Abstract
HIV infection has changed from an acute devastating disease to a more chronic illness due to combination anti-retroviral treatment (cART). In the cART era, the life expectancy of HIV-infected (HIV+) individuals has increased. More HIV+ individuals are aging with current projections suggesting that 50% of HIV+ individuals will be over 50 years old by 2015. With advancing age, HIV+ individuals may be at increased risk of developing other potential neurodegenerative disorders [especially Alzheimer’s disease (AD)]. Pathology studies have shown that HIV increases intra and possibly extracellular amyloid beta (Aβ42), a hallmark of AD. We review the synthesis and clearance of Aβ42 and the effects of HIV on the amyloid pathway, and contrast the impact of AD and HIV on Aβ42 metabolism. Biomarker studies (cerebrospinal fluid AB and amyloid imaging) in HIV+ have shown mixed results. CSF Aβ42 has been shown to be either normal or diminished in HIV+ patients with HIV associated neurocognitive disorders (HAND). Amyloid imaging using [11C] PiB has also not demonstrated increased extracellular amyloid fibrillar deposits in HAND patients. We further demonstrate that Aβ42 deposition is not increased in older HIV+ patients using [11C] PiB amyloid imaging. Together, these results suggest that HIV and aging each independently affect Aβ42 deposition with no significant interaction present. Older HIV+ patients are probably not at increased risk for developing AD. However, future longitudinal studies of older HIV+ patients using multiple modalities (including the combination of CSF markers and amyloid imaging) are necessary for investigating the effects of HIV on Aβ42 metabolism.
Keywords: HIV, CNS, Amyloid, cerebrospinal fluid (CSF), amyloid imaging, combination anti-retroviral therapy (cART), HIV associated neurocognitive disorders (HAND)
The human immunodeficiency virus (HIV) enters the brain soon after seroconversion causing neuronal dysfunction that can lead to HIV associated neurocognitive disorder (HAND) (Kaul et al. 2001). While the more severe forms of HAND have decreased due to combination anti-retroviral therapy (cART), milder forms of impairment are still prevalent (~40%) (Heaton et al. 2010). HAND can occur despite good virologic control with cART due to irreversible injury prior to starting therapy; persistent HIV RNA reservoirs in the central nervous system (CNS) (Ellis et al. 2002); persistent low level inflammation in the CNS (Hagberg et al. 2010); and/or cART toxicity (Akay et al. 2014; Robertson et al. 2010; Robertson et al. 2012). Living with HIV infection has now changed from an acute and fatal disease to a more chronic and treatable condition (Clifford and Ances 2013).
The face of HIV has now greatly changed due to cART. A larger proportion of the HIV-infected (HIV+) community is graying. If current trends continue, by 2015 more than half of all HIV+ individuals in the United States will be greater than fifty years old (Valcour 2013). As the HIV+ population continues to grow older it may become increasingly difficult for clinicians to differentiate the chronic effects of HIV from other neurodegenerative diseases associated with aging [e.g. Alzheimer’s disease (AD)].
AD is characterized by the pathological hallmarks of Aβ42 plaques and neurofibrillary tangles that ultimately lead to neuronal death (Holtzman 2011). Pathological changes in amyloid and synaptic dysfunction often occur more than 20 years before the onset of clinical symptoms of AD (Morris and Price 2001; Bateman et al. 2012). Early surrogate biomarkers are actively being investigated in order to accurately diagnose AD and evaluate therapeutic interventions (Aisen 2009). In particular, changes in amyloid can be measured in the cerebrospinal fluid (CSF) and by positron emission tomography (PET) imaging using [11C] PiB (N-methyl-[11C]2-(4-methylaminophenyl)-6-hydroxybenzothiazole) (Klunk et al. 2004). Observed changes in amyloid using CSF or [11C] PiB may reflect either increased production or decreased clearance of Aβ42.
Cellular level pathology similarities exist between AD and HAND. Both animal and human studies suggest that HIV alters amyloid metabolism (Aksenov et al. 2010; Green et al. 2005; Rempel and Pulliam 2005). Previous autopsy studies of HIV+ individuals demonstrated an increase in diffuse extracellular amyloid plaques in the pre-cART era (Anthony et al. 2010; Rempel and Pulliam 2005). We review the key synthesis and clearance mechanisms involved in Aβ42 and the effects of HIV on the amyloid pathway as measured by CSF Aβ42 and [11C] PiB.
Multiple amyloid synthesis and degradation pathways exist in the brain
Aβ42 is created by site-specific cleavage of the amyloid precursor protein (APP) found in cell membranes. While the exact role of APP remains unknown, animal model neurons lacking APP demonstrate impaired neurite growth, cell to cell adhesion, and intracellular trafficking (Braidy et al. 2012). APP is created in the endoplasmic reticulum, and is transported between the cell membrane and the Golgi apparatus (Zheng et al. 2012). Within the neuron, increases in synaptic transmission and oxidative stress promote an increase in endocytosis of APP from the cell membrane into endolysosomes (Matsuda et al. 2003; Ramaker et al. 2013; Zheng et al. 2012). Within the endolysosomes, APP is cleaved by secretases [including alpha (α), beta (β) (aka BACE-1), and gamma (γ)] to create amyloid-beta monomers of various lengths (between 39–43 amino acids) including the 42 amino acid length protein Aβ42 and soluble APP (sAPP) peptide fragments (LaFerla et al. 2007).
There are two major pathways for cleaving APP into soluble fragments, the amyloidogenic which sequentially uses β and γ secretases to create toxic Aβ42, and a non-amyloidogenic pathway that produces sAPP from α secretase cleavage (Haughey et al. 2010). Under normal circumstances, sAPP peptides are thought to be beneficial to the cell stimulating axonal outgrowth, and neuroprotection (Chasseigneaux and Allinquant 2012). In contrast, an overabundance of Aβ42 monomers can coalesce to form fibrillar Aβ42 plaques, which area pathologic characteristic of AD. For this reason, additional cellular mechanisms are needed for removing Aβ42 from the brain. Insulin degrading enzyme (IDE) or neprilysin can proteolyze Aβ42 and prevent deleterious Aβ42 aggregations in neurons (Madani et al. 2006). In order to evacuate Aβ42 from neural tissue, active shuttling of Aβ42 from the brain into the bloodstream occurs through receptors for advanced glycation end-products (RAGE) and lipoprotein 1 receptors (LRP1) located on endothelial cells in the brain vasculature (Hickman et al. 2008; Kim et al. 2013). Altogether these systems help regulate Aβ42 levels in a sustainable balance that is necessary for normal cellular metabolism.
HIV disrupts multiple steps of amyloid synthesis and clearance
Two mechanisms have been proposed for the entry of HIV across the blood brain barrier (BBB). One view suggests that HIV enters through a Trojan-horse mechanism, whereby an activated T-cell containing HIV migrates across the BBB (Kaul et al. 2001). The other method of entry is based on evidence that HIV directly infects brain vascular endothelial cells, causing a “leaky” BBB that allows uninhibited entry of virus into the brain (Strelow et al. 1998). Once inside the CNS, HIV does not directly infect neurons but instead disrupts astrocyte and glial metabolism leading to neuronal dysfunction and death (Achim et al. 2009; Anthony and Bell 2008; Crews et al. 2009; Gougeon and Piacentini 2009; Gray et al. 1996; Kaul et al. 2001; Mattson et al. 2005).
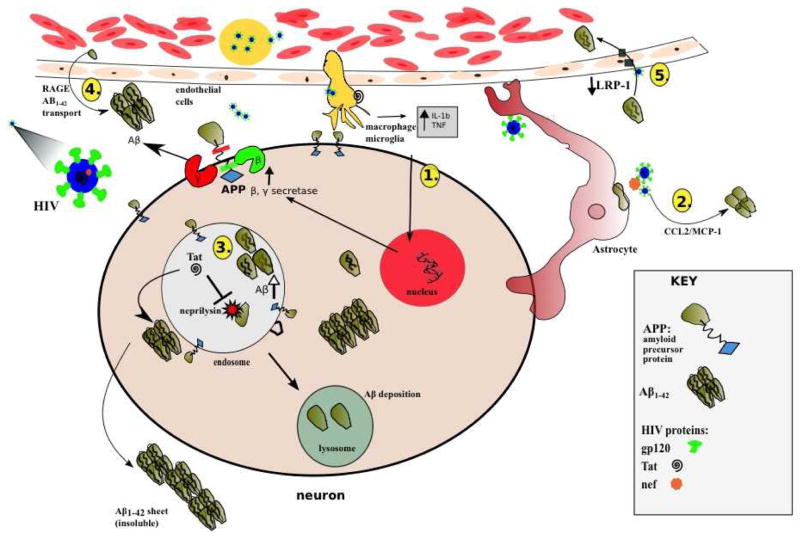
HIV can disrupt several steps in the amyloid synthesis and clearance pathway (András and Toborek 2013) (Figure 1). The viral coat protein of HIV, Gp120, causes infected microglia to release tumor necrosis factor-α (TNFα) and interleukin 1β (IL1β). These inflammatory cytokines increase APP transcription and stimulate increased cleavage of APP by β and γ secretases (Haughey et al. 2010). Another HIV protein that causes dysfunction in multiple cellular pathways is the HIV transactivator of transcription (tat) protein. Several studies have demonstrated that tat inhibits the proteolytic functions of neprilysin (Rempel and Pulliam 2005; Daily et al. 2006; Aksenov et al. 2010; Lan et al. 2011). Tat is also implicated in the increase of intracellular Aβ42 and sAPP within the neuron. Specifically, Tat increases the aggregation of Aβ42 into neuronal endolysosomes through endocytosis (Liu et al. 2000). In microvasculature endothelium cells, Tat binds to the LRP and reduces Aβ42 clearance from the brain (András and Toborek 2013). Tat also attaches to RAGE on endothelial cells causing increased translocation of Aβ42 from the blood into the brain (András and Toborek 2013; Xu and Ikezu 2009).
Figure 1.
Purported pathways for amyloid accumulation in the HIV infected brain. After HIV enters past the blood brain barrier, a chronic state of viral replication causes an upregulation of the amyloidogenic pathology through the following mechanisms. 1) Infected microglia release pro-inflammatory cytokines; Tumor Necrosis Factor alpha (TNFα) & Interleukin 1-beta (IL-1b) that up regulate β (BACE), and γ (gamma) secretases and lead to increases in Aβ42. 2) HIV nef proteins within and around infected astrocytes cause upregulation of Aβ42 leading to accumulation of chemokine ligand-2 (CCL2). 3) Once Aβ42 is invaginated into an endolysosome, HIV tat protein directly inhibits the activity of neprilysin from degrading the amyloid oligomers into inert fragments. These amyloid monomers can then coalesce into intracellular and extracellular oligomers and plaques. 4) HIV proteins can cause the caveolar transport of Aβ42 across the endothelial cells using the receptor for advanced glycation end products (RAGE). 5) HIV indirectly reduces the efflux of Aβ42 by lipoprotein receptor protein (LRP-1).
Astrocytes are key components of the brain, shuttling metabolites between the blood and the neurons (Strazza et al. 2011). HIV also causes astrocyte dysfunction that alters amyloid metabolism. Specifically, the HIV protein nef stimulates astrocytes to release inflammatory cytokines [including chemokine ligand 2 (CCL2)]. An inflammatory cascade can occur leading to an increase in extracellular Aβ42 (White et al. 2005). Altogether, HIV may potentially utilize multiple mechanisms to increase Aβ42 generation.
Controversy exists concerning cerebrospinal fluid (CSF) Aβ42 in HIV+ individuals
Aβ42 levels can be measured within the CSF and maybe a sensitive biomarker of AD. Increased deposition of Aβ42 plaques in AD individuals corresponds to decreased CSF Aβ42 (Fagan et al. 2006; Roe et al. 2013). According to the amyloid cascade hypothesis; a reduction in CSF Aβ42 is one of the earliest changes associated with the onset of AD (Dickerson and Wolk 2013). The reduction in CSF Aβ42, is thought to be caused by increased deposition of fibrillar Aβ42, with less soluble Aβ42 effectively transported out of the brain across the BBB (Hardy and Higgins 1992). A number of studies have confirmed decreased CSF Aβ42 in both prodromal and symptomatic AD individuals[e.g. (Blennow et al. 2010; Dickerson and Wolk 2013; Engler et al. 2006; Hölttä et al. 2013; Jack et al. 2010; Knopman et al. 2013; Mattsson et al. 2009; Morris et al. 2010; Olsson et al. 2003; Potter et al. 2013; Reiman et al. 2012; Rosén et al. 2013)]. These studies suggest that 1) CSF Aβ42 levels are lower in both symptomatic and asymptomatic AD, with decreases of up to 50% seen in AD individuals compared to cognitively normal individuals (Blennow et al. 2010) 2) CSF Aβ42 may be a reliable accurate biomarker for AD (Jack et al. 2010; Roe et al. 2013).
A small set of studies have observed mixed results when quantifying CSF Aβ42 in HIV+ individuals. Three studies have found reductions in CSF Aβ42 in HAND individuals (Clifford et al. 2009a; Krut et al. 2013), or HIV+ patients with CNS co-infections (Gisslén et al. 2009). More recently, a study has shown an increase in CSF Aβ42 with early HIV infection compared to HIV− controls (Peluso et al. 2013). In contrast, three other studies have observed no changes in CSF Aβ42 in HIV+ individuals relative to HIV− controls (Ances et al. 2012; Gisslén et al. 2009; Steinbrink et al. 2013).
Brain pathology studies have also demonstrated that HIV increases intracellular Aβ42 and APP precursors, potentially affecting CSF Aβ42 levels. Post-mortem tissue staining has shown an accumulation of APP within axons of rats (Mankowski et al. 2002) and humans (An et al. 1997; Nebuloni et al. 2001). HIV tat increases cell-bound Aβ42 in the hippocampus (Aksenov et al. 2010). Furthermore, elevated concentrations of intra-neuronal Aβ42 were observed in HIV+ autopsy cases of HAND (Achim et al. 2009).
Several considerations should be considered when interpreting any CSF study of AD or HAND. In particular, Aβ42 levels can be influenced by the time of sample collection (Bateman et al. 2007), storage of the CSF (e.g. thawing and rethawing), and analytical platform used for CSF analysis[multi-analyte Luminex assay (INNO-BIA AlzBio3) vs. single-analyte ELISA tests (INNOTEST)](Le Bastard et al. 2013; Mattsson et al. 2011). For example, the intra-class correlation (same individual) CSF Aβ42 can vary two to six-fold depending on analytic method (Fagan et al. 2011). It is possible that conflicting results seen in HIV+ patients concerning CSF Aβ42 may also reflect the relatively small samples used for analysis (often < 50 HIV+ patients). Future studies should ensure uniform CSF collection and analysis in a large HIV+ cohort in order to understand the effects of the virus on the amyloid pathway.
Aβ42 fibrillar deposition is not increased in HIV+ individuals using [11C] PiB
Positron emission tomography (PET) amyloid imaging using [11C] PiB has been used to measure in vivo amyloid deposits in AD (for review see Cohen et al. 2012). Numerous studies have demonstrated increased amyloid deposition within the medial frontal lobes, inferior temporal lobes, and precuneus regions of AD patients (Benzinger et al. 2013; Cho et al. 2013; Cohen et al. 2012; Klunk et al. 2004; Marchant et al. 2013; Mintun et al. 2006; Reiman et al. 2009; Roe et al. 2013). In contrast, relatively few [11C] PiB studies have been performed in HIV+ patients (Ances et al. 2010; Ances et al. 2012). We have previously shown that HAND individuals do not have increased fibrillar Aβ42 deposits compared to age matched HIV− controls (Ances et al. 2010). In a follow-up study, HIV+ individuals with and without HAND were compared to HIV− controls with and without cognitive impairment. Both HIV+ groups did not have significant elevations in amyloid deposition using [11C] PiB. Only HIV− controls with cognitive impairment (AD) had significant deposition (Ances et al. 2012). A limitation of these studies was that relatively younger HIV+ subjects were primarily included.
Aβ42 fibrillar deposition is similar between HIV+ and HIV− individuals across the lifespan
It remains unknown whether Aβ42 deposition is elevated in older HIV+ individuals. Here we present results from an additional investigation that studied the relationship between aging and Aβ42 in HIV+ (n=26, 69% male, 36–67 years old) and HIV− individuals (n=23, 65% male, 32–62 years old) using [11C] PiB. For this comparison, high resolution structural magnetic resonance imaging (MRI) was co-registered with [11C] PiB images using in-house methods to calculate regional atlas based specific uptake value ratios (SUVR) (Su et al. 2013). Briefly, the SUVR is a regional quantitative estimate of amyloid burden with correction for atrophy and tissue (gray/white matter) compartment distribution (Lopresti et al. 2005; Su et al. 2013). The regions of interest were generated using Freesurfer software aligned to a common atlas (FS v5.1 found at https://surfer.nmr.mgh.harvard.edu)
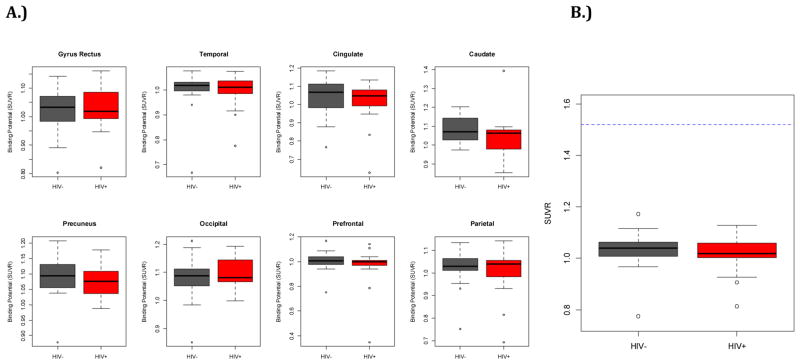
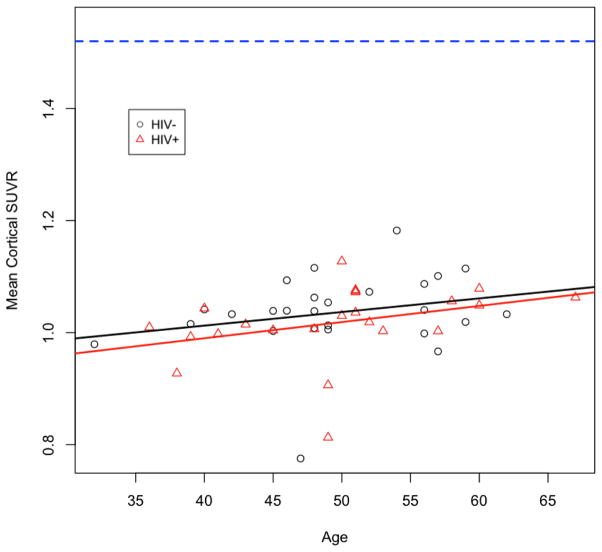
The SUVR for any region analyzed did not differ between the HIV− and HIV+ groups (Figure 2A). Similar results were also seen for the total mean-cortical SUVR (Figure 2B). For both groups the SUVR measurements did not exceed previously defined threshold criteria for amyloid positivity (i.e. SUVR < 1.52)(Roe et al. 2008). When comparing SUVR and aging between groups we observed a slight increase in mean cortical SUVR with aging for both HIV+ and HIV− individuals. The correlation between age and SUVR was not significant (p=0.12) for either group. Interestingly, over the entire lifespan studied, HIV+ individuals had lower SUVR than HIV− individuals. However, these differences were not significant (p=0.85) (Figure 3). These observations suggest that elevated extracellular fibrillar amyloid deposition is not observed with HIV. From this small study it does not appear that HIV accelerates amyloid pathology.
Figure 2.
Regional and mean cortical specific value uptake ratios (SUVR) for HIV infected (HIV+) (red) and HIV− controls (gray). A. SUVR in the gyrus rectus, lateral temporal, cingulate, caudate, precuneus, occipital, prefrontal, and parietal were similar for both groups. Average values were lower than known thresholds for any region of interest (ROI). B. Mean cortical SUVR for the two groups were similar. Average SUVR ratios were less than established amyloid positive threshold criteria (>1.52 arbitrary units) shown as the blue line.
Figure 3.
Effects of aging on mean cortical SUVR in HIV+ and HIV− individuals. An increase in mean cortical SUVR was seen with aging for both groups but values did not exceed threshold criteria (>1.52). The slopes for HIV+ and HIV− individuals were 0.0028/year and 0.0025/year, respectively. No significant differences, or interactions were found between groups (p=0.86). Average SUVR ratios for both groups were less than established amyloid positive threshold criteria (>1.52 arbitrary units) shown as the blue dotted line.
Summary of current findings
HIV causes abnormalities in cellular mechanisms that lead to increases of Aβ42 in the brain (Figure 1). However in-vivo CSF studies show conflicting evidence (Ances et al. 2012; Brew et al. 2005; Clifford et al. 2009a; Gisslén et al. 2009; Peluso et al. 2013; Steinbrink et al. 2013; Vehmas et al. 2004). In particular, some CSF studies have shown a consistent decrease in Aβ42 while others have seen no changes. In vivo amyloid imaging using [11C] PiB has not shown increases in Aβ42 fibrillar deposition. Additional studies using Florbetapir (a fluorine-18 compound that measures Aβ42 deposition) have not observed increased amyloid deposition in older HIV+ individuals (personal communication with Dr. Ned Sacktor). Even in our oldest HIV+ individuals, there was no significant elevation of Aβ42 measured through 11C-PiB (Figure 3). These potential discrepancies in CSF and amyloid imaging could reflect differences in the location and type of amyloid affected by HIV. In cognitively normal HIV+ patients, intracellular amyloid may primarily accumulate within the neuron (Aksenov et al. 2010). A more recent study that included both pre and post-cART era HIV+ patients observed an increase in intracellular and not extracellular Aβ42 (Gelman and Schuenke 2004). In HAND [especially HIV associated dementia (HAD)], CSF Aβ42 is decreased and soluble APP peptides are normal or diminished (Gisslén et al. 2009; Krut et al. 2013). The amyloid hypothesis would suggest increases in extracellular Aβ42 plaques. However, current amyloid imaging methods, such as[11C] PiB, have shown an absence of Aβ42 plaques. This technique primarily measures extracellular fibrillar deposits. The lack of extracellular Aβ42 in HIV may reflect: 1) decreased Aβ42 production due to decreased synaptic activity (Mawuenyega et al. 2010); 2) increased intracellular Aβ42 accumulation (Achim et al. 2009; Chen et al. 2013; Nebuloni et al. 2001;); 3) increased extracellular Aβ42 that is diffuse and not fibrillar in nature (Anthony et al. 2006; Cairns et al. 2009; Green et al. 2005); or 4.) decreased upstream production of sAPP precursors (Gisslén et al. 2009; Krut et al. 2013; Peluso et al. 2013). Arguably, it is possible that a combination of these aforementioned reasons could lead to a lack in extracellular Aβ42 seen with HIV.
Potential differences exist between HAND and AD in regards to Aβ42 pathology
The observed differences between AD and HAND could reflect distinct pathologic effects of these two diseases on the amyloid pathway. First, CSF sAPP peptide levels (both α and β) are often diminished in HIV, especially in HAD (Angel et al. 2012; Gisslén et al. 2009; Krut et al. 2013). These decreases may reflect down-regulation of upstream pathways involved in APP production. In contrast, sAPP (both α and β) remains stable or often elevated in AD due to increased upregulation and/or increased cleavage by secretases (Blennow et al. 2012). Observed increases in CSF sAPP peptides seen with AD are correlated with increased Aβ42 plaques (Lewczuk et al. 2010; Olsson et al. 2003). However, this is not observed in HIV (Gisslén et al. 2009; Krut et al. 2013). Second, differences may exist in the location of Aβ42 deposition. In HAND, amyloid is primarily intracellular (Green et al. 2005; Achim et al. 2009) compared to extracellular for AD (Rosen et al 2013). Extracellular plaques that are seen in HAND are typically diffuse (Rempel and Pulliam 2005) and not fibrillar. This is contrast to AD where typically fibrillar plaques are observed (Ubhi and Masliah 2013). This may lead to observed PiB differences as this compound primarily binds to fibrillar plaques (Ances et al. 2010; Ances et al. 2012). Finally, differences may exist in clearance of plaques in AD vs. HAND. While both diseases cause chronic inflammatory responses (Xu and Ikezu 2009; Lynch 2014), recent studies suggest HIV proteins alters Aβ42 degradation pathways by microglia (Lan et al. 2011).
Recent advances in CSF biomarkers, proteomics, and neuroimaging are potentially promising
While the current diagnostic value of CSF and 11C-PiB measurements of amyloid remain less clear for HIV compared to AD, many in-vitro studies still suggest that neuro-inflammation, as seen with HIV, can affect amyloid metabolism (Kim et al. 2013a). A promising method to investigate differences is CSF proteomics. A recent review has highlighted potential endothelial, neural and inflammatory proteomic markers associated with HIV (see (Price et al. 2013). Productive inflammation can lead to extensive protein-protein interactions that involve the amyloid pathway (Angel et al. 2012). More recent neuroimaging studies using neuro-inflammatory (e.g. PK1195) markers could elucidate potential interactions (Garvey et al. 2014). Additional studies combining these modalities and protein interactions (Haughey et al. 2013) are needed to adequately understand the complex interaction between amyloidogenesis and neurodegeneration seen with HIV infection (Xu and Ikezu 2009).
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01NR014449, R01NR012657, R01NR012907, R21MH099979, and the Alzheimer’s Association (BMA).
Footnotes
Conflict of interest: The authors have no conflicts of interest related to this work to report.
Bibliography
- Achim CL, Adame A, Dumaop W, et al. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–9. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen PS. Alzheimer’s disease therapeutic research: the path forward. Alzheimers Res Ther. 2009;1:2. doi: 10.1186/alzrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akay C, Cooper M, Odeleye A, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014 doi: 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci Lett. 2010;475:174–8. doi: 10.1016/j.neulet.2010.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SF, Giometto B, Groves M, et al. Axonal damage revealed by accumulation of beta-APP in HIV-positive individuals without AIDS. J Neuropathol Exp Neurol. 1997;56:1262–8. doi: 10.1097/00005072-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Ances BM, Benzinger TL, Christensen JJ, et al. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol. 2012;69:72–7. doi: 10.1001/archneurol.2011.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Christensen JJ, Teshome M, et al. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case-control study. Neurology. 2010;75:111–5. doi: 10.1212/WNL.0b013e3181e7b66e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- András IE, Toborek M. Amyloid beta accumulation in HIV-1-infected brain: The role of the blood brain barrier. IUBMB Life. 2013;65:43–9. doi: 10.1002/iub.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel TE, Jacobs JM, Spudich SS, et al. The cerebrospinal fluid proteome in HIV infection: change associated with disease severity. Clin Proteomics. 2012;9:3. doi: 10.1186/1559-0275-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Bell JE. The Neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20:15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Norrby KE, Dingwall T, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133:3685–98. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, et al. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111:529–38. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- Le Bastard N, Coart E, Vanderstichele H, et al. Comparison of two analytical platforms for the clinical qualification of Alzheimer’s disease biomarkers in pathologically-confirmed dementia. J Alzheimers Dis. 2013;33:117–31. doi: 10.3233/JAD-2012-121246. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68:666–9. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. 120711140017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger TLS, Blazey T, Jack CR, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013;110:E4502–9. doi: 10.1073/pnas.1317918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- Braidy N, Muñoz P, Palacios AG, et al. Recent rodent models for Alzheimer’s disease: clinical implications and basic research. J Neural Transm. 2012;119:173–95. doi: 10.1007/s00702-011-0731-5. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, et al. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–2. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Ikonomovic MD, Benzinger T, et al. Absence of Pittsburgh compound B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch Neurol. 2009;66:1557–62. doi: 10.1001/archneurol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasseigneaux S, Allinquant B. Functions of Aβ, sAPPα and sAPPβ: similarities and differences. J Neurochem. 2012;120(Suppl):99–108. doi: 10.1111/j.1471-4159.2011.07584.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Hui L, Geiger NH, et al. Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol Aging. 2013;34:2370–8. doi: 10.1016/j.neurobiolaging.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Seo SW, Kim J-H, et al. Amyloid deposition in early onset versus late onset Alzheimer’s disease. J Alzheimers Dis. 2013;35:813–21. doi: 10.3233/JAD-121927. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13:976–86. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Fagana M, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–7. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Rabinovici GD, Mathis Ca, et al. Using Pittsburgh Compound B for in vivo PET imaging of fibrillar amyloid-beta. Adv Pharmacol. 2012;64:27–81. doi: 10.1016/B978-0-12-394816-8.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Patrick C, Achim CL, et al. Molecular pathology of neuro-AIDS (CNS-HIV) Int J Mol Sci. 2009;10:1045–63. doi: 10.3390/ijms10031045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily A, Nath A, Hersh LB. Tat peptides inhibit neprilysin. J Neurovirol. 2006;12:153–60. doi: 10.1080/13550280600760677. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Wolk Da. Biomarker-based prediction of progression in MCI: Comparison of AD signature and hippocampal volume with spinal fluid amyloid-β and tau. Front Aging Neurosci. 2013;5:55. doi: 10.3389/fnagi.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59:923–8. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–66. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–9. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Shaw LM, Xiong C, et al. Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1–42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol. 2011;68:1137–44. doi: 10.1001/archneurol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey LJ, Pavese N, Politis M, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28:67–72. doi: 10.1097/01.aids.0000432467.54003.f7. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Schuenke K. Brain aging in acquired immunodeficiency syndrome: increased ubiquitin-protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirol. 2004;10:98–108. doi: 10.1080/13550280490279816. [DOI] [PubMed] [Google Scholar]
- Gisslén M, Krut J, Andreasson U, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon M-L, Piacentini M. New insights on the role of apoptosis and autophagy in HIV pathogenesis. Apoptosis. 2009;14:501–8. doi: 10.1007/s10495-009-0314-1. [DOI] [PubMed] [Google Scholar]
- Gray F, Scaravilli F, Everall I, Chretien F. Neuropathology of Early HIV-1 Infection. Brain Pathol. 1996;6:1–12. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Green Da, Masliah E, Vinters HV, et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005a;19:407–11. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005b;19:407–11. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Bandaru VVR, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim Biophys Acta. 2010;1801:878–86. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Zhu X, Bandaru VVR. A biological perspective of CSF lipids as surrogate markers for cognitive status in HIV. J Neuroimmune Pharmacol. 2013;8:1136–46. doi: 10.1007/s11481-013-9506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–60. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä M, Hansson O, Andreasson U, et al. Evaluating amyloid-β oligomers in cerebrospinal fluid as a biomarker for Alzheimer’s disease. PLoS One. 2013;8:e66381. doi: 10.1371/journal.pone.0066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM. CSF biomarkers for Alzheimer’s disease: current utility and potential future use. Neurobiol Aging. 2011;32(Suppl 1):S4–9. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–48. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon J-H, Kim Y-S. HIV-1 Tat Interacts with and Regulates the Localization and Processing of Amyloid Precursor Protein. PLoS One. 2013a;8:e77972. doi: 10.1371/journal.pone.0077972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-J, Ahn J-W, Kim H, et al. Two β-strands of RAGE participate in the recognition and transport of amyloid-β peptide across the blood brain barrier. Biochem Biophys Res Commun. 2013b;439:252–7. doi: 10.1016/j.bbrc.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, et al. Imaging Brain Amyloid in Alzheimer’s Disease with Pittsburgh Compound-B. 2004. pp. 306–319. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Jack CR, Wiste HJ, et al. Selective Worsening of Brain Injury Biomarker Abnormalities in Cognitively Normal Elderly Persons With β-Amyloidosis. JAMA Neurol. 2013;70:1030–8. doi: 10.1001/jamaneurol.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krut JJ, Zetterberg H, Blennow K, et al. Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. J Neurol. 2013;260:620–6. doi: 10.1007/s00415-012-6688-y. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lan X, Xu J, Kiyota T, et al. HIV-1 reduces Abeta-degrading enzymatic activities in primary human mononuclear phagocytes. J Immunol. 2011;186:6925–32. doi: 10.4049/jimmunol.1100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, et al. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–7. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Kamrowski-Kruck H, Peters O, et al. Soluble amyloid precursor proteins in the cerebrospinal fluid as novel potential biomarkers of Alzheimer’s disease: a multicenter study. Mol Psychiatry. 2010;15:138–45. doi: 10.1038/mp.2008.84. [DOI] [PubMed] [Google Scholar]
- Lopresti BJ, Klunk WE, Mathis Ca, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–72. [PubMed] [Google Scholar]
- Lynch Ma. The impact of neuroimmune changes on development of amyloid pathology; relevance to Alzheimer’s disease. Immunology. 2014;141:292–301. doi: 10.1111/imm.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani R, Poirier R, Wolfer DP, et al. Lack of Neprilysin Suffices To Generate Murine Amyloid-Like Deposits in the Brain and Behavioral Deficit In Vivo. 2006;1878:1871–1878. doi: 10.1002/jnr. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Tarwater PM, et al. Accumulation of beta-amyloid precursor protein in axons correlates with CNS expression of SIV gp41. J Neuropathol Exp Neurol. 2002;61:85–90. doi: 10.1093/jnen/61.1.85. [DOI] [PubMed] [Google Scholar]
- Marchant NL, Reed BR, Sanossian N, et al. The aging brain and cognition: contribution of vascular injury and aβ to mild cognitive dysfunction. JAMA Neurol. 2013;70:488–95. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Matsuda Y, D’Adamio L. Amyloid beta protein precursor (AbetaPP), but not AbetaPP-like protein 2, is bridged to the kinesin light chain by the scaffold protein JNK-interacting protein 1. J Biol Chem. 2003;278:38601–6. doi: 10.1074/jbc.M304379200. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath a. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Persson S, et al. The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7:386–395.e6. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–93. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun Ma, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci. 2001;17:101–18. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–31. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebuloni M, Pellegrinelli a, Ferri a, et al. Beta amyloid precursor protein and patterns of HIV p24 immunohistochemistry in different brain areas of AIDS patients. AIDS. 2001;15:571–5. doi: 10.1097/00002030-200103300-00005. [DOI] [PubMed] [Google Scholar]
- Olsson A, Höglund K, Sjögren M, et al. Measurement of α- and β-secretase cleaved amyloid precursor protein in cerebrospinal fluid from Alzheimer patients. Exp Neurol. 2003;183:74–80. doi: 10.1016/S0014-4886(03)00027-X. [DOI] [PubMed] [Google Scholar]
- Peluso MJ, Meyerhoff DJ, Price RW, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207:1703–12. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter R, Patterson BW, Elbert DL, et al. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med. 2013;5:189ra77. doi: 10.1126/scitranslmed.3005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Peterson J, Fuchs D, et al. Approach to Cerebrospinal Fluid (CSF) Biomarker Discovery and Evaluation in HIV Infection. J Neuroimmune Pharmacol. 2013;8:1147–58. doi: 10.1007/s11481-013-9491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker JM, Swanson TL, Copenhaver PF. Amyloid precursor proteins interact with the heterotrimeric G protein Go in the control of neuronal migration. J Neurosci. 2013;33:10165–81. doi: 10.1523/JNEUROSCI.1146-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:6820–5. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Quiroz YT, Fleisher AS, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11:1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–35. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18:388–99. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Su Z, Margolis DM, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74:1260–6. doi: 10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Fagan AM, Grant Ea, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–91. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D’Angelo G, et al. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65:1467–71. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén C, Hansson O, Blennow K, Zetterberg H. Fluid biomarkers in Alzheimer’s disease - current concepts. Mol Neurodegener. 2013;8:20. doi: 10.1186/1750-1326-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink F, Evers S, Buerke B, et al. Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. Eur J Neurol. 2013;20:420–8. doi: 10.1111/ene.12006. [DOI] [PubMed] [Google Scholar]
- Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelow LI, Watry DD, Fox HS, Nelson JA. Efficient infection of brain microvascular endothelial cells by an in vivo-selected neuroinvasive SIVmac variant. J Neurovirol. 1998;4:269–80. doi: 10.3109/13550289809114528. [DOI] [PubMed] [Google Scholar]
- Su Y, D’Angelo GM, Vlassenko AG, et al. Quantitative Analysis of PiB-PET with FreeSurfer ROIs. PLoS One. 2013;8:e73377. doi: 10.1371/journal.pone.0073377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Masliah E. Alzheimer’s disease: recent advances and future perspectives. J Alzheimers Dis. 2013;33(Suppl 1):S185–94. doi: 10.3233/JAD-2012-129028. [DOI] [PubMed] [Google Scholar]
- Valcour VG. HIV, aging, and cognition: emerging issues. Top Antivir Med. 2013;21:119–23. [PMC free article] [PubMed] [Google Scholar]
- Vehmas A, Lieu J, Pardo Ca, et al. Amyloid precursor protein expression in circulating monocytes and brain macrophages from patients with HIV-associated cognitive impairment. J Neuroimmunol. 2004;157:99–110. doi: 10.1016/j.jneuroim.2004.08.035. [DOI] [PubMed] [Google Scholar]
- White Ja, Manelli AM, Holmberg KH, et al. Differential effects of oligomeric and fibrillar amyloid-beta 1–42 on astrocyte-mediated inflammation. Neurobiol Dis. 2005;18:459–65. doi: 10.1016/j.nbd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol. 2009;4:200–12. doi: 10.1007/s11481-008-9136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Cedazo-Minguez A, Hallbeck M, et al. Intracellular distribution of amyloid beta peptide and its relationship to the lysosomal system. Transl Neurodegener. 2012;1:19. doi: 10.1186/2047-9158-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]