Abstract
The Bacillus subtilis gltAB operon, encoding glutamate synthase, requires a specific positive regulator, GltC, for its expression and is repressed by the global regulatory protein TnrA. The factor that controls TnrA activity, a complex of glutamine synthetase and a feedback inhibitor, such as glutamine, is known, but the signal for modulation of GltC activity has remained elusive. GltC-dependent gltAB expression was drastically reduced when cells were grown in media containing arginine or ornithine or proline, all of which are inducers and substrates of the Roc catabolic pathway. Analysis of gltAB expression in mutants with various defects in the Roc pathway indicated that rocG-encoded glutamate dehydrogenase was required for such repression, suggesting that the substrates or products of this enzyme are the real effectors of GltC. Given that RocG is an enzyme of glutamate catabolism, the main regulatory role of GltC may be prevention of a futile cycle of glutamate synthesis and degradation in the presence of arginine-related amino acids or proline. In addition, high activity of glutamate dehydrogenase was incompatible with activity of TnrA.
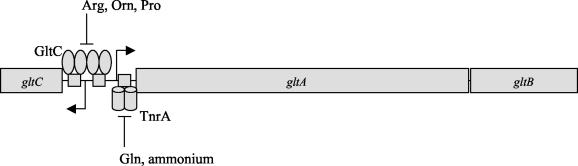
Glutamate is the major donor of nitrogen groups and the principal anion in bacterial cells. The balance between the rates of glutamate synthesis and degradation must be carefully maintained in order to preserve the cellular pool. In Bacillus subtilis cells grown in minimal medium, glutamate is synthesized by glutamate synthase, encoded by the gltAB genes (4) (Fig. 1). Expression of the gltAB operon is subject to nitrogen source-dependent regulation. Transcription is high when cells are grown in glucose-ammonium medium and low when cells are grown in glucose-glutamate or glucose-proline medium; an intermediate to high level of transcription is seen if glutamine or glutamate plus ammonium or proline plus ammonium serves as the nitrogen source (5, 14, 15). In previous work, we showed that glutamate-dependent repression of gltAB is mediated principally by the TnrA protein (12), a global regulator of many genes of nitrogen metabolism that is active under conditions of nitrogen limitation (20, 22, 33, 36) (Fig. 1). When nitrogen is in excess, glutamine and other inhibitors of glutamine synthetase accumulate and bind to glutamine synthetase, inactivating it as an enzyme and causing it to form a complex with TnrA. This TnrA-glutamine synthetase complex is unable to activate TnrA-dependent genes or repress TnrA-inhibited genes (21, 34).
FIG. 1.
gltCAB locus. Binding of the regulatory proteins GltC and TnrA to the gltCA regulatory region is depicted, and the nutritional factors affecting the activity of these proteins are indicated. The gltCA regulatory region is not drawn to scale.
The gltAB operon requires for its regulation, in addition to TnrA, a specific regulator, GltC, a member of the LysR family of bacterial transcription factors (5, 15, 28). The gltC gene is located upstream of and is transcribed divergently from the gltAB operon (Fig. 1). GltC activates the gltA promoter and is essential for cell growth in the absence of glutamate in the medium (5, 15). GltC is also a negative autoregulator (5, 15). The activity of most LysR-type proteins is modulated by interaction with low-molecular-weight effectors (28). GltC is likely to interact with such effectors as well, since mutants with partially constitutive GltC activity have been isolated (10). The nature of the GltC effector remains unknown, however, and its identification by in vitro assays has been hampered by the insolubility of purified GltC (our unpublished results).
When grown in the presence of exogenous amino acids, B. subtilis cells can produce glutamate not only by the glutamate synthase reaction but also by α-ketoglutarate-utilizing aminotransferase reactions and by degradation of certain amino acids, e.g., glutamine, proline, and arginine. Catabolism of arginine to glutamate proceeds by the first three steps of the Roc pathway through ornithine and γ-glutamic semialdehyde (22) (Fig. 2). The last of these three steps is shared with the pathway of proline utilization. Enzymes of the arginine utilization (RocA) and proline utilization (YcgN) pathways both contribute to the penultimate reaction of degradation of these amino acids (Fig. 2) (our unpublished results). The final step of arginine and proline utilization, from glutamate to α-ketoglutarate and ammonium, is catalyzed by glutamate dehydrogenase (GlutDH), the product of the rocG gene (Fig. 2) (11).
FIG. 2.
Roc pathway of utilization of arginine-related amino acids and proline. The enzymes in this figure are indicated by the name of the corresponding gene, as follows: UreABC, urease; RocF, arginase; RocD, ornithine aminotransferase; RocA and YcgN, Δ1-pyrroline-5-carboxylate dehydrogenases; RocG, glutamate dehydrogenase; RocB, citrullinase(?); YcgM and YusM, proline oxidases (proline dehydrogenases).
The rocG gene encodes the only active GlutDH in B. subtilis cells. RocG is strictly catabolic in vivo (11). Another gene, gudB, codes for an inactive form of a second catabolic GlutDH, which can be activated by a short deletion mutation (11).
rocG, like other genes of the Roc pathway, is transcribed by the σL-containing form of RNA polymerase and requires the transcription factors RocR and AhrC for its expression (8, 22). Arginine and proline as well as ornithine and citrulline induce the genes of the Roc pathway (3, 11, 16, 24). The rocG gene, in contrast to the other roc genes, is repressed by the catabolite control protein, CcpA, when glucose is available (6). Therefore, the Roc pathway has a dual function with respect to the glutamate pool; it produces glutamate from arginine or proline whether cells are growing in the presence or absence of glucose, and it degrades glutamate but only does so efficiently when glucose is absent.
In this work, we show that GltC activity is decreased when arginine, arginine-related amino acids, or proline serves as the sole nitrogen or carbon source. A metabolite created as an intermediate or product of the Roc pathway and whose level depends on the activity of GlutDH appears to be an effector of GltC activity.
MATERIALS AND METHODS
Bacterial strains and culture media.
The B. subtilis strains used in this study are described in Table 1 or in the table footnotes. Escherichia coli strain JM107 (35) was used for propagation of most plasmids. E. coli strain BU1255 (dam-3 dcm-6 gal lac ara thr leu/F+) was used for experiments requiring unmethylated DNA. Media and growth conditions were described previously (11). TSS minimal medium was supplemented with 0.5% glucose, 0.4% succinate, or other carbon and nitrogen sources supplied at 0.2%.
TABLE 1.
B. subtilis strains used
| Strain | Genotype | Source or reference |
|---|---|---|
| SMY | Wild type | P. Schaeffer |
| SMY-S | ΔgltC::spc | 5 |
| SF62 | tnrA62::(Tn917 erm) trpC2 | 33 |
| SF168U | ureC::spc trpC2 | 17 |
| BR151MA ccpA::spc | ccpA::[(Tn917-lacZ)::spc] trpC2 lys-3 | T. Henkin |
| QB5521 | rocA::cat trpC2 | M. Débarbouillé |
| QB5577 | rocF::aphA3 trpC2 | 24 |
| QB5618 | rocD::aphA3 trpC2 ΔamyE::[cat Φ(rocD′-lacZ)] | 25 |
| QB5626 | rocR(T120I) trpC2 ΔamyE::[cat Φ(rocD′-lacZ)] | 25 |
| SMB32 | ycgN::kan pheA1 trpC2 | E. Bremer |
| LG203 | ΔamyE::[cat Φ(gltC′p3-gusA) Φ(gltA′p3-lacZ)] | 12 |
| LG219 | ΔamyE::[cat Φ(gltC′p19-gusA) Φ(gltA′p19-lacZ)] | 12 |
| LG219-T | tnrA62::(Tn917 erm) ΔamyE::[cat Φ(gltC′p19-gusA)Φ(gltA′p19-lacZ)] | 12 |
| LG219/24 | ΔamyE::[cat Φ(gltC′p19/24-gusA) Φ(gltA′p19/24-lacZ)] | 12 |
| LG219/24-T | tnrA62::(Tn917 erm) ΔamyE::[cat Φ(gltC′p19/24-gusA) Φ(gltA′p19/24-lacZ)] | 12 |
| LG286 | ΔamyE::[cat Φ(gltC′p86-gusA) Φ(gltA′p86-lacZ)] | 5 |
| LG286-T | tnrA62::(Tn917 erm) ΔamyE::[cat Φ(gltC′p86-gusA) Φ(gltA′p86-lacZ)] | LG286 × SF62 DNA |
| LG419 | ΔamyE::[cat Φ(gltC′p19-gusA) Φ(gltA′p19-lacZ)] | SMY × pLG419 |
| BB603 | ΔgltC::spc gltR24 | 7 |
| BB1218 | rocA::cat | SMY × QB5521 DNA |
| BB1225 | rocA::(cat::tet) | BB1219 × pPS28 |
| BB1271 | ΔrocG::ble ΔgudB::tet | 11 |
| BB1284 | ΔrocG::ble gudB1 | 11 |
| BB1628 | yweA::pBB1013 (′yweA′ neo) | 6 |
| BB1744 | rocGp1 ΔgudB::tet | 6 |
DNA manipulations and transformation.
Methods for plasmid isolation, agarose gel electrophoresis, use of restriction and DNA-modification enzymes, DNA ligation, PCR, and electroporation of E. coli cells were as described by Sambrook et al. (27). Isolation of chromosomal DNA and transformation of B. subtilis cells by chromosomal or plasmid DNA were done as described before (11).
Plasmid pPS28, used to replace a chloramphenicol resistance marker with a tetracycline resistance marker, was constructed by P. Serror (unpublished results) from pCm::Tc (29) by replacing the 2-kb PvuI-SmaI fragment containing the origin of replication of pE194 with the 435-bp PvuI-SspI fragment of pBR322.
To construct a gltA-lacZ fusion containing the strong ribosome-binding site of the B. subtilis spoVG gene, the 0.29-kb BclI-BamHI fragment containing the gltC-gltA intergenic regulatory region of pIPC119, a pIPC100 derivative with the gltAp19 mutation (5), was cloned in two orientations in the BglII site of a bidirectional fusion vector, pLG103 (9). The two resulting plasmids, pLG419 and pLG519, contained gltAp19-lacZ and gltCp19-gusA or gltAp19-gusA and gltCp19-lacZ fusions, respectively. The gltAp19 mutation does not affect expression or regulation from the gltA promoter but strongly increases expression from the gltC promoter and was used to elevate the activity of the gltC-gusA fusion. The plasmids were integrated at the amyE locus of B. subtilis as described previously (5).
Enzyme assays.
β-Galactosidase and β-glucuronidase activities, in Miller units, were determined as described previously (5). All activities reported are the averages of at least two experiments, and the mean errors did not exceed 30%.
RESULTS
Arginine, ornithine, and proline-mediated repression of gltA is independent of TnrA.
Addition of either arginine or proline to the growth medium has long been known to decrease the specific activity of B. subtilis glutamate synthase to roughly the same extent as addition of glutamate (18, 26). After the role of TnrA in glutamate-mediated repression of gltA had been recognized (12), we reexamined the arginine effect to see whether it was at the transcriptional level and whether it depended on TnrA. Expression of a gltA-lacZ transcriptional fusion was decreased in arginine-grown cells compared to ammonium-grown cells, although less severely than in glutamate-grown cells (Table 2, strain LG219). Importantly, arginine-mediated gltA “repression” (the term repression is used operationally simply to indicate reduced gltA expression) remained intact in a tnrA null mutant (Table 2, strain LG219-T). gltA-lacZ expression was decreased even more strongly in ornithine-grown cells; again this effect was independent of TnrA (Table 2). To confirm the TnrA independence of gltA repression mediated by the substrates of the Roc pathway, we used the gltAp24-lacZ fusion containing a TnrA-insensitive version of the gltA promoter (12). This fusion was still repressed by arginine or ornithine or proline, another substrate of the Roc pathway (Fig. 2), although no repression by glutamate was detected (Table 2, strains LG219/24 and LG219/24-T, and data not shown).
TABLE 2.
Expression of the gltA gene in the presence of different nitrogen sourcesa
| Strain | Relevant genotype | gltA/gltC region of the fusion | β-Galactosidase activity (gltA-lacZ fusion) (U)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Medium with glucose plus:
|
Medium without glucose but with ornithine | ||||||||
| Ammonium | Glutamate | Arginine | Ornithine | Ornithine, ammonium | Citrulline | ||||
| LG219 | Wild type | gltAp+ | 74.2 | 2.0 | 5.4 | 1.1 | 3.8 | 34.9 | 0.8 |
| LG219-T | tnrA | gltAp+ | 73.2 | 36.5 | 3.4 | 1.2 | 2.3 | 29.1 | 0.9 |
| BB1118 | ureC | gltAp+ | 1.3 | ||||||
| BB1119 | ureC tnrA | gltAp+ | 1.8 | ||||||
| LG219/24 | Wild type | gltAp24 | 74.4 | 32.6 | 6.5 | 0.8 | |||
| LG219/24-T | tnrA | gltAp24 | 78.0 | 31.2 | 4.6 | 0.9 | |||
Strains BB1118 and BB1119 are derivatives of LG219. The strains were grown to mid-exponential phase in the indicated minimal media and assayed for β-galactosidase activity. Strains of the LG219 series contain the gltAp19 mutation (4) within their fusions. This mutation does not effect expression or regulation from the gltA promoter but strongly increases expression from the gltC promoter and was used to increase the activity of the gltC-gusA fusion.
The proline effect on the wild-type gltA promoter, although obvious in tnrA+ strains (5), was obscured in tnrA null mutant strains (data not shown). It is possible that proline uptake (or degradation) is impaired in tnrA cells, and therefore the intracellular proline concentration does not reach the level required for gltA repression.
Role of ammonium in modulation of TnrA-independent gltA repression.
Arginine degradation by rocF-encoded arginase produces ornithine and urea (Fig. 2). In a ureC mutant that is unable to convert urea to ammonium and CO2 (17), gltA repression in arginine-grown cells was more pronounced than in ureC+ cells and was similar to that in ornithine-grown cells (Table 2, strains BB1118 and BB1119). This result might suggest that ammonium counteracts gltA repression. In accord with this result, gltA repression was less severe in the presence of ornithine plus ammonium than in ornithine-grown cells (Table 2, strains LG219 and LG219-T). In a more extreme case, growth with citrulline, another substrate of the Roc pathway, did not cause repression of the gltA-lacZ fusion (Table 2, strains LG219 and LG219-T). Although the pathway of citrulline utilization has not been established in B. subtilis, our unpublished results suggest that citrulline is converted to ornithine and carbamate by the product of the rocB gene, which is distantly related to some carbamoylases. Because carbamate can be rapidly hydrolyzed to ammonium and CO2, citrulline degradation probably provides copious amounts of endogenous ammonium in addition to ornithine. We hypothesize that a high intracellular ammonium concentration counteracts the repressing effect of the substrates of the Roc pathway on gltA expression.
Most of the experiments to be presented below have been done with ornithine-grown cells.
Roc pathway-mediated repression requires GltC.
Since the effects of arginine, ornithine, and proline on gltA expression could be separated from repression by TnrA, we sought to test the involvement of GltC in regulation by Roc pathway substrates. It is difficult to study gltAB expression in the absence of GltC because the residual level of transcription is very low. Three different strategies allowed us to increase this residual expression and thereby study GltC-independent expression. First, the up-promoter mutation gltAp3 makes gltAB expression partially independent of GltC (12). Second, a gain-of-function mutation in gltR (gltR24) allows GltR, a transcription factor that is independent of the nitrogen source, to substitute for GltC (7, 12). Third, by using a lacZ fusion with a very strong ribosome-binding site, we can increase the sensitivity of the expression assay. In all three cases, gltA-lacZ fusions showed no TnrA-independent ornithine-mediated repression in the absence of GltC even though they were still repressed by glutamate and by ornithine (which is converted to glutamate as a result of catabolism) in a TnrA-dependent manner (Table 3). Thus, GltC seems to be the target of Roc pathway-mediated regulation. Moreover, the apparent TnrA-independent repression of gltA seen in ornithine-grown wild-type cells probably reflects inactivity of GltC, a positive regulator.
TABLE 3.
Requirement for GltC for ornithine-mediated gltA repressiona
| Strain | Relevant genotype | gltA/gltC region of the fusion | β-Galactosidase activity (gltA-lacZ fusion) (U)
|
|||
|---|---|---|---|---|---|---|
| Medium with glucose plus:
|
Medium without glucose but with ornithine | |||||
| Ammonium | Glutamate | Ornithine | ||||
| LG203-S | gltC | gltAp3 | NGb | 1.1 | 2.3 | 11.2 |
| LG203-ST | gltC tnrA | gltAp3 | NG | 12.3 | 10.4 | |
| BB660 | gltC gltR24 | gltAp+ | 26.5 | 1.5 | 3.1 | 22.6 |
| BB873 | gltC gltR24 tnrA | gltAp+ | 32.0 | 28.8 | 21.5 | |
| LG419-S | gltC | gltAp+ | NG | 4.0 | 7.8 | 19.3 |
| LG419-ST | gltC tnrA | gltAp+ | NG | 24.1 | 23.4 | |
The strains were grown to mid-exponential phase in the indicated minimal media and assayed for β-galactosidase activity. Strains LG203-S and LG203-ST contain a gltAp3-lacZ fusion as in LG203. Strains BB660 and BB873 contain a gltA-lacZ fusion as in LG219. Strains LG419-S and LG419-ST contain a gltA-lacZ fusion as in LG419. Strains of the LG419 series contain the B. subtilis spoVG ribosome-binding site upstream of the lacZ gene. All other gltA fusions used in this work have the E. coli lpp ribosome-binding site.
NG, no growth. gltC mutants cannot grow if a source of glutamate is not present.
Constitutively active GltC mutants.
Previously, we reported the isolation of three gltC mutants with partially constitutive GltC activity. In these mutants, gltA-lacZ expression is much less reduced, compared to the wild-type strain, in the presence of glutamate, proline, or ornithine as the nitrogen source (10) (Table 4, strains BB917, BB947, and BB977). Little or no effect of ornithine on gltA expression was observed in such mutants if TnrA-dependent repression was eliminated by a tnrA null mutation (Table 4, strains BB1051 to BB1053). This result confirms that GltC is the target of ornithine-mediated repression and shows that GltC proteins previously described as partially constitutively active are fully or almost fully insensitive to ornithine-mediated repression.
TABLE 4.
Role of GltC in ornithine-mediated gltA repressiona
| Strain | Relevant genotype | gltA/gltC region of the fusion | β-Galactosidase activity (gltA-lacZ fusion) (U)
|
|||
|---|---|---|---|---|---|---|
| Medium with glucose plus:
|
Medium without glucose but with ornithine | |||||
| Ammonium | Glutamate | Ornithine | ||||
| LG219 | Wild type | gltAp+ | 74.2 | 2.0 | 1.1 | 0.8 |
| BB917 | gltC(P88L) | gltAp+ | 100.5 | 13.5 | 26.2 | 75.6 |
| BB1051 | gltC(P88L) tnrA | gltAp+ | 104.7 | 125.8 | 76.0 | |
| BB947 | gltC(T99A) | gltAp+ | 167.6 | 27.9 | 29.1 | 208.8 |
| BB1052 | gltC(T99A) tnrA | gltAp+ | 203.5 | 203.5 | 222.8 | 208.3 |
| BB977 | gltC(I160K) | gltAp+ | 104.6 | 5.8 | 8.0 | 61.0 |
| BB1053 | gltC(I160K) tnrA | gltAp+ | 124.1 | 99.0 | 52.6 | |
| LG286 | Wild type | gltAp86 | 166.5 | 21.3 | 56.9 | 198.5 |
| LG286-T | tnrA | gltAp86 | 192.0 | 191.0 | 126.0 | 195.8 |
Strains BB917, BB947, BB977, and BB1051 to BB1053 contain a gltA-lacZ fusion as in LG219 and the proJ::pBB559 (′proJ′ neo) insertion linked to the gltC locus. The strains were grown to mid-exponential phase in the indicated minimal media and assayed for β-galactosidase activity.
The intermediate level of gltA expression observed in tnrA+ versions of strains with constitutively active forms of GltC in the presence of glutamate, proline, or ornithine was the result of a balance between TnrA-mediated repression and activation by GltC (Table 4 and data not shown). In fact, it is the ability of constitutively active GltC to partially override TnrA repression that allowed us to find such mutants in the TnrA+ background (10).
Mutations in the GltC binding site.
Several insertion mutations that increase the distance between the two putative GltC binding sites in the gltA regulatory region (Fig. 1) were thought to make gltA expression partially independent of the GltC activation state (5, 10). In fact, once TnrA-mediated repression was removed, lacZ fusions to these mutant promoters, e.g., gltAp86-lacZ, were highly expressed in ornithine-grown cells (Table 4, strains LG286 and LG286-T).
Effect of mutations in the Roc pathway on GltC activity.
No effect of arginine on gltA expression was observed in a rocF null mutant (Table 5, strain BB1219), which is deficient in the first step of arginine utilization (Fig. 2). Thus, the reduction in GltC activity in arginine-grown cells is not due to arginine per se but either requires RocF protein or reflects, at a minimum, the conversion of arginine to ornithine. The effect of ornithine was not significantly altered by the rocF mutation, suggesting that RocF protein by itself is not responsible for altering GltC activity (Table 5, strain BB1219).
TABLE 5.
Role of the Roc pathway in gltA repressiona
| Strain | Relevant genotype | β-Galactosidase activity (gltA-lacZ fusion) (U) in medium with glucose plus:
|
|||
|---|---|---|---|---|---|
| Ammonium | Arginine, ammonium | Ornithine | Ornithine, ammonium | ||
| LG219-T | tnrA | 73.2 | 4.8 | 1.2 | 2.3 |
| BB1219 | rocF tnrA | 84.8 | 58.5 | 2.2 | 7.1 |
| BB1221 | rocD tnrA | 76.1 | 52.5 | NGb | 73.2 |
| BB2050 | rocA ycgN tnrA | 50.7 | 98.4 | NG | NG |
| BB1282 | rocG tnrA | 72.6 | 32.4 | 27.3 | 36.2 |
| BB1320 | rocG gudB1 tnrA | 106.0 | 2.7 | 0.9 | 0.6 |
All strains have a gltA-lacZ fusion as in LG219. Strain BB1282 contains the ΔgudB::tet mutation, which prevents the emergence of spontaneous gudB1 mutants. The strains were grown to mid-exponential phase in the indicated glucose minimal media and assayed for β-galactosidase activity.
NG, no growth. The rocD mutant cannot grow with ornithine as the only nitrogen source. The double rocA ycgN null mutant does not grow in the presence of ornithine or proline, apparently due to accumulation of a toxic metabolite, presumably γ-glutamic semialdehyde.
No effect of ornithine or arginine on gltA-lacZ expression was detected in rocD mutant cells (Table 5, strain BB1221). Therefore, ornithine itself does not provide the signal for modulating GltC activity, but RocD is required in order to convert ornithine to γ-glutamic semialdehyde (Fig. 2).
Similarly, in the double rocA ycgN null mutant, which is unable to convert Δ1-pyrroline carboxylic acid to glutamate (Fig. 2), gltA-lacZ expression also remained high in arginine-containing medium (Table 5, strain BB2050), indicating that the interconvertible pools of γ-glutamic semialdehyde and Δ1-pyrroline carboxylic acid do not affect GltC activity directly, but rather by way of their oxidation to glutamate.
Finally, the inhibitory effects of arginine and ornithine on gltA expression were mostly relieved in a rocG null mutant (Table 5, strain BB1282). Thus, NAD+-dependent oxidative deamination of glutamate to α-ketoglutarate and NH4+ (Fig. 2) or GlutDH protein itself affects GltC activity. Though other steps of the Roc pathway are also required for Roc pathway-induced inactivity of GltC, their likely role is to provide ornithine, the apparent coactivator for transcription of the rocG gene (24), and glutamate, the substrate for GlutDH.
Interestingly, in a mutant that lacks RocG but produces a different GlutDH by virtue of a gain-of-function mutation in gudB (11), GltC inactivation was also seen in the presence of arginine or ornithine (Table 5, strain BB1320). Thus, it appears that it is more likely to be the enzymatic activity of GlutDH that is important than the protein per se.
Effect of high expression of GlutDH.
To explore further the correlation between GlutDH and gltA expression, we introduced into cells three mutations affecting GlutDH expression and activity. The rocR(T120I) mutation (25) makes rocG expression independent of the Roc pathway inducers, i.e., increases rocG gene expression in media containing ammonium or glutamate but does not affect catabolite repression of rocG (6, 8). The rocGp1 mutation alters the CcpA-binding cre site within the rocG promoter region and relieves CcpA-mediated catabolite repression but does not abolish the requirement for the Roc pathway inducers (6). The gudB1 mutation activates an alternative catabolic GlutDH activity under all growth conditions tested (11). In strains lacking TnrA, none of these mutations caused any substantial reduction in gltA-lacZ expression in glucose-ammonium medium (Table 6). In glucose-glutamate medium, the slightly elevated expression of rocG in the rocGp1 mutant and the moderately elevated expression of rocG in the rocR(T120I) mutant caused a significant decrease in gltA expression (Table 6, strains BB1338 and BB1753), indicating that increased GlutDH activity and glutamate excess are both required for GltC inactivation. However, no decrease in gltA-lacZ expression was observed if high or very high GlutDH activity was present, as in the case of a gudB1 mutant or the rocR(T120I) rocGp1 double mutant, respectively (Table 6, strains BB1320 and BB2070). More surprisingly, gltA expression in the rocGp1 mutant cells (strains BB1753 and BB2070) grown in glucose-ornithine medium was significantly increased compared to that in rocGp+ cells, apparently due to an increase in GltC activity (Table 6). These effects of high GlutDH activity remain to be explained (see Discussion).
TABLE 6.
Effect of GlutDH overexpression on gltA expression in the tnrA backgrounda
| Strain | Relevant genotype | β-Galactosidase activity (gltA-lacZ fusion) (U) in medium with glucose plus:
|
||
|---|---|---|---|---|
| Ammonium | Glutamate | Ornithine | ||
| LG219-T | tnrA | 73.2 | 36.5 | 1.2 |
| BB1282 | rocG tnrA | 72.6 | 41.2 | 27.3 |
| BB1320 | rocG gudB1 tnrA | 106.0 | 33.7 | 0.9 |
| BB1338 | rocR(T120I) tnrA | 50.4 | 10.1 | 1.3 |
| BB1753 | rocGp1 tnrA | 59.6 | 15.8 | 21.9 |
| BB2070 | rocR(T120I) rocGp1 tnrA | 72.3 | 64.5 | 13.7 |
All strains have a gltA-lacZ fusion as in LG219. Strains BB1282 and BB2070 contain the ΔgudB::tet mutation, which prevents the emergence of spontaneous gudB1 mutants. Strain BB2070 contains the yweA::pBB1013 (′yweA′ neo) insertion linked to the rocGp1 allele. The strains were grown to mid-exponential phase in the indicated glucose minimal media and assayed for β-galactosidase activity.
Utilization of arginine-related amino acids and proline as the sole carbon and nitrogen source.
No expression of the gltA-lacZ fusion was observed when arginine, ornithine, proline, and citrulline were each used as the sole carbon and nitrogen source in the growth medium (Table 2, strains LG219 and LG219-T, and data not shown). The lack of GltC activity was responsible for this effect, since high gltA expression was seen when constitutively active forms of GltC were present or when versions of the gltA promoter that are independent of the GltC activation state were used (Table 4). Little or no effect of glucose was seen when gltA expression was monitored in the absence of GltC (Table 3).
The low gltA expression and apparent GltC activity seen when arginine-related amino acids or proline was the sole carbon and nitrogen source were most likely due to the high activity of RocG-GlutDH that results from the absence of glucose repression (6). We could not use the rocG null mutant to test this suggestion directly because GlutDH is essential for utilization of arginine-related amino acids and proline as sole carbon sources (11). If ornithine medium was supplemented with succinate and glutamate, which do not cause catabolite repression, TnrA-independent gltA repression was relieved by the rocG mutation (Table 7, strains LG219-T and BB1282).
TABLE 7.
gltA-lacZ expression in succinate-glutamate mediuma
| Strain | Genotype | β-Galactosidase activity (gltA-lacZ fusion) (U) in medium with succinate plus:
|
||||
|---|---|---|---|---|---|---|
| Glutamate | Glutamate, glucose | Glutamate, ammonium | Glutamate, ammonium, glucose | Glutamate, ornithine | ||
| LG219 | Wild type | 3.4 | 2.2 | 6.1 | 29.2 | |
| LG219-T | tnrA | 20.9 | 33.2 | 6.9 | 30.0 | 1.1 |
| BB1282 | rocG tnrA | 35.0 | 30.9 | 19.9 | ||
| BB2185 | yweA::pBB1013 tnrA | 28.7 | 25.3 | 0.7 | ||
All strains have a gltA-lacZ fusion as in LG219. Strain BB1282 contains the ΔgudB::tet mutation, which prevents the emergence of spontaneous gudB1 mutants. The strains were grown to mid-exponential phase in the indicated minimal succinate-glutamate media and assayed for β-galactosidase activity.
Role of catabolite repression of RocG in gltA expression in glutamine and glutamate media.
It was recently reported that glucose stimulates gltA expression by a mechanism mediated by the catabolite control protein CcpA (13, 19, 30). We confirmed that gltA-lacZ expression was severalfold lower in succinate-glutamate-ammonium medium or succinate-glutamine medium than in the same media with glucose added or with glucose replacing succinate in both tnrA+ and tnrA strains (Table 7, strains LG219 and LG219-T, and data not shown). Expression from the gltA promoter in the absence of glucose was at an intermediate level and was reduced about sixfold if ornithine was included in succinate-glutamate medium (Table 7, strain LG219-T and data not shown). We also confirmed that a ccpA null mutant underexpressed gltA in glucose-glutamate-ammonium or glucose-glutamine medium (Table 8, strains BB1142 and BB1153). Since rocG expression is subject to CcpA-mediated catabolite repression (6), we tested whether RocG is involved in glucose-dependent stimulation of gltA expression. In all cases, gltA expression was restored to nearly the normal level by introduction of a rocG null mutation (Tables 7 and 8, strains BB1282 and BB1577), suggesting that gltA expression requires glucose and CcpA because catabolite repression of rocG reduces GlutDH activity and results in higher activity of GltC.
TABLE 8.
gltA-lacZ expression in the ccpA mutanta
| Strain | Genotype | β-Galactosidase activity (gltA-lacZ fusion) (U) in medium with glucose plus:
|
||
|---|---|---|---|---|
| Glutamate | Glutamate, ammonium | Glutamine | ||
| LG219 | Wild type | 2.0 | 30.8 | 32.7 |
| BB1142 | ccpA | 1.4 | 5.4 | 5.1 |
| BB1153 | ccpA tnrA | 9.1 | 6.0 | 4.1 |
| BB1577 | ccpA rocG tnrA | 27.8 | 19.9 | 15.6 |
| BB2186 | ccpA yweA::pBB1013 tnrA | 14.8 | 12.2 | 11.3 |
All strains have a gltA-lacZ fusion as in LG219. Strain BB1577 contains the ΔgudB::tet mutation, which prevents the emergence of spontaneous gudB1 mutants. The strains were grown to mid-exponential phase in the indicated minimal glucose media and assayed for β-galactosidase activity.
In an accompanying paper, we reported that, although the rocG promoter is inactive in the absence of the RocR inducers, a low level of rocG expression occurs due to transcriptional readthrough from a promoter located upstream of the adjacent gene, yweA (6). This readthrough transcription is noticeably elevated if catabolite repression of rocG is relieved by a mutation in the cre site responsible for interaction with CcpA (6). Elimination of readthrough transcription of rocG by insertion of plasmid DNA between the rocG gene and the yweA promoter (as in strain BB2185) made gltA-lacZ expression high even in the absence of glucose (Table 7), confirming the role of rocG expression in glucose-mediated gltA regulation. The same insertion significantly increased gltA expression in a ccpA mutant (Table 8, strain BB2186).
Unexpectedly, gltA expression in a tnrA mutant strain was much higher in succinate-glutamate medium than in succinate-glutamate-ammonium medium despite the absence of glucose in both media (Table 7, strain LG219-T). To explain this phenomenon, we tested expression of the rocG gene in the two succinate media. rocG-lacZ expression, measured as described in the accompanying paper (6), was 1.7 Miller units in succinate-glutamate-ammonium medium and only 0.2 Miller units in succinate-glutamate medium. This difference in rocG expression presumably reflects activation by ammonium of readthrough transcription of rocG from the upstream promoter. The inverse correlation between the activities of the gltA and rocG fusions suggests again that the role of glucose in gltA expression is to reduce expression of RocG. Glucose apparently has no effect on gltA expression if RocG activity is low.
Effect of GlutDH expression on TnrA activity.
TnrA activity is regulated by its interaction with glutamine synthetase in such a way that feedback-inhibited glutamine synthetase sequesters TnrA and prevents its binding to DNA (21, 34). For in vivo experiments, the most common way to have TnrA in its active state is to grow cells with glutamate or proline as the sole nitrogen source. TnrA activity is low whenever ammonium or ammonium-generating compounds are present in the growth medium (1, 20, 33). Therefore, TnrA activity should be reduced when GlutDH, which generates ammonium, is highly active. In fact, glutamate-mediated, TnrA-dependent gltA repression was much reduced if GudB1-GlutDH was present in cells and completely eliminated when RocG-GlutDH was highly expressed in glucose-glutamate-grown cells due to the presence of the rocR(T120I) and rocGp1 mutations (Table 9, strains BB1310 and BB1793). A similar inverse correlation between the activities of GlutDH and TnrA was detected when the positively regulated amtB (formerly nrgA) promoter (32) was used to monitor TnrA activity. High activity of RocG-GlutDH in rocR(T120I) rocGp1 mutant cells virtually abolished amtB-lacZ expression (Table 10, strain BB2250). A similar effect was observed if GudB-GlutDH was activated by the gudB1 mutation (Table 10, strain BB1479). In wild-type cells, and especially in rocGp1 cells, proline does not activate rocG to the same extent as does ornithine (6). Correspondingly, TnrA activity remained high in proline-grown cells but was reduced in ornithine-grown cells (Table 10, strains BB1405 and BB2249).
TABLE 9.
Effect of GlutDH overexpression on gltA expression in the tnrA+ backgrounda
| Strain | Relevant genotype | β-Galactosidase activity (gltA-lacZ fusion) (U) in medium with glucose plus:
|
|
|---|---|---|---|
| Ammonium | Glutamate | ||
| BB1281 | rocG | 68.8 | 2.5 |
| BB1310 | rocG gudB1 | 122.2 | 23.2 |
| BB1318 | rocR(T120I) | 51.5 | 2.6 |
| BB1749 | rocGp1 | 59.8 | 2.2 |
| BB1793 | rocR(T120I) rocGp1 | 57.2 | 82.4 |
All strains have a gltA-lacZ fusion as in LG219. Strains BB1281 and BB1749 contain the ΔgudB::tet mutation, which prevents the emergence of spontaneous gudB1 mutants. Strain BB1793 contains the yweA::pBB1013 (′yweA′ neo) insertion linked to the rocGp1 allele. The strains were grown to mid-exponential phase in the indicated minimal glucose media and assayed for β-galactosidase activity.
TABLE 10.
Effect of GlutDH expression on amtB-lacZ expressiona
| Strain | Relevant genotype | β-Galactosidase (amtB-lacZ fusion) activityb (%)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Medium with glucose plus:
|
Medium without glucose but with ornithine | |||||||
| Ammonium | Glutamate | Proline | Ornithine | Arginine | Citrulline | |||
| BB1405 | Wild type | 0.1 | 100.0 | 73.0 | 31.3c | 0.1 | 0.1 | 0.1 |
| BB2248 | rocR(T120I) | 73.1 | 58.8 | 48.1 | ||||
| BB2249 | rocGp1 | 87.4 | 62.4 | 0.1 | ||||
| BB2250 | rocR(T120I) rocGp1 | 0.3 | 3.5 | 0.1 | ||||
| BB1479 | rocG gudB1 | 1.0 | 2.4 | 0.6 | ||||
| BB1484 | rocG | 75.7 | 71.2 | 43.2 | 14.2 | 30.7 | NGd | |
| BB1599 | rocG ureC | 69.1 | NG | |||||
All strains have a ΔamyE::(amtB-lacZ)402 fusion (obtained from S. Fisher). Strains BB1484, BB1599, and BB2249 contain the ΔgudB::tet mutation, which prevents the emergence of spontaneous gudB1 mutants. Strain BB2250 contains the yweA::pBB1013 (′yweA′ neo) insertion linked to the rocGp1 allele. The strains were grown to mid-exponential phase in the indicated minimal media and assayed for β-galactosidase activity.
Expressed as a percentage of wild-type activity in glutamate medium (378.2 U).
Low activity was detected during early exponential growth.
NG, no growth. The rocG null mutant cannot grow with ornithine as the sole carbon source.
TnrA was not active in cells growing in glucose-arginine or glucose-citrulline media, presumably because ammonium production from arginine-derived urea and glutamate and from citrulline-derived carbamate is too high (Table 10, strain BB1405). Interestingly, in a rocG null mutant, TnrA was partially active in glucose-arginine and glucose-citrulline media and almost full TnrA activity was observed in glucose-arginine medium for the rocG ureC double mutant, in which ammonium generation from both glutamate and urea was abolished (Table 10, strains BB1484 and BB1599).
No TnrA activity was detected when cells were utilizing ornithine or proline as the sole nitrogen and carbon source, as reflected by low amtB-lacZ expression (Table 10, strain BB1405, and data not shown) and high gltA-lacZ activity in strains expressing gltA independently of GltC or its activation state (Tables 3 and 4 and data not shown). This effect is undoubtedly due to high activity of RocR-GlutDH, which produces ammonium from glutamate generated by degradation of ornithine and proline.
Effect of the Roc pathway on gltC expression.
Expression of the gltC-gusA fusion was reduced about twofold in the presence of ornithine or arginine as the sole nitrogen source and even less or not at all in the presence of proline and citrulline (data not shown). The lower gltC expression could reflect tighter binding of GltC to its binding sites that overlap parts of both the gltC and gltA promoters (5) (Fig. 1). Expression from the gltC promoter in ornithine medium was not affected by the rocG null mutation, which greatly increased expression from the gltA promoter (data not shown). Therefore, it is unlikely that small variations in gltC expression contribute significantly to regulation of the gltAB operon.
DISCUSSION
Transcription of the gltA operon is controlled by two regulatory proteins, GltC and TnrA. GltC is required for any significant expression of the gltAB operon, but TnrA can repress transcription even if GltC is fully active. The activities of these proteins depend on the nature of the nitrogen and carbon sources in the medium and respond to a variety of nutritional factors, primarily to availability of ammonium and amino acids of the glutamate family. The physiological role of both proteins seems to be reduction of unneeded gltA expression in the presence of excess glutamate or glutamate-related amino acids; this may be the only function of GltC, while TnrA is a global regulator of gene expression in B. subtilis cells (20, 22, 33, 36).
TnrA is inactivated as both a positive and negative regulator by interaction with the feedback-inhibited form of glutamine synthetase under conditions of nitrogen excess (21, 34); the default state of TnrA is its active, DNA-binding state. In the default state, TnrA represses gltA. GltC is inactivated as a positive regulator by a component of the Roc pathway, or induction of the Roc pathway reduces the concentration of a coactivator of GltC. We suggest that the default state of GltC is its active form, i.e., in its default state GltC activates gltA transcription. If so, modulation of gltAB operon expression can be achieved through inactivation of TnrA as a repressor, leading to elevation of expression, or by inactivation of GltC as a positive regulator, causing reduction of expression.
The activities of most LysR-type proteins are modulated by low-molecular-weight compounds (28). The substrates and the products of the GlutDH reaction are the primary candidates for the coeffectors of GltC. Glutamate is an unlikely effector of GltC, since the glutamate pool is very high (31). It seems plausible that GltC activity would respond to the intracellular levels of NAD(H) or α-ketoglutarate. Since the glutamate synthase reaction links nitrogen and carbon-energy metabolism, it is logical that gltAB transcription appears to respond to signals from nitrogen metabolism mediated by the glutamine synthetase reaction acting on TnrA and from carbon-energy metabolism mediated by the GlutDH reaction and acting on GltC. By placing rocG-encoded GlutDH under the control of RocR, the cell has arranged to limit the use of glutamate as a carbon source to situations where it is derived from arginine, ornithine, or proline. Because the activity of RocG is inversely related to the activity of GltC, such an arrangement prevents a futile cycle of simultaneous glutamate synthesis and degradation in medium containing arginine, ornithine, or proline.
A delicate balance between the availability of glutamate and its precursors, the ability to utilize glutamate through GlutDH activity, and the availability of intracellular ammonium seems to be very important for regulation of nitrogen metabolism in B. subtilis. High activity of TnrA is responsible for low expression from the gltA promoter in glucose-glutamate medium, and low activity of GltC causes the same result in glucose-arginine medium. Both GltC and TnrA serve to reduce gltA expression when proline or ornithine is the sole nitrogen source in glucose-containing medium, and the two mechanisms of gltA regulation appear to be redundant under these conditions. On the other hand, because both TnrA- and GltC-mediated mechanisms of gltA regulation appear to be negated by high ammonium levels, the gltA promoter is expressed at a high level in glucose-citrulline medium. In cells grown with proline, arginine, ornithine, or citrulline as the sole carbon and nitrogen source, TnrA activity is low but the lack of GltC activity prevents gltA expression.
The inverse correlation between GlutDH and GltC activities described in this work is not perfect and can be broken by mutations that increase GlutDH activity. GltC activity is high if GlutDH is very low (as in glucose-ammonium or glucose-glutamate medium), low if GlutDH activity is moderate (as in glucose-ornithine medium), and rather high again if high activity of RocG-GlutDH is achieved (as in glucose-ornithine medium for rocGp1 mutants), apparently due to increased ammonium production. Inhibition of TnrA when RocG-GlutDH or GudB1-GlutDH activity is high is also apparently due to ammonium accumulation. A similar effect of incompatibility of ammonium production with TnrA activity, although at a more moderate level, was observed when expression of another ammonium-generating enzyme, aspartase, was highly increased in glutamate-grown cells (23). Strong carbon catabolite repression of rocG prevents extensive ammonium production from glutamate and allows TnrA to be active in glucose-proline medium and to some extent in glucose-ornithine medium. It may be significant that B. subtilis cells evolved in such a way that one gene for GlutDH, rocG, is not induced in glutamate-grown cells, in which TnrA needs to be highly active, and the other GlutDH gene, gudB, although expressed in glutamate-grown cells, produces an enzyme that is inactive in the wild-type strain (8, 11).
It is possible that the danger of excessive glutamate drain due to high GlutDH activity contributed to the evolution of the mechanisms of modulation of TnrA and GltC activities in other ways. Lack of TnrA activity when arginine-related amino acids or proline serves as the sole carbon and nitrogen source may allow some gltA expression that could compensate for glutamate drain. A similar need for additional glutamate synthesis may explain why, in the rocGp1 mutants, GltC activity is apparently not completely inhibited in glucose-ornithine medium.
While ammonium accumulation most likely inactivates TnrA by its rapid conversion to glutamine and other inhibitors of glutamine synthetase, the role of ammonium availability in modulation of GltC activity in minimal glucose medium remains unclear. Interestingly, ammonium accumulation may slow down the ammonium-producing catabolic GlutDH reaction, which, in turn, could affect GltC activity and explain increased gltA expression. It is also unclear why the cell permits rather high gltA expression in glucose medium if glutamine or a combination of glutamate plus ammonium serves as the nitrogen source, conditions under which glutamate synthesis appears to be superfluous. Perhaps the cyclic reactions of glutamate synthase and glutamine synthetase are involved in removal of excess ammonium from the cells. The toxic effect of ammonium on mammalian cells is well known; enzymes of the urea cycle and glutamate metabolism are responsible for ammonia removal in animals (2). Alternatively, glutaminase activity of glutamate synthase may be required for cells to deal with excess glutamine.
In the absence of glucose, it is in the cells' interest to support gluconeogenesis by feeding glutamate and other Roc intermediates into the Krebs cycle rather than synthesizing glutamate from α-ketoglutarate. Low expression of gltAB can be achieved in several ways. In succinate-glutamate medium, low gltA expression is mostly due to repression by TnrA, but expression of rocG from an upstream promoter also contributes. In succinate-ornithine medium or in cells grown with arginine-related amino acids or proline as the sole carbon and nitrogen source, TnrA fails to repress, but gltA expression is abolished due to induction of the rocG promoter and high activity of RocG, causing inactivity of GltC. In succinate-glutamate-ammonium or succinate-glutamine medium, TnrA is again inactive and rocG is not expressed from its own promoter, but gltAB expression is still rather low (13, 19, 30) because rocG expression from the upstream promoter is derepressed.
Acknowledgments
We are grateful to S. Fisher and M. Nakano for many helpful discussions, to E. Bremer, M. Débarbouillé, S. Fisher, R. Gardan, P. Serror, and L. Wray, Jr., for gifts of strains or plasmids, and to E. Bremer and J. Stülke for communicating their results before publication.
This work was supported by a grant from the U.S. Public Health Service to A.L.S. (GM36718) and from the National Science Foundation (MCB-0110651) to B.R.B.
REFERENCES
- 1.Atkinson, M. R., and S. H. Fisher. 1991. Identification of genes and gene products whose expression is activated during nitrogen-limited growth in Bacillus subtilis. J. Bacteriol. 173:23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, C. 2002. Mechanisms of hyperammonemia. Clin. Chem. Lab. Med. 40:653-662. [DOI] [PubMed] [Google Scholar]
- 3.Baumberg, S., and C. R. Harwood. 1979. Carbon and nitrogen repression of arginine catabolic enzymes in Bacillus subtilis. J. Bacteriol. 137:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belitsky, B. R. 2002. Biosynthesis of amino acids of the glutamate and aspartate families, alanine, and polyamines, p. 203-231. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 5.Belitsky, B. R., P. J. Janssen, and A. L. Sonenshein. 1995. Sites required for GltC-dependent regulation of Bacillus subtilis glutamate synthase expression. J. Bacteriol. 177:5686-5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belitsky, B. R., H.-J. Kim, and A. L. Sonenshein. 2004. CcpA-dependent regulation of Bacillus subtilis glutamate dehydrogenase gene expression. J. Bacteriol. 186:3392-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belitsky, B. R., and A. L. Sonenshein. 1997. Altered transcription activation specificity of a mutant form of Bacillus subtilis GltR, a LysR family member. J. Bacteriol. 179:1035-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belitsky, B. R., and A. L. Sonenshein. 1999. An enhancer element located downstream of the major glutamate dehydrogenase gene of Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 96:10290-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belitsky, B. R., and A. L. Sonenshein. 2002. GabR, a member of a novel protein family, regulates utilization of γ-aminobutyrate in Bacillus subtilis. Mol. Microbiol. 45:569-583. [DOI] [PubMed] [Google Scholar]
- 10.Belitsky, B. R., and A. L. Sonenshein. 1995. Mutations in GltC that increase Bacillus subtilis gltA expression. J. Bacteriol. 177:5696-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belitsky, B. R., and A. L. Sonenshein. 1998. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J. Bacteriol. 180:6298-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belitsky, B. R., L. V. Wray, Jr., S. H. Fisher, D. E. Bohannon, and A. L. Sonenshein. 2000. Role of TnrA in nitrogen source-dependent repression of Bacillus subtilis glutamate synthase gene expression. J. Bacteriol. 182:5939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blencke, H. M., G. Homuth, H. Ludwig, U. Mader, M. Hecker, and J. Stulke. 2003. Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab. Eng. 5:133-149. [DOI] [PubMed] [Google Scholar]
- 14.Bohannon, D. E., M. S. Rosenkrantz, and A. L. Sonenshein. 1985. Regulation of Bacillus subtilis glutamate synthase genes by the nitrogen source. J. Bacteriol. 163:957-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohannon, D. E., and A. L. Sonenshein. 1989. Positive regulation of glutamate biosynthesis in Bacillus subtilis. J. Bacteriol. 171:4718-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calogero, S., R. Gardan, P. Glaser, J. Schweizer, G. Rapoport, and M. Debarbouille. 1994. RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J. Bacteriol. 176:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz-Ramos, H., P. Glaser, L. V. Wray, Jr., and S. H. Fisher. 1997. The Bacillus subtilis ureABC operon. J. Bacteriol. 179:3371-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desphande, K. L., J. R. Katze, and J. F. Kane. 1980. Regulation of glutamate synthase from Bacillus subtilis by glutamine. Biochem. Biophys. Res. Commun. 95:55-60. [DOI] [PubMed] [Google Scholar]
- 19.Faires, N., S. Tobisch, S. Bachem, I. Martin-Verstraete, M. Hecker, and J. Stulke. 1999. The catabolite control protein CcpA controls ammonium assimilation in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1:141-148. [PubMed] [Google Scholar]
- 20.Fisher, S. H. 1999. Regulation of nitrogen metabolism in Bacillus subtilis: vive la difference! Mol. Microbiol. 32:223-232. [DOI] [PubMed] [Google Scholar]
- 21.Fisher, S. H., J. L. Brandenburg, and L. V. Wray, Jr. 2002. Mutations in Bacillus subtilis glutamine synthetase that block its interaction with transcription factor TnrA. Mol. Microbiol. 45:627-635. [DOI] [PubMed] [Google Scholar]
- 22.Fisher, S. H., and M. Débarbouillé. 2002. Nitrogen source utilization and its regulation, p. 181-191. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 23.Fisher, S. H., and L. V. Wray, Jr. 2002. Bacillus subtilis 168 contains two differentially regulated genes encoding l-asparaginase. J. Bacteriol. 184:2148-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardan, R., G. Rapoport, and M. Debarbouille. 1995. Expression of the rocDEF operon involved in arginine catabolism in Bacillus subtilis. J. Mol. Biol. 249:843-856. [DOI] [PubMed] [Google Scholar]
- 25.Gardan, R., G. Rapoport, and M. Debarbouille. 1997. Role of the transcriptional activator RocR in the arginine-degradation pathway of Bacillus subtilis. Mol. Microbiol. 24:825-837. [DOI] [PubMed] [Google Scholar]
- 26.Pan, F. L., and J. G. Coote. 1979. Glutamine synthetase and glutamate synthase activities during growth and sporulation in Bacillus subtilis. J. Gen. Microbiol. 112:373-377. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. J. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 29.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142:79-83. [DOI] [PubMed] [Google Scholar]
- 30.Wacker, I., H. Ludwig, I. Reif, H. M. Blencke, C. Detsch, and J. Stulke. 2003. The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA. Microbiology 149:3001-3009. [DOI] [PubMed] [Google Scholar]
- 31.Whatmore, A. M., J. A. Chudek, and R. H. Reed. 1990. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J. Gen. Microbiol. 136:2527-2535. [DOI] [PubMed] [Google Scholar]
- 32.Wray, L. V., Jr., M. R. Atkinson, and S. H. Fisher. 1994. The nitrogen-regulated Bacillus subtilis nrgAB operon encodes a membrane protein and a protein highly similar to the Escherichia coli glnB-encoded PII protein. J. Bacteriol. 176:108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wray, L. V., Jr., A. E. Ferson, K. Rohrer, and S. H. Fisher. 1996. TnrA, a transcription factor required for global nitrogen regulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:8841-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wray, L. V., Jr., J. M. Zalieckas, and S. H. Fisher. 2001. Bacillus subtilis glutamine synthetase controls gene expression through a protein-protein interaction with transcription factor TnrA. Cell 107:427-435. [DOI] [PubMed] [Google Scholar]
- 35.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida, K., H. Yamaguchi, M. Kinehara, Y. H. Ohki, Y. Nakaura, and Y. Fujita. 2003. Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box. Mol. Microbiol. 49:157-165. [DOI] [PubMed] [Google Scholar]