Abstract
The atypical cadherins, Fat (Ft) and Dachsous (Ds) control tissue growth via the Salvador-Warts-Hippo (SWH) pathway, and also regulate planar cell polarity (PCP), proximo-distal (PD) patterning and morphogenesis. Ft and Ds engage in reciprocal signalling as both proteins can serve as receptor and ligand for each other. The intracellular domains (ICD) of Ft and Ds regulate activity of the key SWH pathway transcriptional co-activator protein Yorkie (Yki). Signalling from the Ft ICD is well characterized and controls tissue growth by regulating abundance of the Ykirepressive kinase Warts (Wts). Here we describe two new regulators of the SWH pathway that function downstream of the Ds ICD: the WD40 domain protein Riquiqui (Riq) and the DYRK-family kinase Minibrain (Mnb). Ds physically interacts with Riq, which forms a complex with both Mnb and Wts. Riq and Mnb promote Ykidependent tissue growth by stimulating phosphorylation-dependent inhibition of Wts. Thus, we describe a new branch of the SWH pathway that promotes tissue growth downstream of Ds.
INTRODUCTION
Organ size is controlled by many factors including morphogens and nutrients. The Salvador-Warts-Hippo (SWH) pathway has been theorized to control organ size based on the fact that modulation of pathway activity influences the size of both D. melanogaster and murine organs1-4. Deregulation of SWH pathway activity has also been linked to carcinogenesis in humans1. The best-defined receptor for the SWH pathway is the large atypical cadherin, Ft5-9, which is activated by binding to its ligand, the related cadherin Ds10, 11. Both Ft and Ds possess several extracellular cadherin repeats and cytoplasmic tails that mediate intracellular signalling events4, 12, 13. Several studies show that Ds not only acts as a ligand for Ft but also functions as a receptor that signals via its intracellular domain (ICD) to regulate PCP and SWH pathway activity10, 14, 15. For example, the Ds ICD is required for D. melanogaster imaginal disc cells to derepress Yki activity in response to ectopic ds expression10. In addition, in a ds mutant background, ds ICD overexpression activates Yki and causes tissue overgrowth in a cell-autonomous fashion14. Ft is likely to act as a ligand for Ds because Ds is partially required for overexpression of the Ft extracellular domain (ECD) to trigger Yki hyperactivation14. Therefore, Ds can both promote Yki activity cell-autonomously, and repress Yki activity non-cell autonomously by signalling via Ft.
The mechanism by which Ft mediates cell-autonomous repression of Yki is relatively well-defined4, 12, 16. Upon binding to Ds on neighbouring cells, Ft controls imaginal disc growth via downstream proteins that include the atypical myosin Dachs9, the LIM-domain protein Zyxin17 and the palmitoyltransferase Approximated18. These proteins influence activity of the SWH pathway core kinase cassette, by regulating abundance of the Wts kinase9, 17. Wts in turn represses tissue growth by phosphorylating and inhibiting the Yorkie (Yki) transcriptional co-activator protein19. Ft also controls stability and subcellular localization of Expanded (Ex)5-7, another upstream regulator of the SWH pathway, while the Four-jointed (Fj) kinase regulates the interaction between the ECDs of Ft and Ds20-22.
In contrast to signalling from the Ft ICD to the SWH pathway, signalling events downstream of the Ds ICD are poorly defined. Ds ICD regulates morphogenesis by polarizing Dachs13, and has been proposed to activate Yki by sequestering SWH pathway proteins at the apical membrane14, but this idea has not been interrogated. Here we describe the identification of a membrane-to-nucleus Ds signalling pathway that promotes Yki activity by repressing Wts. Unlike the Ft branch of the SWH pathway, Ds-mediated regulation of Wts and Yki occurs independent of Dachs. By contrast, Ds promotes Yki activity by signalling via the WD40 domain protein Riq and the DYRK family kinase Mnb to induce phosphorylation-mediated repression of Wts.
RESULTS
Riquiqui, a newly-identified Dachsous-interacting protein
The atypical cadherins Ds and Ft act as a ligand-receptor pair to control tissue growth, PCP and PD patterning10, 14, 15, 23-25. The ECDs of these proteins form physical complexes and initiate signalling events between neighbouring cells21, 22. Signalling downstream of the Ft ICD has begun to be elucidated in recent years4, 12, 16. Ds controls morphogenesis by influencing the apical membrane polarity of Dachs13, but the mechanism by which it controls Yki activity and tissue growth is poorly understood. To address this knowledge gap we attempted to identify proteins that signal downstream of the Ds ICD. Using affinity purification in Drosophila melanogaster S2 cells followed by mass spectrometry26, we identified proteins that interact with the Ds ICD. An abundant Ds-interacting protein was the uncharacterized protein CG14614, hereafter referred to as Riquiqui (Riq) referring to its small size phenotype, as described below. Riq is a 343 amino acid protein and contains a WD40 domain predicted to mediate protein-protein interactions. Riq homologues are present throughout the animal kingdom and in plants, and are highly conserved; D. melanogaster Riq and its human homologue (known as DCAF7 or Han11) are 85% identical and 91% similar. The zebrafish Riq homologue Wdr68 has been implicated in craniofacial development27, but Riq function has not been studied in vivo in other organisms. To confirm Riq as a Ds-interacting protein we performed immunoprecipitation experiments using transfected S2 cell lysates. As shown in Fig. 1a, a robust physical interaction was detected between Riq and the Ds ICD.
Figure 1. Riquiqui forms a physical complex with Dachsous and controls tissue growth.
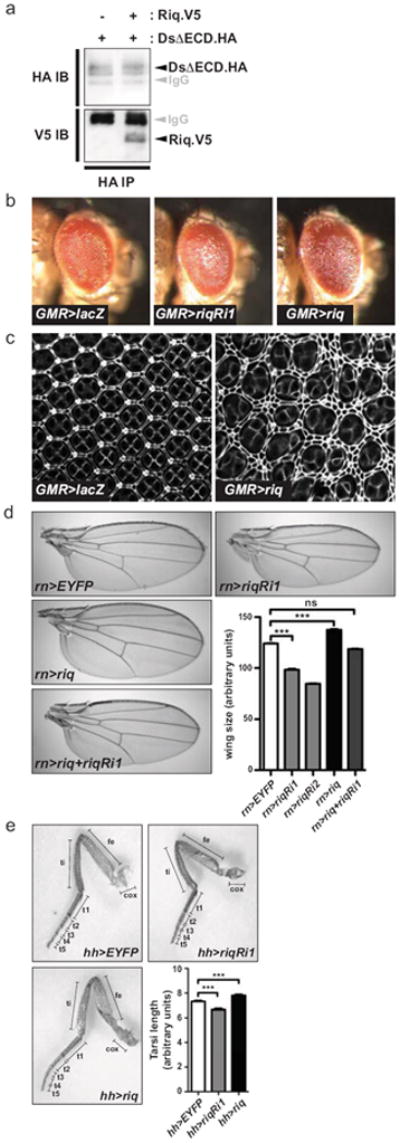
(a) Lysates from S2 cells transfected with DsΔECD.HA, alone or in combination with Riq.V5, were immunoprecipitated with anti-HA antibodies. Western blots were performed using anti-HA and anti-V5 antibodies to reveal Ds and Riq, respectively. (b) Lateral views of adult female eyes (anterior is to the right) expressing UAS-lacZ, UAS-riqRi1 or UAS-riq under the control of GMR-Gal4. (c) Eyes 44 hours APF expressing UAS-lacZ or UAS-riq under the control of GMR-Gal4 that have been stained with anti-Discs-large antibody. (d) Representative images of wings from adult female flies expressing UAS-EYFP, UAS-riqRi1, UAS-riqRi2, UAS-riq or UAS-riqRi1 concomitantly with UAS-riq under the control of rn-Gal4. Wing size was quantified for each genotype (n=20 for each). (e) Right rear legs from adult female flies expressing UAS-EYFP, UAS-riqRi1 or UAS-riq under the control of hh-Gal4. Legend of leg segments is as follows, coxa (cox), femur (fe), tibia (ti), tarsal segments 1 to 5 (t1, t2, t3, t4, t5). The length of the entire tarsal segment was quantified (n=15 for each genotype). In d and e, data are presented as mean ± SEM, *** = p<0.001, ns = non-significant.
Riquiqui regulates tissue growth
In conjunction with Ft, Ds controls tissue growth by modulating activity of the SWH pathway10, 11. In order to investigate a potential role for Riq in tissue growth, we modulated its expression in the developing eye, wing and leg by generating UASinducible riq transgenes, and with the use of independent riq RNAi transgenes that target different regions of the riq gene. A slight decrease in adult eye size was observed when UAS-riq RNAi was expressed using GMR-Gal4, compared to control eyes expressing a UAS-LacZ transgene (Fig. 1b). In the converse experiment, a modest increase in eye size was observed when riq was overexpressed using GMR-Gal4. Alterations in eye size are often accompanied by changes in interommatidial cell (IOC) number in pupal eye discs when assessed 44 hours after puparium formation (APF). When riq was overexpressed we observed an increase in IOC number compared to control (Fig. 1c).
We further explored a role for Riq in tissue growth by modulating its expression in developing wings using rotund-Gal4 (rn-Gal4). RNAi-mediated depletion of Riq caused a 21% or 32% decrease in wing size depending on the RNAi line used (Fig. 1d). Conversely, riq overexpression caused an 11% increase in wing size. We confirmed that the reduced wing size observed when riq RNAi was expressed was due to Riq depletion by showing expression of a riq transgene rescued the reduction in wing size (Fig. 1d). Further, expression of riq RNAi lines substantially reduced expression of endogenous Riq protein in developing wing imaginal discs, as determined using an antibody against the human Riq homologue, DCAF7 (Supplementary Fig. S1a). Riq knockdown was also confirmed by real time PCR; riq transcript was depleted by almost 50% in wing discs expressing UAS-riq RNAi under control of hedgehog (hh)-Gal4 (Supplementary Fig. S1b). We also assessed a role for Riq in leg growth by driving expression of riq RNAi or the riq transgene using hh-Gal4. Legs expressing riq RNAi were 9% shorter, while riq overexpression increased leg length by 7% (Fig. 1e). Collectively, these data suggest that Riq regulates IOC number in the eye, and the size of wings, eyes and legs.
The Minibrain kinase forms a physical complex with Riquiqui and is functionally related
The human Riq homologue, DCAF7 has been shown to physically complex with the human Dyrk1a kinase (homologous to D. melanogaster Mnb)28. Given the high degree of conservation of Riq, we investigated the possibility that Riq and Mnb physically and functionally interact in D. melanogaster. Initially, we tested whether Riq and Mnb physically interact by performing co-immunoprecipitation experiments on S2 cell lysates expressing epitope-tagged versions of Mnb and Riq. As shown in Fig. 2a, a robust physical association was observed between Riq and Mnb.
Figure 2. Minibrain interacts with Riquiqui and phenocopies its effect on tissue growth.
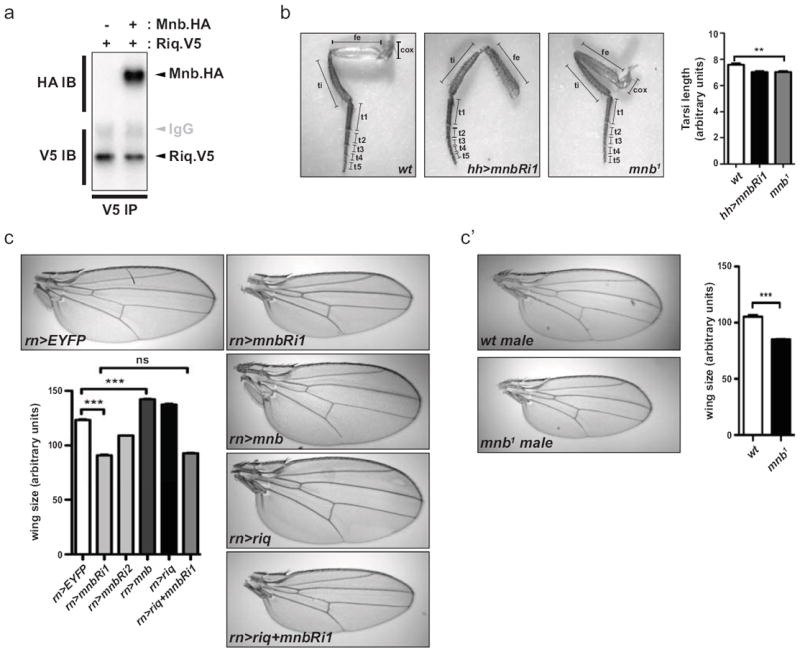
(a) Lysates from S2 cells transfected with Riq.V5 alone or together with Mnb.HA, were immunoprecipitated with anti-V5 antibodies. Western blots were performed using anti-HA and anti-V5 antibodies to reveal Mnb and Riq, respectively. (b) Images of right rear legs from adult female flies expressing hh-Gal4 driven UAS-EYFP or UAS-mnbRi1, or legs from mnb1 flies. The entire tarsal segment of individual legs was quantified (n=15 for each genotype). Legend of leg segments is as follow, coxa (cox), femur (fe), tibia (ti), tarsal segment 1 to 5 (t1, t2, t3, t4, t5). (c) Wing area from adult female flies expressing UAS-EYFP as control, UAS-mnbRi1, UAS-mnbRi2, UAS-mnb, UAS-riq or UAS-mnbRi1 concomitantly with UAS-riq under the control of rn-Gal4. Representative imagess are shown and wing size was quantified (n=20 for each genotype). (c’) Area of adult male wings of mnb1 mutant flies were quantified and compared to adult male wild-type wings. Representative images are shown. Results from leg and wing quantification (b, c and c’) are presented as mean ± SEM, *** = p<0.001, ** = p<0.01.
Subsequently, we investigated a potential role for Mnb in leg and wing growth. To characterize the role of Mnb in leg development, we measured the size of legs from flies expressing a mnb RNAi transgene or flies that were hemizygous for a mutant allele of mnb called mnb1 that has been shown to express approximately 60% to 70% less Mnb protein29. In each case, the length of the tarsal segments of these flies was 7% smaller than control flies (Fig. 2b). To investigate a role for Mnb in wing growth we expressed mnb RNAi with rn-Gal4 and found that adult wings were significantly smaller (Fig. 2c), mirroring our earlier observation with Riq. Importantly, this reduction in size was observed using two independent mnb RNAi transgenes directed against independent regions of the mnb gene, thus ruling out off-target effects (Fig. 2c). Consistent with a role for Mnb as a growth-promoting protein, rn-Gal4 driven mnb overexpression led to a 15% increase in wing size. A role for mnb in wing growth was further substantiated by assessing wing size in flies that were hemizygous for mnb1; these flies possessed wings that were 19% smaller than wild-type flies (Fig. 2c’). These findings show that Mnb, like Riq, is required for normal wing growth.
To begin to assess whether Mnb and Riq control wing growth together we used rn-Gal4 to simultaneously overexpress Riq, and deplete Mnb by RNAi. Mnb depletion completely suppressed Riq’s ability to promote wing overgrowth as these wings were undergrown and were of similar size to wings expressing the mnb RNAi alone (Fig. 2c). Collectively, our biochemical and genetic data support a model whereby Riq and Mnb function together to promote tissue growth.
Riquiqui and Mnb regulate activity of the SWH pathway oncoprotein Yorkie
Ds has been postulated to control SWH pathway activity by acting both as a receptor and ligand for Ft10, 14, 23. Based on our above data, we hypothesized that Riq and Mnb control tissue growth downstream of the Ds ICD by modulating SWH pathway activity. To address this hypothesis we modulated expression of Mnb and Riq and assessed Yki activity using lacZ enhancer traps in the ex, thread (th or Diap1) and bantam (ban) genes. Both riq and mnb overexpression caused Yki activity to increase, as indicated by increases in ex-lacZ, th-lacZ or ban-lacZ (Fig. 3a-d). Consistent with these findings, RNAi-mediated depletion of either Riq or Mnb led to reduced Yki activity, as determined by th-lacZ (Fig. 3e,f). Collectively, these experiments suggest that Riq and Mnb regulate tissue growth by controlling SWH pathway activity.
Figure 3. Riquiqui and Minibrain regulate SWH pathway activity.
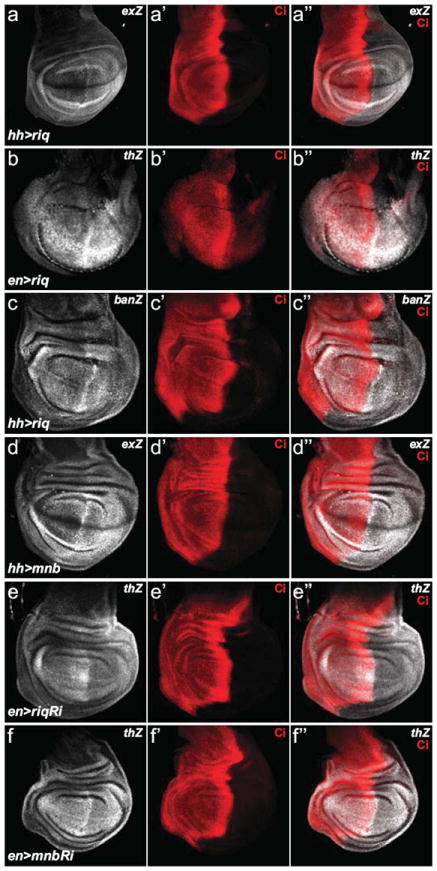
Wing imaginal discs from third instar D. melanogaster larvae harbouring the following transgenes: (a) hh-Gal4, UAS-riq and ex-lacZ; (b) en-Gal4, UAS-riq and th-lacZ; (c) hh-Gal4, UAS-riq and ban-lacZ; (d) hh-Gal4, UAS-mnb and ex-lacZ; (e), en-Gal4, UAS-riqRi and th-lacZ; and (f) en-Gal4, UAS-mnbRi and th-lacZ. Yki activity (grey in a-f) was reported by ex-lacZ (a and d), th-lacZ (b, e and f) or ban-lacZ (c). All transgenes were expressed in the posterior compartment of wing imaginal discs; Cubitus interruptus (Ci) expression (red in a’-f’) marked the anterior compartment. Merged images are shown in (a”-f”).
Riquiqui and Minibrain repress the SWH pathway by phosphorylating and inhibiting the Warts kinase
Ft controls SWH pathway activity by polarizing the localization of Dachs at the apical membrane of epithelial cells9, while Ds polarizes Dachs to regulate morphogenesis13. However we found no evidence that Ds controls tissue growth via Dachs, as modulating the expression of either Riq or Mnb had no influence on Dachs levels or localization in wing imaginal discs, and these proteins appeared to control wing size in parallel to each other (Supplementary Fig. S2). To further investigate the mechanism by which Mnb and Riq regulate the SWH pathway we used a biochemical approach. Given that Mnb is a kinase, we hypothesized that it phosphorylates a component(s) of the SWH pathway. To test this notion we co-expressed Mnb with different SWH pathway proteins in S2 cells and assessed their mobility by SDS-PAGE. When co-expressed with Mnb the mobility of Wts was decreased (Fig. 4a), while Hpo and Sav were unaffected (data not shown). Mnb’s ability to alter Wts mobility was potentiated by Riq because when S2 cells co-expressed Wts, Mnb and Riq, Wts mobility was further decreased (Fig. 4a). Riq alone had no impact on Wts mobility showing that it requires exogenous Mnb to influence Wts mobility. Mnb’s ability to influence Wts mobility was also observed when these proteins were co-expressed with Sav and Hpo, which are known to regulate Wts phosphorylation (Supplementary Fig. S3a).
Figure 4. Minibrain phosphorylates Warts.
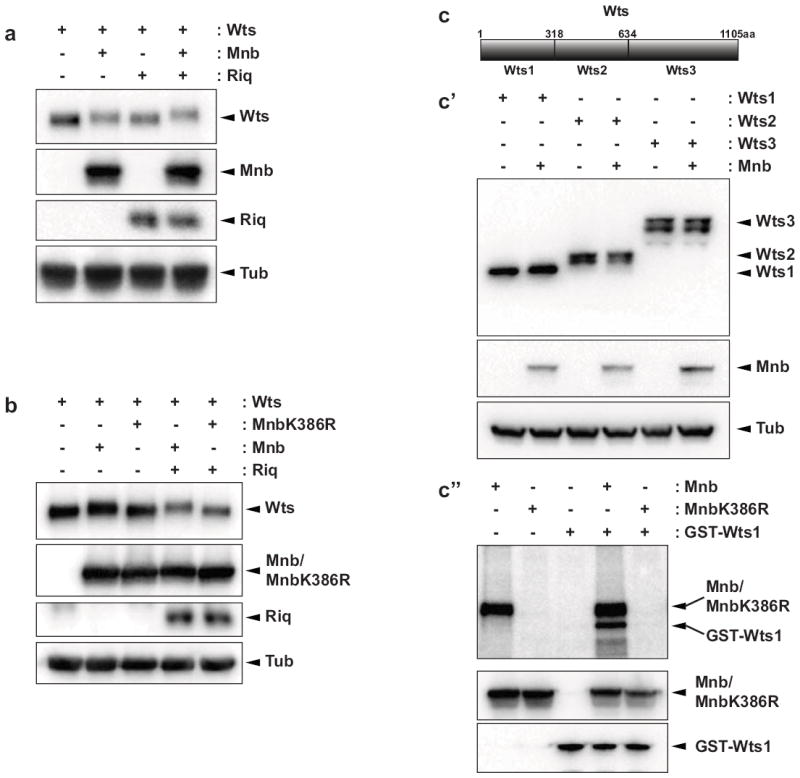
(a and b) Western blot analysis of lysates from S2 cells transfected with the indicated plasmids. Wts and Riq were revealed with anti-V5 and Mnb or Mnb K386R with anti-HA. Tubulin levels were assessed to ensure even protein loading. Note that tubulin displayed no change in mobility. (c) Schematic diagram of the Wts protein and the fragments of Wts (Wts1, Wts2 and Wts3) that were expressed in S2 cells alone or together with Mnb. (c’) Western Blot analysis was performed with antibodies to V5 (to reveal Wts), HA (to reveal Mnb) and Tubulin. (c’’) A kinase assay performed using recombinant GST-Wts1 as a substrate and either Mnb or Mnb K386R immunoprecipitated from S2 cells. Isolated proteins were incubated alone or together in kinase buffer containing γ32P-ATP and subjected to SDS-PAGE (higher panel). Western blotting was used to detect input proteins (lower panels).
To investigate whether the observed change in Wts mobility was the result of phosphorylation, we generated a kinase-dead version of Mnb by mutating the ATP-binding site (Mnb K386R). Co-expression of Mnb K386R with Wts failed to modify Wts mobility in the presence or absence of Riq (Fig. 4b), showing that Mnb requires its kinase activity to modulate Wts mobility. For several reasons, we also investigated whether Homeodomain-interacting protein kinase (Hipk) could alter the mobility of Wts; Hipk is the closest Mnb homologue in D. melanogaster; Hipk’s human orthologue (HIPK2) can also physically interact with the human Riq orthologue (DCAF7)30; and we and others have recently shown that Hipk regulates tissue growth via the SWH pathway31, 32. However, in contrast to Mnb, Hipk had no effect on Wts mobility, suggesting that it does not phosphorylate Wts (Supplementary Fig. S3b).
We then explored which region of Wts was phosphorylated by Mnb, by expressing three portions of Wts protein with or without Mnb. Only Wts1, representing amino acids 1-318, showed a clear mobility change in the presence of Mnb (Fig. 4c’). To determine whether Mnb could indeed phosphorylate this portion of Wts we performed in vitro kinase assays using recombinant GST-Wts1 as a substrate. Immunoprecipitations were performed on lysates from untransfected cells or cells expressing either Mnb or Mnb K386R. After incubation in kinase buffer supplemented with γ32P-ATP Mnb, but not Mnb K386R, displayed strong autophosphorylation confirming that the K386R mutation renders Mnb inactive (Fig. 4c”). Mnb stimulated strong phosphorylation of GST-Wts1, whereas Mnb K386R did not. These data show that Mnb indeed phosphorylates Wts, and does so by phosphorylating one or more residues between amino acids 1-318.
Given that Mnb could phosphorylate Wts in vitro we sought to determine whether Mnb and/or Riq physically interact with Wts. Immunoprecipitations were performed on lysates obtained from S2 cells transfected with different combinations of epitope-tagged versions of Mnb, Riq and Wts. Initially, we found that Wts and Mnb could form a physical complex in S2 cells (Fig. 5a). We then investigated whether Riq and Wts could interact by immunoprecipitating Riq with an antibody directed against human DCAF7, which we previously found to cross-react with Riq in imaginal discs (Supplementary Fig. S1a). We were able to detect a weak but consistent physical interaction between Riq and Wts (Fig. 5a’). We also co-transfected Wts, Mnb and Riq together in S2 cells and detected physical interactions between each protein (Fig. 5a”). These experiments suggest that Mnb, Wts and Riq can form a complex that allows Mnb to phosphorylate Wts.
Figure 5. Minibrain and Riquiqui form a physical complex with Warts and inhibit its activity.
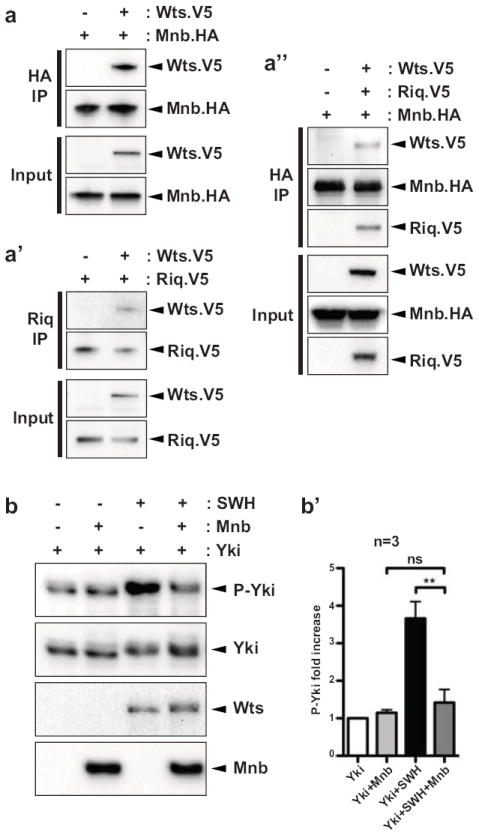
(a) V5-tagged Wts was expressed alone or together with HA-tagged Mnb or V5-tagged Riq or both (as labeled above the blot) in S2 cells. Cells were lysed and lysates were subjected to immunoprecipitation using the anti-HA antibody or the anti-DCAF7 antibody (homologues of Riq in mammals). Proteins were then revealed using the corresponding antibody. (b) Yki was expressed alone or together with Mnb or a combination of Salvador/Warts/Hippo (SWH) or both in S2 cells. Yorkie phosphorylation was assessed by Western Blot using an anti-PhosphoS168-Yorkie antibody. Fold increase from Phospho-Yki/total Yki over three independent transfections (n=3) has been quantified. **p<0.01, ns = non-significant.
Next, we investigated the functional consequence of Riq/Mnb-dependent phosphorylation of Wts. Based on our observations that Riq and Mnb normally promote tissue growth and Yki activity, we hypothesized that Riq and Mnb repress Wts activity. To interrogate this hypothesis we assayed Wts activity in the presence or absence of Mnb by assessing Yki phosphorylation. Wts stimulates phosphorylation of Yki on at least three residues, with S168 being the major site33, 34. Co-expression of Mnb with Yki did not influence basal phosphorylation of Yki at S168, as detected by a phospho-Yki-S168 antibody (Fig. 5b). In the presence of Sav, Wts and Hpo, Yki was strongly phosphorylated at S168; when quantified across three independent experiments, a 3.7-fold increase was observed (Fig. 5b’). When Mnb was also expressed together with Sav, Wts and Hpo a strong reduction of Yki-S168 phosphorylation was observed; in this setting Yki-S168 phosphorylation was similar to that observed in cells expressing Yki alone (Fig. 5b,b’). These data are consistent with the idea that Riq and Mnb promote tissue growth by repressing Wts, thus derepressing Yki.
Dachsous requires Riquiqui and Minibrain to activate Yorkie
To determine whether Riq and Mnb function downstream of the Ds ICD to regulate the ability of Wts to limit Yki activity, we performed a series of experiments in developing D. melanogaster tissues. We first overexpressed ds in the posterior compartment of wing imaginal discs using hh-Gal4 and found that Yki activity, as assessed by ex-lacZ, was strongly increased especially in the wing pouch (Fig. 6a). Interestingly, and as reported previously, non-ds overexpressing cells at the AP border also showed a strong increase in ex-lacZ (Fig. 6a”)14. We hypothesized that depletion of Riq or Mnb would suppress the ability of ds overexpression to induce Yki activity without affecting non-cell autonomous Yki elevation in anterior wing disc cells. Our experiments showed that indeed ex-lacZ levels in the posterior wing disc were greatly reduced when either Riq or Mnb were depleted by RNAi, but that Yki hyperactivation in anterior cells near the AP boundary was unaffected (Fig. 6b-c”). Mnb and Riq depletion also affected Ds-induced effects on tissue morphology: ds overexpression caused wing disc convolution and this was suppressed by Mnb or Riq depletion (X-Z sections in Fig. 6a-c). Suppression of Ds-induced Yki activation was not caused by a reduced Ds expression as depletion of neither Mnb nor Riq, affected Ds levels (Supplementary Fig. S4).
Figure 6. Riquiqui and Minibrain function downstream of Dachsous to promote Yorkie activity.
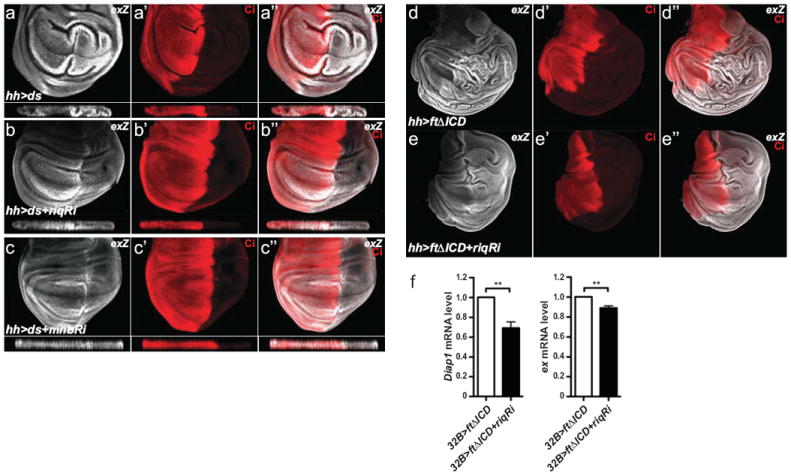
Wing imaginal discs from third instar D. melanogaster larvae harbouring the following transgenes: (a) hh-Gal4, UAS-ds and ex-lacZ; (b) hh-Gal4, UAS-ds, UAS-riqRi and ex-lacZ; (c) hh-Gal4, UAS-ds, UAS-mnbRi and ex-lacZ; (d) hh-Gal4, UAS-ftΔICD and ex-lacZ; (e) hh-Gal4, UAS-ftΔICD, UAS-riqRi and ex-lacZ. Yki activity was reported by ex-lacZ levels (grey in a-e). Cubitus interruptus (Ci) expression (red in a’-e’) marked the anterior compartment of wing imaginal discs. Merged images are shown in (a’’-e’’). X-Z sections through the wing pouch are shown below planar sections of wing discs in a-c”. (f) Levels of ex mRNA and Diap1 mRNA relative to the control actin5C mRNA, assessed by QRT-PCR, in wing imaginal discs harbouring the following transgenes: 32B-Gal4 and UAS-ftΔICD; and 32B-Gal4, UAS-ftΔICD and UAS-riqRi. Data is presented as mean ± SEM, n=3, ** = p<0.01.
Consistent with the idea that signalling downstream of the Ds ICD regulates Yki activity upon Ds-Ft binding, expression of the Ft ECD (ftΔICD) can hyperactivate Yki in a Ds-dependent fashion14. In order to determine if Ds-dependent activation of Yki requires Riq and Mnb, we overexpressed ftΔICD in the posterior compartment of the wing and assessed Yki activity in the absence or presence of riq RNAi. ftΔICD expression strongly increased ex-lacZ and caused substantial overgrowth and folding of the posterior wing disc (Fig. 6d). RNAi mediated depletion of Riq in ftΔICD-expressing tissues partially suppressed both of these phenotypes (Fig. 6e). To further characterize this effect, we used the 32B-Gal4 driver to express ftΔICD with or without riq RNAi throughout wing imaginal discs. Depletion of Riq in ftΔICDexpressing discs caused an 11% decrease in ex and a 31% decrease in Diap1, as assessed by RT-PCR (Fig. 6f). These data support the idea that, in response to Ft-Ds binding, the Ds ICD promotes Yki-dependent tissue growth by signalling via Riq and Mnb to alleviate Wts-dependent repression of Yki (Fig. 7).
Figure 7. Model of signalling from Dachsous and Fat to the SWH pathway.
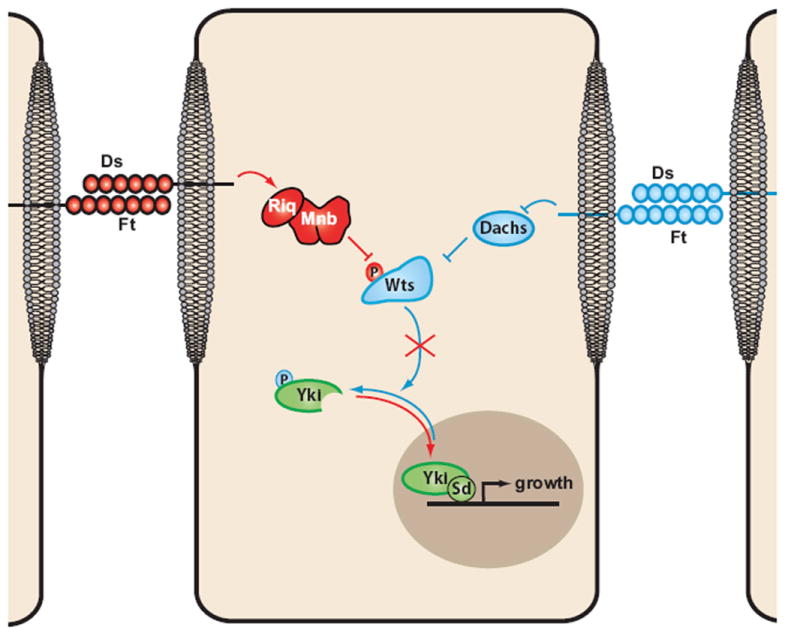
Signalling from the Ds ICD is represented in red (positively regulating growth) and signalling from the Ft ICD in blue (negatively regulating growth). (Ds: Dachsous, Ft: Fat, Riq: Riquiqui, Mnb: Minibrain, Wts: Warts, Yki: Yorkie, Sd: Scalloped)
DISCUSSION
The related cadherins, Ft and Ds, control tissue growth by regulating SWH pathway activity5-7, 9-11, and also control PCP and PD patterning12, 23. Intriguingly, Ft and Ds regulate SWH pathway activity by engaging in reciprocal signalling as a ligand-receptor pair. Signalling downstream of Ft is reasonably well defined, but signalling downstream of Ds has remained relatively obscure until the discovery of a membrane-to-nucleus signalling pathway controlling SWH pathway activity downstream of the Ds ICD, described here. Our genetic and biochemical data implies that, upon ligation of the Fat and Ds ECDs, the WD40 domain protein Riq complexes with the Ds ICD and the Mnb and Wts kinases. Riq promotes Mnb-dependent phosphorylation and inhibition of Wts, and thereby promotes Yki-dependent tissue growth.
Therefore, Ds-Ft ligation induces two seemingly opposing growth-regulatory events: Ds activates Ft, which represses Yki by modulating Dachs 10, 11; while Ft signals via Ds, Riq and Mnb to activate Yki. At first glance it seems counterintuitive that Ft-Ds binding would both promote, and repress Yki-dependent tissue growth but raises several interesting possibilities. One option is that the timing of signalling from both the Ds ICD and the Ft ICD is different and varies throughout the cell cycle. For example, Ds ICD might deliver a pulse of Yki activity to induce transcriptional events associated with tissue growth. Subsequently, to ensure that Yki activity does not perdure and cause tissue overgrowth, it could be repressed by signalling from Ft ICD. Alternatively, Ds ICD or Ft ICD signalling might predominate over the other in different regions of imaginal discs or at different stages of development, to regulate Yki. How such regulation could occur is unclear, but could possibly: 1) stem from polarized activity of Ft and Ds that occurs in cells of growing imaginal discs in response to graded expression of Ds and Fj21, 22; 2) occur if the influence of signalling downstream of Ft ICD or Ds ICD on Wts activity was quantitatively different; 3) result from non-uniform activity of additional proteins that mediate Ft and Ds signalling. Alternatively, repression of Yki by the Ft ICD, and activation by the Ds ICD, could quantitatively oppose each other and serve to set a fine threshold of Yki activity that is highly sensitive to regulation by other branches of the SWH pathway such as the Kibra-Expanded-Merlin proteins35-38, the Hpo activating kinase Tao-139, 40, or apicobasal polarity proteins41-44. In future studies it will be important to define the spatiotemporal activity profile of Ft ICD and Ds ICD signalling and the relative influence of the Ds and Ft branches of the SWH pathway on tissue growth.
Given that Ft and Ds also engage in bi-directional signaling to control PCP15 and PD patterning24, it will be important to determine whether Riq and Mnb control these processes downstream of the Ds ICD. Finally, given the emergence of the SWH pathway as an important regulator of different human tumours1, the present study raises the possibility that the human orthologues of Riq (DCAF7) and Mnb (Dyrk1a and Dyrk1b) could function as oncogenes.
METHODS
Drosophila melanogaster stocks
Riquiqui knockdown was performed using two RNAi lines: UAS-riqRi1 (KK 107076) from Vienna Drosophila RNAi Center (VDRC) and UAS-riqRi2 (14614R-1) from the Japanese National Institute of Genetic (NIG). Minibrain knockdown was performed using two RNAi lines: UAS-mnbRi1 (KK 107066) and UAS-mnbRi2 (GD 28628) both from VDRC. The riq overexpression line was engineered by injection of a pUAST-riq construct cloned by PCR from the CG14614 cDNA obtained from DGRC (clone LD15927). UAS-mnb was provided by K. Yu. Other stocks were: UAS-Ds, UAS-EYFP, ex-lacZ; hh-Gal4, ban-lacZ; hh-Gal4, en-Gal4; th-LacZ, UAS-dachs, UAS-dachs RNAi, salm-Gal4, rn-Gal4, hh-Gal4 and GMR-Gal4. The mnb1 allele was provided by F. Tejedor. Clones of UAS-riqRi1 and UAS-mnbRi1 were obtained by crossing UAS lines with the actin-Gal4 flip out stock: hsflp; actin>CD2>Gal4, UAS-GFP.
Quantification of wing size and leg length
Wing area from female or male (as specified) flies reared at 25°C was quantified using Adobe Photoshop. The tarsal segment (comprising T1 to 5) of the right back leg of female adult flies was quantified using Image J. The mean and S.E.M values of wing area or tarsal length were determined using GraphPad Prism. To assess statistical significance, each genotype was compared using one-way ANOVA followed by a Bonferroni post-test. P values <0.05 were considered significant.
Immunostaining
We used the following primary antibodies: mouse anti-β-galactosidase (Sigma), rat anti-Cubitus-Interruptus, mouse anti-Disc-large (both from Developmental Studies Hybridoma Bank), rabbit anti-DCAF7 (Novus Biologicals), rat anti-Dachs (gift from D. Strutt) and rat anti-Dachsous (gift from M Simon). Secondary antibodies (anti-rat, anti-mouse and anti-rabbit) were from Invitrogen. Third instar larval imaginal discs and pupal eye discs (44 hours APF) were stained as in45, 46.
Plasmids
The Ds ICD (DsΔECD) was amplified by PCR from pUAST-DsΔECD47 and cloned into the pAc5.1 and GS-TAP vectors26. The Riq open reading frame was PCR-amplified from DGRC clone LD15927, epitope-tagged and cloned into pAc5.1. pMT-Mnb. HA was obtained from I.Edery48. pMT-Mnb K386R.HA was generated by site-directed mutagenesis. pAc5.1-Wts.V5 and pAc5.1-Yki.HA were from D. Pan19. pAc5.1-Hpo.FLAG and pAc5.1-Sav.myc were from N. Tapon49. Portions of Wts were amplified by PCR and cloned into pAc5.1. pAc5.1-myc.HipK was described in31. Wts1 was cloned into pGEX-4T1 for bacterial expression.
Cell culture, transfection and western blot analysis
S2 cells were cultured in Schneider’s Drosophila medium (Gibco) supplemented with 10% FBS (Gibco) and 1% Penicillin/streptomycin (Gibco). Cells were transfected with the indicated plasmids using the Effectene transfection reagent (Qiagen). CuSo4 was added to culture media at a final concentration of 0.35mM when using pMT based plasmids. After 48 hours, cells were lysed using a RIPA buffer (10 mm NaPi buffer, pH 7.8, 60 mM NaCl, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS, 10% glycerol, 25 mM β-glycerol phosphate, 50 mM sodium fluoride, 2 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and protease inhibitor Complete, Roche) and subjected to SDS-PAGE then transferred to a PVDF membrane (Millipore). In order to visualize differences in Wts mobility, 3-8% Tris Acetate gels were used (Invitrogen). 4-12% Bis-Tris gels were used for other experiments. Membranes were immunobloted with the following antibodies: rabbit ant-HA, mouse anti-V5, mouse anti-Tubulin (all from Invitrogen), rabbit anti-Phospho-S168-Yorkie (gift from N. Tapon). HRP coupled secondary antibodies were from Dako. Detection was performed using the ECL prime Western Blotting Detection form Amersham. Images were taken using the ChemiDoc MP (Biorad). Quantification of bands was performed with Image J.
Kinase assay
S2 cells were transfected with Mnb or Mnb K386R. Two days after transfection, cells were lysed in DISC lysis buffer [150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 10% glycerol and 20 mM Tris pH 7.5, 10 mM NaF, 2 mM Na pyrophosphate, 5 mM β-glycerophosphate and PhosStop phosphatase inhibitor cocktail (Roche) and Complete Mini protease inhibitor cocktail (Roche)] and immunoprecipitated using anti-HA (Invitrogen). BL-21 bacteria transformed with pGEX-4T1-GST-Wst1 were incubated with 1mM IPTG at 37°C to induce GST-Wts1. Recombinant protein was purified using Glutathione sepharose, and eluted in 50mM Tris-HCl, 10mM Glutathione, 0.05% Triton X-100, pH8. Equivalent amounts of GST-Wts1 were used in each kinase assay. Equivalent amounts of immunoprecipitates were divided into the combinations noted in the figure legend in kinase buffer [50 mM Hepes pH 7.4, 10 mM MgCl2, 1mM DTT, 10% glycerol, 1 mM EDTA, 1 mM EGTA, 100 mM NaCl, 1 mM NaF, 5 mM β-glycerophosphate, 100 μM ATP, PhosStop phosphatase inhibitor cocktail and Complete Mini protease inhibitor cocktail]. 5μCi γ32P-ATP (Perkin Elmer) was added to each sample and incubated at 30°C for 30 minutes as in50. The reaction was stopped by the addition of 5X protein loading buffer, boiled at 95°C and subjected to SDS-PAGE. Gels were dried and exposed to a Phosphorimager screen (Molecular Dynamic) and analysed using the Typhoon Trio Phosphorimager and ImageQuant software (GE Healthcare) as in39.
Quantitative Real Time PCR
RNA was extracted from wing imaginal discs by Trizol (Invitrogen), and used to generate cDNA with Superscript III (Invitrogen). Quantitative PCR reactions were performed on Applied Biosystems Step One Plus software with Fast SYBR Green Master Mix (Applied Biosystems) and primers designed to detect ex, Diap1 and Actin 5C mRNA as in51.
Supplementary Material
Acknowledgments
We thank J. Lin for expertise with kinase assays and S. Blair, I. Edery, K. Irvine, D. Pan, M. Simon, D. Strutt, N. Tapon, F. Tejedor, K. Yu, the Developmental Studies Hybridoma Bank, the Vienna Drosophila RNAi Center, the Australian Drosophila Research Support Facility (www.ozdros.com), the National Institute of Genetics and the Bloomington Stock Centre for fly stocks, plasmids and antibodies. K.F.H is a Sylvia and Charles Viertel Senior Medical Research Fellow. This research was supported by a Project Grant from the National Health and Medical Research Council of Australia, and by NIH grants GM097727 and CA156734 and NSF grant 0640700 to A.V. Mass spectrometry was performed at the Taplin Mass Spectrometry Facility, Harvard Medical School.
Footnotes
CONTRIBUTIONS
J.D. and C.C.M. performed Drosophila genetic experiments. J.D. and E.Y. carried out biochemistry and molecular biology experiments. M.T. and A.V. performed affinity pruification followed by mass spectrometry. F.B. and Y.B. analyzed Dachs localization in the pupal notum. J.D. and K.F.H. designed experiments, analyzed data and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nature reviews Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 2.Badouel C, Garg A, McNeill H. Herding Hippos: regulating growth in flies and man. Curr Opin Cell Biol. 2009;21:837–843. doi: 10.1016/j.ceb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy BV, Irvine KD. The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development. 2008;135:2827–2838. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- 5.Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 6.Willecke M, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumorsuppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Tyler DM, Baker NE. Expanded and fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Dev Biol. 2007;305:187–201. doi: 10.1016/j.ydbio.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 10.Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci U S A. 2008;105:14897–14902. doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sopko R, McNeill H. The skinny on Fat: an enormous cadherin that regulates cell adhesion, tissue growth, and planar cell polarity. Curr Opin Cell Biol. 2009;21:717–723. doi: 10.1016/j.ceb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Bosveld F, et al. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science. 2012;336:724–727. doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]
- 14.Matakatsu H, Blair SS. Separating planar cell polarity and Hippo pathway activities of the protocadherins Fat and Dachsous. Development. 2012;139:1498–1508. doi: 10.1242/dev.070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:R574–582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Rauskolb C, Pan G, Reddy BV, Oh H, Irvine KD. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 2011;9:e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matakatsu H, Blair SS. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr Biol. 2008;18:1390–1395. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of Fat:Dachsous Binding by the Cadherin Domain Kinase Four-Jointed. Curr Biol. 2010 doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-Jointed Modulates Growth and Planar Polarity by Reducing the Affinity of Dachsous for Fat. Curr Biol. 2010 doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence PA, Struhl G, Casal J. Do the protocadherins Fat and Dachsous link up to determine both planar cell polarity and the dimensions of organs? Nat Cell Biol. 2008;10:1379–1382. doi: 10.1038/ncb1208-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark HF, et al. Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev. 1995;9:1530–1542. doi: 10.1101/gad.9.12.1530. [DOI] [PubMed] [Google Scholar]
- 25.Thomas C, Strutt D. The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Developmental dynamics : an official publication of the American Association of Anatomists. 2012;241:27–39. doi: 10.1002/dvdy.22736. [DOI] [PubMed] [Google Scholar]
- 26.Kyriakakis P, Tipping M, Abed L, Veraksa A. Tandem affinity purification in Drosophila: the advantages of the GS-TAP system. Fly (Austin) 2008;2:229–235. doi: 10.4161/fly.6669. [DOI] [PubMed] [Google Scholar]
- 27.Nissen RM, Amsterdam A, Hopkins N. A zebrafish screen for craniofacial mutants identifies wdr68 as a highly conserved gene required for endothelin-1 expression. BMC developmental biology. 2006;6:28. doi: 10.1186/1471-213X-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skurat AV, Dietrich AD. Phosphorylation of Ser640 in muscle glycogen synthase by DYRK family protein kinases. J Biol Chem. 2004;279:2490–2498. doi: 10.1074/jbc.M301769200. [DOI] [PubMed] [Google Scholar]
- 29.Tejedor F, et al. minibrain: a new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron. 1995;14:287–301. doi: 10.1016/0896-6273(95)90286-4. [DOI] [PubMed] [Google Scholar]
- 30.Ritterhoff S, et al. The WD40-repeat protein Han11 functions as a scaffold protein to control HIPK2 and MEKK1 kinase functions. EMBO J. 2010;29:3750–3761. doi: 10.1038/emboj.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poon CL, Zhang X, Lin JI, Manning SA, Harvey KF. Homeodomain-interacting protein kinase regulates hippo pathway-dependent tissue growth. Curr Biol. 2012;22:1587–1594. doi: 10.1016/j.cub.2012.06.075. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Verheyen EM. Homeodomain-interacting protein kinase regulates yorkie activity to promote tissue growth. Curr Biol. 2012;22:1582–1586. doi: 10.1016/j.cub.2012.06.074. [DOI] [PubMed] [Google Scholar]
- 33.Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamaratoglu F, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 36.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra Is a Regulator of the Salvador/Warts/Hippo Signaling Network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW Domain Protein Kibra Acts Upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, et al. Kibra Functions as a Tumor Suppressor Protein that Regulates Hippo Signaling in Conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poon CL, Lin JI, Zhang X, Harvey KF. The Sterile 20-like Kinase Tao-1 Controls Tissue Growth by Regulating the Salvador-Warts-Hippo Pathway. Dev Cell. 2011;21:896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 Phosphorylates Hippo/MST Kinases to Regulate the Hippo-Salvador-Warts Tumor Suppressor Pathway. Dev Cell. 2011;21:888–895. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 42.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling C, et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CL, et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milton CC, Zhang X, Albanese NO, Harvey KF. Differential requirement of Salvador-Warts-Hippo pathway members for organ size control in Drosophila melanogaster. Development. 2010;137:735–743. doi: 10.1242/dev.042309. [DOI] [PubMed] [Google Scholar]
- 46.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 47.Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 48.Chiu JC, Ko HW, Edery I. NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell. 2011;145:357–370. doi: 10.1016/j.cell.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 50.Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 51.Harvey KF, et al. FOXO-regulated transcription restricts overgrowth of Tsc mutant organs. J Cell Biol. 2008;180:691–696. doi: 10.1083/jcb.200710100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


