Abstract
Nielsen et al (2013 Jun 28, p.1577) characterized their purified RNA polymerase (pol) III preparation and concluded that it requires an RNA hairpin/duplex structure for terminating transcription in vitro and in vivo. We could not corroborate their findings using pol III from two laboratory sources. Moreover, we show that a hairpin/duplex does not appear to be necessary for RNA production by pol III in vivo.
Nielsen et al (2013 Jun 28, p.1577) presented a biochemical analysis of their S. cerevisiae-derived RNA polymerase (pol) III and concluded that although it pauses at the known termination signal, oligo(T), transcript release requires a terminator-proximal RNA hairpin or other RNA duplex structures. Since the authors did not address prior reports that concluded that no hairpin or dyad structure was required for termination by pol III (1, 2), we examined this issue and were unable to corroborate Nielsen’s findings using pol III from two independent laboratories. Soren Nielsen and Nikolay Zenkin graciously provided a sample of their pol III in order to shed light on this dilemma.
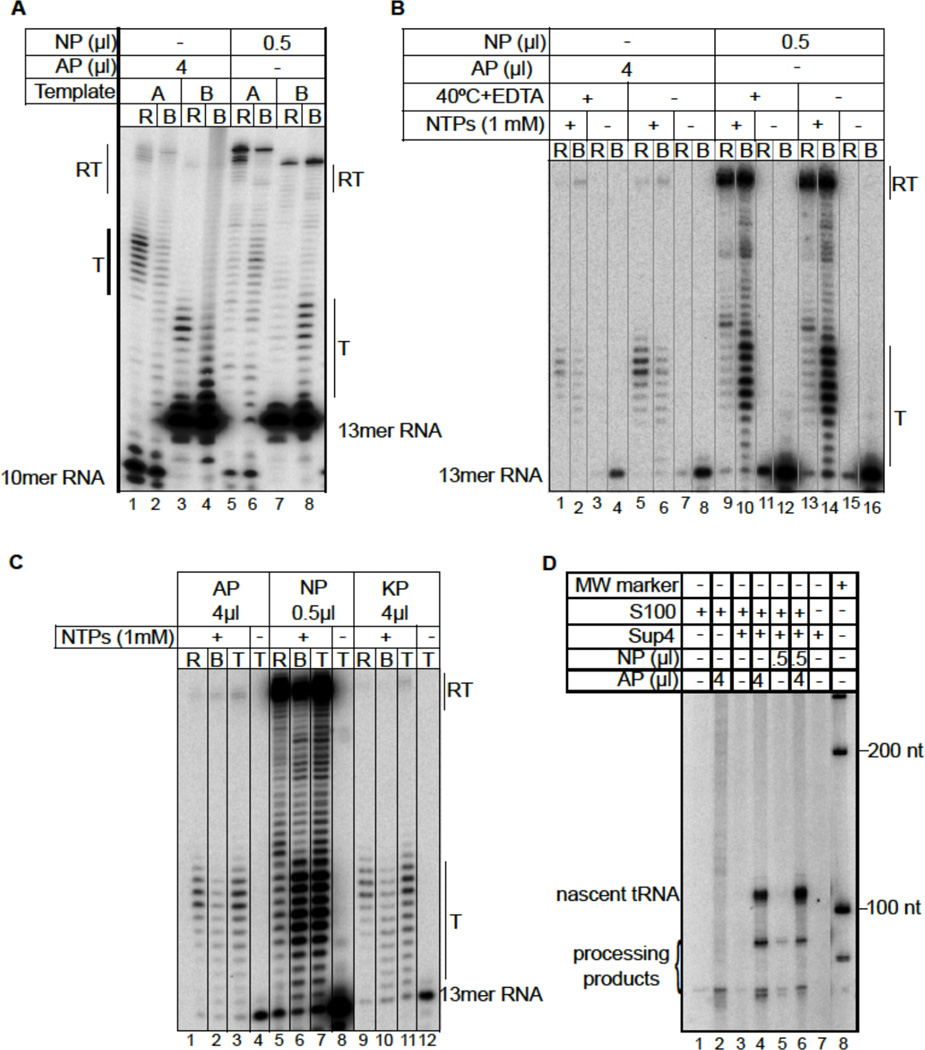
Using the template specified by Nielsen to produce a transcript lacking secondary structure we found that our S. cerevisiae pol III efficiently released transcripts at oligo(T). Further analyses indicated that the disparity must lie in the polymerases rather than variables of the assays. This was confirmed by direct comparisons of the two polymerases, the Nielsen pol III (NP) and ours (AP) (3), using two different templates, and two immobilization methods: through the polymerase His6 tag (3) (Fig. 1A) or through the 5'-biotin-tagged non-template strand DNA (Fig. 1B) (4). For the experiment shown in Fig. 1A, templates A and B contain differently positioned 9T and 10T terminators, respectively, producing 5'-32P-labeled RNA primer-directed transcripts predicted to lack secondary structure; AP efficiently released transcripts at the T-tracts of both templates (lanes 1 & 3). In contrast, NP produced more read-through transcripts (RT) and most of the transcripts with 3’ ends at the T tract were in the bound fractions (lanes 6 & 8). For the experiment shown in Fig. 1B, we used immobilized DNA, unlabeled RNA primer and α-[32P]GTP incorporation (4) and the same amounts of polymerases as in Fig. 1A. In this assay, NP was far more active than AP and also produced more RT. Transcripts paused at oligo(T) were not released by NP but efficiently released by AP (Fig. 1B). It is noteworthy that substantial RT was also produced by NP for transcripts with a hairpin and on even longer T-tracts (Figure 2C in (4)), reflecting unexpectedly low termination efficiency relative to what would result with other preparations of pol III (with comparable NTP concentrations, see 2, 3, 5)). We also compared NP and AP with an untagged preparation of pol III, KP (6), using immobilized DNA (Fig. 1C). KP was nearly indistinguishable from AP, while NP again produced mostly bound transcripts, arrested at oligo(T). It is likely significant that NP was substantially more active than AP in the immobilized DNA assay relative to the immobilized polymerase assay. NP also differed in read-through beyond its oligo(T) elongation-arrest site compared to read-through beyond the oligo(dT) pause/release site by AP and KP. Thus, NP is very different from AP and KP. Its activity also differs very significantly from pol IIIΔ, a form that lacks its C53/C37 subcomplex, since pol IIIΔ efficiently releases transcripts at long, 9T terminators (3).
Figure 1. Direct comparisons of Nielsen (NP) polymerase and our (AP) pol III for termination and TFIIIB+TFIIC-dependent initiation.
A) Elongation complexes were assembled on templates A and B and immobilized, through the RNA polymerase His6 tag, on NiNTA agarose. Ribonucleoside triphosphates (NTPs) were added, incubated for 10 minutes and the released (R) and bound (B) transcripts separated, as indicated above each lane. B) Comparison of EC formation and transcription by AP and NP on immobilized template B. Quantities of AP and NP used are the same as in A; reactions were for 1 minute. Lanes 1–4, 9–12 were done as described (3) while lanes 5–8, 13–16 were done in the absence of EDTA and 40°C incubation. C) Comparison of transcript release by three different preparations of pol III. AP was purified on NiNTA agarose (3) (used in other figures) while untagged KP was highly purified by sequential ion exchange chromatography (6). Quantities of AP and NP used are the same as in A and B. Released, bound and total (T) transcripts are indicated. D) Pol III-specific transcription assay. Preinitiation complexes were formed by incubating a SUP4 tRNA gene plasmid with S. cerevisiae S100 extract deficient in endogenous pol III activity (see text) followed by addition of AP or NP as indicated above the lanes. Reactions were started by the addition of NTPs (with α-[32P] GTP) and MgCl2, and incubated for 20 minutes.
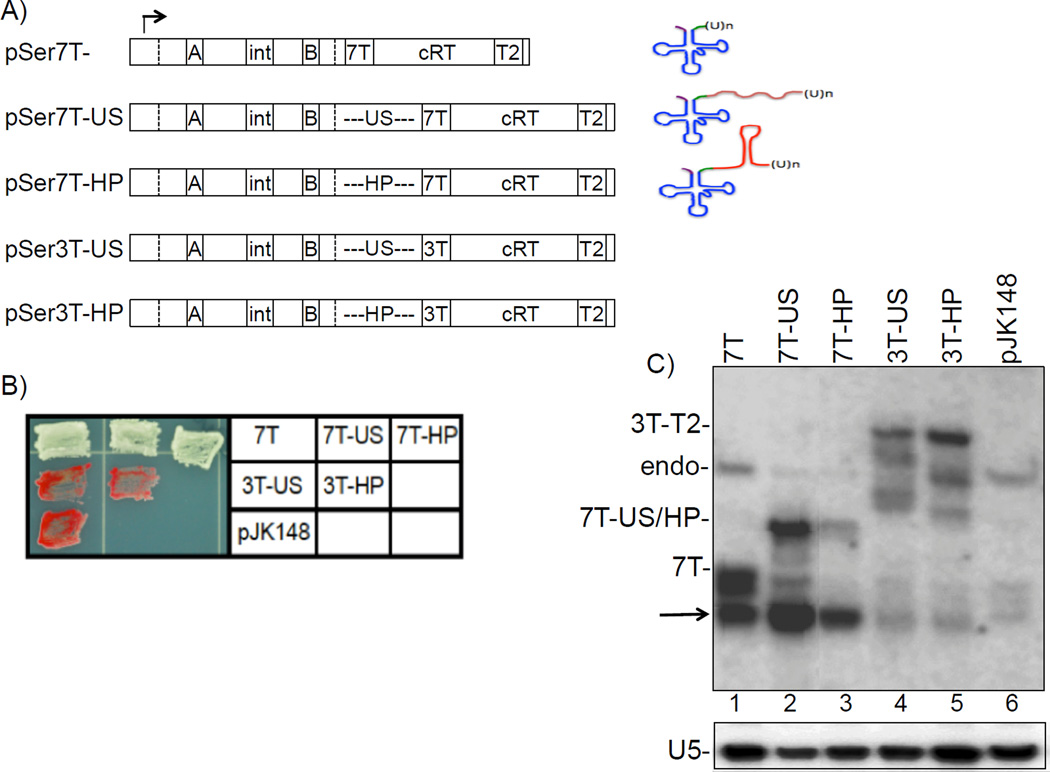
Figure 2. Pol III termination is independent of transcript terminator-proximal RNA structure in vivo.
A) Schematic of the different suppressor tRNA constructs used. pSer7T is the parent construct (8) (see text). Promoter elements and other features are indicated (→, start site; box A; intron; box B; 7T or 3T terminators; cRT, complementary read-through region; T2, read-through terminator). The dashed vertical lines delineate the 5' and 3' matured/processed ends of the tRNA. An unstructured spacer (US) or a spacer that forms a stable hairpin (HP) was introduced within the 3’ trailer, immediately upstream of the terminator. On the right side is a schematic of the resultant predicted pretRNA products. B) Patch suppression assay analysis of S. pombe colonies carrying the different suppressor constructs. Red color indicates lack of suppression and white color indicates efficient suppression. C) Northern blot analysis of the nascent tRNAs produced by the different suppressor constructs using an intron-specific probe. 7T- and 7T-US/HP-designations to the left side indicate the position of the nascent 7T-terminated pre-tRNAs of pSer7T and pSer7T-US/HP constructs respectively. ‘Endo’ indicates the nascent transcript of the endogenous dimeric tRNAser-tRNAi-met gene. 3T-T2- indicates the 3' extended transcript generated by "read through" transcription of the constructs with the 3T terminator. The arrow indicates a partially processed pre-tRNA intermediate prior to nuclear export (see text). The differences in the profiles in lanes 2 and 3 likely reflect alternative 3' processing pathways (15). The lower panel shows the same blot probed for the pol II-transcribed U5 snRNA as a sample recovery control.
We also compared AP and NP for factor-dependent, specifically initiating transcription of the SUP4 tRNA gene by assembling initiation complexes with an S. cerevisiae S100 extract deficient in pol III activity (from pol IIIΔ-expressing cells (3)). Addition of AP produced the expected 104 nt transcript (6) and smaller products consistent with pre-tRNA processing in cellular extracts (7), whereas NP produced substantially less (Fig. 1D, lanes 4 & 5). Mixing AP and NP yielded levels similar to AP alone (lane 6). Since NP terminates efficiently on the SUP4 gene (4), the low ratio of specific to nonspecific transcription observed with NP indicates an additional difference between the pol III preparations.
Examining the 280 pol III transcribed genes in the S. cerevisiae genome for potential RNA secondary structure within twelve nucleotides upstream of their terminators, Nielsen et al. proposed that their RNA structure model is relevant to pol III termination in vivo (4). We employed an S. pombe suppressor-tRNA reporter system to examine this issue directly (8, 9). Fig. 2A depicts pSer7T, which has a short 3' trailer between the end of the tRNA module and the 7T terminator. The nearest RNA duplex structure for pSer7T has its 3’-end 9 nt upstream from the terminator. Construct pSer7T-US contains a 26 nt unstructured CA-repeat spacer (US) in the 3' trailer, and pSer7T-HP contains a 26 nt spacer containing a G+C-rich dyad sequence forming an 8 bp hairpin. Structure prediction of the full length sequences (10) confirmed that the US would be single stranded while HP would form an independent hairpin. The control constructs pSer3T-US and pSer3T-HP have only 3 Ts in the terminator region and should produce RT transcripts only; the downstream cRT region is complementary to, and can base pair with, the tRNA sequence thereby blocking normal processing (8). The three 7T constructs produced high levels of suppression, reflected by white colony/patch color (Fig. 2B, top row). The 3T constructs demonstrated that suppression is not achieved in the absence of termination. Northern blot analysis using an intron-based probe of the suppressor-tRNA pSer constructs in Fig. 2B displayed the expected primary nascent RNAs of different lengths reflected in Fig. 2A (Fig. 2C). Prior characterizations indicate that the major, i.e. lowest, band designated by the arrow is an intron-containing intermediate substrate for mature tRNA production ((11), and refs therein). Comparable levels of this band in lanes 1–3 reflect the comparable suppression activities in Fig. 2B. These findings provide strong evidence that a terminator-proximal hairpin is largely inconsequential to suppressor tRNA production by pol III in vivo.
A mechanistic link between termination and reinitiation is widely believed to underlie the high productivity of pol III, which is important for proliferation, growth and development (12, 13). High productivity and additional features also make pol III a compelling choice for therapeutic expression of siRNAs for RNAi and other purposes (14) and refs. therein). In this regard, our data indicate that a terminator-proximal hairpin is not essential for termination, either in vivo or in vitro.
REFERENCES
- 1.Bogenhagen DF, Brown DD. Nucleotide sequences in Xenopus 5S DNA required for transcription termination. Cell. 1981;24:261. doi: 10.1016/0092-8674(81)90522-5. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Folk WR. Termination of transcription by RNA polymerase III from wheat germ. J Biol Chem. 1994;269:4993. [PubMed] [Google Scholar]
- 3.Arimbasseri AG, Maraia RJ. Distinguishing core and holoenzyme mechanisms of transcription termination by RNA polymerase III. Mol Cell Biol. 2013;33:1571. doi: 10.1128/MCB.01733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen S, Yuzenkova Y, Zenkin N. Mechanism of eukaryotic RNA polymerase III transcription termination. Science. 2013;340:1577. doi: 10.1126/science.1237934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuzaki H, Kassavetis GA, Geiduschek EP. Analysis of RNA chain elongation and termination by Saccharomyces cerevisiae RNA polymerase III. J. Mol. Biol. 1994;235:1173. doi: 10.1006/jmbi.1994.1072. [DOI] [PubMed] [Google Scholar]
- 6.Kassavetis GA, Braun BR, Nguyen LH, Geiduschek EP. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell. 1990;60:235. doi: 10.1016/0092-8674(90)90739-2. [DOI] [PubMed] [Google Scholar]
- 7.Engelke DR, Gegenheimer P, Abelson J. Nucleolytic processing of a tRNAArg-tRNAAsp dimeric precursor by a homologous component from Saccharomyces cerevisiae. J Biol Chem. 1985;260:1271. [PubMed] [Google Scholar]
- 8.Hamada M, Sakulich AL, Koduru SB, Maraia R. Transcription termination by RNA polymerase III in fission yeast: A genetic and biochemical model system. J Biol Chem. 2000;275:29076. doi: 10.1074/jbc.M003980200. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Intine RV, Mozlin A, Hasson S, Maraia RJ. Mutations in the RNA Polymerase III Subunit Rpc11p That Decrease RNA 3' Cleavage Activity Increase 3'-Terminal Oligo(U) Length and La-Dependent tRNA Processing. Mol Cell Biol. 2005;25:621. doi: 10.1128/MCB.25.2.621-636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuker M. On Finding All Suboptimal Foldings of an RNA Molecule. Science. 1989;244:48. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- 11.Cherkasova V, Bahler J, Bacikova D, Pridham K, Maraia RJ. Altered nuclear tRNA metabolism in La-deleted S. pombe is accompanied by a nutritional stress response involving Atf1p and Pcr1p that is suppressible by Xpo-t/Los1p. Mol Biol Cell. 2011;23:480. doi: 10.1091/mbc.E11-08-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arimbasseri AG, Rijal K, Maraia RJ. Comparative overview of RNA polymerase II and III transcription cycles, with focus on RNA polymerase III termination and reinitiation. Transcription. 2013;4 doi: 10.4161/trns.27369. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall L, Goodfellow SJ, White RJ. Diminished Activity of RNA Polymerase III Selectively Disrupts Tissues with the Most Actively Dividing Cells. PLoS Biol. 2007;5:e286. doi: 10.1371/journal.pbio.0050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yen MC, et al. An HDAC inhibitor enhances cancer therapeutic efficiency of RNA polymerase III promoter-driven IDO shRNA. Cancer Gene Ther. 2013;20:351. doi: 10.1038/cgt.2013.27. [DOI] [PubMed] [Google Scholar]
- 15.Maraia RJ, Lamichhane TN. 3' processing of eukaryotic precursor tRNAs. WIRES RNA. 2011;2:362. doi: 10.1002/wrna.64. [DOI] [PMC free article] [PubMed] [Google Scholar]