)51017-3. [DOI] [PubMed] [Google Scholar]
- Shupert CL, Horak FB. Effects of vestibular loss on head stabilization in response to head and body perturbations. J Vestib Res. 1996;6:423–437. [PubMed] [Google Scholar]
- Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci. 2002;3:952–964. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- Sugiuchi Y, Kakei S, Izawa Y, Shinoda Y. Functional synergies among neck muscles revealed by branching patterns of single long descending motor-tract axons. Prog Brain Res. 2004;143:411–421. doi: 10.1016/S0079-6123(03)43039-2. [DOI] [PubMed] [Google Scholar]
- Suzuki JI, Cohen B. Head, eye, body and limb movements from semicircular canal nerves. Exp Neurol. 1964;10:393–405. doi: 10.1016/0014-4886(64)90031-7. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Premotor correlates of integrated feedback control for eye-head gaze shifts. J Neurosci. 2006;26:4922–4929. doi: 10.1523/JNEUROSCI.4099-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentagothai J. The elementary vestibulo-ocular reflex arc. J Neurophysiol. 1950:395–407. doi: 10.1152/jn.1950.13.6.395. [DOI] [PubMed] [Google Scholar]
- Tabak S, Smeets JB, Collewijn H. Modulation of the human vestibuloocular reflex during saccades: probing by high-frequency oscillation and torque pulses of the head. J Neurophysiol. 1996;76:3249–3263. doi: 10.1152/jn.1996.76.5.3249. [DOI] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Terzuolo CA, Soechting JF, Viviani P. Studies on the control of some simple motor tasks. I. Relations between parameters of movements and EMG activities. Brain Res. 1973;58:212–216. doi: 10.1016/0006-8993(73)90834-2. [DOI] [PubMed] [Google Scholar]
- Thomson DB, Ikegami H, Wilson VJ. Effect of MLF transection on the vertical vestibulocollic reflex in decerebrate cats. J Neurophysiol. 1995;74:1815–1818. doi: 10.1152/jn.1995.74.4.1815. [DOI] [PubMed] [Google Scholar]
- Tomko DL, Peterka RJ, Schor RH, O’Leary DP. Response dynamics of horizontal canal afferents in barbiturate-anesthetized cats. J Neurophysiol. 1981;45:376–396. doi: 10.1152/jn.1981.45.3.376. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate. II. Interactions between saccades and the vestibuloocular reflex. J Neurophysiol. 1986;56:1558–1570. doi: 10.1152/jn.1986.56.6.1558. [DOI] [PubMed] [Google Scholar]
- Uchino YSM, Sato H, Bai R, Kawamoto E. Otolith and canal integration on single vestibular neurons in cats. Brain Res. 2005:271–285. doi: 10.1007/s00221-005-2341-7. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Das reafferenzprinzip. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- Welgampola MS, Colebatch JG. Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology. 2005;64:1682–1688. doi: 10.1212/01.WNL.0000161876.20552.AA. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Maeda M. Connections between semicircular canals and neck motoneurons in the cat. J Neurophysiol. 1974;37:346–357. doi: 10.1152/jn.1974.37.2.346. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Schor RH. The neural substrate of the vestibulocollic reflex. What needs to be learned. Exp Brain Res. 1999;129:483–493. doi: 10.1007/s002210050918. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Monosynaptic inhibition of neck motoneurons by the medial vestibular nucleus. Brain Res. 1969;11:365–380. doi: 10.1007/BF00235245. [DOI] [PubMed] [Google Scholar]
- Winklhofer M, Kirschvink JL. A quantitative assessment of torque-transducer models for magnetoreception. J R Soc Interface. 2010;7(2):S273–S289. doi: 10.1098/rsif.2009.0435.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters JM, Stark L. Analysis of fundamental human movement patterns through the use of in-depth antagonistic muscle models. IEEE Trans Biomed Eng. 1985;32:826–839. doi: 10.1109/TBME.1985.325498. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. Are arm trajectories planned in kinematic or dynamic coordinates? An adaptation study. Exp Brain Res. 1995;103:460–470. doi: 10.1007/BF00241505. [DOI] [PubMed] [Google Scholar]
- Zangemeister WH, Stark L. Active head rotations and eye-head coordination. Ann N Y Acad Sci. 1981;374:540–55. doi: 10.1111/j.1749-6632.1981.tb30899.x. [DOI] [PubMed] [Google Scholar]


