Abstract
Objective
The objective of this study is to systematically review the literature on worldwide numbers of leukodystrophy patients undergoing hematopoietic stem cell transplantation (HSCT) as well as the safety and efficacy of the procedure in this patient population.
Materials and Methods
A PubMed and EMBASE search up to June 2012 was conducted with a manual search of references from relevant articles. Selected studies were evaluated using internationally accepted criteria. The effect estimates of HSCT upon survival in early-stage disease versus late-stage disease were compared.
Results
One hundred and fifty-two studies qualified for inclusion and reported on a total of 689 patients. Study quality ranged from poor to good; no study was rated excellent. Small sample sizes limited most studies. Meta-analysis in a subset of larger studies indicates that transplantation in earlier stages of disease fairs better than in the late stages. Beyond survival, little longitudinal data on functional outcome is reported and neurological outcome is sparse.
Conclusion
Further studies are needed to determine the neurological outcome following HSCT in the leukodystrophies. HSCT in the early stages of cerebral disease is still recommended for select leukodystrophies.
Keywords: leukodystrophy, bone marrow transplantation, white matter diseases
Leukodystrophies are heritable disorders affecting the white matter of the central nervous system (CNS). They can affect multiple functional domains over time, and are often progressive, resulting in significant disability and impaired quality of life. Hematopoietic stem cell transplantation (HSCT) has been employed to arrest disease progression for more than 20 years. Yet, the number of leukodystrophy patients transplanted worldwide, the optimal timing for transplant, the selection criteria, and outcome remain largely unknown. We systematically reviewed studies reporting HSCT performed in leukodystrophy patients.
Materials and Methods
Consensus Definition
On the basis of a definition of leukodystrophies arrived at by consensus among 13 investigators using a Delphi method, an interactive method based on a structured communication among a panel of experts (Adeline Vanderver, MD, personal written communication, January 11, 2013).
Research Questions
What have been the numbers of leukodystrophy patients reported worldwide and what is known about the safety and efficacy of the procedure? How does outcome in patients at early stages of cerebral disease compare with those transplanted in advanced cerebral disease?
Search Strategy
A literature review of PubMed and EMBASE from 1990 to 2012 was performed. The search terms (leukodystrophy and transplant) were based on the consensus definition mentioned above. Data extraction was performed by two blinded investigators excluding animal studies, review articles, and duplicates.
Inclusion and Exclusion Criteria
For the purpose of outcome analysis, peer-reviewed original articles written in English with a sample size of more than 10 patients that reported on the outcome after HSCT intervention were included. No relevant systematic reviews or meta-analysis were identified. Patients were categorized as late-stage versus early-stage at the time of transplant based on symptoms or magnetic resonance imaging (MRI) (e.g., the cut-off for Loes Score of 9 for adrenoleukodystrophy [ALD]) unless otherwise specified. The principle outcome for this review was defined as survival after having the transplant. To include the outcome for all the included patients, no time limit was considered for the analysis.
Data Analysis
The level and quality of evidence were determined by the study design, sample size, potential bias, statistical analysis, use of controls, and data collection strategy. Potential conflicts of interest were noted but were not included in the quality assessment. Articles were independently selected and reviewed by F.E. and A.P., and consensus on disagreements was reached between P.M., F.E., and A.P. Articles were assessed regarding the comparison of HSCT in early-stage and late-stage leukodystrophies. Discerning measures for early-stage versus late-stage disease as well as neurological outcome measures were extracted.
Standard Protocol Approvals, Registrations, and Patient Consents
Published data were used for this systematic review; hence, no ethical approval was sought.
Results
Search Results
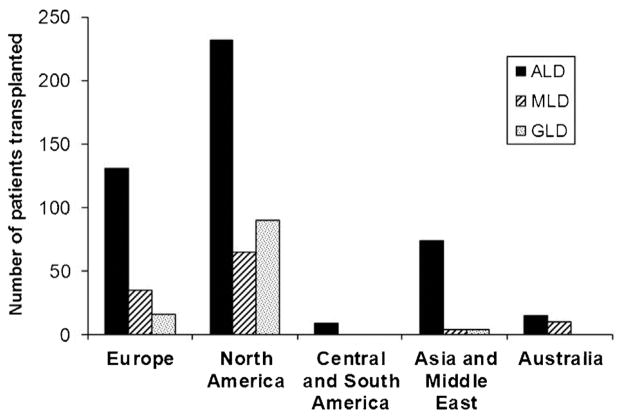
PubMed yielded 1,381 hits and an additional 233 hits in EMBASE, with 536 unique citations identified. After excluding animal studies, review articles and duplicates, 152 studies qualified for inclusion, reporting on a total of 689 patients. Fig. 1 shows the numbers of transplanted leukodystrophy patients across continents. Two articles were collaborative international efforts (see, Peters et al7 and Shapiro et al8). The majority of cases were ALD patients (461). Most studies included small numbers (n < 5) and only 10 articles reported on more than 10 patients of a specific disorder. Of these, only six articles reported survival events for both groups and only one reported on transplanted versus nontransplanted leukodystrophy patients. Transplantation occurred mostly in pediatric but was also reported in adult-onset leukodystrophies: 6 ALD, 13 metachromatic leukodystrophy (MLD), and 2 globoid leukodystrophy (GLD).
Fig. 1.
Hematopoietic stem cell transplantation in leukodystrophy patients reported across continents from 1999 to 2011 (number of articles, 152; number of patients, 689). The two international collaborative efforts account for 104 adrenoleukodystrophy patients and have been equally divided among European, North American, and Asian columns.
Donor Source for Transplantation
In most reported cases, the donor was unrelated (Table 1). However, in up to half of cases with MLD and GLD, and up to one-third of cases of ALD, the donor was not reported. The source of donor was most often bone marrow for ALD and MLD patients (31 and 36%, respectively) and most often umbilical cord blood (UCB) for GLD patients (32%). A small minority of cases had peripheral blood stem cell as donor source.
Table 1.
Summary of donor source reported
| Source of donor | ||||
|---|---|---|---|---|
| Bone marrow, n (%) | Umbilical cord blood, n (%) | Peripheral blood stem cell, n (%) | Unknown, n (%) | |
| ALD (n = 461) | 145 (31) | 77 (17) | 21 (5) | 218 (47) |
| MLD (n = 114) | 41 (36) | 16 (14) | 2 (2) | 55 (48) |
| GLD (n = 110) | 16 (15) | 35 (32) | 1 (1) | 58 (53) |
| Relation to donor | ||||
| Related to donor, n (%) | Unrelated to donor, n (%) | Unknown, n (%) | ||
| ALD (n = 461) | 126 (27) | 195 (42) | 140 (30) | |
| MLD (n = 114) | 19 (17) | 34 (30) | 61 (54) | |
| GLD (n = 110) | 8 (7) | 44 (40) | 58 (53) | |
Abbreviations: ALD, adrenoleukodystrophy; GLD, globoid leukodystrophy; MLD, metachromatic leukodystrophy.
Transplant-Related Complications
On the majority of patients, there was no information regarding complications (Table 2). A third or fewer studies reported explicitly on transplant-related complications. Graft-versus-host (GvHD) was the most commonly reported complication followed by infection and graft failure. The type (acute or chronic) and severity of GvHD were inconsistently reported. This was true for ALD, MLD, and GLD.
Table 2.
Summary of complications reported
| Reported presence of complications, n (%) | Reported absence of complications, n (%) | Unknown, n (%) | ||
|---|---|---|---|---|
| ALD (n = 461) | 79 (17) | 9 (2) | 373 (81) | |
| MLD (n = 114) | 34 (30) | 4 (4) | 76 (67) | |
| GLD (n = 110) | 27 (25) | 4 (4) | 79 (72) | |
| GvHD, n (%) | Infection, n (%) | Graft failure, n (%) | Other, n (%) | |
| ALD (n = 88) | 44 (50) | 23 (26) | 14 (16) | 13 (15) |
| MLD (n = 38) | 27 (71) | 8 (21) | 6 (16) | 8 (21) |
| GLD (n = 31) | 28 (90) | 2 (6) | 0 (0) | 6 (19) |
Abbreviations: ALD, adrenoleukodystrophy; GLD, globoid leukodystrophy; MLD, metachromatic leukodystrophy.
Neurological Outcome and Survival
Approximately 67 to 80% of articles reported functional outcome, but up to half of the articles only listed “death” or “deterioration” as outcome measure. Only 20 to 25% of the articles made a reference to neuroimaging outcome. The subset of articles that listed more than 10 patients and neurological outcome were assessed for quality and nine articles judged as “good” or “fair” used for further analysis (see Table 3).1–9 All but one of the articles employed neurological outcome measures that allowed for a comparison of HSCT in early-stage and late-stage leukodystrophies (see Table 4).
Table 3.
Summary of unique studies examining hematopoietic stem cell transplantation in the leukodystrophiesa
| Country (publication year) | Study design | Target population (numbers of patients) | Source of HSCT | Comparison | Neurological outcome measure |
|---|---|---|---|---|---|
| Germany (Baumann et al, 2003) | Prospective cohort | ALD (n = 12) | BM (n = 10), PBSC (n = 2) | Early vs. late transplant | Brain MRI |
| United States (Beam et al, 2007) | Prospective cohort | ALD (n = 12) | UCB (n = 12) | Early vs. late transplant | Survival, neurodevelopmental outcome |
| United States (Escolar et al, 2005) | Prospective cohort | GLD (n = 25) | UCB (n = 25) | asymptomatic vs. symptomatic | Survival, neurodevelopmental outcome |
| United States (Martin et al, 2005) | Prospective cohort | ALD (n = 8), MLD (n = 6), GLD (n = 16) | UCB (n = 20) | 2 different transplant regimens | None |
| United States (Mahmood et al, 2007) | Retrospective cohort | ALD (n = 19) | BM (n = 19) | Early vs. late transplant, transplanted vs. nontransplanted | Survival |
| United States (Miller et al, 2011) | Retrospective cohort | ALD (n = 60) | BM (n = 60) | Early vs. late transplant | Survival, neurological outcome, brain MRI |
| International (Peters et al, 2004) | Retrospective cohort | ALD (n = 94) | BMC or UBC | Early vs. late transplant | Survival |
| International (Shapiro et al, 2000) | Prospective cohort | ALD (n = 12) | BM (n = 12) | Early vs. late transplant | Survival, neurological outcome, brain MRI |
| Canada (Siddiqi et al, 2006) | Prospective cohort | GLD (n = 12) | Not listed | Early vs. late transplant | Neurophysiology |
Abbreviations: ALD, adrenoleukodystrophy; BM, bone marrow; GLD, globoid leukodystrophy; HSCT, hematopoietic stem cell transplantation; MLD, metachromatic leukodystrophy; MRI, magnetic resonance imaging; PBSC, peripheral blood stem cells; UCB, umbilical cord blood.
Studies reporting on more than 10 patients.
Note: All but one of the articles employed neurological outcome measures that allowed for a comparison of HSCT in early-stage and late-stage leukodystrophies.
Table 4.
Comparative measures and outcome in early-stage versus late-stage leukodystrophies
| Country (publication year) | Target population (numbers of patients) | Discerning measure | Outcome measure | Outcome in advanced patients | Outcome in early patients |
|---|---|---|---|---|---|
| Germany (Baumann et al, 2003) | ALD (n = 12) | MRI Score (Loes) ≤ 9 vs. > 9 | Clinical reporting | 3 deteriorated, 1 stable | 3 deteriorated, 5 stable |
| United States (Beam et al, 2007) | ALD (n = 12) | MRI Score (Loes) ≤ 9 vs. > 9 | Event-free survival | 3 long-term survival (> 800 d), 2 early death (< 200 d) | 5 long-term survival (> 800 d), 2 early death (< 200 d) |
| United States (Escolar et al, 2005) | GLD (n = 25) | Neurodevelopmental, MRI, and neurophysiology assessment | Clinical reporting | Minimal neurologic improvement | Progressive central myelination, appropriate cognitive function |
| United States (Mahmood et al, 2007) | ALD (n = 19) | Compares early transplant to no transplant | 5-year survival | 51% | 61% |
| United States (Miller et al, 2011) | ALD (n = 60) | MRI Score (Loes) < 10 vs. ≥ 10 | 5-year survival | 60% | 89% |
| International (Peters et al, 2004) | ALD (n = 94) | MRI Score (Loes) ≤ 9 vs. > 9 | 5-year survival | 45% | 92% |
| International (Shapiro et al, 2000) | ALD (n = 12) | MRI Score (Loes) ≤ 9 vs. > 9 | Cognitive evaluation | 2 deteriorated, 1 stabilized | 5 improved, 3 stable, 1 deteriorated |
| Canada (Siddiqi et al, 2006) | GLD (n = 12) | age at transplant (first month vs. later in first year) | Nerve conduction velocities | Motor conduction velocity improved by 3 m/sec | Motor conduction velocity improved by 13 m/sec |
Abbreviations: ALD, adrenoleukodystrophy; GLD, globoid leukodystrophy; MLD, metachromatic leukodystrophy; MRI, magnetic resonance imaging.
Discussion
Transplantations for leukodystrophies occur worldwide and have been reported from all continents apart from Africa. Most reported cases come from North America and Europe, although a publication bias may favor English-speaking countries.
Small samples sizes limited most studies. A systematic review in a subset of larger studies confirms that patients transplanted earlier in the process of neurologic decline fair better than those in advanced stages. This appears true for both ALD and GLD, and likely applies to other leukodystrophies as well. The difference may be based on the degree of normal brain that can be preserved. However, little longitudinal data or functional outcome is reported. Often the reason for “death” is unclear. Only rarely is brain imaging commented on and only a few images are shown.
Despite the commonalities among the leukodystrophies, important differences in the management of individual leukodystrophies remain. The crucial decision for the pediatric neurologist is at what stage to recommend transplantation. In ALD, mutation status does not predict the conversion to the cerebral form of the disease. However, MRI is an exquisitely sensitive marker of brain involvement—particularly, the presence of contrast enhancement—that can be used to determine who will develop progressive brain disease.10,11 In GLD and MLD, on the contrary, concordance rates among siblings are usually high and progression of cerebral disease is more predictable, allowing for transplantation to be performed in presymptomatic or minimally symptomatic patients based on firm biochemical and genetic testing. A recent article on the neurodevelopmental outcome of UCB transplantation in MLD emphasizes this point.12 Overall, conventional imaging in GLD and MLD correlates less well with disease severity compared with ALD, but advanced MRI in these disorders adds prognostic power.11,13
Despite improvements with early transplantation reported in the majority of large studies, it is still unclear whether these are sustained. There are reports on deterioration of peripheral nerve conduction velocities after initial improvements.9 Reasons for this may include long-term toxicity from chemotherapy as well as defective remyelination by mutant Schwann cells. These findings are likely not related to the peripheral nerve alone but may apply to the CNS as well.
There is limited experience on the overall long-term neurological development and outcome of children after bone marrow transplantation in the leukodystrophies. The vast majority of studies have not reported on neurological outcome. We know that pediatric patients who receive HSCT for hematologic malignancies have neurocognitive deficiencies that can be both acute and chronic.14,15 The challenge in assessing leukodystrophies is separating residual toxic effects of myeloablation versus that of the disease itself.
There is heterogeneous and inconsistent reporting of HSCT complications and transplant-related mortality in the literature. However, we found the rate and nature of complications, including GvHD and infection, in children with leukodystrophies to be comparable to that of other inherited metabolic disorders cited in the literature.16 Graft failure appears to be greater in this population than in HSCT for childhood malignancy. However, as graft failure is not well-described, conclusions cannot be drawn from the published literature. Overall, complication rates may be underestimated as poor outcomes are generally under-reported. Hemorrhagic cystitis and septicemia are among the most common infections reported.
Fully myeloablative conditioning regimens are used in the large majority of transplants in the literature. Over the years, effort has been made to use reduced intensity conditioning (RIC) regimens. There are case reports and small series of RIC in this population. However, there are no large, published prospective trials of RIC in childrenwith leukodystrophies. In some cases, this has come at the risk of engraftment problems (personal experience). We anticipate that in future, RIC may lead to improved transplant-related morbidity/mortality, more rapid engraftment, lessened neurotoxicity, and fewer late effects of transplant.
Several limitations to our systematic review exist. We cannot exclude that the difference in survival between patients transplanted with early-stage versus late-stage leukodystrophies is partially explained by lead-time bias. Furthermore, a formal meta-analysis was not possible because of heterogeneity in reported outcome measures.
In our review, we found that most articles on HSCT for leukodystrophies report on either transplant measures alone and do not include neurological or brain imaging scales or report neurologic outcomes with little detail about transplant complication or toxicity.
Prospective studies of the role and efficacy in HSCT for leukodystrophies are needed that include both transplant and neurologic clinical outcome measures and imaging scoring systems. Although scales have been developed for some diseases such as ALD, this yet has to be done for most of the other leukodystrophies.
Acknowledgments
We thank Razina Aziz-Bose and Tracey Suter for their help in data collection.
Footnotes
Author Contributions
Articles were independently selected and reviewed by F.E., J.P., and A.P., and consensus on disagreements was reached between P.M., F.E., and A.P., F.E., P.M., and C.D. drafted the manuscript. Critical review and editing was performed by C.D., T.L., and M.E.
Disclosure
Florian Eichler and Asif Paker have served as consultants to Bluebird Bio (Cambridge, Massachusetts, United States). Florian Eichler and Christine Duncan are both coinvesti-gators of a clinical trial of gene therapy for X-linked ALD sponsored by Bluebird Bio.
Conflict of Interest
The authors have reported no conflicts of interest.
References
- 1.Beam D, Poe MD, Provenzale JM, et al. Outcomes of unrelated umbilical cord blood transplantation for X-linked adrenoleukodystrophy. Biol Blood Marrow Transplant. 2007;13(6):665–674. doi: 10.1016/j.bbmt.2007.01.082. [DOI] [PubMed] [Google Scholar]
- 2.Baumann M, Korenke GC, Weddige-Diedrichs A, et al. Haematopoietic stem cell transplantation in 12 patients with cerebral X-linked adrenoleukodystrophy. Eur J Pediatr. 2003;162(1):6–14. doi: 10.1007/s00431-002-1097-3. [DOI] [PubMed] [Google Scholar]
- 3.Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352(20):2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 4.Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011;118(7):1971–1978. doi: 10.1182/blood-2011-01-329235. [DOI] [PubMed] [Google Scholar]
- 5.Mahmood A, Raymond GV, Dubey P, Peters C, Moser HW. Survival analysis of haematopoietic cell transplantation for childhood cerebral X-linked adrenoleukodystrophy: a comparison study. Lancet Neurol. 2007;6(8):687–692. doi: 10.1016/S1474-4422(07)70177-1. [DOI] [PubMed] [Google Scholar]
- 6.Martin PL, Carter SL, Kernan NA, et al. Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. 2006;12(2):184–194. doi: 10.1016/j.bbmt.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Peters C, Charnas LR, Tan Y, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104(3):881–888. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro E, Krivit W, Lockman L, et al. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet. 2000;356(9231):713–718. doi: 10.1016/S0140-6736(00)02629-5. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqi ZA, Sanders DB, Massey JM. Peripheral neuropathy in Krabbe disease: effect of hematopoietic stem cell transplantation. Neurology. 2006;67(2):268–272. doi: 10.1212/01.wnl.0000230156.01228.33. [DOI] [PubMed] [Google Scholar]
- 10.Melhem ER, Loes DJ, Georgiades CS, Raymond GV, Moser HW. X-linked adrenoleukodystrophy: the role of contrast-enhanced MR imaging in predicting disease progression. AJNR Am J Neuroradiol. 2000;21(5):839–844. [PMC free article] [PubMed] [Google Scholar]
- 11.Musolino PL, Rapalino O, Caruso P, Caviness VS, Eichler FS. Hypoperfusion predicts lesion progression in cerebral X-linked adrenoleukodystrophy. Brain. 2012;135(Pt 9):2676–2683. doi: 10.1093/brain/aws206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin HR, Poe MD, Provenzale JM, Kurtzberg J, Mendizabal A, Escolar ML. Neurodevelopmental outcomes of umbilical cord blood transplantation in metachromatic leukodystrophy. Biol Blood Marrow Transplant. 2013;19(4):616–624. doi: 10.1016/j.bbmt.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Escolar ML, Poe MD, Smith JK, et al. Diffusion tensor imaging detects abnormalities in the corticospinal tracts of neonates with infantile Krabbe disease. AJNR Am J Neuroradiol. 2009;30(5):1017–1021. doi: 10.3174/ajnr.A1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin M, Epport K, Azen C, Parkman R, Kohn DB, Shah AJ. Long-term neurocognitive function of pediatric patients with severe combined immune deficiency (SCID): pre- and post-hematopoietic stem cell transplant (HSCT) J Clin Immunol. 2009;29(2):231–237. doi: 10.1007/s10875-008-9250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah AJ, Epport K, Azen C, et al. Progressive declines in neurocognitive function among survivors of hematopoietic stem cell transplantation for pediatric hematologic malignancies. J Pediatr Hematol Oncol. 2008;30(6):411–418. doi: 10.1097/MPH.0b013e318168e750. [DOI] [PubMed] [Google Scholar]
- 16.Prasad VK, Kurtzberg J. Cord blood and bone marrow transplantation in inherited metabolic diseases: scientific basis, current status and future directions. Br J Haematol. 2010;148(3):356–372. doi: 10.1111/j.1365-2141.2009.07974.x. [DOI] [PubMed] [Google Scholar]