Abstract
In gram-negative bacteria, the RNA-binding protein Hfq has emerged as an important regulatory factor in a variety of physiological processes, including stress resistance and virulence. In Escherichia coli, Hfq modulates the stability or the translation of mRNAs and interacts with numerous small regulatory RNAs. Here, we studied the role of Hfq in the stress tolerance and virulence of the gram-positive food-borne human pathogen Listeria monocytogenes. We present evidence that Hfq is involved in the ability of L. monocytogenes to tolerate osmotic and ethanol stress and contributes to long-term survival under amino acid-limiting conditions. However, Hfq is not required for resistance to acid and oxidative stress. Transcription of hfq is induced under various stress conditions, including osmotic and ethanol stress and at the entry into the stationary growth phase, thus supporting the view that Hfq is important for the growth and survival of L. monocytogenes in harsh environments. The stress-inducible transcription of hfq depends on the alternative sigma factor σB, which controls the expression of numerous stress- and virulence-associated genes in L. monocytogenes. Infection studies showed that Hfq contributes to pathogenesis in mice, yet plays no role in the infection of cultured cell lines. This study provides, for the first time, information on the role of Hfq in the stress tolerance and virulence of a gram-positive pathogen.
In recent years, small RNA (sRNA) molecules have attracted increasing attention as important regulatory elements in various biological processes (15, 30). In Escherichia coli, the RNA-binding protein Hfq has emerged as a key modulator of riboregulation, and mutants lacking Hfq are defective in growth and survival under various conditions, such as high-temperature, oxidative, acid, and osmotic stress conditions (19, 32). In E. coli, Hfq displays several modes of action. It interacts with numerous regulatory sRNAs, including Spot42, OxyS, DsrA, and RprA RNAs, that control gene expression at the posttranscriptional level by base pairing with complementary sequences present in the target mRNAs (15, 36, 39). Hfq modulates the activity of several of these regulatory sRNAs by stimulating the pairing between sRNAs and their target mRNAs or by stabilizing some of the sRNAs (18, 38). In addition, Hfq affects other processes that appear not to involve the action of sRNAs, such as the stability of mRNAs (33, 35) and the elongation of poly(A) tails (7).
Analysis of available genome sequences has revealed that Hfq homologues are present in both gram-negative and gram-positive bacteria. Structural analysis of Hfq from Staphylococcus aureus showed that this protein forms a hexameric ring-shaped structure and that it belongs to the large family of Sm-like proteins that participate in a variety of RNA processing reactions (28). In several gram-negative pathogens, including Yersinia enterocolitica, Pseudomonas aeruginosa, and Brucella abortus, Hfq has been implicated in virulence (20, 26, 29). B. abortus is an intracellular pathogen that is capable of establishing and maintaining long-term residence in the phagosomal compartment of host macrophages. Within this compartment, B. abortus has to resist harsh conditions, such as nutrient deprivation, oxidative stress, and acid stress. In this environment, a B. abortus mutant lacking Hfq is unable to survive and replicate (26). Hfq regulates a number of genes related to stationary-phase-induced stress resistance in B. abortus, suggesting that the gene products support survival of this pathogen in the phagosomal compartment (27). However, the regulatory mechanism of Hfq in B. abortus remains to be investigated.
In gram-positive bacteria the role of Hfq has not been addressed yet. Therefore, we chose to study Hfq in the gram-positive food-borne pathogen Listeria monocytogenes, which causes serious infections in humans. The clinical symptoms of human listeriosis include febrile gastroenteritis, abortion, life-threatening septicemia, and meningitis (34). Like B. abortus, L. monocytogenes is a facultative intracellular bacterium that is capable of invading, surviving, and growing within living host cells. Following the invasion of host cells, L. monocytogenes escapes from the phagocytic vacuole and replicates within the host cytosol. Subsequently, the bacteria spread to neighboring cells, and a new infection cycle begins. Key virulence factors mediating these events include the pore-forming toxin LLO and the phospholipase PlcA, both of which mediate the escape from phagocytic vacuoles. Transcription of the virulence genes is tightly controlled in response to various stimuli by the regulatory protein PrfA (14).
In the present study, we sought to clarify the role of Hfq in stress tolerance and virulence in L. monocytogenes. Using an in-frame hfq deletion mutant, we found that Hfq is important for the tolerance of L. monocytogenes to osmotic stress and ethanol stress and for long-term survival under amino acid-limiting conditions but that it does not contribute to resistance to acid stress and oxidative stress. We also found that the alternative stress sigma factor σB regulates the transcription of hfq. Finally, we found that Hfq contributes to the pathogenicity of L. monocytogenes in mice but is not required for intracellular multiplication of this pathogen in cell lines J774A.1 and INT-407. These results demonstrate, for the first time, that Hfq of a gram-positive pathogen plays a role in stress tolerance and virulence in mice.
MATERIALS AND METHODS
Bacterial strains and growth media.
The L. monocytogenes EGD serotype 1/2a strain (obtained from W. Goebel, University of Wurzburg, Wurzburg, Germany) and the EGDΔsigB mutant (3) were routinely grown at 37°C with shaking in brain heart infusion (BHI) medium (Oxoid), Luria-Bertani (LB) medium, or improved minimal medium (IMM) (22). When required, erythromycin and kanamycin were added to final concentrations of 5 and 50 μg ml−1, respectively.
E. coli strain TOP10 (Invitrogen) was grown in LB medium. When required, 150 μg of erythromycin ml−1 was added to the medium. Hemolysis and lecithinase activity tests were performed on blood agar plates (blood agar base; Oxoid) or egg yolk agar plates supplemented with charcoal, as described previously (5). Utilization of different carbon sources was determined by the API 50 CHL method as recommended by the manufacturer (BioMérieux).
Construction of hfq deletion strain.
For in-frame deletion of the hfq gene, L. monocytogenes EGD chromosomal DNA was used as the template for PCR amplification of DNA fragments containing either the 5′ end of the gene and upstream sequences or the 3′ end of the gene and downstream sequences. Primers HfqA and HfqB, giving a 495-bp PCR fragment, and primers HfqC and HfqD, resulting in a 505-bp PCR fragment, were used for this procedure (Table 1). Splicing by the overlap extension method was used to join the hfq fragments, which created a PCR fragment containing a 213-bp in-frame deletion in the hfq gene (9). The Δhfq fragment was digested with XbaI and BamHI, and the fragment was cloned into the temperature-sensitive shuttle vector pAUL-A (4). The resulting plasmid was introduced into L. monocytogenes EGD by electroporation (21), and integration of the plasmid on the chromosome was achieved by growing the transformed strains at 42°C in the presence of erythromycin. To allow allelic exchange between the intact gene and the deleted gene to take place, cells containing the integrated plasmid were grown at 30°C in the absence of erythromycin. Finally, mutant strains carrying a deletion of the hfq gene were identified by PCR. One-half of the erythromycin-sensitive candidates tested were found to carry an in-frame hfq deletion. Correct deletion events were verified by DNA sequence analysis of the resulting PCR products (CEQ2000; Beckman).
TABLE 1.
PCR primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| HfqA | GGGGTCTAGAGAAGGTTTAGTGACAGAAGCGa |
| HfqB | CATAATTTCCCTCTCCAATCTC |
| HfqC | GAGATTGGAGAGGGAAATTATGCCTGATGCGGAATAAGCACd |
| HfqD | CCCCGGATCCAGCCGAAATATTGCGCACb |
| Hfq 5 | GGGGGAATTCGCGGGTGTTAGTACTACCGc |
| Hfq 6 | CCCCGGATCCCAGACAAGACTAATCGTAGCb |
| Hfq 9 | GACTTGATTTGGACAGAGCG |
| Hfq 10 | GCACACATAGCAAAGTAGTC |
| Hfq 11 | GGGGGAATTCGAAGAAATAAAGGGTATTTGAAATTAGc |
| Hfq 12 | CCCCGGATCCCCTGTAACCCTTGTCCACCb |
| Hfq 13 | CAAAACTTACAACGCGTCCTC |
| Hfq 14 | CCCCGGATCCTCGGTAGTACTAACACCCGCb |
| Hfq N | GGTGGTTGCTCTTCCAACATGAAACAAGGTGGACAAGGGTTACAGG |
| Hfq C | TTATTCCGCATCAGGATTTAAAGCGAC |
| MiaA 1 | GGGGGAATTCTGATGCTAGAGAACCGGCTGc |
| Lmo1296-1 | CAAGCGTCATTCATTGGTTCTG |
| Lmo1296-2 | CACTAGCGCAGCGAGTTCATC |
The underlined sequence is an XbaI restriction enzyme site.
The underlined sequence is a BamHI restriction enzyme site.
The underlined sequence is an EcoRI restriction enzyme site.
The underlined sequence anneals to primer HfqB.
Computer analyses of protein sequences.
Homology searches were performed with BLAST (1).
Stress tolerance assays.
For growth experiments, overnight cultures or exponential-phase cultures (optical density at 600 nm [OD600], 0.6) grown in BHI medium were diluted 1:100 in fresh BHI media containing various concentrations of NaCl (4 to 7%), ethanol (2 to 5%), lactic acid (media acidified to pH 4.5 to 6.5), H2O2 (0.003 to 0.15%), or Triton X-100 (0.01 to 2%). Bacteria were exposed to thermal stress by inoculation into BHI medium at 4 or 43.5°C. Bacterial growth was monitored by measuring the OD600.
For viability tests, cultures were grown in BHI medium to the exponential phase or the stationary phase. Cells were harvested by centrifugation, and the pellets were suspended in 0.9% saline containing various concentrations of H2O2 (0.1 to 4%), in BHI medium acidified to pH 2.5 to 4 with lactic acid, or in BHI medium at a lethal temperature (50°C). Cell viability was measured by standard plate counting in duplicate on BHI medium plates by using samples taken before exposure to stress and at various times after exposure to stress.
Long-term nutrient deprivation.
Overnight cultures of wild-type and Δhfq strains grown in BHI medium were diluted 1:100 in BHI medium. Alternatively, overnight cultures of wild-type and Δhfq strains grown in IMM were diluted 1:100 in IMM, IMM containing 0.002% l-leucine, 0.002% dl-isoleucine, 0.002% dl-valine, 0.002% dl-methionine, 0.002% l-arginine, 0.002% l-tryptophan, 0.002% l-phenylalanine, and 0.002% l-histidine (normal concentration, 1%), or IMM containing 0.1% glucose (normal concentration, 2.0%). The cultures were then incubated at 37°C for several weeks. The number of surviving bacteria was determined at specified times. The cells were harvested, washed, and suspended in 0.9% saline, and then 10-fold serial dilutions were prepared, which were plated onto BHI agar plates. Colonies were counted after overnight incubation at 37°C.
Disk diffusion assays.
The sensitivities of the wild-type and Δhfq mutant strains to a variety of antibiotics and chemical reagents were determined by agar diffusion assays as described previously (11). The filter disks contained 30 μg of the following antibiotics: ampicillin, penicillin G, cefuroxime, chloramphenicol, kanamycin, tetracycline, erythromycin, vancomycin, and streptomycin. The following membrane-active chemicals were tested: 10 μl of 100% Triton X-100, 10 μl of 50% Tween 20, and 10 μl of 1% sodium dodecyl sulfate. For each antibiotic or chemical, at least three independent disk diffusion assays were performed for each strain.
RNA techniques.
Total RNA was prepared by using a hot acid-phenol procedure (23). Primer extension analysis was performed as described previously (17) by using 15 μg of total RNA per reaction mixture. Primer Hfq13 labeled at the 5′ end with 32P was used for detection of hfq transcription start sites. Wild-type and ΔsigB cell cultures were grown to an OD600 of 0.3 and subjected to various stress conditions. RNA was prepared from cells collected after 20 min of treatment with 4% NaCl, after 20 min of treatment with 2% ethanol, after 20 min of treatment with 1 mM EDTA, after 20 min of incubation at 45°C, after 20 min of treatment with 0.15% H2O2, after 20 min of incubation at pH 5.5 (medium acidified with lactic acid), or after 24 h of incubation at 4°C. A control without stress treatment was included. To perform reverse transcription (RT)-PCR, the purified RNA was treated with RNase-free DNase I (Amersham) according to the manufacturer's recommendations. For cDNA synthesis, 0.1 pmol of primer Hfq14, Hfq13, HfqC, or Hfq10 (Table 1) was allowed to anneal to 0.5 μg of RNA in AMW buffer (Finnzymes) and 1.1 pmol of each deoxynucleoside triphosphate in a 10-μl (total volume) mixture. To initiate cDNA synthesis, 2 U of AMW reverse transcriptase (Finnzymes) was added, and the reaction was allowed to proceed for 30 min at 42°C. Then 0.25 μl of the cDNA reaction mixture was used as the template in a 25-μl PCR amplification reaction mixture with forward primer MiaA1, Hfq9, or HfqN, reverse primer Hfq14, Hfq13, HfqC, or Hfq10 (Table 1), and Taq DNA polymerase (Promega), as described by the supplier. In control reactions, RNA or chromosomal DNA was used as the template. For quantitative RT-PCRs, DNase I-treated RNA from nonstressed or salt-stressed cells was reverse transcribed by using Taq-Man RT reagents with the supplied hexamers according to the protocol recommended by the supplier (Applied Biosystems). SYBR Green PCR Master Mix was added to cDNA obtained from RT of 100 ng of RNA, together with 300 nM primer Lmo1296-1 and 300 nM primer Lmo1296-2 (Table 1). The primers hybridized to the 5′ end of lmo1296 and produced a 51-bp DNA fragment. The RT-PCR was performed with an ABI PRISM 7700 sequence detection system with the standard setup. Primers were designed by using PrimerExpress as recommended by the supplier of the ABI PRISM 7700 sequence detection system (Applied Biosystems).
Construction of lacZ fusion to miaA and hfq and β-galactosidase assays.
DNA fragments containing regions of the miaA promoter, the miaA gene, and the hfq promoter were amplified by PCR. For a fragment containing the miaA promoter region, the miaA gene, and the hfq promoter region, a 1,232-bp fragment (ranging from position −1125 to position 107 relative to the hfq transcription start site) was amplified by using primers MiaA1 and Hfq12. Primers MiaA1 and Hfq14 were used for amplification of a 1,016-bp miaA fragment (ranging from position −1125 to position −109 relative to the hfq transcription start site). For the full-length hfq promoter fragment, a 216-bp fragment (ranging from position −109 to position 107 relative to the hfq transcription start site) was amplified by using primers Hfq5 and Hfq12 (Table 1). Primers Hfq11 and Hfq12 were used for amplification of a truncated 132-bp hfq promoter fragment (ranging from position −25 to position 107 relative to the hfq transcription start site) (Table 1). To construct plasmids phfq(−109)-lacZ, phfq(−25)-lacZ, pmiaA-hfq-lacZ, and pmiaA-lacZ, the PCR fragments were digested with EcoRI and BamHI and cloned into EcoRI-BamHI-digested pTCV-lac, a shuttle vector used for construction of transcriptional fusions to lacZ (25). Correct insertion of the fragments into pTCV-lac was verified by DNA sequencing analyses. The resulting plasmids were electroporated into the wild-type and ΔsigB mutant strains. For measurement of hfq expression under various stress conditions, wild-type and ΔsigB mutant strains carrying the lacZ fusions were grown in BHI medium to an OD600 of 0.3. The cultures were split, and the stress factors were added. Cells were collected 1 h after the addition of stress factors and assayed for β-galactosidase activity. The stress conditions tested were ethanol (final concentration, 2%), NaCl (final concentration, 4%), EDTA (final concentration, 1 mM), lactic acid (final pH 4.0 to 5.5), H2O2 (final concentration, 0.003 to 0.15%), and Triton X-100 (final concentration, 0.5%). For the β-galactosidase assay, cells were permeabilized by treatment with 0.5% toluene and 4.5% ethanol, and β-galactosidase activities were determined as described previously (16). The specific activity of β-galactosidase was calculated as follows: (OD420 of the reaction mixture − OD550 of the reaction mixture)/(reaction time in minutes × OD600 of cells used in the reaction mixture). The β-galactosidase activities presented below are the averages of three independent experiments in which the observed variations did not exceed 10%.
Intracellular infection assay.
The murine macrophage-like cell line J774A.1 (= ATCC TIB-67) was propagated in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen). The embryonic intestinal epithelial cell line INT-407 (= ATCC CCL 6) was propagated in Eagle's minimal essential medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum. Cells were incubated in the presence of 5% CO2 at 37°C. For the infection assays, the concentration of cells was adjusted to 5 × 105 cells per ml, and the cultures were grown in 24-well tissue culture plates to obtain monolayers (20 h at 37°C). Bacteria grown to the mid-log phase or overnight were washed, and the concentration was adjusted to approximately 5 × 105 (J774A.1) or 1 × 107 (INT-407) CFU per ml of cell culture medium. Bacteria were added to the wells so that the multiplicities of infection were 1 and 20 bacteria per cell for J774A.1 and INT-407 cells, respectively. The tissue culture plates were centrifuged for 2 min at 150 × g to bring the bacteria into contact with the cell monolayers and were subsequently incubated at 37°C. After 1 h of incubation, each infected monolayer was washed twice with phosphate-buffered saline (PBS) and then overlaid with cell culture medium containing 100 μg of gentamicin ml−1 to kill extracellular bacteria. After 1 h, the monolayer was washed twice and then overlaid with cell culture medium containing 10 μg of gentamicin ml−1 and incubated at 37°C. The monolayer was washed with PBS and lysed with 0.1% Triton X-100 either immediately (time zero) or at a later time. The number of bacteria released was expressed in CFU per milliliter by plating appropriate dilutions on BHI agar plates. Each experiment was carried out in triplicate and repeated twice.
Mouse virulence assay.
Overnight cultures of L. monocytogenes strains were diluted 1:100 in BHI medium and grown until the OD600 was 0.6. The bacteria were washed and suspended in PBS prior to infection. Six-week-old female BALB/c mice were infected intraperitoneally with 2 × 104 bacteria. Three days after infection, the mice were sacrificed, and the liver and spleen of each mouse were homogenized. Tenfold serial dilutions of the homogenized spleen or liver in physiological saline buffer were plated onto BHI agar plates. CFU were counted following overnight incubation at 37°C. Significance was determined by using the Student t test. The experiment was done in accordance with the legal notices of the Danish Animal Experiments Inspectorate.
RESULTS
In-frame deletion of the hfq gene in L. monocytogenes.
Analysis of the L. monocytogenes EGD-e genome sequence revealed that the lmo1295 gene is predicted to encode a protein similar to host factor 1 protein, also known as Hfq (6). The protein encoded by lmo1295 (designated hfq) consists of 77 amino acids and is 62% similar and 46% identical to the E. coli Hfq protein. According to a structure-based sequence alignment of Hfq proteins (28), the residues that mediate RNA interactions in S. aureus Hfq (e.g., Gln8, Tyr42, Lys57, and His58) are conserved in L. monocytogenes Hfq (corresponding to Gln9, Phe43, Lys58, and His59, respectively). L. monocytogenes hfq is located downstream from miaA, which encodes a protein similar to tRNA isopentenylpyrophosphate transferase, and upstream from lmo1296, which encodes the putative GTP-binding protein HflX (Fig. 1). A similar gene organization has been observed at the hfq locus in E. coli (31). Putative transcription terminators are found upstream from miaA and downstream from hfq in L. monocytogenes. RT-PCR analyses indicated that miaA and hfq are cotranscribed in L. monocytogenes and suggested that there is a transcription terminator downstream from hfq (Fig. 1). To analyze the function of Hfq in L. monocytogenes, an in-frame deletion in the hfq gene was constructed in L. monocytogenes EGD. The resulting Δhfq strain lacked a region predicted to encode amino acids 4 to 74 of the Hfq protein. To test whether in-frame deletion of hfq perturbs the expression of the downstream gene lmo1296, quantitative RT-PCR analyses were performed with total RNA isolated from wild-type and Δhfq cells (see Materials and Methods). We found similar levels of lmo1296 mRNA in the two strains, showing that in-frame deletion of hfq has no downstream effects on lmo1296 expression (data not shown).
FIG. 1.
Genetic organization of the hfq locus in L. monocytogenes EGD-e. See the text for a detailed description of the gene products. Putative transcriptional terminators are indicated by lollipop structures. The locations and designations of the primer pairs used for RT-PCR analyses are indicated. Regions that were successfully amplified by RT-PCR are indicated by solid lines, whereas the dashed lines indicate the specific regions that could not be amplified by RT-PCR.
L. monocytogenes wild-type and Δhfq strains were grown in BHI medium, LB medium, a defined minimal medium (IMM), and IMM with reduced levels of amino acids or glucose. In all four media, the growth and morphology of the Δhfq mutant were indistinguishable from the growth and morphology of the parent strain (data not shown). In addition, there were no differences in carbohydrate utilization (as determined by API 50 CHL tests) between the two strains (data not shown).
Hfq contributes to the tolerance of L. monocytogenes to osmotic stress, ethanol stress, and Triton X-100.
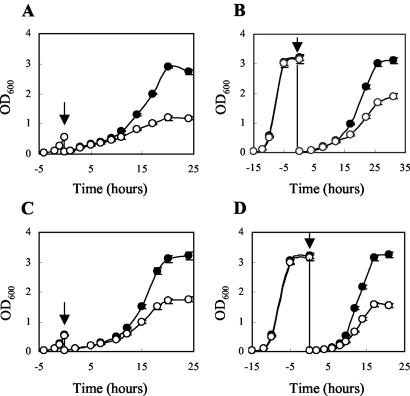
Wild-type and Δhfq cells were compared with respect to their abilities to grow and survive during exposure to various stress conditions. When cells grown in BHI medium to the exponential or stationary phase were diluted in BHI medium containing various concentrations of NaCl, ethanol, lactic acid, or H2O2, growth of the Δhfq strain was clearly impaired in the presence of 4.5% ethanol or 7% NaCl (Fig. 2), while growth was not affected by lactic acid (pH values ranging from 5.0 to 6.5) or H2O2 (0.0125 to 0.15%) (data not shown). Furthermore, growth of the wild-type and Δhfq strains in BHI medium was compared at low (4°C) and high (43.5°C) temperatures. At 4°C, the Δhfq strain displayed a slightly prolonged lag phase (approximately 1 day) compared to the wild-type strain, but the growth rates of the two strains in the exponential phase were similar (0.27 and 0.29 doublings per day for the wild-type strain and the Δhfq strain, respectively). At the high temperature (43.5°C) the growth of the mutant strain was indistinguishable from that of the wild-type strain. We also examined the viability of the two strains at 50°C, at various pH values (ranging from pH 2.5 to 4.5), and in the presence of H2O2 (0.1 to 4%). Under all conditions tested we observed no differences in the survival of the wild-type and Δhfq strains, suggesting that Hfq is not required for the resistance of L. monocytogenes to high temperature, acid, or H2O2 (data not shown).
FIG. 2.
Ethanol and osmotic stress tolerance of wild-type and Δhfq strains. (A) Cells grown in BHI medium to the early exponential phase were diluted in BHI medium containing 4.5% ethanol. (B) Cells grown in BHI medium to the stationary phase were diluted in BHI medium containing 4.5% ethanol. (C) Cells grown in BHI medium to the early exponential phase were diluted in BHI medium containing 7% NaCl. (D) Cells grown in BHI medium to the stationary phase were diluted in BHI medium containing 7% NaCl. The arrows indicate the time of dilution. Symbols: •, wild-type strain; ○, Δhfq mutant. The error bars indicate standard deviations based on duplicate experiments.
The resistance of the wild-type and Δhfq mutant strains to three membrane-perturbing agents was tested in disk diffusion assays and in growth experiments. No difference between the two strains was observed when resistance to sodium dodecyl sulfate and resistance to Tween 20 were examined (data not shown). However, the Δhfq strain proved to be significantly more sensitive to the nonionic surfactant Triton X-100 (P < 0.05). The size of the zone of inhibition for the wild-type strain was 22.1 ± 1.4 mm, whereas the size of the zone of inhibition for the Δhfq strain was 26.1 ± 0.8 mm. These results were supported by growth experiments performed with different concentrations of Triton X-100, which showed that there was reduced growth of the Δhfq strain compared to the growth of the wild-type strain (data not shown). In disk diffusion assays, the wild-type and Δhfq strains displayed similar levels of resistance to various antibiotics acting on the cell wall (ampicillin, penicillin G, vancomycin, cefuroxime, bacitracin) or protein synthesis (chloramphenicol, erythromycin, kanamycin, streptomycin, tetracycline) (data not shown).
In summary, our results suggest that Hfq contributes to the tolerance to osmotic stress, ethanol stress, and Triton X-100 but does not contribute to acid and oxidative stress resistance or to thermotolerance in L. monocytogenes.
Hfq enhances survival during long-term amino acid starvation.
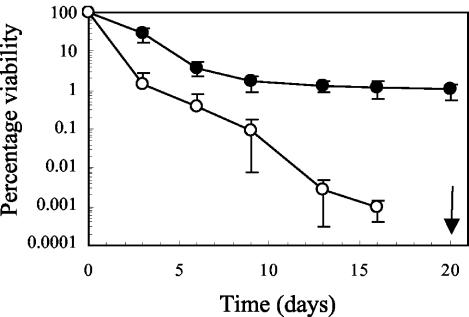
Studies with E. coli and B. abortus have shown that inactivation of hfq results in an inability to withstand long-term nutrient deprivation (19, 26). To assess whether Hfq plays a similar role in L. monocytogenes, we analyzed survival of the wild-type and Δhfq strains during long-term incubation in BHI medium, IMM, and IMM with reduced levels of glucose or amino acids. In IMM with reduced levels of amino acids, the viability of the Δhfq mutant declined more rapidly than the viability of the wild-type strain declined (Fig. 3). Within the first 6 days, about 90 to 99% of the wild-type cells lost viability. After 6 days the surviving population remained relatively constant, and there was only a slight decrease in viability. In contrast to the wild-type strain, for the Δhfq mutant there was a continuous decline in viability, and survivors were not detectable at day 19 (Fig. 3). The pH of the IMM with reduced levels of amino acids remained constant during growth and long-term survival experiments for both the Δhfq mutant and wild-type cells (pH 7.0) (data not shown). No significant difference in viability between the wild-type and Δhfq strains was observed in BHI medium, IMM, or IMM with reduced levels of glucose (data not shown). These results indicate that under amino acid-limiting conditions in IMM, a starvation survival response is induced in L. monocytogenes, and this response allows the wild-type strain to remain viable for a prolonged period of time. Apparently, Hfq is important for this starvation survival response.
FIG. 3.
Viability during long-term amino acid starvation of wild-type and Δhfq strains. Cells were grown in IMM containing amino acids at a concentration of 0.002%. After the cells entered the stationary phase, they were incubated for 20 days. Samples were taken at the times indicated to determine viability. The arrow indicates the sampling time for the Δhfq mutant when no survivors were detected. The limit of detection was 25 CFU ml−1. Symbols: •, wild-type strain; ○, Δhfq mutant. The error bars indicate standard deviations based on duplicate experiments.
Alternative sigma factor σB regulates the transcription of hfq in L. monocytogenes.
The RT-PCR analyses (Fig. 1) indicated that hfq is cotranscribed with the upstream gene miaA. To investigate the transcription of miaA and hfq, a 1,243-bp fragment containing the miaA promoter region, the entire miaA gene, and the hfq promoter region was fused to lacZ in the transcriptional fusion vector pTCV-lac (25). The resulting plasmid was introduced into the wild-type strain, and the levels of β-galactosidase activity in cells growing in BHI medium and under osmotic stress conditions were determined. We observed that in the presence of 4% NaCl the expression of the miaA-hfq-lacZ fusion was approximately twofold greater than the expression under nonstress conditions (Table 2). To test whether the increased expression of the miaA-hfq-lacZ fusion under osmotic stress conditions depended on the alternative sigma factor σB, we examined the expression of the miaA-hfq-lacZ fusion in a ΔsigB strain. We found that the twofold induction of pmiaA-hfq-lacZ expression by osmotic stress was eliminated in the ΔsigB background (Table 2). When we inspected the DNA region between miaA and hfq, we noticed putative −10 and −35 sequences exhibiting high levels of similarity with σB-regulated promoters (12) (Fig. 4A). To define the region responsible for the σB-dependent expression, two fragments, one containing the miaA promoter region and one containing the hfq promoter region, were fused to lacZ in pTCV-lac and introduced into wild-type and ΔsigB strains. Under osmotic stress conditions, the expression levels of miaA-lacZ were similar in the two strains, whereas the level of expression of hfq-lacZ was approximately 27-fold higher in the wild-type strain than in the ΔsigB strain (Table 2). These results indicate that a σB-regulated promoter is located in the hfq promoter region. The higher levels of expression of the miaA-lacZ fusion than of the miaA-hfq-lacZ fusion suggest that a large proportion of the transcripts that initiate upstream from miaA may not proceed into hfq (Table 2).
TABLE 2.
Transcription analysis of miaA and hfq by using lacZ reporter fusionsa
| lacZ fusionb | β-Galactosidase activitiesc
|
|||
|---|---|---|---|---|
| BHI medium
|
BHI medium + 4% NaCl
|
|||
| Wild-type strain | ΔsigB strain | Wild-type strain | ΔsigB strain | |
| miaA-hfq-lacZ (−1125 and 107) | 21.5 ± 0.2 | 20.0 ± 0.5 | 39.9 ± 0.2 | 21.4 ± 0.5 |
| miaA-lacZ (−1125 and −109) | 178.9 ± 3.5 | 172.4 ± 7.5 | 138.4 ± 3.2 | 144.8 ± 8.1 |
| hfq-lacZ (−109 and 107) | 0.65 ± 0.05 | 0.65 ± 0.04 | 18.1 ± 0.1 | 0.65 ± 0.04 |
The wild-type and ΔsigB strains containing the lacZ fusions were grown in BHI medium until the OD600 was 0.3. The cultures were split, and NaCl was added to one-half of each culture at a final concentration of 4%. After 1 h, cell pellets were harvested and subjected to β-galactosidase assays.
The numbers in parentheses are the starting and ending positions of the fragment fused to lacZ relative to the hfq transcription start site (position 1).
β-Galactosidase activities were determined as described in Materials and Methods.
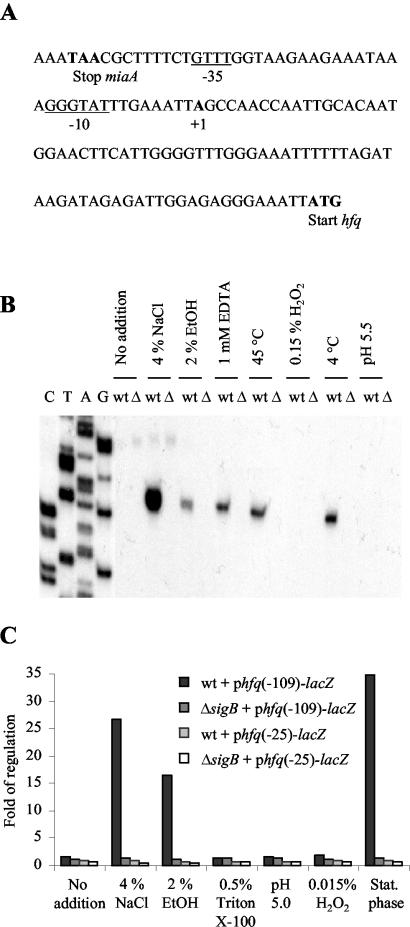
FIG. 4.
Transcription of hfq is induced by various stress conditions in a σB-dependent manner. (A) Sequence of the hfq promoter region. The translation start and stop codons are indicated by boldface type. The transcription start site is indicated by boldface type. Putative −35 and −10 sequences for σB are underlined. (B) Primer extension analysis of transcription originating from the hfq promoter under various stress conditions. The analysis was performed by using RNA purified from the wild-type strain or the ΔsigB strain. Cells were grown in BHI medium to an OD600 of 0.3. The cell cultures were split and stressed as indicated at the top. Controls without stress treatment were included. Δ, EGDΔsigB; wt, wild type; EtOH, ethanol. (C) Expression of hfq-lacZ transcriptional fusions in response to various stress conditions. The wild-type and ΔsigB strains containing phfq(−109)-lacZ or phfq(−25)-lacZ were grown in BHI medium until the OD600 was 0.3. The cell cultures were split and subjected to stresses as indicated at the bottom for 1 h. For stationary-phase cells, cultures were grown in BHI medium until the OD600 was 2.7. Cell pellets were harvested and subjected to β-galactosidase assays. Controls without stress treatment were included. The data are the means for three experiments in which the observed variation did not exceed 10%. Stat. phase, stationary phase.
To identify potential σB-dependent transcription start sites upstream from hfq, we performed a primer extension analysis with RNA samples from wild-type and ΔsigB strains exposed to various stress conditions. In the early exponential growth phase, no primer extension products were observed in the wild-type or ΔsigB strain (Fig. 4B). After addition of 4% NaCl, 2% ethanol, or 1% EDTA or after a temperature up- or downshift (from 37°C to 45 and 4°C, respectively), a transcript was observed for the wild-type strain. Importantly, the extension product was not observed in the ΔsigB strain, suggesting that induction of hfq transcription depends on σB (Fig. 4B). The transcriptional start site maps just downstream from the putative binding sequences for σB in the hfq promoter region (Fig. 4A). Addition of hydrogen peroxide (0.15%) and acidification to pH 5.5 did not induce transcription of hfq (Fig. 4B).
To investigate the role of σB in hfq expression in more detail, the wild-type and ΔsigB strains containing the full-length hfq-lacZ fusion were exposed to various types of stress, and β-galactosidase activity was measured. Consistently, osmotic and ethanol stress strongly induced expression of hfq in a σB-dependent manner (Fig. 4C), while acidification of the growth medium with lactic acid to obtain pH values ranging from 4.0 to 5.5 or the addition of H2O2 to final concentrations ranging from 0.003 to 0.15% had no effect on hfq expression (Fig. 4C; data not shown). Furthermore, Triton X-100 did not act as an inducer of hfq expression (Fig. 4C). The β-galactosidase activity was considerably higher in wild-type cells that reached the stationary phase than in exponential-phase cells (Fig. 4C). Importantly, growth phase-dependent induction was not found in the ΔsigB background (Fig. 4C).
To determine if the putative σB binding determinants observed in the hfq promoter region are important for the σB-dependent induction of hfq, a truncated version of the hfq promoter was fused to lacZ in pTCV-lac. In phfq(−25)-lacZ, the −35 box of the putative σB binding site and upstream sequences were deleted. As shown in Fig. 4C, the conditions that induce β-galactosidase activity at the hfq promoter in a σB-dependent manner (i.e., salt, ethanol, and entry into stationary phase) all failed to induce hfq(−25)-lacZ expression.
The results of the primer extension analysis and the β-galactosidase assays show that transcription of hfq is induced under specific stress conditions in a σB-dependent manner. The stress-inducible expression of hfq depends on sequences in the hfq promoter region that may be utilized by the σB-RNA polymerase holoenzyme. Curiously, in both experimental setups we observed that only some of the stress conditions known to induce σB activity in L. monocytogenes (2) were capable of inducing the σB-dependent transcription of hfq.
Hfq contributes to the pathogenesis of L. monocytogenes in mice.
To determine if Hfq is important for the virulence of L. monocytogenes, we initially tested the activities of the virulence factors LLO and PlcB on blood agar and egg yolk agar plates, respectively. The wild-type and Δhfq strains displayed similar degrees of hemolytic activity and lecithinase activity, suggesting that hfq does not influence the expression of LLO and PlcB under these conditions.
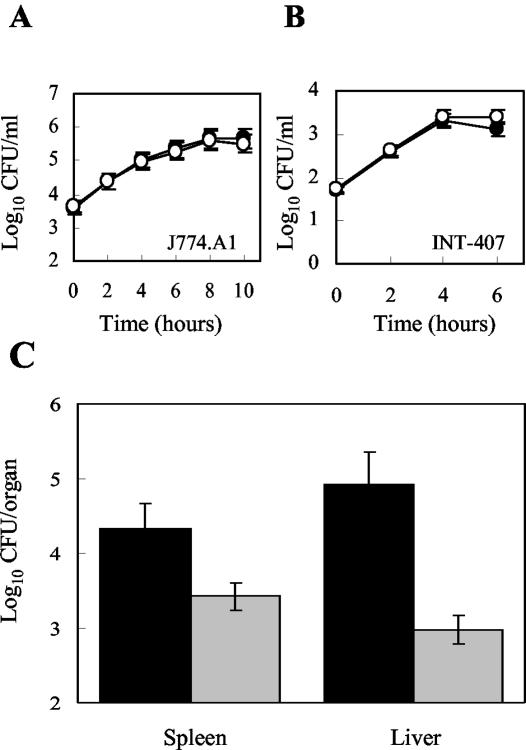
Infection studies were performed in vitro by using the murine macrophage-like cell line J774A.1 and the embryonic intestinal epithelial cell line INT-407. Wild-type and Δhfq cells in the stationary or exponential growth phase were used for infection of cell monolayers. The intracellular infection assays showed that entry and replication of stationary-phase Δhfq cells in J774A.1 and INT-407 were similar to entry and replication of stationary-phase wild-type cells (Fig. 5A and B). Similar results were obtained when J774A.1 was infected with exponential-phase cells (data not shown). These experiments indicated that hfq has no immediate effect on the proliferation of L. monocytogenes in the intracellular environment represented by J774A.1 and INT-407.
FIG. 5.
Infection studies of wild-type and Δhfq strains: effect of hfq on the intracellular replication of L. monocytogenes in the murine macrophage-like cell line J774A.1 (A) or the intestinal epithelial cell line INT-407 (B). Cell monolayers were infected with approximately 1 bacterium per cell (A) or 20 bacteria per cell (B). After 1 h of incubation, cells were incubated for 1 h in the presence of gentamicin (time zero). The data are the means for two independent experiments, each performed in triplicate. The error bars indicate standard deviations. Symbols: •, wild-type strain; ○, Δhfq strain. (C) Intraperitoneal infection of mice with L. monocytogenes: growth and survival of the wild-type strain (black bars) and the Δhfq strain (grey bars) in the spleens and livers of infected mice on day 3 after injection. The log10 CFU in the organs are averages for five mice. The experiments were repeated twice with similar results.
For in vivo infection studies, the mouse model of tissue colonization was used. Groups of five mice were infected by the intraperitoneal route with the wild type or the Δhfq strain. On day 3, the numbers of bacteria in the spleen and liver were estimated. Compared to the wild-type strain, the Δhfq mutant displayed significant decreases in bacterial counts (1 and 2 log10 units in the spleen and liver, respectively; P < 0.03) (Fig. 5C). These results suggest that Hfq contributes to the pathogenesis of L. monocytogenes in mice.
DISCUSSION
In order to survive and grow within the hostile environments of host organisms, pathogenic bacteria must be able to monitor changes in the environment and respond accordingly by adjusting the expression of stress- and virulence-associated genes. In bacteria, sRNA molecules and RNA-binding proteins that modulate their actions have emerged as important players in the coordinate responses to environmental changes (15, 30). In this study we investigated the role of the RNA-binding protein Hfq in the stress tolerance and virulence of a gram-positive organism, the human pathogen L. monocytogenes, for the first time.
The importance of L. monocytogenes Hfq under stress conditions was examined by comparing the growth characteristics of an in-frame hfq deletion mutant to those of a wild-type strain. We found that the mutant cells without Hfq were more sensitive to salt, ethanol, and Triton X-100 than the wild-type cells, suggesting that Hfq affects the membrane integrity of L. monocytogenes (10, 24). The demand for Hfq during environmental stress was further supported by the increase in hfq transcription when cells were exposed to various stress conditions, including salt and ethanol, and during entry into the stationary phase. Inspection of the hfq promoter region revealed a putative binding sequence for the general stress sigma factor σB, suggesting that the stress induction of hfq is σB dependent. This notion was supported when we examined hfq expression in an L. monocytogenes mutant lacking the sigB gene and found that stress induction was eliminated in the absence of σB. We found that ethanol and salt, but not oxidative stress and acid stress, induced the σB-dependent expression of hfq. Interestingly, Hfq contributes to the tolerance to ethanol and salt, but not to the tolerance to oxidative stress and acid stress, indicating that the σB-dependent control of hfq is an important regulatory event. In a recent study, DNA microarray analysis of L. monocytogenes 10403S revealed 55 genes dependent on σB for expression under osmotic stress conditions and in the stationary growth phase; however, hfq was not among these genes (12). We speculate that this discrepancy may be due to the different experimental approaches used and differences between the two L. monocytogenes strains used (10403S and EGD).
When the availability of nutrients is insufficient for growth, bacteria develop a starvation survival response. The starvation response is characterized by an initial decrease in the viability of the bacterial population, followed by long-term maintenance of cell viability (13, 37). The death of the majority of the population results in the release of valuable nutrients that is thought to promote establishment of a small population of surviving cells. In long-term starvation experiments with IMM, we observed that limitation of the amino acid content reduced the viability of the wild-type strain to about 99% of the population, whereas the viability of the Δhfq mutant declined to below the detection level within 19 days. These results suggest that in the IMM used in the present study L. monocytogenes developed a starvation survival response in response to amino acid limitation and Hfq contributed to this response.
In E. coli, the highly pleiotropic phenotype of an hfq mutant has been explained by the central role of hfq in translational activation of rpoS, which encodes the general stress sigma factor σS (8). In gram-positive bacteria, σB is the functional homologue of σS, and therefore we investigated whether the phenotypes associated with the hfq mutant could be ascribed to altered σB activity. Since σB controls the transcription of hfq, a transcriptional hfq-lacZ fusion could be used as a tool for examining the σB activity in a Δhfq background. When we compared the β-galactosidase activities of the wild-type and Δhfq mutant strains carrying the hfq-lacZ fusion, we found that the σB-dependent promoter activity of the hfq-lacZ fusion was not affected by the absence of Hfq (Christiansen and Kallipolitis, unpublished data). Thus, our data suggest that Hfq does not affect the activity of the general stress sigma factor σB in L. monocytogenes.
Previously, it was shown that hfq mutant cells of the gram-negative pathogen B. abortus had reduced tolerance to acid and oxidative stress conditions (26). Interestingly, we found that although transcription of hfq in L. monocytogenes is controlled by σB, it was not induced by acid or oxidative stress, and accordingly hfq did not contribute to acid and H2O2 resistance. Therefore, unlike Hfq in B. abortus, L. monocytogenes Hfq is not expected to be part of the protective response of this pathogen against acid and oxidative attack by the host in the host cell phagocytic compartment. However, the decreased tolerance of the Δhfq mutant to other stress conditions prompted us to investigate its ability to establish an infection. We observed that Hfq contributes to the growth and survival of L. monocytogenes during infection in mice but is not required for invasion and multiplication in the macrophage-like cell line J774A.1 or in the intestinal epithelial cell line INT-407. During a systemic infection in mice, L. monocytogenes encounters a variety of microenvironments. We speculate that Hfq may be important under specific conditions present in a subset of these microenvironments. Apparently, J774A.1 and INT-407 do not represent these conditions.
The ability of a pathogen to sense signals from the host organism and mount a coordinated response to these signals is closely linked to its virulence potential. sRNA molecules represent an interesting alternative to the classic protein regulators. E. coli possesses at least 50 sRNAs, and several studies have suggested that the sRNAs are used by E. coli to adapt to environmental changes (15, 36, 39). sRNAs may control the expression of genes at the level of mRNA transcription, translation, or stability, and many of the sRNAs require Hfq for activity (15, 38, 39). The results of our study suggest that Hfq of L. monocytogenes plays a role in stress tolerance and pathogenesis in mice. Since Hfq of E. coli is implicated in a variety of RNA interactions, it is tempting to speculate that Hfq of L. monocytogenes contributes to these processes through interactions with RNA molecules. In future research, we will focus on clarifying the molecular mechanisms by which Hfq contributes to stress tolerance and pathogenesis of L. monocytogenes.
Acknowledgments
We thank P. Valentin-Hansen for inspiring discussions, C. Kirkegaard and J. Pedersen for excellent technical assistance, L. Brøndsted for providing the EGDΔsigB strain, and W. Goebel for providing the EGD strain.
This work received financial support from the FREJA Foundation and the Danish Natural Science Research Council.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. Schaffer, J. Zhang, S. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, L. A., M. S. Cetin, R. W. Hutkins, and A. K. Benson. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brøndsted, L., B. H. Kallipolitis, H. Ingmer, and S. Knöchel. 2003. kdpE and a putative RsbQ homologue contribute to growth of Listeria monocytogenes at high osmolarity and low temperature. FEMS Microbiol. Lett. 219:233-239. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty, T., M. Leimeister-Wachter, E. Domann, M. Hartl, W. Goebel, T. Nichterlein, and S. Notermans. 1992. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 174:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ermolaeva, S., T. Karpova, S. Novella, M. Wagner, M. Scortti, I. Tartakovskii, and J. A. Vazquez-Boland. 2003. A simple method for the differentiation of Listeria monocytogenes based on induction of lecithinase activity by charcoal. Int. J. Food Microbiol. 82:87-94. [DOI] [PubMed] [Google Scholar]
- 6.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. G. Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 7.Hajndsorf, E., and P. Regnier. 2000. Host factor Hfq of Escherichia coli stimulates elongation of poly(A) tails by poly(A) polymerase I. Proc. Natl. Acad. Sci. USA 97:1501-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 10.Ingram, L. O., N. S. Vreeland, and L. C. Eaton. 1980. Alcohol tolerance in Escherichia coli. Pharmacol. Biochem. Behav. 13:191-195. [DOI] [PubMed] [Google Scholar]
- 11.Kallipolitis, B. H., H. Ingmer, C. G. Gahan, C. Hill, and L. Søgaard-Andersen. 2003. CesRK, a two-component signal transduction system in Listeria monocytogenes, responds to the presence of cell wall-active antibiotics and affects β-lactam resistance. Antimicrob. Agents Chemother. 47:3421-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazmierczak, M. J., S. C. Mithoe, K. J. Boor, and M. Wiedmann. 2003. Listeria monocytogenes σB regulates stress response and virulence functions. J. Bacteriol. 185:5722-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolter, R., D. A. Siegele, and A. Tormo. 1993. The stationary phase of the bacterial life cycle. Annu. Rev. Microbiol. 47:855-874. [DOI] [PubMed] [Google Scholar]
- 14.Kreft, J., and J. A. Vazquez-Boland. 2001. Regulation of virulence genes in Listeria. Int. J. Med. Microbiol. 291:145-157. [DOI] [PubMed] [Google Scholar]
- 15.Massé, E., N. Majdalani, and S. Gottesman. 2003. Regulatory roles for small RNAs in bacteria. Curr. Opin. Microbiol. 6:120-124. [DOI] [PubMed] [Google Scholar]
- 16.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Moazed, D., S. Stern, and H. F. Noller. 1986. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30S ribosomal subunits using primer extension. J. Mol. Biol. 187:399-416. [DOI] [PubMed] [Google Scholar]
- 18.Møller, T., T. Franch, P. Højrup, D. R. Keene, H. P. Bachinger, R. G. Brennan, and P. Valentin-Hansen. 2002. Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol. Cell 9:23-30. [DOI] [PubMed] [Google Scholar]
- 19.Muffler, A., D. D. Traulsen, D. Fischer, R. Lange, and R. Hengge-Aronis. 1997. The RNA-binding protein HF-I plays a global regulatory role which is largely, but not exclusively, due to its role in expression of the σS subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 179:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakao, H., H. Watanabe, S. Nakayama, and T. Takeda. 1995. yst gene expression in Yersinia enterocolitica is positively regulated by a chromosomal region that is highly homologous to Escherichia coli host factor 1 gene (hfq). Mol. Microbiol. 18:859-865. [DOI] [PubMed] [Google Scholar]
- 21.Park, S. F., and G. S. A. B. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 22.Phan-Thanh, L., and T. Gormon. 1997. A chemically defined minimal medium for the optimal culture of Listeria. Int. J. Food Microbiol. 35:91-95. [DOI] [PubMed] [Google Scholar]
- 23.Podbielski, A., A. Glosdorff, and J. Weber-Heynemann. 1995. The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect. Immun. 63:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poolman, B., P. Blount, J. H. Folgering, R. H. Friesen, P. C. Moe, and T. van der Heide. 2002. How do membrane proteins sense water stress? Mol. Microbiol. 44:889-902. [DOI] [PubMed] [Google Scholar]
- 25.Poyart, C., and P. Trieu-Cuot. 1997. A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to β-galactosidase in Gram-positive bacteria. FEMS Microbiol. Lett. 156:193-198. [DOI] [PubMed] [Google Scholar]
- 26.Robertson, G. T., and R. M. Roop II. 1999. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol. Microbiol. 34:690-700. [DOI] [PubMed] [Google Scholar]
- 27.Roop, R. M., II, J. M. Gee, G. T. Robertson, J. M. Richardson, W. L. Ng, and M. E. Winkler. 2003. Brucella stationary-phase gene expression and virulence. Annu. Rev. Microbiol. 57:57-76. [DOI] [PubMed] [Google Scholar]
- 28.Schumacher, M. A., R. F. Pearson, T. Moller, P. Valentin-Hansen, and R. G. Brennan. 2002. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 21:3546-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnleitner, E., S. Hagens, F. Rosenau, S. Wilhelm, A. Habel, K. E. Jager, and U. Blasi. 2003. Reduced virulence of a hfq mutant of Pseudomonas aeruginosa O1. Microb. Pathog. 35:217-228. [DOI] [PubMed] [Google Scholar]
- 30.Storz, G. 2002. An expanding universe of noncoding RNAs. Science 296:1260-1263. [DOI] [PubMed] [Google Scholar]
- 31.Tsui, H. C., and M. E. Winkler. 1994. Transcriptional patterns of the mutL-miaA superoperon of Escherichia coli K-12 suggest a model for posttranscriptional regulation. Biochimie 76:1168-1177. [DOI] [PubMed] [Google Scholar]
- 32.Tsui, H. C., H. C. Leung, and M. E. Winkler. 1994. Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli K-12. Mol. Microbiol. 13:35-49. [DOI] [PubMed] [Google Scholar]
- 33.Tsui, H. C., G. Feng, and M. E. Winkler. 1997. Negative regulation of mutS and mutH repair gene expression by the Hfq and RpoS global regulators of Escherichia coli K-12. J. Bacteriol. 179:7476-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft, J. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:1-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vytvytska, O., I. Moll, V. R. Kaberdin, A. von Gabain, and U. Blasi. 2000. Hfq (HF1) stimulates ompA mRNA decay by interfering with ribosome binding. Genes Dev. 14:1109-1118. [PMC free article] [PubMed] [Google Scholar]
- 36.Wassarman, K. M., F. Repoila, C. Rosenow, G. Storz, and S. Gottesman. 2001. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 15:1637-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson, S. P., M. O. Clements, and S. J. Foster. 1998. Characterization of the starvation-survival response of Staphylococcus aureus. J. Bacteriol. 180:1750-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, A., K. M. Wassarman, J. Ortega, A. C. Steven, and G. Storz. 2002. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol. Cell 9:11-22. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, A., K. M. Wassarman, C. Rosenow, B. C. Tjaden, G. Storz, and S. Gottesman. 2003. Global analysis of small RNA and mRNA targets of Hfq. Mol. Microbiol. 50:1111-1124. [DOI] [PubMed] [Google Scholar]