Abstract
Hesperidin, a flavanone glycoside, and its aglycone hesperetin, are potential candidates for the treatment of diabetic retinopathy and macular edema. The objective of this study was to delineate the vitreal pharmacokinetics of hesperidin and hesperetin and the hydrophilic derivative hesperidin G (glucosyl hesperedin), following intravitreal administration in anaesthetized rabbits. Concentration changes in the vitreous humor were monitored using microdialysis sampling procedure. All three molecules were administered intravitreally at three dose levels (50 μl injection volume containing 1.5, 4.5 and 15 µg of the drug, resulting in a final vitreal concentration of 1, 3 and 10 μg/mL). Vitreal microdialysis samples were collected every 20 minutes over a period of 10 h. All three molecules exhibited linear pharmacokinetics, within the dose range tested, since AUC and Cmax increased linearly with increasing dose and a significant difference in the elimination parameters, like clearance or half-life, was not observed. The vitreal elimination half-life of these three compounds was observed to correlate with the molecular weight and lipophilicity of the molecules. The findings from this study provide practical information that will be useful in the future design of ocular drug delivery strategies for the bioflavonoids.
Keywords: Hesperidin, Hesperetin, Hesperidin G, Vitreal, Ocular, Pharmacokinetics, Elimination, Dose proportionality, Microdialysis, Physicochemical
INTRODUCTION
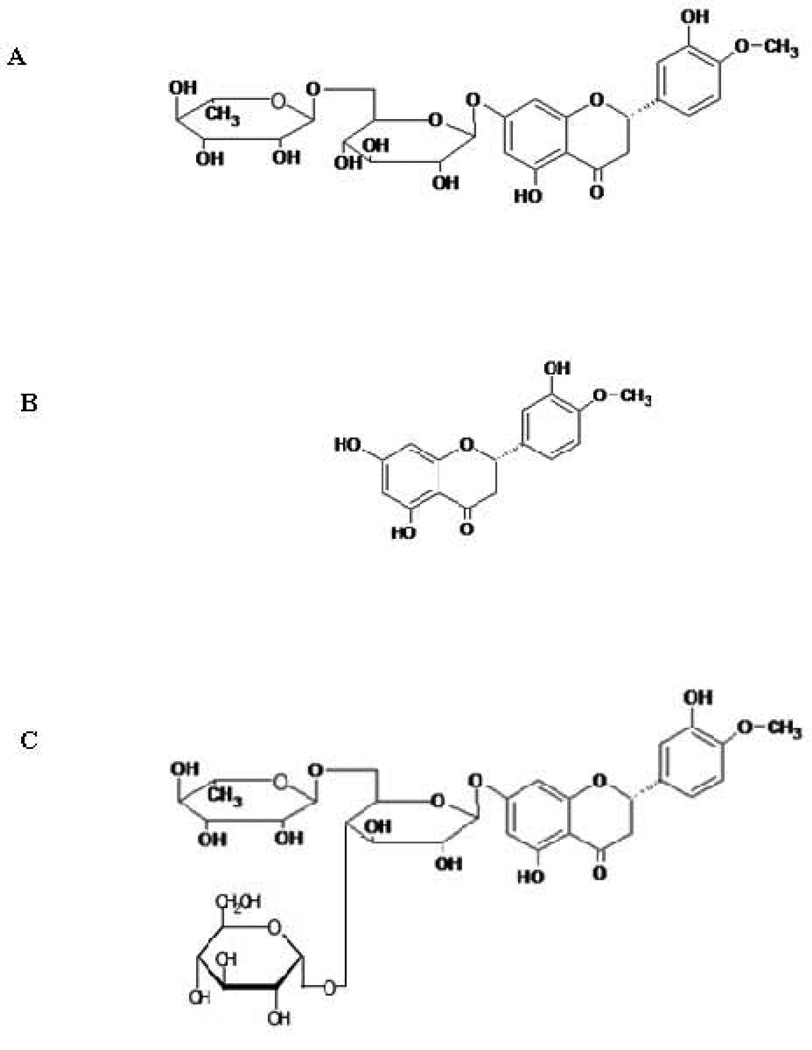
Diabetic retinopathy and diabetic macular edema are the leading causes of acquired blindness, especially in Western countries 1. Hesperidin, a flavanone glycoside and its aglycone, hesperetin (Fig. 1) have beneficial effects in the prevention or treatment of diseases of the posterior segment of the eye, like diabetic retinopathy, diabetic macular edema and other ocular disorders, because of their favorable pharmacological properties. These molecules can act as potential antioxidants, can increase the ocular blood flow and minimize ischemic injury to the retina, can decrease vascular permeability, and act as a neuroprotectant, anti-inflammatory agent and anticancer agent 2–4. Hesperidin G (glucosyl hesperidin) is a water soluble derivative of hesperidin (Fig. 1). In hesperidin G, a glucose molecule is attached to the rutinose moiety of hesperidin. This improved hesperidin’s solubility several folds 5. Hesperidin G is also reported to have some beneficial effects 6–8. It suppressed the oxidative stress in the vasculature and thereby ameliorated endothelial dysfunction and hypertension in spontaneously hypertensive rats. However, the observed pharmacological effects could be because of conversion of hesperidin G into hesperidin and hesperetin by the intestinal or hepatic metabolic enzymes 9.
Figure 1.
Chemical structure of A: Hesperidin; B: Hesperetin and C: Hesperidin G (Glucosyl Hesperidin)
Ameer et al., 10 reported that hesperidin’s oral bioavailability is very low (<25%). Over and above all the barriers to systemic bioavailability, in order to exert a therapeutic effect in diabetic retinopathy, these compounds must penetrate into the neural retina from the systemic circulation. For this, they need to permeate across the blood-retinal barrier (BRB), formed by the retinal pigmented epithelium (RPE) and the endothelial cells of the retinal blood vessels. The diffusion limiting capabilities of the BRB has been well established for both hydrophilic (limited by tight junctions) as well as lipophilic (through efflux mechanism) compounds. Thus, following oral administration very little, if any, amounts can reach the neural retina.
Intravitreal therapy has brought about a paradigm shift in the management of posterior segment ocular diseases. Currently, it is approved by the US FDA to target submacular choroidal neovascularization in patients with AMD 11,12. Considering back-of-the eye drug delivery, the major advantage of IVI is that it generates higher concentrations of the therapeutic agent in the vitreous with minimal or negligible systemic exposure. These injections have been used to deliver many types of medications into the vitreous cavity, for example anti-infective (antibiotic, antifungal, and antiviral), anti-inflammatory (nonsteroidal anti-inflammatory, steroids, and immunomodulators), anticancer and anti-VEGF agents. However, IVIs may introduce further complications, such as the progression of endophthalmitis and cataract due to repeated injections. Additionally, repeated injections may cause extreme patient discomfort and may lead to complications such as vitreal hemorrhage, infection, and lens or retinal injury 11,12. In spite of the above, IVIs still represent the most effective route of delivery for the back-of-the eye diseases.
The duration of effect of an intravitreally administered drug depends on the retention of the injected drug at the site of administration. Disposition of the intravitreally administered drug from the vitreous humor mainly depends on the physicochemical properties of the molecule and metabolism by ocular metabolizing enzymes. A scientific understanding of the elimination kinetics of the drug from posterior chamber and its relationship with physicochemical properties such as molecular weight, lipophilicity and solubility is essential for the development of intravitreal drug delivery technologies with desired pharmacokinetic properties 11–14.
The three molecules hesperidin, hesperetin, and hesperidin G, differ in their physicochemical properties and molecular weight. Hesperetin has the lowest molecular weight (C16H14O6, 302.27 g/mole) followed by hesperidin (C28H34O15, 610.57 g/mole) and hesperidin G (C34H44O20, 772.70 g/mole). Hesperidin G is significantly more hydrophilic than the other two molecules with a log P of < −3.2 while hesperidin and hesperetin have a log P of 1.78 and 2.9, respectively 2,4. Thus, the specific objective of the current project was to evaluate the dose dependent vitreal disposition kinetics of the selected three compounds and to evaluate the role of log P, solubility and molecular weight on the vitreal elimination kinetics. In this study, vitreal microdialysis was used to estimate the concentration of the compounds in the vitreous humor. It is an invasive sampling technique, which involves surgical implantation of the probes into the organ/tissue of choice. It has become an important tool for dynamic in vivo sampling and in recent times has been effectively used in characterizing intraocular disposition of drugs in both the anterior and the posterior chambers of the eye 15–18.
MATERIALS AND METHODS
Animals
New Zealand male white rabbits were procured from Myrtle’s Rabbitry (Thompson Station, TN). Experiments conformed to the tenets of the Association for Research in Vision and Ophthalmology (ARVO) statement on the Use of Animals in Ophthalmic and Vision Research and followed the University of Mississippi Institutional Animal Care and Use Committee (IACUC) approved protocol.
Materials
Microdialysis probes (CMA/20; 20,000 Da molecular mass weight and 10 mm shaft) were obtained from CMA/Microdialysis Inc. (North Chelmsford, MA). Hesperidin and hesperetin were purchased form Sigma-Aldrich (St. Louis, MO). Hesperidin G was obtained as a gift sample from Hayashibara International Inc. (Broomfield, CO). Ketamine hydrochloride and xylazine were procured from Fort Dodge Animal Health (Fort Dodge, IA) and Lloyd Laboratories (Shenandoah, IA), respectively. Pentobarbital was obtained from Virbac AH, Inc. (Fort Worth, TX). All other chemicals and solvents (HPLC grade) used were purchased from Thermo Fisher Scientific (Waltham, MA) and used as such.
In vitro Probe Recovery
Microdialysis probe recovery was determined following a previously published report17. Briefly, recovery values were obtained by placing the probe in an isotonic phosphate-buffered saline (IPBS) solution (pH 7.4) at 37°C, containing a known concentration (1, 3, 10 µg/mL) of the compound; hesperidin, hesperetin or hesperidin G. The probe was perfused with sterile IPBS at different flow rates (1.8, 3 and 4 µL/min), and the dialysate was collected every 20 min, to choose optimal conditions. Relative recovery was calculated using eq. 1:
| (1) |
where Cdis the concentration of the respective compound in the dialysate and Cs is the concentration in IPBS. The concentration of the respective compound in the vitreous humor samples was calculated by dividing the dialysate concentration by the in vitro recovery factor obtained as described above.
Initial studies were conducted to determine an optimum flow rate and it was found to be 3µL/min (supplementary data). The recovery factor for each probe is individually determined before and after the experiment and the in vivo samples obtained from each probe is uniquely coded. The mean recovery factor for that particular probe is then used to obtain the actual vitreous humor levels from the vitreous humor sample concentrations. If a significant difference in the recovery factor is observed between the in vitro recovery values obtained at the beginning and at the end of each experiment, data from that probe is not considered. Determination of the recovery factors were carried out for each individual compound separately. In order to validate the microdialysis probe recovery factor estimation in IPBS (pH 7.4), in vitro recovery was also determined in pooled rabbit vitreous humor (collected at the end of other experiments involving New Zealand white rabbits, from the same or other protocols). The same probes were used in both media. The concentration used for the compounds in these studies was 1 µg/mL. The recovery factor obtained from IPBS was found to be consistent with that obtained from the vitreous humor for all the three molecules (supplementary data). Thus, IPBS was used for estimating the probe recovery factor, before and after each experiment, for each compound.
Probe Implantation
Probe implantation was done following previously published reports 17. Briefly, rabbits (weighing 2–2.5 kg) were anesthetized using ketamine (35 mg/kg)/xylazine (3.5 mg/kg) administered intramuscularly and were maintained under anesthesia throughout the duration of the experiment (ketamine/xylazine administered intramuscularly every 40 min). Before probe implantation, 1% tropicamide was applied topically to dilate the pupil. A 22-guage needle was then inserted into the posterior chamber of the eye. The point of insertion was approximately 3 mm below the corneal-scleral limbus. The needle was withdrawn, and the vitreal probe was implanted immediately. The position of the probe was adjusted so that the semipermeable membrane was in the mid-vitreous section. The probes were continuously perfused with sterile IPBS (pH 7.4) at a flow rate of 3 µL/min using a CMA/100 microinjection pump (CMA/Microdialysis Inc.). After probe implantation, animals were allowed to stabilize for a period of 2 h before the administration of respective compound. Vitreal samples were collected every 20 min for a period of 10 h post intravitreal administration. Samples were collected in microcentrifuge tubes and stored at −80°C until further analysis. At the end of the study, animals were euthanized, under deep anesthesia, with an overdose of sodium pentobarbital administered through the marginal ear vein.
Intravitreal Administration
Hesperidin, hesperetin or hesperidin G was administered intravitreally (respective dose (1.5, 4.5 or 15 µg) in 50 µL of IPBS (pH 7.4)). In the case of hesperidin and hesperetin, initial stock was prepared using 5 % DMSO and further dilutions were made with sterile IPBS (pH 7.4). Final concentration of DMSO in the vitreous humor ranged between 0.005 to 0.05 %.
Analytical Methods
Hesperidin, hesperetin and hesperetin G content in the samples was estimated using an analytical method based on reversed phase HPLC An HPLC system equipped with Waters 600 pump controller, 2470 dual wavelength UV detector, refrigerated 717 plus auto-sampler and Agilent 3394B integrator was used. The detector was operated at 284 nm. Mobile phase consisted of 20 mM monobasic potassium phosphate (pH adjusted to 2.5 with ortho-phosphoric acid) and acetonitrile in a ratio of 75:25 for hesperidin and glucosyl hesperidin while a ratio of 50:50 was used for hesperetin. The flow rate was maintained at 1 mL/min. A Phenomenex Luna, 250 × 4.6 mm, 5µ, C18(2) column was used. Samples were injected (30 µL) on to the column as such. Calibration curve concentrations used were 0.02, 0.2, 0.5, 1.0, 5.0, 10.0, 15.0 µg/mL for hesperetin and 0.05, 0.2, 0.5, 1.0, 5.0, 10.0, 15.0 µg/mL for hesperidin and hesperidin G. The calibration curve had to have a correlation coefficient (r2) of 0.99 or better. Accuracy and precision was analyzed using four QC samples, at 0.02, 0.2, 1.0, 10.0 for hesperetin and 0.05, 0.2, 1.0, 10.0 for hesperidin and hesperidin G. The acceptance criterion for each back-calculated standard concentration was 15% deviation from the nominal value except at LLOQ, which was set at 20%. Inter-day and intra-day accuracy and precision (CV %) were within 80 −120 % of nominal concentration at LLOQ and 85 −115 % at remaining quality control levels (supplementary data).
Data Analysis
Vitreal pharmacokinetic parameters were determined using WinNonlin® software (version 5.2; Pharsight, Mountain View, CA). Data was modeled according to one-compartment and two-compartment pharmacokinetic models. Akaike Information Criterion (AIC) and Schwarz Bayesian Criterion (SBC) were used for the selection of the most appropriate model. Additionally, the goodness of fit was assessed graphically by evaluation of the agreement between the observed and the predicted concentrations and on the correlation coefficient (R2) for observed vs predicted values. Statistical analysis was carried out using JMP software (Version 5.0.1). ANOVA was used to check for difference among different groups and Student t-test was used to evaluate difference between two groups. A p value less than 0.05 is considered statistically significant.
RESULTS AND DISCUSSION
Hesperidin and its aglycone, hesperetin are potential candidates for the prevention or treatment of diseases of the posterior segment of the eye like diabetic retinopathy and diabetic macular edema 2–4. Effective concentrations of hesperidin and hesperetin for various pharmacological activities have been reported by several researchers. These compounds exhibited dose dependent activities in different in vitro (1–100 µM) 19–25 and in vivo studies (10–80 mg/Kg BW) 26–30. Hesperidin G is also reported to have some beneficial effects 6–8. However, the observed pharmacological effects could be because of conversion of hesperidin G into hesperidin and hesperetin by the metabolic enzymes present in the intestine or liver 9.
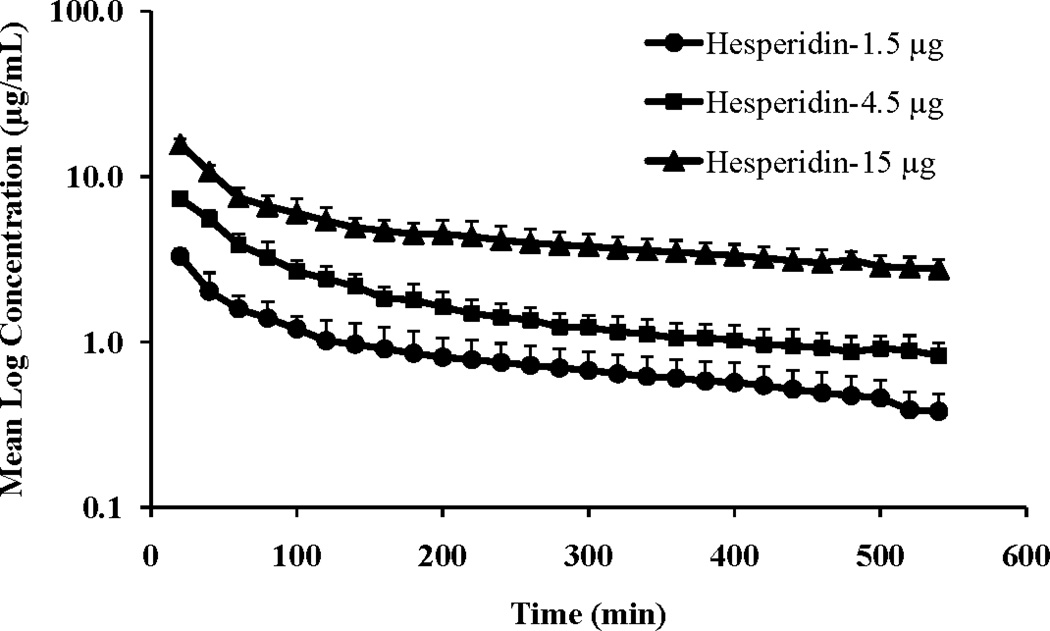
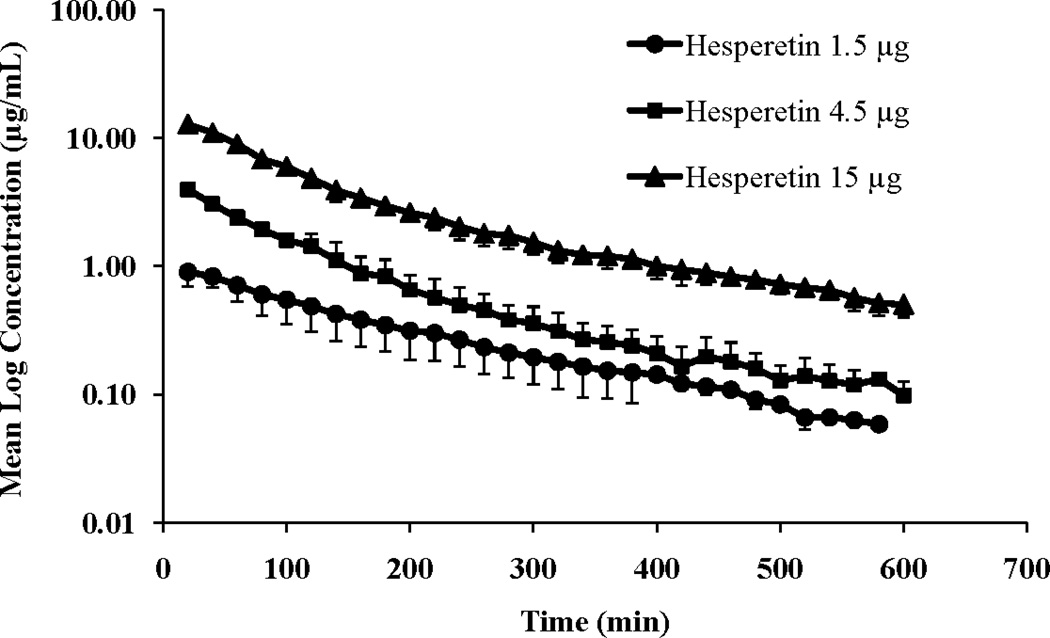
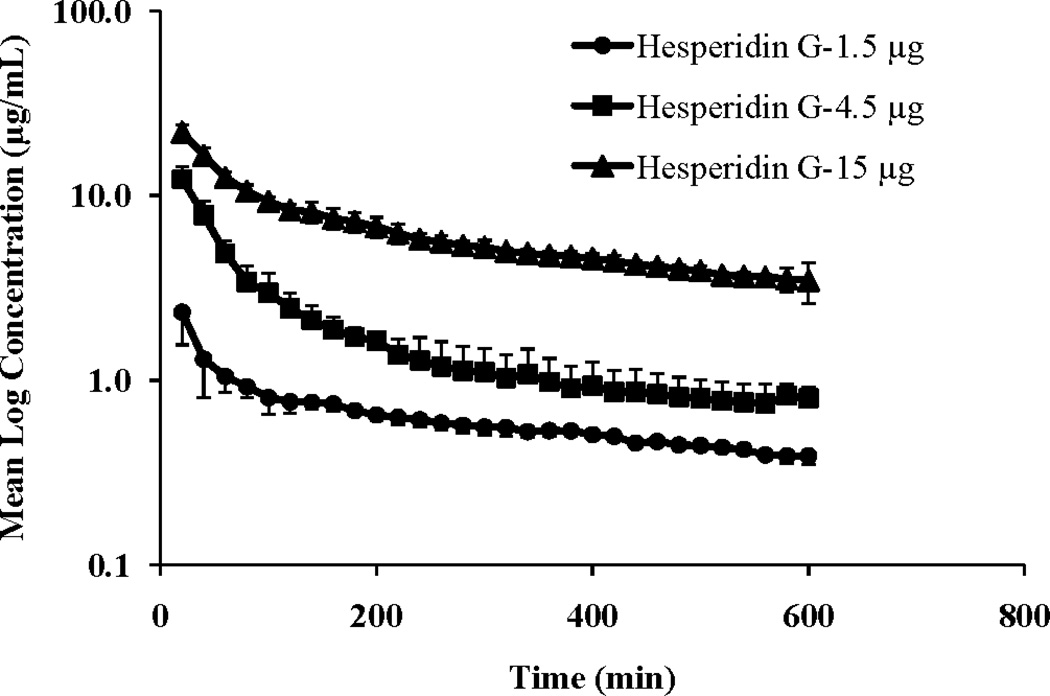
Vitreal kinetics of hesperidin and hesperetin and a water soluble derivative of hesperidin, hesperidin G, were determined at three dose levels (1.5, 4.5 or 15 µg in 50 µL injection volume) following intravitreal administration in rabbits. The vitreal concentration-time profiles of hesperidin, hesperetin and hesperidin G at the three dose levels are presented in Figures 2, 3 and 4, respectively.
Figure 2.
Mean vitreal log concentration vs. time profile of hesperidin following intravitreal administration of hesperidin at three doses (1.5, 4.5 and 15 μg resulting in vitreal concentration of 1, 3 and 10 μg/mL, respectively). Injection volume was 50 μL. Values represent Mean ± SD (n=4).
Figure 3.
Mean vitreal log concentration vs. time profile of hesperetin following intravitreal administration of hesperetin at three doses (1.5, 4.5 and 15 μg resulting in vitreal concentration of 1, 3 and 10 μg/mL, respectively). Injection volume was 50 μL. Values represent Mean ± SD (n=4).
Figure 4.
Mean vitreal log concentration vs. time profile of hesperidin G following intravitreal administration of hesperidin G at three doses (1.5, 4.5 and 15 μg resulting in vitreal concentration of 1, 3 and 10 μg/mL, respectively). Injection volume was 50 μL. Values represent Mean ± SD (n=4).
The vitreal concentration vs. time profile of hesperidin and hesperidin G suggested a two-compartment pharmacokinetic model. Data was modeled according to one-compartment and two-compartment pharmacokinetic models. Akaike Information Criterion (AIC) and Schwarz Bayesian Criterion (SBC) were used for the selection of the most appropriate model, in addition to visual (graphical) estimation (supplemental data). Model selection criteria also indicated a two-compartment pharmacokinetic model for hesperidin and hesperidin G vitreal concentration-time profile, at all the dose levels tested. Thus, i.v. bolus two-compartment pharmacokinetic model was used for calculating the different pharmacokinetic parameters. However, in case of hesperetin, vitreal concentration vs. time profiles at the three doses suggested a one-compartment pharmacokinetic model. Additionally, AIC and SBC criteria also confirmed one-compartment pharmacokinetic model as the best fit. Thus, i.v. bolus one-compartment pharmacokinetic model was used for calculating the pharmacokinetic parameters. Vitreal pharmacokinetic parameters of hesperidin, hesperetin and hesperidin G are presented in Tables 1, 2 and 3, respectively.
Table 1.
Calculated pharmacokinetic parameters of hesperidin at three doses (1.5, 4.5 and 15 µg resulting in vitreal concentration of 1, 3 and 10 µg/mL, respectively) following intravitreal administration. Parameters were calculated following two-compartment pharmacokinetic model. Injection volume was 50 µL. Values represent mean (SD) (n=4).
| Parameter | Units | Intravitreal dose
(µg) |
||
|---|---|---|---|---|
| 1.5 | 4.5 | 15 | ||
| AUC | min.µg/mL | 651 ± 88 | 1575 ± 164 | 4835 ± 861 |
| Cmax | µg/mL | 3.26 ± 1.35 | 10.82 ± 1.12 | 25.23 ± 3.02 |
| CL | mL/min | 0.0027 ± 0.0003 | 0.0029 ± 0.0003 | 0.0032 ± 0.0005 |
| CLD2 | mL/min | 0.0058 ± 0.0010 | 0.0054 ± 0.0026 | 0.0130 ± 0.0026 |
| Vss | mL | 1.48 ± 0.42 | 1.29 ± 0.21 | 2.07 ± 0.35 |
| V2 | mL | 0.75 ± 0.62 | 0.87 ± 0.21 | 1.46 ± 0.40 |
| Alpha | 1/min | 0.0267 ± 0.0077 | 0.0249 ± 0.0109 | 0.0358 ± 0.0084 |
| Beta | 1/min | 0.0016 ±0.0004 | 0.0017 ± 0.0003 | 0.0014 ± 0.0004 |
| Alpha_HL | min | 28 ± 7 | 31 ± 11 | 20 ± 5 |
| Beta_HL | min | 386 ± 116 | 407 ± 58 | 466 ± 98 |
| MRT | min | 545 ± 106 | 448 ± 55 | 668 ± 184 |
Table 2.
Calculated pharmacokinetic parameters of hesperetin at three doses (1.5, 4.5 and 15 µg resulting in vitreal concentration of 1, 3 and 10 µg/mL, respectively) following intravitreal administration. Parameters were calculated following one-compartment pharmacokinetic model. Injection volume was 50 µL. Values represent mean (SD) (n=4).
| Parameter | Units | Intravitreal dose
(µg) |
||
|---|---|---|---|---|
| 1.5 | 4.5 | 15 | ||
| AUC | min.µg/mL | 179 ± 58 | 475 ± 70 | 1746 ± 146 |
| Cmax | µg/mL | 1.02 ± 0.20 | 4.51 ± 0.57 | 14.67 ± 1.99 |
| CL | mL/min | 0.0093 ± 0.0040 | 0.0096 ± 0.0015 | 0.0086 ± 0.00070 |
| VVH | mL | 1.52 ± 0.29 | 1.01 ± 0.13 | 1.04 ± 0.16 |
| T1/2 | min | 110 ± 28 | 89 ± 14 | 83 ± 10 |
Table 3.
Calculated pharmacokinetic parameters of hesperidin G at three doses (1.5, 4.5 and 15 µg resulting in vitreal concentration of 1, 3 and 10 µg/mL, respectively) following intravitreal administration. Parameters were calculated following two-compartment pharmacokinetic model. Injection volume was 50 µL. Values represent mean (SD) (n=4).
| Parameter | Units | Intravitreal dose
(µg) |
||
|---|---|---|---|---|
| 1.5 | 4.5 | 15 | ||
| AUC | min.µg/mL | 688 ± 47 | 1811 ± 269 | 6167 ± 168 |
| Cmax | µg/mL | 3.79 ± 1.06 | 20.16 ± 6.12 | 32.05 ± 4.39 |
| CL | mL/min | 0.0022 ± 0.0002 | 0.0025 ± 0.0004 | 0.0024 ± 0.0002 |
| CLD2 | mL/min | 0.011 ± 0.002 | 0.003 ± 0.001 | 0.007 ± 0.001 |
| Vss | mL | 1.41 ± 0.14 | 1.05 ± 0.013 | 1.25 ± 0.16 |
| V2 | mL | 1.00 ± 0.07 | 0.81 ± 0.05 | 0.78 ± 0.16 |
| Alpha | 1/min | 0.045 ± 0.019 | 0.028 ± 0.011 | 0.027 ± 0.007 |
| Beta | 1/min | 0.0014 ± 0.0002 | 0.0015 ± 0.0002 | 0.0017 ± 0.0003 |
| Alpha_HL | min | 17 ± 6 | 29 ± 16 | 27 ± 8 |
| Beta_HL | min | 495 ± 66 | 468 ± 82 | 414 ± 70 |
| MRT | min | 648 ± 90 | 418 ± 21 | 517 ± 80 |
Following intravitreal administration of a compound, it will be distributed within the vitreous humor and into the surrounding ocular tissues (retina, choroid, lens and aqueous humor), from where elimination may take place 14. Distribution of an intravitreally administered drug is governed by 1) the gel structure of the vitreous humor, 2) the time taken by the compound to set up equilibrium with the peripheral ocular tissues. The vitreous humor is composed of water (~ 98–99%) and solids (~ 1%, mainly collagen and glycosaminoglycans) 31. This solid content is responsible for maintaining the gel structure and acts as a molecular barrier to the diffusion of administered compounds with factors such as molecular weight and interactions between the drug and collagen through H-bonds for example, playing significant roles. Establishing of equilibrium between the peripheral ocular tissues and vitreous humor largely depends on the partition coefficient of the respective compounds 12. In our study hesperetin is observed to follow a one-compartment pharmacokinetic model (Fig. 3), indicating rapid distribution within the vitreous gel. Additionally, volume of the vitreous humor in the rabbits is around 1.5 mL and correspondingly, hesperetin volume of distribution (VVH) is in the range of 1 – 1.5 mL (Table 3). The other two larger molecules with higher molecular weight, hesperidin and glucosyl hesperidin, exhibited a two-compartment pharmacokinetic model (Fig. 2 and 4), probably because of slower distribution within the vitreous humor and into peripheral ocular tissues (RCS, lens and aqueous humor). The Vss for these compounds is close to 1–2 mL and peripheral volume of distribution (V2) ranged between 075–1.5 mL. It was also reported that, distribution of large molecules depends on the convection, generated by pressure and temperature difference between anterior chamber and the retinal surface 12.
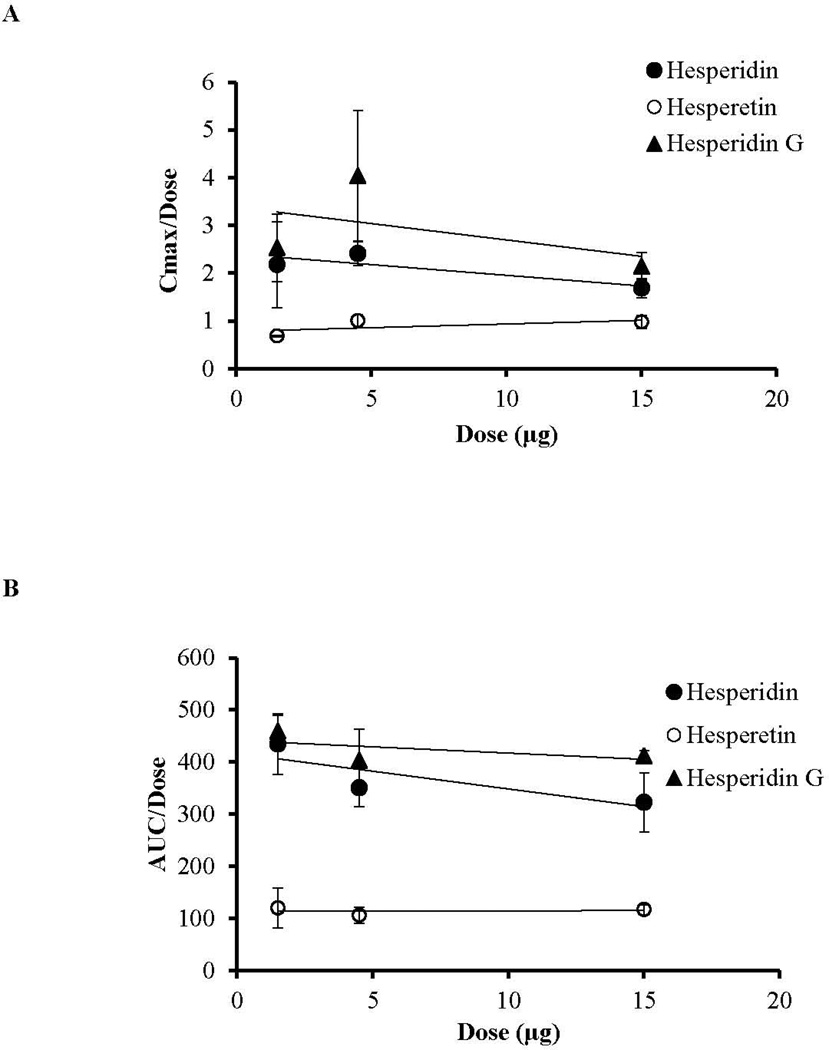
It has been established that hepatic/intestinal beta-glucosidase and/or alpha-rhamnosidase converts hesperidin into hesperetin and hesperetin then undergoes phase-II metabolism to yield hesperetin-glucoronides and hesperetin-sulphates. Additionally hesperetin can be converted into eriodictyol 32–34. In the case of hesperidin G, apha-glucosidase converts hesperidin G into hesperidin. All these enzymes are reported to be present in the various ocular tissues, however, the expression levels are low compared to that present in the liver 35–37. In our study levels of metabolites, if any, following intravitreal administration of these compounds were not detected. This could be because of small sample volumes and/or low metabolite concentrations generated. In a majority of the cases, elimination of a drug molecule from the body has been assumed to follow first order kinetics. Nevertheless, it is also possible that elimination may display non linear pharmacokinetics. In such cases ‘concentration’ or 'dose' dependent kinetics are observed 38. In this study, all three compounds exhibited linear pharmacokinetics, within the dose range tested as indicated by a linear increase in the AUC and Cmax with administered dose (Fig. 5A and 5B) without a significant difference in the elimination parameters like clearance and elimination half-life across the doses (Tables 1, 2 and 3).
Figure 5.
Linear increase in vitreal pharmacokinetic parameters Cmax (A) and AUC (B) following intravitreal administration of hesperidin, hesperetin, and hesperidin G at three doses. Values represent mean ± SD
Elimination of intravitreally administered drug can take place by two major routes 12–14. Direct pathway; the vitreous humor is enclosed by the retina, therefore the most direct pathway is through this tissue. The other route of elimination would be passage of the drug forward via the anterior elimination route through the aqueous humor 12. Taken together, the results of this study indicate that, among the three molecules, hesperetin is eliminated more rapidly, lowest elimination half-life (T1/2: 83.41 – 110.66 min) and highest clearance rate (CL: 0.0086 – 0.0096 mL/min), from the vitreous humor, followed by hesperidin (T1/2: 385.68 – 466.15 min; CL: 0.0027 – 0.0032 mL/min) and hesperidin G (T1/2: 413.83 – 495.07 min; CL: 0.0024 – 0.0025 mL/min) (Tables 1, 2 and 3). This is probably because of higher lipophilicity (Log P: 2.9) and lower molecular weight (MW: 302.27) of hesperetin compared to hesperidin (Log P: 1.79; MW: 610.57) and hesperidin G (Log P: −3.2; MW: 772.70). Therefore, hesperetin can easily permeate across the retinal barrier. Furthermore, our previous in vitro permeation studies across isolated rabbit retina-choroid-sclera (RCS) indicated that the apparent permeability of hesperetin in the retina to scleral (R-S) direction (2.52 ± 0.51 × 10−6 cm/s) is higher compared to hesperidin (1.51 ± 0.78 × 10−6 cm/s) 4. The terminal elimination of a molecule from the vitreous humor is significantly dependent on the molecular weight 12. However, the difference between hesperidin and glucosyl hesperidin with respect to elimination half-life and clearance is not significant, indicating the role of H-bonding, polar surface area and other molecular characteristics on transretinal elimination.
The other important information that can be drawn from this study, from a drug delivery perspective, is that these compounds are exhibiting short half-life in the vitreous humor. Thus, in order to maintain the therapeutic levels for longer duration frequent administration would be necessary. Thus, development of a sustained or controlled ocular drug delivery system is warranted.
CONCLUSION
Overall, in this study vitreal pharmacokinetics of hesperidin, hesperetin and hesperidin G were evaluated. All three molecules exhibit a linear vitreal pharmacokinetic profile within the dose range tested. Additionally, it was observed that vitreal kinetics of these molecules is dependent on their lipophilicity and molecular weight. The results provide practical information that will be useful in the future design of ocular drug delivery platforms for these molecules.
Supplementary Material
ACKNOWLEDGMENT
This project was supported in part by Grant Numbers, P20RR021929 from the National Center for Research Resources (NIH/NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Eye Institute, National Institutes of Health.
REFERENCES
- 1.Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26(9):2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 2.Majumdar S, Srirangam R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: a natural bioflavonoid. Pharm Res. 2009;26(5):1217–1225. doi: 10.1007/s11095-008-9729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majumdar S, Srirangam R. Potential of the bioflavonoids in the prevention/treatment of ocular disorders. J Pharm Pharmacol. 2010;62(8):951–965. doi: 10.1211/jpp.62.08.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srirangam R, Majumdar S. Passive asymmetric transport of hesperetin across isolated rabbit cornea. Int J Pharm. 2010;394(1–2):60–67. doi: 10.1016/j.ijpharm.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hijiya H, Miyake T. Patent US. United States: Kabushiki Kaisha Hayashibara, Okayama, Japan; 1997. alpha-glycosyl hesperidin, and its preparation and uses. editor, ed., [Google Scholar]
- 6.Yamamoto M, Suzuki A, Hase T. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo) 2008;54(1):95–98. doi: 10.3177/jnsv.54.95. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Suzuki A, Jokura H, Yamamoto N, Hase T. Glucosyl hesperidin prevents endothelial dysfunction and oxidative stress in spontaneously hypertensive rats. Nutrition. 2008;24(5):470–476. doi: 10.1016/j.nut.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsuki K, Abe A, Mitsuzumi H, Kondo M, Uemura K, Iwasaki Y, Kondo Y. Glucosyl hesperidin improves serum cholesterol composition and inhibits hypertrophy in vasculature. J Nutr Sci Vitaminol (Tokyo) 2003;49(6):447–450. doi: 10.3177/jnsv.49.447. [DOI] [PubMed] [Google Scholar]
- 9.Yamada M, Tanabe F, Arai N, Mitsuzumi H, Miwa Y, Kubota M, Chaen H, Kibata M. Bioavailability of glucosyl hesperidin in rats. Biosci Biotechnol Biochem. 2006;70(6):1386–1394. doi: 10.1271/bbb.50657. [DOI] [PubMed] [Google Scholar]
- 10.Ameer B, Weintraub RA, Johnson JV, Yost RA, Rouseff RL. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin Pharmacol Ther. 1996;60(1):34–40. doi: 10.1016/S0009-9236(96)90164-2. [DOI] [PubMed] [Google Scholar]
- 11.Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. 2009;29(7):875–912. doi: 10.1097/IAE.0b013e3181a94f01. [DOI] [PubMed] [Google Scholar]
- 12.Laude A, Tan LE, Wilson CG, Lascaratos G, Elashry M, Aslam T, Patton N, Dhillon B. Intravitreal therapy for neovascular age-related macular degeneration and inter-individual variations in vitreous pharmacokinetics. Prog Retin Eye Res. 2010;29(6):466–475. doi: 10.1016/j.preteyeres.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Maurice D. Review: practical issues in intravitreal drug delivery. J Ocul Pharmacol Ther. 2001;17(4):393–401. doi: 10.1089/108076801753162807. [DOI] [PubMed] [Google Scholar]
- 14.Tojo KJ, Ohtori A. Pharmacokinetic model of intravitreal drug injection. Math Biosci. 1994;123(1):59–75. doi: 10.1016/0025-5564(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 15.Duvvuri S, Rittenhouse KD, Mitra AK. Microdialysis assessment of drug delivery systems for vitreoretinal targets. Adv Drug Deliv Rev. 2005;57(14):2080–2091. doi: 10.1016/j.addr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Macha S, Mitra AK. Ocular pharmacokinetics in rabbits using a novel dual probe microdialysis technique. Exp Eye Res. 2001;72(3):289–299. doi: 10.1006/exer.2000.0953. [DOI] [PubMed] [Google Scholar]
- 17.Majumdar S, Hippalgaonkar K, Srirangam R. Vitreal kinetics of quinidine in rabbits in the presence of topically coadministered P-glycoprotein substrates/modulators. Drug Metab Dispos. 2009;37(8):1718–1725. doi: 10.1124/dmd.108.026450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hippalgaonkar K, Srirangam R, Avula B, Khan IA, Majumdar S. Interaction between topically and systemically coadministered P-glycoprotein substrates/inhibitors: effect on vitreal kinetics. Drug Metab Dispos. 2010;38(10):1790–1797. doi: 10.1124/dmd.110.032672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi EM, Lee YS. Effects of hesperetin on the production of inflammatory mediators in IL-1beta treated human synovial cells. Cell Immunol. 2010;264(1):1–3. doi: 10.1016/j.cellimm.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38(3):133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 21.Kalpana KB, Devipriya N, Srinivasan M, Menon VP. Investigation of the radioprotective efficacy of hesperidin against gamma-radiation induced cellular damage in cultured human peripheral blood lymphocytes. Mutat Res. 2009;676(1–2):54–61. doi: 10.1016/j.mrgentox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Kalpana KB, Srinivasan M, Menon VP. Evaluation of antioxidant activity of hesperidin and its protective effect on H2O2 induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol Cell Biochem. 2009;323(1–2):21–29. doi: 10.1007/s11010-008-9960-9. [DOI] [PubMed] [Google Scholar]
- 23.Choi EJ, Kim GD, Chee KM, Kim GH. Effects of hesperetin on vessel structure formation in mouse embryonic stem (mES) cells. Nutrition. 2006;22(9):947–951. doi: 10.1016/j.nut.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Chen MC, Ye YY, Ji G, Liu JW. Hesperidin Upregulates Heme Oxygenase-1 To Attenuate Hydrogen Peroxide-Induced Cell Damage in Hepatic L02 Cells. J Agric Food Chem. 2010;58(6):3330–3335. doi: 10.1021/jf904549s. [DOI] [PubMed] [Google Scholar]
- 25.Choi IY, Kim SJ, Jeong HJ, Park SH, Song YS, Lee JH, Kang TH, Park JH, Hwang GS, Lee EJ, Hong SH, Kim HM, Um JY. Hesperidin inhibits expression of hypoxia inducible factor-1 alpha and inflammatory cytokine production from mast cells. Mol Cell Biochem. 2007;305(1–2):153–161. doi: 10.1007/s11010-007-9539-x. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama S, Katsumata S, Suzuki K, Ishimi Y, Wu J, Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J Clin Biochem Nutr. 2010;46(1):87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoclet JC, Chataigneau T, Ndiaye M, Oak MH, El Bedoui J, Chataigneau M, Schini-Kerth VB. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004;500(1–3):299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Balakrishnan A, Menon VP. Antioxidant properties of hesperidin in nicotine-induced lung toxicity. Fundam Clin Pharmacol. 2007;21(5):535–546. doi: 10.1111/j.1472-8206.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 29.Gandhi C, Upaganalawar A, Balaraman R. Protection against in vivo focal myocardial ischemia/reperfusion injury-induced arrhythmias and apoptosis by hesperidin. Free Radic Res. 2009;43(9):817–827. doi: 10.1080/10715760903071656. [DOI] [PubMed] [Google Scholar]
- 30.Choi EJ, Ahn WS. Neuroprotective effects of chronic hesperetin administration in mice. Arch Pharm Res. 2008;31(11):1457–1462. doi: 10.1007/s12272-001-2130-1. [DOI] [PubMed] [Google Scholar]
- 31.Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19(3):323–344. doi: 10.1016/s1350-9462(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 32.Garg A, Garg S, Zaneveld LJ, Singla AK. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother Res. 2001;15(8):655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 33.Brand W, Boersma MG, Bik H, Hoek-van den Hil EF, Vervoort J, Barron D, Meinl W, Glatt H, Williamson G, van Bladeren PJ, Rietjens IM. Phase II metabolism of hesperetin by individual UDP-glucuronosyltransferases and sulfotransferases and rat and human tissue samples. Drug Metab Dispos. 2010;38(4):617–625. doi: 10.1124/dmd.109.031047. [DOI] [PubMed] [Google Scholar]
- 34.Breinholt VM, Offord EA, Brouwer C, Nielsen SE, Brosen K, Friedberg T. In vitro investigation of cytochrome P450-mediated metabolism of dietary flavonoids. Food Chem Toxicol. 2002;40(5):609–616. doi: 10.1016/s0278-6915(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 35.Lentrichia B, Bruner W, Kean E. Glycosidases of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17(9):884. [PubMed] [Google Scholar]
- 36.Hawkins LA, Garg HS, Awasthi YC, Srivastava SK. Distribution of lysosomal hydrolases in human and bovine ocular tissues. Curr Eye Res. 1981;1(9):497–500. doi: 10.3109/02713688109069174. [DOI] [PubMed] [Google Scholar]
- 37.Duvvuri S, Majumdar S, Mitra AK. Role of metabolism in ocular drug delivery. Curr Drug Metab. 2004;5(6):507–515. doi: 10.2174/1389200043335342. [DOI] [PubMed] [Google Scholar]
- 38.Gibaldi M, Perrier D. Nonlinear pharmacokinetics. In: Gibaldi M, Perrier D, editors. Pharmacokinetics. 2 ed. New York: M. Dekker Inc.; 1975. pp. 271–315. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.