Abstract
Background
While methamphetamine (MA) and alcohol are often used in combination, little is known about the pattern of co-use between these substances. The goal of the present study is to examine the relationship between MA use and alcohol use in a community sample of non-treatment seeking regular MA users.
Methods
Participants completed a face-to-face assessment battery, which included a diagnostic interview for MA dependence and the Timeline Follow-Back interview for both alcohol and MA use over the past 30 days. Sixty regular MA and alcohol users supplied data for 1800 person-days.
Results
Compared with non-drinking days, drinking days and binge drinking days increased the odds of same day MA use by 4.22 and 4.50 times, respectively (p’s < 0.0001). Further, binge drinking incrementally increased risk for MA use above and beyond the effects of drinking itself (p < 0.0001). Lagged models revealed previous day MA use to predict following day MA use (p < 0.0001), yet, after controlling for this relationship, neither previous day alcohol use nor previous day binge drinking predicted following-day MA use. Finally, the effect of binge drinking on MA use was stronger among individuals with lower MA dependence severity or higher alcohol problem severity (p’s < 0.05).
Conclusions
These results suggest that alcohol and MA are co-used in predictable patterns, and in particular, that binge drinking may be incrementally associated with the likelihood of MA use. Future studies are needed to explore the temporal relationship between alcohol and MA use within a given episode.
Keywords: Methamphetamine, Alcohol, Co-Use
1. INTRODUCTION
Methamphetamine (MA) use remains a significant public health concern in the United States and worldwide. Estimates from 2012 suggest over 12 million people in the United States have used MA in their lifetimes and 1.2 million people (0.4%) reported using MA in the past year (Substance Abuse and Mental Health Services Administration, 2013). Amphetamine-type simulants, of which MA is the most frequently used, were found to be the second most commonly used class of illicit drugs worldwide (UNODC, 2012). Furthermore, chronic MA use is associated with increased risk for a number of serious health problems, including psychiatric disorders (e.g., depression, psychosis, etc.), abnormal brain morphology and function, and cardiovascular disease (Darke et al., 2008; Panenka et al., 2012). Given that chronic, heavy alcohol use is also associated with a host of negative health consequences (Harwood, 2000), the consistent co-use of alcohol and MA may exacerbate the health risks of each substance alone. Therefore, understanding patterns of alcohol and MA co-use may have important implications for intervention and public health.
While a paucity of studies to date have examined the co-use of MA and alcohol, the few that have done so indicated a positive association between these substances in cross-sectional epidemiological designs and laboratory-based experimental studies. For example, individuals reporting frequent alcohol intoxication are five times more likely to report using MA as compared to non-drinkers (Furr et al., 2000) and MA use is more frequent among individuals with higher severity of alcoholism (Caetano and Weisner, 1995). Among college students, heavier alcohol consumption is associated with the co-use of alcohol and a psychostimulant (i.e., cocaine or methylphenidate; Barrett et al., 2006). Additionally, frequent alcohol use (≥ 16 days in the past month) is associated with greater odds of psychotic symptoms among chronic MA users (McKetin et al., 2013). And while the biological mechanisms mediating the acute response to alcohol and MA co-use remain unclear, the concurrent administration of MA and alcohol produces greater changes in cardiovascular function and subjective ratings of drug effects compared to either drug taken alone (Mendelson et al., 1995; Kirkpatrick et al., 2012).
In summary, the available epidemiologic data suggest a significant association between MA use and alcohol co-use, primarily that heavy and frequent drinkers are more likely to use MA. Additionally, the experimental findings, while limited, indicate that the co-administration of MA and alcohol may produce synergistic cardiovascular and subjective effects, thereby simultaneously and problematically potentiating the reinforcing and hazardous effects of each drug. However, most studies to date examining the prevalence of MA and alcohol co-use have been cross-sectional. Thus, the pattern and predictive relationship of MA and alcohol co-use within a given day or across multiple days remains unknown. Finally, as the majority of clinical trials for MA dependence have excluded alcohol dependent participants or did not report data on alcohol use (e.g., Anderson et al., 2012; Galloway et al., 2011; Rawson et al., 2004) and most studies reporting the co-use rates of these substances have been in community samples, there is limited information available on the relationship between alcohol and MA use among regular MA users. Therefore, the goal of this study is to examine the relationship between MA use and alcohol use in non-treatment seeking regular MA users who report regular alcohol consumption. Designs which analyze data at the level of individual days for each participant can determine whether days in which co-use of MA and alcohol occur more frequently than would be expected by chance and also reduce confounds that typically affect cross-sectional epidemiological studies, such as sociodemographic and environmental factors. Logistic multilevel models were conducted to test whether (a) alcohol use and MA use were correlated on a given day, and (b) previous day alcohol use or binge drinking was predictive of following day MA use. A secondary aim of this study was to account for severity of MA and alcohol use problems as plausible moderators of the relationship between alcohol and MA co-use.
2. METHODS
2.1 Participants and Procedures
Non-treatment seeking MA users (N=126; 33 females) were recruited from the Los Angeles community via print and online advertisements as part of a larger study investigating the effects of naltrexone and response to MA in the laboratory. The study protocol and all related procedures were approved by the University of California, Los Angeles Institutional Review Board. Initial study eligibility was determined via telephone interview. Inclusion criteria included: (1) Self-reported use of MA; and (2) age between 18 and 50. Exclusion criteria included: (1) currently in treatment for MA use problems or currently seeking treatment; (2) self-reported history of bipolar disorder or any other major psychiatric disorder; (3) self-reported used of drugs other than MA, alcohol, or marijuana; or (4) serious medical conditions or self-reported use of contraindicated medications for the parent study (e.g., opioids).
After the initial telephone screening interview, participants were invited to the laboratory for an in-person screening session from which data were culled. After providing written informed consent, participants provided a urine sample for a toxicology screen. Participants with a positive toxicology screen for drugs other than MA or marijuana were excluded from the screening session (N=6), and a small number of participants self-withdrew from the screening session (N=3). In addition, 57 participants reported drinking less than monthly in the last year and were thus excluded from analyses resulting in a final sample of N=60 (13 females).
2.2 Individual Difference Measures
The following individual questionnaires and interviews were administered during the study: (1) The 30-day Timeline Follow-Back (TLFB) was administered in interview format to capture daily MA and alcohol use over the 30 days prior to the visit by trained masters level clinical psychology graduate students (Sobell et al., 1988); (2) the Structured Clinical Interview for DSM-IV (SCID) was also administered by a masters’ level clinician to assess criteria for MA dependence and abuse (First, 2005); and (3) the self-report Alcohol Use Disorders Identification Test (AUDIT) was administered in order to assess for drinking severity with a cutoff score of 8 being used to identify hazardous alcohol use (Allen et al., 1997). Participants also completed a smoking history questionnaire and alcohol consumption questionnaire (Glovannucci et al., 1991) measuring cigarette smoking and general alcohol use, respectively. Lastly, participants completed a demographics questionnaire reporting, among other variables, age, sex, and level of education, in addition to the Beck Depression Inventory-II (BDI-II; Beck et al., 1996) and the Beck Anxiety Inventory (BAI; Beck and Steer, 1990) to assess for symptoms of depression and anxiety, respectively.
2.3 Data Analytic Strategy
To improve the clinical applicability of our results, alcohol use variables included a binary daily drinking variable (Drink: coded 0 for non-drinking day, 1 for drinking day), and a binary daily binge drinking variable (Binge: coded 0 for four [three for women] or fewer drinks, and 1 for five [four for women] or more drinks on a given day). Given the lack of reliability regarding a quantitative index of retrospective MA use quantity, MA use was coded as a binary variable indicating whether or not MA was used at all on a given day (MA Use: coded 1 or 0 respectively). Binary MA use on a given day was the primary outcome in all analyses presented. Owing to a binary outcome variable and daily data nested within individuals, a series of logistic multilevel models were implemented using PROC GLIMMIX in SAS version 9.4 for Windows with Satterthwaite approximated degrees of freedom. Odds ratios with 95% confidence intervals were computed as indicators of effect size. Initial models examined main effects of drinking and binge drinking on likelihood of same day MA use on concurrent days. Clinically relevant person-level covariates included MA abuse/dependence symptom count derived from the SCID (Count), severity of alcohol use problems indicated by square root-transformed (to improve normality) AUDIT score (AUDIT). The effect of all covariates was examined after a statistically significant main effect was observed in order to determine whether the risk associated with alcohol use would survive statistical control while providing accurate assessments of overall main effects. A series of lagged models were conducted to examine whether alcohol use on a prior day was associated with a greater likelihood of MA use on the following day after controlling for previous day MA use, or vice versa (e.g. MA use on previous day predicting alcohol use subsequently, controlling for previous day alcohol use). Lastly, MA SCID Count and AUDIT score were investigated as potential moderators of concurrent and lagged MA and alcohol use associations.
3. RESULTS
3.1 Sample Characteristics
Demographic and other descriptive data for the final study sample (N = 60) are presented in Table 1. On average, participants endorsed 5.68 (SD = 2.55, Range: 0 – 11) DSM-IV symptoms of MA abuse and dependence and used MA on 17.00 (SD = 8.87) of the previous 30 days. Data on preferred route of MA administration was available for 52 subjects, the vast majority of whom identified smoking as their preferred route (71%) with 19% preferring snorting, and the remaining 10% preferring intravenous injection. Mean number of drinking days over the past 30 days was 11.67 (SD = 9.95), with an average of 4.88 (SD = 3.33) standard drinks per drinking day. On average, participants binge drank on 4.87 (SD = 6.72) of the past 30 days and had an AUDIT score of 10.52 (SD = 8.50, Range: 1 – 35, square-root transformed and centered range: −2.00 – 2.91). 53% of subjects reported an AUDIT score ≥ 8, which is indicative of a hazardous pattern of alcohol use (Allen et al., 1997). Only 1 subject reported frequent (i.e. weekly) use of 3 (or more) substances, specifically Marijuana, Methamphetamine, and MDMA. Thus this sample represents a population of MA users for whom MA is their drug of choice. Furthermore, considerable variability was found with respect to smoking status (Table 1), thus additional models were conducted to assess the impact of smoking status on these results. Overall no significant differences were observed between smoking groups in terms of number of drinking days (F(2,57) = 2.39, p = 0.10), percentage binge drinking days (F(2,57) = 0.33, p = 0.72), or number of MA using days (F(2,57) = 0.40, p = 0.67). All results reported below were remained significant after controlling for smoking status.
Table 1.
Sample characteristics (N = 60)
| Mean (SD) |
|
|---|---|
| Age | 35.38 (8.89) |
| BDI-II | 11.82 (9.23) |
| BAI | 8.95 (9.00) |
| Age of First MA Use | 23.27 (8.23) |
| Years of Education | 12.52 (3.55) |
| Current AUD | 28.33% |
| Lifetime AUD | 36.67% |
| Smoking Status | |
| Non-Smoker | 25% |
| 1–9 Cigarettes Per Day | 46.67% |
| ≥10 Cigarettes Per Day | 28.33% |
| ≥Monthly Marijuana Use | 56.67% |
| Meth SCID Symptom Count | 5.68 (2.55) |
| AUDIT Score | |
| Raw | 10.52 (8.50) |
| Sqrt Transformed | 3.00 (1.24) |
3.2 Concurrent Alcohol and MA Use
Analyses revealed that both drinking any alcohol (B = 1.44, SE = 0.16, p < 0.0001, OR = 4.22, 95% CI: 3.07 – 5.79) and binge drinking (B = 1.50, SE = 0.20, p < 0.0001, OR = 4.50, 95% CI: 3.02 – 6.70) were associated with a significant increase in the likelihood of concurrent MA use. Furthermore, a multivariate model revealed that binge drinking was associated with an incremental increase in MA use likelihood over and above the effect of drinking in general (Drink: B = 1.10, SE = 0.19, p < 0.0001, OR = 3.00, 95% CI: 2.07 – 4.35; Binge: B = 0.79, SE = 0.24, p = 0.001, OR = 2.20, 95% CI: 1.37 – 3.51, Figure 1). Controlling for Count and AUDIT score did not impact the results presented in terms of either significance or magnitude. Age, sex, BDI-II, BAI, years of education, and age of first MA use were not significantly associated with the likelihood of MA use (p’s ≥ 0.15), and statistically controlling for these factors did not alter the impact of alcohol use factors on the likelihood of MA use.
Figure 1.
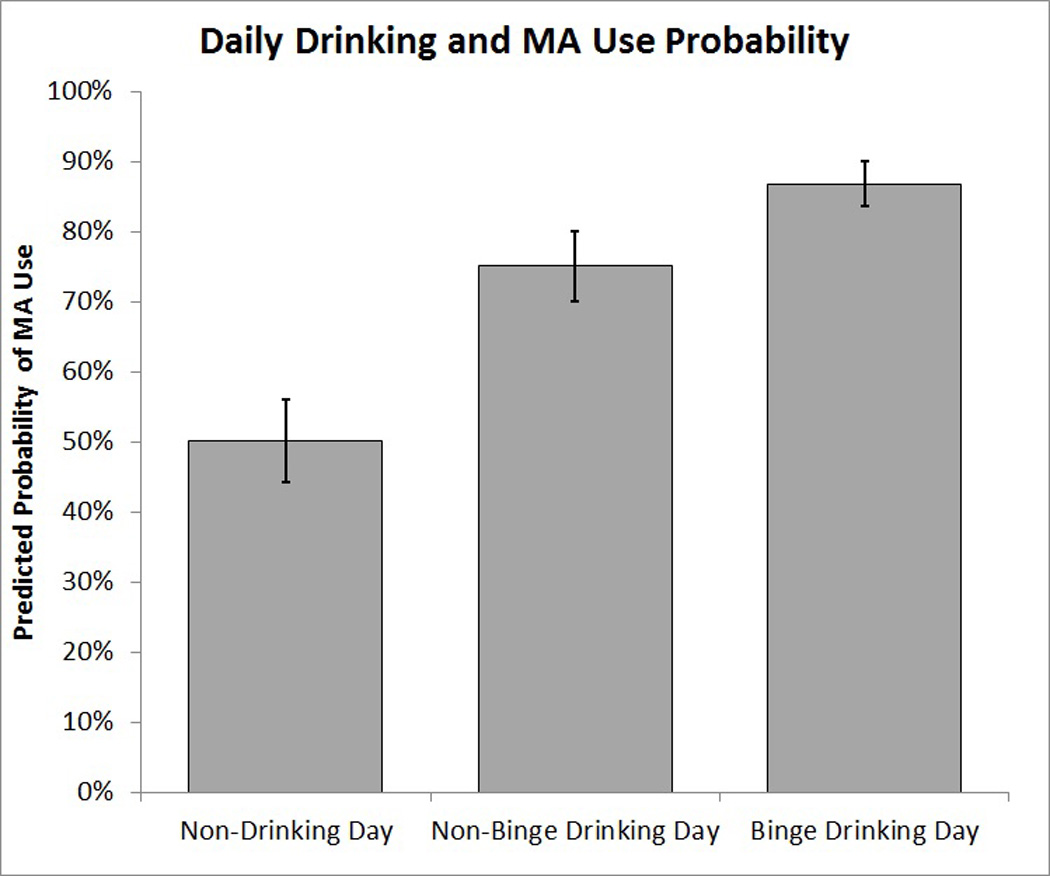
Predicted probability of MA use on non-drinking days, non-binge drinking days and binge drinking days derived from a multivariate model logistic model with standard error bars.
In the full sample of regular MA users (N = 117), which included participants who reported zero drinking days, the results were marginally smaller in magnitude, yet the pattern of statistical significance was identical to the final sample of regular drinkers (Univariate Drink: B = 1.24, SE = 0.15, p < 0.0001, OR = 3.46, 95% CI: 2.55 – 4.68; Univariate Binge: B = 1.33, SE = 0.20, p < 0.0001, OR = 3.78, 95% CI: 2.56 – 5.57; Multivariate model: Drink: B = 0.94, SE = 0.18, p < 0.0001, OR = 2.57, 95% CI: 1.80 – 3.67; Binge: B = 0.70, SE = 0.23, p < 0.01, OR = 2.01, 95% CI: 1.27 – 3.18).
3.3 Lagged Effects of Alcohol and MA Use
To test whether previous day alcohol use or binge drinking was predictive of following day MA use, a series of lagged models was conducted. In univariate models, previous day drinking (B = 0.62, SE = 0.15, p < 0.0001, OR = 1.87, 95% CI: 1.38 – 2.52) and binge drinking (B = 0.65, SE = 0.19, p < 0.001, OR = 1.92, 95% CI: 1.33 – 2.78) were each significant predictors of following day MA use. However, neither effect survived controlling for previous day MA use (p’s ≥ 0.06). Previous day MA use was significantly predictive of following day MA use (B = 1.22, SE = 0.13, p < 0.0001, OR = 3.39, 95% CI: 2.65 – 4.34), and this result was unaffected by controlling for alcohol use variables. Similarly, previous day MA use was predictive of following day alcohol use (B = 0.50, SE = 0.15, p < 0.001, OR = 1.66, 95% CI: 1.23 – 2.23) and following day binge drinking (B = 0.43, SE = 0.18, p < 0.05, OR = 1.54, 95% CI: 1.07 – 2.20); however, neither of these effects survived controlling for previous day alcohol use (p’s ≥ 0.23).
3.4 Moderation by MA Severity and Alcohol Problem Severity
As an exploratory aim, a series of models were conducted to examine whether Count and AUDIT moderated effects reported thus far. Results revealed a significant interaction between Count and binge drinking on likelihood of MA use (Count × Binge: B = −0.28, SE = 0.094, p < 0.01, Figure 2). This interaction was such that, on binge drinking days, MA symptom count was negatively associated with likelihood of MA use (B = −0.27, SE = 0.12, p < 0.05, OR = 0.76, 95% CI: 0.60 – 0.97), yet no simple effect of Count was observed on non-binge drinking days (B = 0.01, SE = 0.10, p = 0.94, OR = 1.01, 95% CI: 0.83 – 1.22). In the same model, a significant interaction between AUDIT and binge drinking was observed (AUDIT × Binge: B = 0.42, SE = 0.18, p < 0.05, Figure 3). This interaction indicated that on binge drinking days there was a significant positive simple effect of AUDIT on MA use likelihood (B = 0.51, SE = 0.25, p < 0.05, OR = 1.66, 95% CI: 1.01 – 2.75), yet no simple effect of AUDIT was found on non-binge days (B = 0.08, SE = 0.20, p = 0.67, OR = 1.09, 95% CI: 0.73 – 1.62). To confirm the presented results were not due to sampling bias, all of the previously described analyses were repeated in the full sample of 117 (i.e., without excluding participants based on alcohol use) and the substantive findings did not differ in terms of statistical significance. Of note, the interaction between MA count and Binge remained significant when AUDIT was removed from the model (B=−0.21, SE=0.09, p < 0.05); However the AUDIT × Binge interaction did not survive removal of MA symptom count (p = 0.18).
Figure 2.
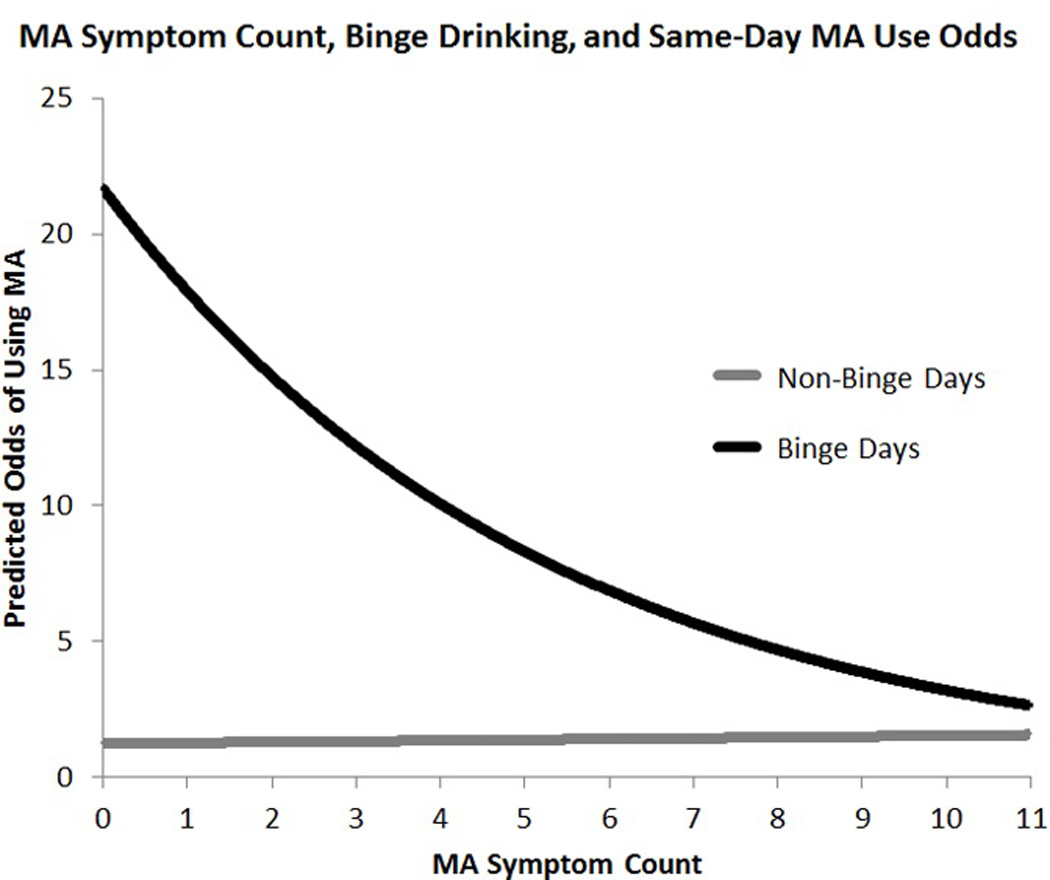
Predicted odds of MA use as predicted by MA symptom count and binge drinking. MA symptom count was found to moderate the effect of binge drinking on the likelihood of MA use.
Figure 3.
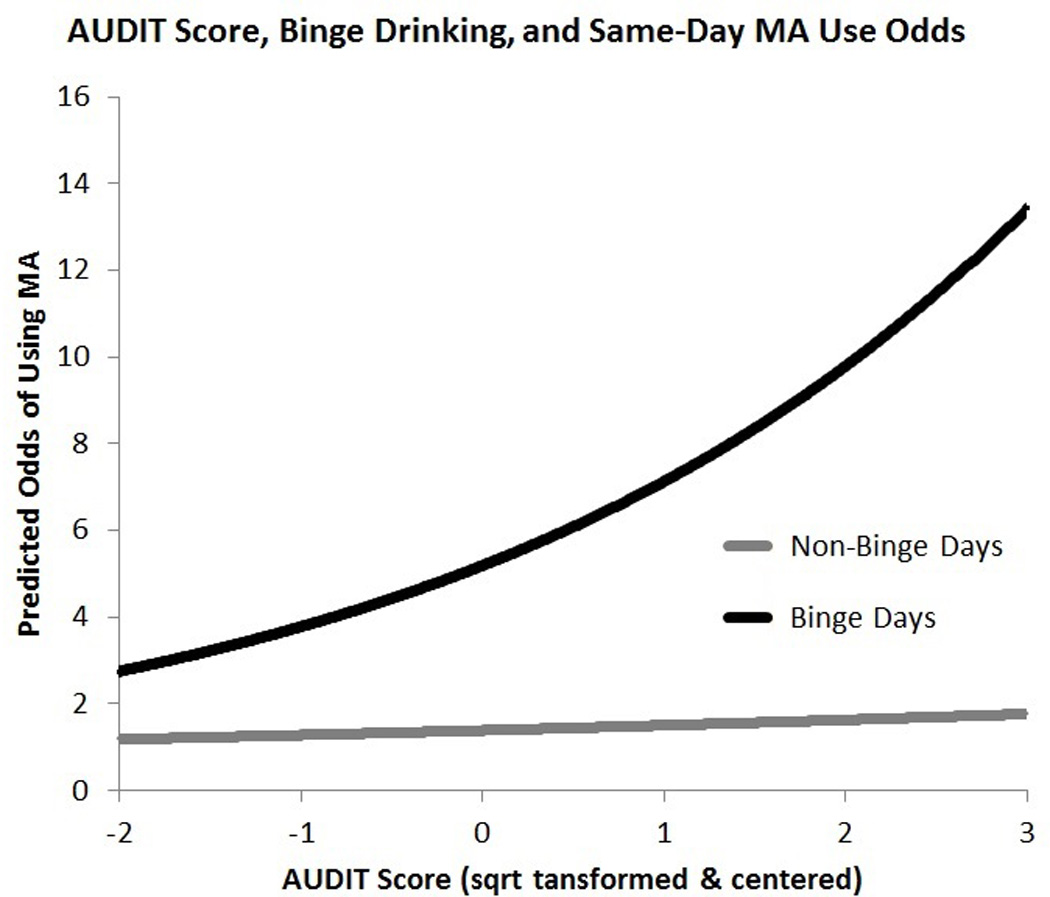
Predicted odds of MA use as predicted by AUDIT score and binge drinking. Alcohol use/problem severity was found to moderate the effect of binge drinking on likelihood of MA use.
4. DISCUSSION
To address the gap in knowledge on patterns of MA and alcohol co-use, the present study examined the relationship between MA use and alcohol use in a community sample of non-treatment seeking regular MA users. Using the Timeline Follow-Back as an interview-based retrospective indicator of alcohol and MA use over the past 30 days, this study found that among regular MA users, odds of MA use were 3.0 times greater on days when alcohol was consumed as compared to non-drinking days, and binge drinking was associated with a further multiplicative increase of 2.2 times (i.e., odds of MA use on binge drinking days was 6.6 times greater than on non-drinking days). These results suggest that alcohol use, and binge drinking in particular, is likely to co-occur with MA use and this co-use may be clinically relevant for the treatment of MA use disorders. To better understand this relationship, and in particular the causal pathway underlying associations of co-use, future studies should examine the temporal relationship between alcohol use and MA use within a given drug use episode.
While alcohol use was associated with a significant and large increase in likelihood of MA use on the same day, in lagged analyses there was no association between previous day alcohol use on MA use likelihood the following day after controlling for previous day MA use, or vice versa. Likely reflecting the multiple day bingeing pattern commonly associated with MA use (Cho et al., 2001), previous day MA use was significantly predictive of following day MA use, and this result was unaffected by controlling for alcohol use variables. Specifically, individuals’ odds of MA use were 3.37 times greater on a given day if they endorsed using MA the previous day. While preliminary, these results suggest that previous day alcohol use may not be a risk factor for MA use over and above the effect of previous day MA use. These results may also indicate that any causal effect of alcohol use on motivation to use MA (or vice versa) are short lived and are perhaps most robust immediately following the initiation of substance use, while the subjective effects of the initial substance are still being experienced. This explanation may be related to the ability of concurrent MA and alcohol use to both magnify the pleasurable subjective effects of each substance and reduce the unwanted behavioral effects of one substance (e.g., MA to reduce alcohol-related sedation; alcohol to reduce MA-related insomnia; Kirkpatrick et al., 2012). Future studies should elucidate the within-episode relationship between alcohol and MA use, which may ultimately be more informative than lagged analyses of sequential day-to-day effects.
As an exploratory aim, this study examined whether severity of alcohol and MA use/problems moderated the effect of alcohol use. This may be an important question in order to identify those for whom the association between MA and alcohol use may be strongest, and thus most clinically meaningful. Analyses revealed that the likelihood of MA use increased more steeply on binge drinking days, versus non binge drinking days, among individuals reporting lower levels of MA dependence. A related finding suggested that the likelihood of MA use on binge drinking days, versus non binge drinking days, was stronger among individuals with higher AUDIT scores. In other words, the concurrent association between binge drinking and MA use was stronger among individuals with lower MA dependence severity and those with higher alcohol problem severity. It may be that for individuals at higher levels of MA dependence severity their use of MA is more habitual and therefore less likely to be influenced by external factors such as alcohol use or other environmental conditions (Everitt and Robbins, 2005). Likewise, individuals at higher levels of alcohol problems (i.e., higher AUDIT scores) may be more sensitive to the effects of alcohol in precipitating MA use or vice versa. Of note however our results suggested that MA count is a robust moderator of the effect of binge drinking on MA use likelihood (i.e., remained significant after AUDIT was removed from the model), whereas the moderating effect of AUDIT score was dependent on controlling for severity of MA dependence.
Outside of cross-sectional epidemiological reports, relatively little research has been conducted on the predictive patterns of use between alcohol and illicit drugs, especially in a treatment setting. For example, both pharmacological and psychosocial clinical trials for MA dependence have often excluded participants with alcohol dependence or not examined alcohol use during the trial (Anderson et al., 2012; Elkashef et al., 2007; Galloway et al., 2011; Rawson et al., 2004), although there are exceptions. (e.g., Roll et al., 2006). Because of this, the role of alcohol use and binge drinking on MA treatment outcomes is largely unknown. As alcohol use has been shown to be temporally and incrementally predictive of smoking relapse, this may not be a trivial matter (Anthony and Echeagaray-Wagner, 2000; Kahler et al., 2010; Shiffman, 1986, 1997). Heavy drinking smokers are 4 times more likely to experience a smoking lapse in the context of a drinking episode and 8 times more likely to lapse in the context of a binge drinking episode (Hendricks et al., 2012; Kahler et al., 2010). If a similar risky pattern of alcohol and MA co-use is observed in a clinical trial setting, we speculate that alcohol use could also be a risk factor for MA lapse and thus, a potentially important clinical consideration in the context of MA treatment.
While these novel results suggest that alcohol use, particularly in a binge drinking pattern, is associated with greater likelihood of MA use, several strengths and limitations of the present study must be considered when interpreting these findings. Strengths include a well-characterized community sample of regular MA users and the use of sophisticated data analytic strategies that allow for identification of co-use patterns within a given day and at the level of individual subjects. The 30-day TLFB procedures are well suited for this study by providing a well-validated measure of alcohol and MA use while also generating multiple observations per subject, thereby increasing statistical power to detect within subject effects. Study limitations include the cross-sectional and retrospective nature of the study design, as well as the lack of temporal resolution within a given episode of alcohol and/or MA use which precludes causal claims about the pattern of same day MA and alcohol co-use (i.e., did the episode start with alcohol or with MA, was there an intention to use MA, etc.). In addition, it is important to consider that a sizeable number of MA users (49%) recruited for this study did not endorse regular drinking (i.e., drinking at least once per month) and therefore were excluded from the analyses. Due to lack of studies describing the prevalence of MA and alcohol co-use in regular MA users, it is unclear whether this relatively high percentage of irregular drinkers is specific to our study sample or a generalizable trait of the MA-using population. Of note, we have repeated all analyses using the full sample who provided valid data (N=117), and the significance and pattern of results remained unchanged. Thus, it is plausible that a significant number of MA users are not regular drinkers, but to the extent to which MA users report alcohol use, our findings suggest that alcohol use increases the odds of MA use by 4.22 times, as compared with non-drinking days, and the likelihood of MA use is further exacerbated by the occurrence of binge drinking. Furthermore, as only alcohol and MA use was collected during the TLFB, we cannot exclude the possibility that other common substances of abuse (e.g., nicotine and marijuana) may also be associated with MA use. Future research into the within-subjects relationship between the co-use of other commonly-used drugs and MA is warranted. Finally, though this paper is novel by being one of the first to establish the link between naturalistic patterns of contemporaneous co-use of MA and alcohol, it will be important for future research to examine factors that may drive co-use in the natural environment.
In conclusion, this study advances the literature by suggesting that alcohol and MA may be co-used in predictable patterns, particularly under conditions of binge drinking. In addition, this association appears to be stronger among individuals with low MA dependence severity and those with higher levels of alcohol problems. If supported by future studies, these findings may inform interventions to reduce MA use by suggesting that alcohol use should also be targeted. In fact, programs such as the Community Reinforcement Approach (CRA) for cocaine dependence routinely address alcohol use by requiring compliance with Disulfiram treatment for alcohol dependence during engagement in CRA for cocaine dependence (Higgins et al., 2003), and it remains to be determined whether a similarly alcohol-focused program would be effective in treating MA dependence.
Acknowledgements
The authors would like to thank Anna Sheng, Brooke Rowland, Nadine Jacquez, Noelle Cunningham, and Suzanna Osuna for their help with participant recruitment and data collection. The authors would also like to thank the UCLA Clinical and Translational Research Center for their support.
Role of Funding Source
This research was supported by a grant from the National Institute on Drug Abuse (DA029831) to LAR. Support for this study was also provided by a grant from the UCLA Clinical and Translational Science Institute (CTSI), grants UL1RR033176 and UL1TR000124. NRM, KEC, and DR were supported by the UCLA Training Program in Translational Neuroscience of Drug Abuse (T32 DA024635). KEC is currently supported by a predoctoral fellowship from the National Institute on Drug Abuse (DA035604). EH is supported by the UCLA Integrated Substance Abuse Program (T32 DA07272-21).
AL is a collaborator and Co-I for the NIDA grant which funded this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors have made substantial contributions warranting authorship on the current manuscript, and all authors have given their approval for the current version to be submitted for peer-review and publication. Lara A. Ray is the PI for the UCLA Addictions Lab, in which SB, NRM, KEC, VA, and EH, are graduate students, DR, is a post-doctoral fellow, and KL and TR are lab managers. LAR was also the PI for the study from which data was culled.
Author Disclosures
Conflict of Interest
LAR is a paid consultant for GSK. None of the authors have any other conflicts of interest to disclose.
REFERENCES
- Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol. Clin. Exp. Res. 1997;21:613–619. [PubMed] [Google Scholar]
- Anderson AL, Li S-H, Biswas K, McSherry F, Holmes T, Iturriaga E, Kahn R, Chiang N, Beresford T, Campbell J, Haning W, Mawhinney J, McCann M, Rawson R, Stock C, Weis D, Yu E, Elkashef AM. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120:135–141. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use. Alcohol Res. Health. 2000;24:201–208. [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, Pihl RO. Patterns of simultaneous polysubstance use in drug using university students. Hum. Psychopharmacol. Clin. Exp. 2006;21:255–263. doi: 10.1002/hup.766. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Vol. 1. San Antonio TX: Psychol. Corp.; 1996. p. 82. [Google Scholar]
- Caetano R, Weisner C. The association between DSM-III-R alcohol dependence, psychological distress and drug use. Addiction. 1995;90:351–359. doi: 10.1046/j.1360-0443.1995.9033515.x. [DOI] [PubMed] [Google Scholar]
- Cho AK, Melega WP, Kuczenski R, Segal DS. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse. 2001;39:161–166. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Darke S, Darke S, Kaye S, Darke S, Kaye S, McKetin R, Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:253–262. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li S-H, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2007;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB. Structured Clinical Interview For DSM-IV-TR Axis I Disorders: Patient Edition. Biometrics Research Department, Columbia University; 2005. [Google Scholar]
- Furr CD, Delva J, Anthony JC. The suspected association between methamphetamine (“ice”) smoking and frequent episodes of alcohol intoxication: data from the 1993 National Household Survey on Drug Abuse. Drug Alcohol Depend. 2000;59:89–93. doi: 10.1016/s0376-8716(99)00078-2. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Buscemi R, Coyle JR, Flower K, Siegrist JD, Fiske LA, Baggott MJ, Li L, Polcin D, Chen CYA, Mendelson J. A Randomized, placebo-controlled trial of sustained-release dextroamphetamine for treatment of methamphetamine addiction. Clin. Pharmacol. Ther. 2011;89:276–282. doi: 10.1038/clpt.2010.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am. J. Epidemiol. 1991;133:810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- Harwood HJ. The United States: Estimates, Update Methods, And Data. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 2000. Updating Estimates Of The Economic Costs Of Alcohol Abuse. [Google Scholar]
- Hendricks PS, Delucchi KL, Humfleet GL, Hall SM. Alcohol and marijuana use in the context of tobacco dependence treatment: impact on outcome and mediation of effect. Nicotine Tob. Res. 2012;14:942–951. doi: 10.1093/ntr/ntr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, Dantona RL, Anthony S. Community reinforcement therapy for cocaine-dependent outpatients. Arch. Gen. Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob. Res. 2010;12:781–785. doi: 10.1093/ntr/ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Levin FR, Foltin RW, Hart CL. Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology (Berl.) 2012;219:191–204. doi: 10.1007/s00213-011-2390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Lubman D, Baker A, Dawe S, Ali R. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70:319–324. doi: 10.1001/jamapsychiatry.2013.283. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Upton R, Jacob P. Methamphetamine and ethanol interactions in humans. Clin. Pharmacol. Ther. 1995;57:559–568. doi: 10.1016/0009-9236(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, Barr AM. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2012;129:167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, Galloway GP, Herrell J, Huber A, McCann MJ. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- Roll J, Petry N, Stitzer M, Brecht M, Peirce J, McCann M, Blaine J, MacDonald M, DiMaria J, Lucero L. Contingency management for the treatment of methamphetamine use disorders. Am. J. Psychiatry. 2006;163:1993–1999. doi: 10.1176/ajp.2006.163.11.1993. [DOI] [PubMed] [Google Scholar]
- Shiffman S. A cluster-analytic classification of smoking relapse episodes. Addict. Behav. 1986;11:295–307. doi: 10.1016/0306-4603(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: predicting smoking lapse from daily urge. J. Abnorm. Psychol. 1997;106:104. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br. J. Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. Results from the 2012 National Survey on Drug Use and Health (NSDUH) (No. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795) [Google Scholar]
- UNODC. World Drug Report 2012. 2012 (No. (United Nations publication, Sales No. E.12.XI.1)). [Google Scholar]


