Abstract
Past research has shown that aggressive behaviors can affect female reproductive outcome in non-human primate captive breeding programs. In this study, aggressive behaviors were recorded in a colony of pigtailed macaque monkeys (Macaca nemestrina) and related to pregnancy outcome. For twenty-two weeks, behavioral data were collected from nine breeding groups, consisting of zero to one male (some males were removed after a cycle of conceptions for husbandry reasons) and four to eight females. Observations included all occurrences of eleven aggressive behaviors during 15-minute observation sessions, one to three times a week. Mean weekly aggression levels during the study period were determined for each group, as well as for each pregnancy. Aggression data were summarized with Principal Components Analyses (PCA). Results indicate that pigtailed macaque aggression falls into five distinctive categories: warn, engage, threaten, pursue, and attack. Breeding groups differed in their levels of aggression, even after controlling for group size, presence of a sire, and group stability. Levels of the five aggression categories were found to affect the probability that a pregnancy ended in either a natural birth of a live infant, a clinical intervention producing a live infant, or a nonviable outcome. The predictive value of aggression was significant when clinical interventions were included as possible reproductive outcomes, Behavioral observation of captive groups could identify “risk” conditions affecting pregnancy outcome and the requirement for clinical intervention.
Keywords: aggression, pregnancy, reproductive, outcome, macaque
Introduction
The breeding of captive non-human primates is essential to a variety of fields including biomedical research [Cohen 1986; Hatch 1987], psychological and developmental research, and management of endangered species populations [Cohen 1986; Hatch 1987; Snowdon 1989]. The management of captive breeding programs requires consideration of the effects that social behavior can have on reproduction, and specifically how exposure to aggressive behavior can have effects during vulnerable phases of pregnancy [Hendrie et al. 1996]. Research has shown that the first trimester of pregnancy is more vulnerable to disruption because of the endocrine changes that are necessary in establishing and maintaining pregnancy [Hendrie et al. 1996; Schneider et al. 1999]. In addition, there is a difference in the degree to which aggression will influence closely related species of non-human primates [Clarke et al. 1987]. For example, variability in stress hormones, immune system function, and time spent from other species-typical behaviors will vary the impact of aggression by species. Increases in aggressive behavior might be related to reproduction and could drastically affect the success of non-human primate captive breeding programs. Therefore, research into the specific effects that aggressive behavior can have on reproductive non-human primate females in social groups is necessary to increase successful management of captive breeding programs and to improve the health and well-being of the animals.
Aggression and Social Groups
Non-human primate social groups in captivity can range from the hundreds, in outdoor corrals, to just a few individuals, in indoor enclosures. The common smaller-group composition of groups consists of a single adult male, several adult females, and their infants. The specifics of such a group composition of pigtailed macaques housed in indoor enclosures has been shown to affect the probability of a viable birth, with viable birth rates positively associated with longer duration of sire presence, increased number of other pregnant females, and decreased number of social moves [Ha et al. 1999]. Social moves were defined as the move of any animal in the group into or out of a group during a female’s pregnancy.
It has been shown that there is a strong positive correlation between contact aggression and the number of females in the group [Erwin & Erwin 1976]. During aggressive interactions, the recipient must mobilize energy reserves [Beehner et al. 2005] through a series of physiological reactions that result in the secretion of glucocortioids from the adrenal cortex [Sapolsky 2002]. While this physiological stress response is adaptive in the short-term (i.e., responding to the current event), chronic activation of the stress response can lead to pathology [Beehner et al. 2005]. As aggressive interactions often involve multiple individuals, this type of physiological stress is not limited to the initial recipient of the aggression, so even high aggression at the level of the group could impact the health of other group members. We hypothesize that high levels of aggression could result in chronic stress and that chronic stress would reduce reproductive success.
Overall, the relationship between aggressive behavior and successful reproduction is obviously complex. If the goal of maximizing the success of captive breeding programs is to be met, we believe that further research must be completed in the following areas: quick and accurate assessment of aggressive behavior, the effects that aggressive behavior can have on reproductive females, and eventually, management techniques to reduce intragroup aggressive behaviors.
In this study, we examined the role of aggressive behavior in influencing pregnancy outcome of captive non-human primates. To achieve our goal, we created a detailed ethogram of biologically relevant aggressive behaviors; recorded intragroup aggressive behaviors over time; statistically condensed our detailed ethogram to a minimum set of measures useful for more efficient future work; and analyzed behavioral data in conjunction with pregnancy records to determine the effect(s) of aggression on pregnancy outcome. Our basic hypothesis was that the probability of a normal live-birth should increase with decreased aggression levels within the social group. To test this hypothesis, we recorded group-wide levels of aggression in social groups of pregnant and non-pregnant females with varying levels of aggression.
Methods
Subjects
Data were collected at the Washington National Primate Research Center (WaNPRC). Subjects included nine groups of socially housed Macaca nemestrina, containing 0–1 males, 3–8 females and 0–4 infants, for a total of 54 animals. There were a total of 41 females in the sample, aged between 4.17 and 13.24 years. There was no difference in ages of females observed between Year 1 (M= 7.22 years, SD−2.76) and Year 2 (M= 7.60, SD=2.95) of the study, t(39)=−0.41, p=0.68. Twenty-seven pregnancies were observed in total, and no female experienced more than one pregnancy in the study period. Prior to the study, females had experienced 0–7 pregnancies; there was no difference in mean parities between Year 1 (M= 1.08, SD=1.72) and Year 2 (M=1.00, SD=1.41) of the study.
Animals were housed in indoor rooms (4.1 m × 3 m × 2.6 m high) under a 12:12hr light cycle. One group was included during the initial 10-weeks of data collection but was unavailable during the later 12-weeks. The WaNPRC is AAALAC-accredited, and all animals were housed under IACUC-approved protocols, and adhered to the legal requirements for animal research in the United States and to the American society of Primatologists’ Principles for the Ethical Treatment of Non Human Primates.
Data Collection
Two distinct types of data were utilized during this study: observations of aggressive behavior and social group demographics, and veterinary records of reproductive status and health. The WaNPRC maintains a detailed database, the Animal Records System (ARS), which contains the social, physiological, reproductive, and clinical history of each non-human primate at the WaNPRC.
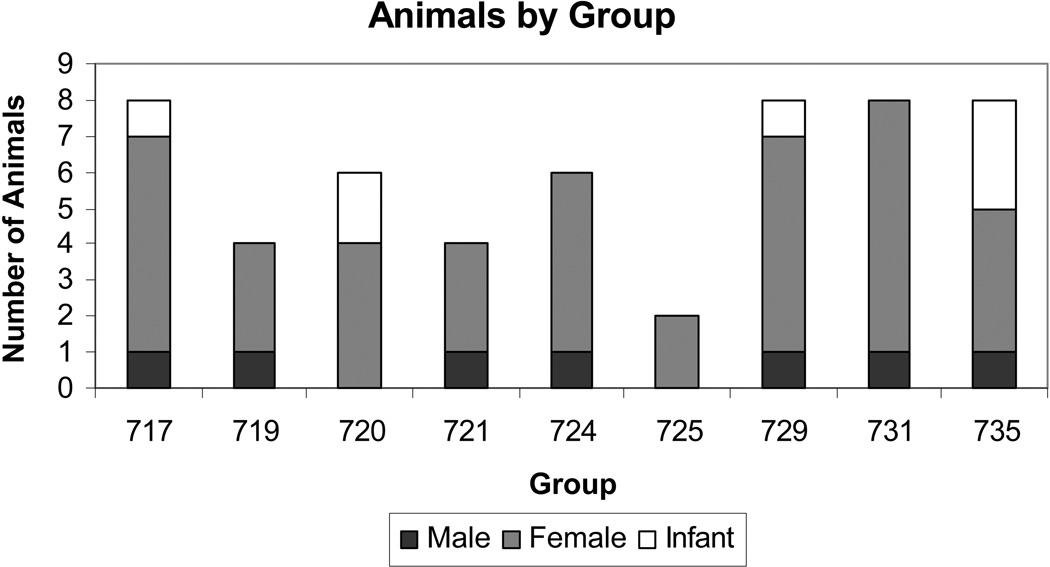
Behavioral data were collected between 08/25/2003 – 12/08/2003 and 08/02/2004 – 12/27/2004; 1–3 times per week; between 1200 and 1800; for a total of 22-weeks. Observations included all occurrences of eleven aggressive behaviors during 15-minute observation sessions. Behavioral observation was made by a single observer, using an all-occurrence method, and recorded using Palm PDA-based software written by the first author. All coding sessions were counter-balanced to account for changes in activity levels during afternoon hours, and similar in relation to feeding and enrichment schedules. Behaviors were calculated as an average rate per hour for each group, each week. Rates were also adjusted for week-to-week and group-to-group differences in group size by dividing by the number of animals per group. Aggressive behaviors recorded included: bite, chase, grab, grunt, hit, lunge, open-mouth, shake, stare, and yawn (Table 1). It is important to note that all of the behaviors included in our ethogram were selected and defined objectively. Behavioral data were recorded from all members of the group except infants (Figure 1). Pregnancy outcome records were obtained from the Animal Records System (ARS). Dates of conception and duration of pregnancies was determined based on timed mating, by observing ovulation cycles and performing ultrasounds of pregnancies. Due to difficulty of detection, first-trimester spontaneous abortions were not included in the data as non-viable births. All pregnancies (first trimester, second trimester, third trimester) that overlapped with the time of behavioral observations were included in the analysis.
Table 1.
Definitions of ethogram behaviors.
| Behavior | Definition |
|---|---|
| Bite, nip | To seize with the teeth or jaws to grip, or wound. |
| Chase | To rapidly follow a recipient that flees. |
| Grab, hold down | To take or seize by a sudden motion or grasp; to keep under restraint. |
| Grunt | Short, rapid vocalization(s) being performed by an animal in an alert posture. |
| Hit, swat | To strike a blow by hand or arm; to strike with a sharp slapping blow. |
| Lunge, charge | A sudden forward rush or reach toward a recipient. |
| Open-mouth threat | To open the eyes widely, raise the eyebrows, pull the ears back, and open the mouth; the mouth corners and lips are tensed forming a rounded opening. |
| Push, shove, lean on | To press against with force in order to drive or impel; to move by forcing away; to apply the pressure of a body upon another. |
| Shake (Cage/Platform) | To move quickly up and down, back and forth, while holding onto a cage wall or platform. |
| Stare | An intent fixed gaze directed at a recipient and accompanied by a tense, rigid posture of the upper body of the actor. |
| Yawn | To open the mouth widely, baring the teeth while tilting the head back. |
Figure 1.
Mean total number of animals in each group over the 22-week observation period, defined by gender and age.
Analyses of aggression data among groups
The first issue was to determine the degree to which aggression varied among groups. To eliminate the need to correct for week-by-week variance in the behavioral data, and to demonstrate variability in aggression among groups, we conducted a General Linear Model (GLM) of group, week, and group-by-week interaction.
The second issue was to reduce our detailed behavioral coding of aggression, summed across all individuals in a group on a week-by-week basis, to a reduced set of biologically-relevant and statistically-independent categories of behaviors which characterize each group in terms of the level of group-wide aggression. To accomplish this, we first performed an overall principal components analysis (PCA) with varimax rotation on all eleven variables to obtain independent factors, the scores of which could then be compared to veterinary records of pregnancy outcome. All PCA components with Eigenvalues >1 were retained, and were defined by behaviors with loading scores of +/− 0.50 or greater. Then, for each pregnancy, factors were calculated for the aggression within the dam’s group for the weeks of her pregnancy, for the reduced set of behavioral factors. These scores, customized for each pregnancy and associated with the ultimate outcome of the pregnancy, set up the following analyses.
To detect which factors might create differences in behavior among groups, we performed an additional hierarchical regression analysis. We included four dependent variables in this analysis: group size, group composition (whether or not an adult male was present in the group), social moves (whether or not a group member had been removed or added since the last observation), and the group itself, as a dummy-coded variable. Variables were entered hierarchically, in the order listed. The components derived from the PCA analysis were used as the dependent variables; this reduced set of dependent variables was used to reduce Type I error (Cohen, 1990).
Analyses of aggression on pregancy outcome
Factor loading scores from the PCA were used to perform logistic regression (LR), with which we assessed the ability of the resulting PCA-based categories of behavior to predict the probability of a viable birth. The LR analyzed pregnancy outcome as one of two possible variables: viable (N=22) or non-viable (N=5). In the analysis, viable was defined to include natural spontaneous birth and clinically-intervened births.
In a second pregnancy-outcome analysis, separation of clinically-intervened births from natural spontaneous births was accomplished through multinomial logistic regression (MLR). The MLR analyzed pregnancy outcome as one of three possible variables: viable (N=14), clinical (N=8), or non-viable (N=5). In this analysis, viable births were defined as natural spontaneous births, while clinical births were those that required clinical intervention, such as cesarean section, but still resulted in a live offspring.
All tests were performed using the SYSTAT and PASW (v. 18, IBM®) statistics programs and were evaluated at the statistical threshold of p=0.05.
Results
Behavioral data were collected for a total of 94.25 hours over 22-total weeks. Compiled behavioral data showed variation in frequency of behaviors by group with some behaviors occurring with higher absolute frequency (e.g. stare vs. push). Two of the eleven behaviors (hit and push) occurred relatively rarely and were removed from this analysis for simplicity of presentation and to reduce Type I error (e.g., Cohen, 1990). The following results apply to the remaining nine behaviors. None of the behaviors showed a significant difference between weeks (Table 2). None of the behaviors showed a significant difference in the group-by-week interaction. Differences among groups explained a significant portion of the variance in open-mouth (R2= 0.295), shake (R2= 0.420), stare (R2=0.329), and yawn (R2= 0.531; Table 2).
Table 2.
Results of General Linear Model Analyses on group, week and group-by-week interaction of ethogram behaviors. Significant results are in bold.
| p-value | ||||
|---|---|---|---|---|
| Behavior | R2 | Group | Week | GrpXWk |
| Bite | 0.166 | 0.985 | 0.922 | 0.969 |
| Chase | 0.369 | 0.996 | 0.775 | 0.606 |
| Grab | 0.149 | 0.952 | 0.978 | 0.964 |
| Grunt | 0.380 | 0.692 | 0.871 | 0.275 |
| Lunge | 0.479 | 0.998 | 0.999 | 0.771 |
| OpnMth | 0.295 | 0.003 | 0.594 | 0.448 |
| Shake | 0.420 | 0.007 | 0.841 | 0.985 |
| Stare | 0.329 | 0.003 | 0.713 | 0.346 |
| Yawn | 0.531 | <.001 | 0.982 | 0.063 |
PCA results indicated that 62.5 percent of the variance among the eleven variables was explained by the five categories of behaviors (Table 3). We defined the reduced categories, in increasing order of intensity, as: warn, engage, threaten, pursue, and attack. Warn includes only the behavior yawn, while Engage includes stare and grunt. Threaten includes shake and push; Pursue includes lunge, open-mouth, and chase; Attack includes grab, bite, and hit.
Table 3.
Results of Principal Components Analyses of ethogram behaviors. Significant groups of behaviors are grouped in bold.
| BEHAVIOR CATEGORIES | |||||
|---|---|---|---|---|---|
| BEHAVIORS | ATTACK | ENGAGE | PURSUE | WARN | THREATEN |
| GRAB | 0.81 | 0.17 | 0.19 | 0.04 | 0.04 |
| BITE | 0.78 | 0.05 | −0.01 | −0.14 | −0.29 |
| HIT | 0.55 | −0.33 | 0.02 | 0.21 | 0.07 |
| STARE | −0.10 | 0.75 | −0.17 | 0.05 | −0.01 |
| GRUNT | 0.15 | 0.61 | 0.07 | 0.30 | 0.02 |
| LUNGE | 0.01 | −0.03 | 0.83 | −0.07 | 0.02 |
| OPNMTH | 0.16 | −0.16 | 0.67 | 0.34 | −0.18 |
| CHASE | 0.13 | 0.49 | 0.51 | −0.33 | 0.19 |
| YAWN | 0.03 | 0.19 | 0.01 | 0.79 | 0.06 |
| SHAKE | −0.06 | −0.01 | 0.11 | 0.13 | −0.82 |
| PUSH | 0.24 | −0.02 | −0.09 | −0.25 | −0.63 |
| Eigenvalue | 2.02 | 1.51 | 1.26 | 1.05 | 1.04 |
| VARIANCE | 1.70 | 1.38 | 1.48 | 1.09 | 1.23 |
| % VARIANCE | 15.48% | 12.52% | 13.44% | 9.87% | 11.18% |
| TOTAL: | 62.49% | ||||
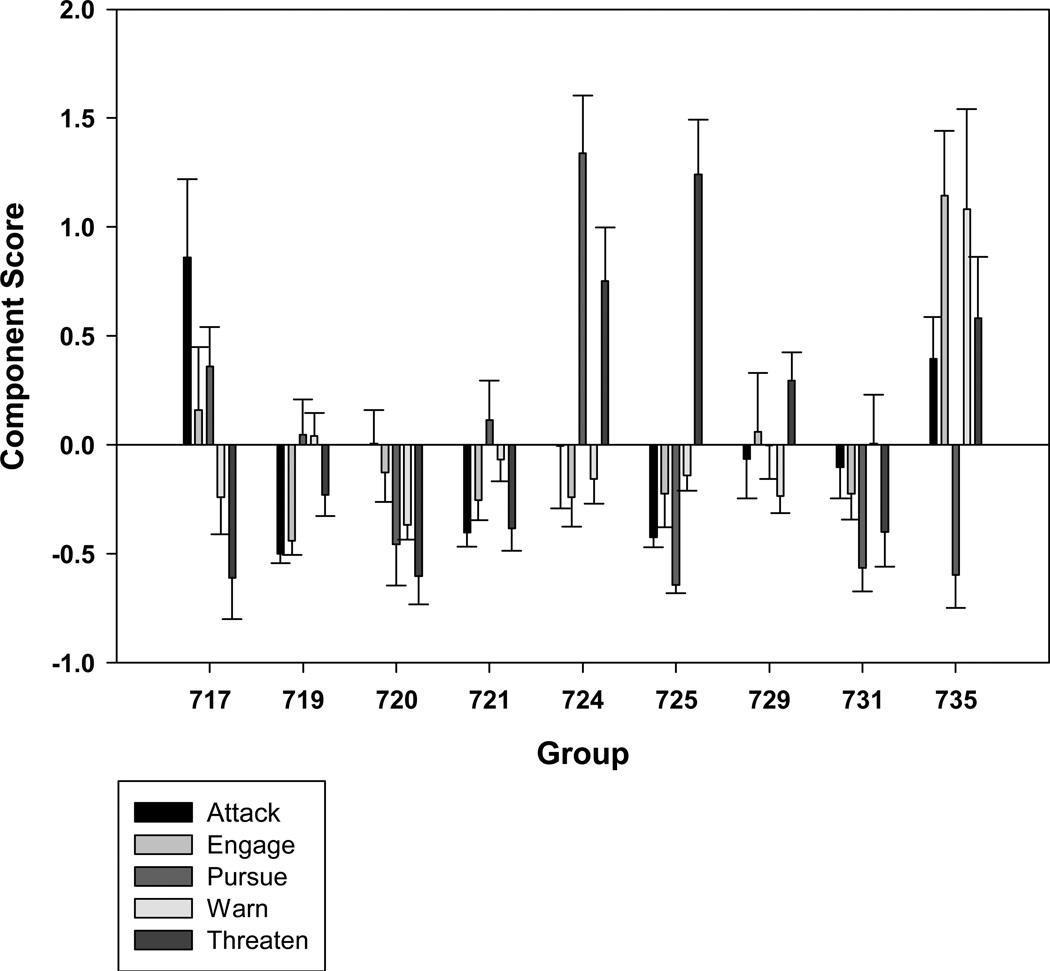
The regression analysis indicated that group size and group composition were significant predictors of aggressive behavior, but that groups differed significantly even after controlling for these factors (Table 4, Figure 2). Whether or not the group had experienced recent social moves was not a significant independent predictor of aggression in this analysis. Specifically, group size was a significant independent predictor of the components Attack and Engage, while group composition was a predictor of Attack, Pursue, and Threaten. Groups with a male present had significantly lower Attack scores (M= − 0.02, SD=1.02) than groups with no male (M= 0.14, SD= 0.81), as well as lower Threaten scores (M= 0.36, SD= 0.97) than groups with no male (M= − 0.05, SD=1.00), but significantly higher Pursue scores (M= 0.10, SD=1.00) than groups with no male (M= − 0.81, SD=0.50; Figure 2). Attack score increased significantly with group size (R=0.34, p<.001), as did Engage scores (R=0.32, p<.001).
Table 4.
Results of a Hierarchical Regression Analysis. Increment in variance explained by each predictor is shown, along with the F-value of the increment. Significant relationships (at the p<.05 level) are shown in bold. Degrees of freedom for each model are shown in the bottom row.
| DV | Model 1: R2change for GROUP SIZE |
Model 2: R2 change COMPOSITION |
Model 3: R2 change for MOVES |
Model 4: R2 change for ROOM |
|---|---|---|---|---|
| ATTACK | R2=0.12, F=23.41 | R2 =0.02, F=4.68 | R2 = 0.01, F=1.59 | R2=0.10 F=2.93 |
| ENGAGE | R2=0.10, F=20.17 | R2 = 0.0001, F=0.03 | R2 = 0.0001, F=0.0.02 | R2=0.12, F=3.30 |
| PURSUE | R2 = 0.0004, F=0.08 | R2=0.10, F=19.07 | R2 = 0.014, F=2.81 | R2=0.27, F=9.28 |
| WARN | R2 = 0.013, F=2.44 | R2 = 0.003, F=0.49 | R2 = 0.003, F=0.63 | R2=0.18, F=4.70 |
| THREATEN | R2 = 0.013, F=2.36 | R2=0.03, F=5.22 | R2 = 0.003, F=0.49 | R2=0.31, F=10.29 |
| DF | (1, 179) | (1, 178) | (1, 177) | (8, 169) |
Figure 2.
Mean scores on PCA-derived aggression components for each group, +/− SE.
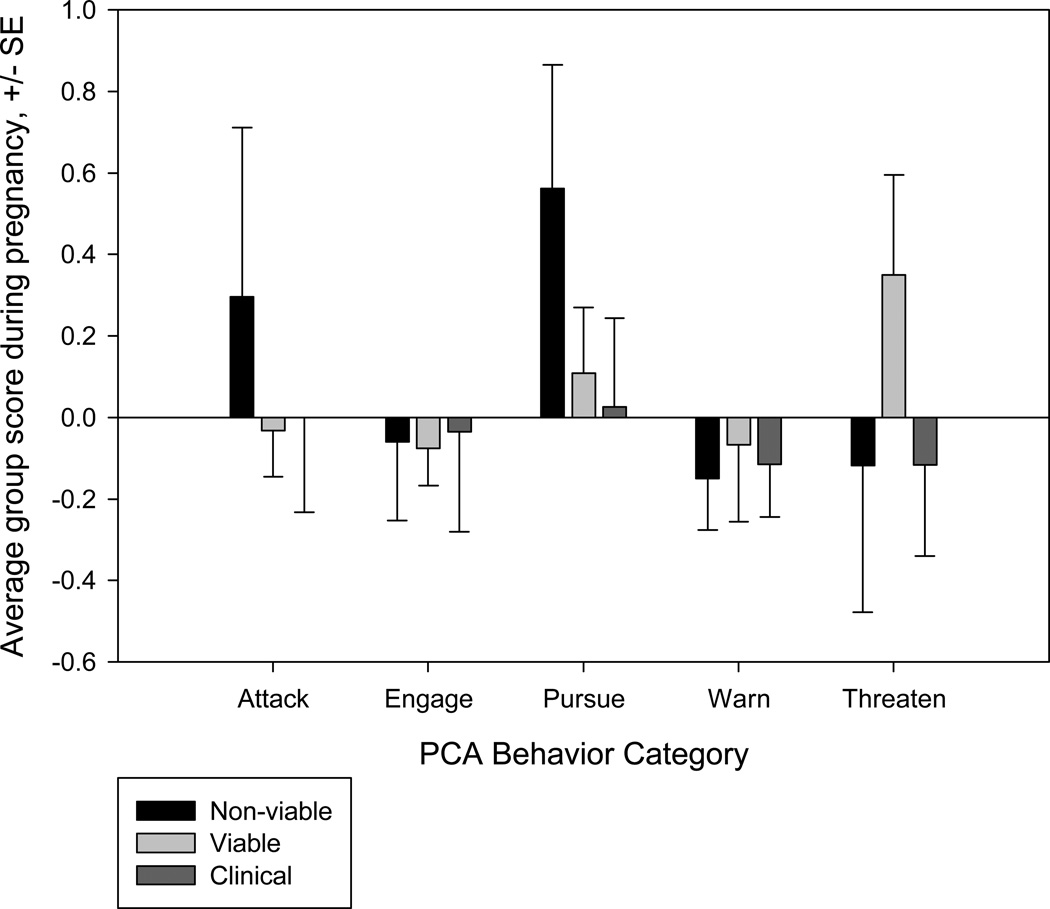
Group differences in pregnancy outcome are shown in Table 5. Logistic regression indicated that the probability of a viable birth was not significantly predicted by the five categories of behavior, when analyzed as one of two possible outcomes (X25=8.734, p= 0.120, R2= 0.337). Multinomial logistic regression indicated that the probability of a viable birth was significantly predicted by the five categories of behavior, when analyzed as one of three outcomes (X210= 21.986, p=0.015, R2=0.402). The separation of natural births from live births requiring clinical intervention indicated that those requiring clinical intervention were positively associated, while natural births were negatively associated with levels of aggression (Figure 3).
Table 5.
Distribution of pregnancy outcomes by social group.
| Room | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | 717 | 719 | 720 | 721 | 724 | 725 | 729 | 731 | 735 | TOTAL |
| Not viable | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 5 |
| Clinical | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 8 |
| Viable | 1 | 0 | 2 | 0 | 3 | 1 | 4 | 2 | 1 | 14 |
| TOTAL | 4 | 3 | 2 | 2 | 5 | 1 | 4 | 4 | 2 | 27 |
Figure 3.
a) Mean group scores for each PCA-derived aggression component across pregnancy outcome categories (Non-viable, Viable, and Clinical Intervention), +/− SE.
Discussion
The GLM results supported our prediction that aggression levels would vary between groups. This result is consistent with our expectations of natural variation among groups, depending in part on group composition. Results indicated that seven behaviors were significantly different between groups, while four behaviors were either infrequent or consistent in frequency across groups (Tables 2).
The practice of collecting behavioral data requires significant commitment of time and concentration. Determination of succinct “molar” categories of biologically relevant behavior can facilitate the collection of quick and accurate behavioral data by a single observer [Coelho & Bramblett 1981a, b]. Preliminary and/or periodic evaluation by PCA enables adjustment of the “molar” ethogram to the current study/subjects. Our PCA reduced the eleven recorded behaviors to five categories of behaviors relevant to this type of research. We chose to label our five categories as: Warn, Engage, Threaten, Pursue, and Attack.
We found that these five categories of aggressive behaviors differed significantly among groups, as shown in Figure 2. The presence of a sire predicted reduced incidence of Attack and Threat, but increases in Pursue. The decrease in Attack behavior is consistent with past studies showing that presence of a sire reduces contact aggression (Ha et al., 1999; Bernstein & Sharpe, 1964). As group size increased, the number of Attack and Engage behaviors increased, but other behaviors were unaffected. Interestingly, groups differed even after controlling for these variables, suggesting that some other difference among groups may account for the difference in aggressive behavior. We suggest that individual differences in temperament among group members may create these differences, as past research has shown such differences to be related to group dynamics in other species [e.g., Sih & Watters, 2005; Capitanio, 2004]. We hope to explore this relationship in future research.
The five categories were then collectively tested against reproductive history to determine the potential role of exposure to aggression in determining pregnancy outcome. Together the five behaviors negatively affected pregnancy outcome, and significantly indicated the probabilityof viable, clinical-intervention, and nonviable outcomes. With a two-way outcome, logistic regression explained a marginally significant 33.7 percent of the variability in the model, while multinomial logistic regression on the three-way outcome explained 40.2 percent. Separation of the clinical treatments in pregnancy outcome led to a significantly improved ability to predict pregnancy outcome from group-level aggression levels. It is common to discuss the outcome of a pregnancy as viable vs. non-viable [Ha et al. 1999; Hendrie et al. 1996]; however, pregnancy results can manifest as a variety of physiological states for both mother and offspring. Specific outcomes can include: death of the fetus during pregnancy [Hertig et al. 1971; Hird et al. 1975], death of the mother during pregnancy or birth [Tarantal & Hendrickx 1988], or complication during pregnancy or birth [Hendrie et al. 1996]. Pregnancy or birth complications that occur in captive housing most often result in clinical intervention. An example of pregnancy complication is placenta previa, which can lead to maternal bleeding and fetal loss. These sorts of clinical complications of pregnancy, some of which have been attributed to prenatal stress, include premature birth, low birth weight newborns [Glynn et al. 2001] and small head circumference [Lou et al. 1994].
Our results suggest that aggression can have subtle effects on pregnancy outcome. The predictive value of aggression improved when clinical interventions were included as possible reproductive outcomes. Specifically, the effects of aggression may manifest themselves as complications during pregnancy rather than overt termination of the pregnancy. This finding makes separation of clinically-intervened births from natural viable births essential to the research on aggressive behavior and its effects on pregnancy outcome, prenatal effects of infant development, and evaluations of breeding colony stock management. It is also important to note that under non-captive conditions, it is likely that many of the births we classify here as “clinical intervention” would be non-viable.
Future research on this topic should focus on data collection of individual pregnant females’ exposure to aggressive behavior, including behavior received prior to conception, during pregnancy, and during the post-partum period to allow for comparison with conception ability, pregnancy outcome, as well as infant development. Tracking first-trimester abortions would also help clarify the effects of aggression observed here, and should be monitored in future studies. Data collection on groups during sequential pregnancies would also allow for comparisons of the effects of variation in group composition over time. Ha et al. [1999] demonstrated a connection between social group composition (presence of the sire and of other pregnant females), as well as individual characteristics (age and parity) in determining pregnancy outcome. Our analysis supports the role of males in mediating pregnancy outcome indirectly, via moderating levels of aggression, and suggests that other social influences, such as temperament of group members, may also have an effect. Simultaneously, at a lower level, the influence of aggression on the endocrine and/or immune system is a predictor of pregnancy outcome.
Finally, we would propose an examination of both behavior between- and within-groups, and a range of endocrinological and immunological measures concurrently with pregnancies and their outcomes. While this study used aggression levels as a predictor of pregnancy outcome, it is possible that the causality of this relationship is reversed, and difficult pregnancies actually affect aggression levels within the group. Better understanding of the physiological relationship between aggression and pregnancy outcome will help clarify this issue.
In summary, we found that aggression in pigtail macaque social groups varies among groups, but is consistent within the group over time, and that as aggression levels increase, the probability of an uncomplicated viable birth decreases. Group composition and size account for a significant portion of the variance in aggression levels, although group behaviors are significantly different even when controlling for these factors. In addition, we noted that distinguishing between viable births requiring clinical intervention and natural viable births increases the predictive ability of a group aggression model. This suggests that higher group-wide levels of aggression may likely produce problems during pregnancy which require clinical intervention to produce a live birth. Principal components analysis revealed that our detailed levels of data collection actually involved only five basic functional categories of aggressive behavior, thus producing a simplified list of behaviors, more suitable for complex statistical analyses.
Acknowledgments
We wish to thank the staff of WaNPRC, especially Steve Kelley, for their cooperation and assistance during this project. We also thank Gene (Jim) Sackett, Kris Coleman, Daniel Ha, Renee Robinette Ha, and especially Carolyn Crockett for helpful comments on earlier drafts of this paper. Support for the subjects and for this research was provided by NIH grant RR00166 to the Washington National Primate Research Center and the U.W. Department of Psychology to Hayley Alloway. The WaNPRC is AAALAC-accredited, and all animals were housed under IACUC-approved protocols, and adhered to the legal requirements for animal research in the United States and to the American society of Primatologists’ Principles for the Ethical Treatment of Non Human Primates.
References
- Alberts SC, Sapolsky RM, Altmann J. Behavioral, endocrine and immunological correlates of immigration by an aggressive male into a natural primate group. Hormones and Behavior. 1992;26:167–178. doi: 10.1016/0018-506x(92)90040-3. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whittens PL. The effect of new alpha males on female stress in free-ranging baboons. Animal Behaviour. 2005;69:1211–1221. [Google Scholar]
- Bernstein IS, Sharpe L. Social roles in a rhesus monkey group. Behaviour. 1964;26:91–104. doi: 10.1163/156853966x00038. [DOI] [PubMed] [Google Scholar]
- Bernstein IS. An investigation of the organization of pigtail monkey groups through the use of challenges. Primates. 1966;7:471–480. [Google Scholar]
- Capitanio JP. Personality factors between and within species. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: A model for the study of social organization. Cambridge, UK: Cambridge University Press; 2004. pp. 13–33. [Google Scholar]
- Clarke S, Line S, Ellman G, Markowitz H. Hormonal and behavioral responses of rhesus macaques to an environmental enrichment apparatus. American Journal of Primatology. 1987;12(3):335–336. [Google Scholar]
- Cohen C. The case for the use of animals in biomedical research. New England Journal of Medicine. 1986;315:865–870. doi: 10.1056/NEJM198610023151405. [DOI] [PubMed] [Google Scholar]
- Cohen J. Things I have learned (so far) American Psychologist. 1990;45:1304–1312. [Google Scholar]
- Erwin N, Erwin J. Social density and aggression in captive groups of pigtailed monkeys (Macaca nemestrina. Applied Animal Ethology. 1976;2:265–269. [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: Effects of earthquake timing on stress responsivity in pregnancy. American Journal of Obstetrics and Gynecology. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Ha JC, Robinette RL, Sackett GP. Social housing and pregnancy outcome in captive pigtailed macaques. American Journal of Primatology. 1999;47:153–163. doi: 10.1002/(SICI)1098-2345(1999)47:2<153::AID-AJP5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Hatch OG. Biomedical research. American Psychologist. 1987;42:591–592. doi: 10.1037/h0092051. [DOI] [PubMed] [Google Scholar]
- Hendrie TA, Peterson PE, Short JJ, Tarantal AF, Rothgarn E, Hendrie MI, Hendrickx AG. Frequency of prenatal loss in a macaque breeding colony. American Journal of Primatology. 1996;40:41–53. doi: 10.1002/(SICI)1098-2345(1996)40:1<41::AID-AJP3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hertig AT, King NE, MacKey J. Spontaneous abortion in wild-caught rhesus monkeys (Macaca mulatta) Laboratory Animal Science. 1971;21:510–519. [PubMed] [Google Scholar]
- Hird DW, Hendrickson RV, Hendrickx AG. Infant mortality in Macaca mulatta: Neonatal and post-neonatal mortality at the California Primate Research Center, 1968–1972. Journal of Medical Primatology. 1975;4:8–22. [PubMed] [Google Scholar]
- Lou HC, Hansen D, Nordentoft M, Pryds O, Jensen F, Nim J, Hemmingsen R. Prenatal stressors of human life affect fetal brain development. Developmental Medicine and Child Neurology. 1994;36:826–832. doi: 10.1111/j.1469-8749.1994.tb08192.x. [DOI] [PubMed] [Google Scholar]
- Michael RP, Zumpe D. A review of hormonal factors influencing the sexual and aggressive behavior of macaques. American Journal of Primatology. 1993;30(3):213–241. doi: 10.1002/ajp.1350300306. [DOI] [PubMed] [Google Scholar]
- Oswald M, Erwin J. Control of intragroup aggression by male pigtail monkeys (Macaca nemestrina) Nature. 1976;262:686–687. [Google Scholar]
- Pereira M. Abortion following the immigration of an adult male baboon (Papio cynocephalus) American Journal of Primatology. 1983;4:93–98. doi: 10.1002/ajp.1350040109. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral endocrinology. Cambridge, Massachusetts: MIT Press; 2002. pp. 409–450. [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: An examination of ontogenetic vulnerability. Child Development. 1999;70:263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Sih A, Watters JV. The mix matters: behavioural types and group dynamics in water striders. Behaviour. 2005;142(9/10):1417–1431. [Google Scholar]
- Snowdon CT. The criteria for successful captive propagation of endangered species. Zoo Biology. 1989;1:149–161. [Google Scholar]
- Tarantal AF, Hendrickx AG. The use of ultrasonography for evaluating pregnancy in macaques. In: Neubert D, Merker HJ, Hendrickx AG, editors. Proceedings of the symposium on non-human primates- developmental biology and toxicology. Wien, Berlin: Ueberreuter Wissenschaft; 1988. pp. 91–99. [Google Scholar]
- Williams MT, Davis HN, Mccrea AE, Long SJ, Hennessy MB. Changes in the hormonal concentrations of pregnant rats and their fetuses following multiple exposures to a stressor during the third trimester. Neurotoxiocology Teratology. 1999;21:403–414. doi: 10.1016/s0892-0362(98)00060-9. [DOI] [PubMed] [Google Scholar]