Abstract
This paper provides a short review of physical principles, technology, biomedical applications and perspectives of the optoacoustic imaging. Ideas that made this rapidly developing field possible include the following: (1) laser pulses may be effectively used to produce acoustic pressure in biological tissues localized to the areas of increased optical absorption, (2) the resulting acoustic (ultrasonic) waves propagate in tissues with minimal distortions and attenuation, (3) 2D and 3D maps (images) of the absorbed optical energy can be reconstructed with high resolution from the detected optoacoustic signals. Modern optoacoustic imaging systems include scanning focused transducers and 2D/3D transducer arrays. The widely accepted 2D arrays are employed either for real-time 2D optoacoustic imaging or for 3D imaging via translational or rotational scanning. A commercial prototype of a 3D OAT system is being developed by TomoWave Labs where major biomedical applications include visualization of specific targeting using exogenous optoacoustic contrast agents and imaging of blood distribution and oxygentaion status can be investigated.
Keywords: gold nanorods, optoacoustic tomography, in-vivo imaging, preclinical research
INTRODUCTION
An optoacoustic (OA) image is provided by optical contrast in tissue while mapping tissue structures with ultrasonic resolution [1, 2]. The main advantage of the optical contrast is its spectroscopic selectivity, which allows to not only map distribution of blood concentration, but also its oxygen saturation (functional imaging) in the given tissue [2, 3, 4]. One example is the visualization of the tumor angiogenesis made possible by high optical contrast of blood (specifically hypoxic blood), which provides functional information for differentiation of malignant and benign tumors [5]. OA imaging also allows mapping the distribution of molecules of interest, such as cancer receptors or other biomarkers (molecular imaging) using nanoparticles as optoacoustic contrast agents [6, 7, 8]. In cases when the tissue molecules poorly absorb in the near-infrared light, application of targeted nanoparticles, such as gold nanorods, strongly absorbing in the near-infrared range of laser wavelengths can dramatically enhance the tissue contrast [6, 9, 10, 11].
History
Investigations into the theoretical and experimental background for the development of biomedical optoacoustics can be traced as far back as 1970s and early 1980s [1]. Given the well-known sensitivity of piezo-electric transducers, it was first proposed to use optoacoustics as a sensitive method for detection of lesions in biological tissues based on analysis of the temporally resolved signals [3]. Later on it became apparent that 2D or even 3D imaging is required to achieve high-resolution spatial mapping and quantitative analysis of the tissue chromophores [5, 12, 13].
Principles of optoacoustic tomography
OA imaging is based on tomographic reconstruction using the measured temporal OA signals, which are generated by the propagating acoustic waves. Under the illumination conditions of temporal pressure confinement [1], the original pressure distribution replicates the distribution of absorbed optical energy. The detection system, including ultrasonic transducers, must be sensitive within an ultra wide band of ultrasonic frequencies in order to detect optoacoustic signals without distortion and to conserve their quantitative information. Filtered radial backprojection is the tomographic procedure, which is most frequently used in optoacoustic imaging [5].
Applications
OA imaging system can be a stand-alone imaging modality or a supporting modality, for example to that of ultrasound, providing additional functional information. The most important biomedical applications for optoacoustic imaging today include cancer detection and diagnosis, angiography, and intravascular imaging [10, 13, 14]. The organs and tissues that are studied are breast, brain, major blood vessels and peripheral vascular networks, joints, lymph nodes, and prostate [5]. In the near infrared spectrum blood has different absorption properties depending on its oxygenation status [1]. This allows one to use multiple laser wavelengths to help determine the blood oxygenation in subjects, which is important for diagnosing of cancer, ischemia, hemorrhage, tissue hypoxia, etc [5]. Modern methods of nanoparticle conjugation, for example gold nanorods, with the molecules targeting specific tissue receptors, allow OA contrasting of the regions and pathological conditions that are otherwise not distinguishable from the background [7, 8, 15].
THREE DIMENSIONAL OPTOACOUSTIC TOMOGRAPHY
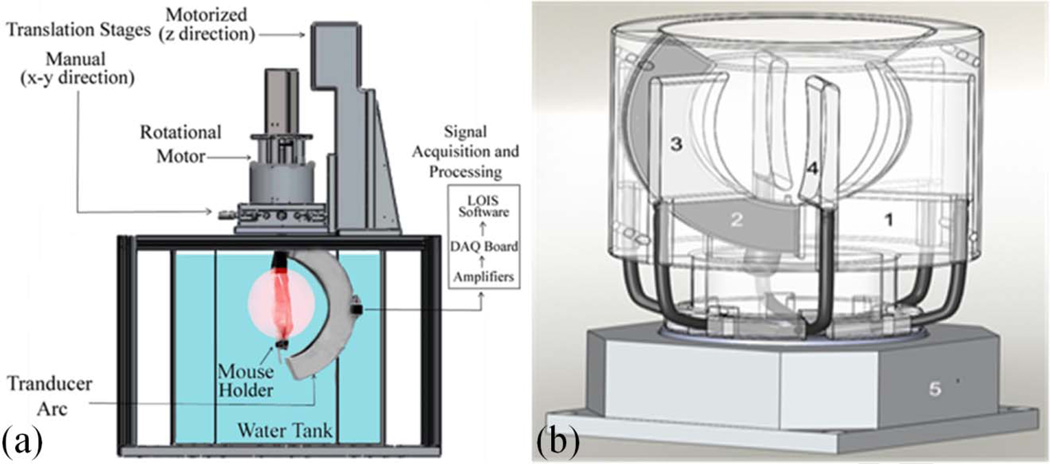
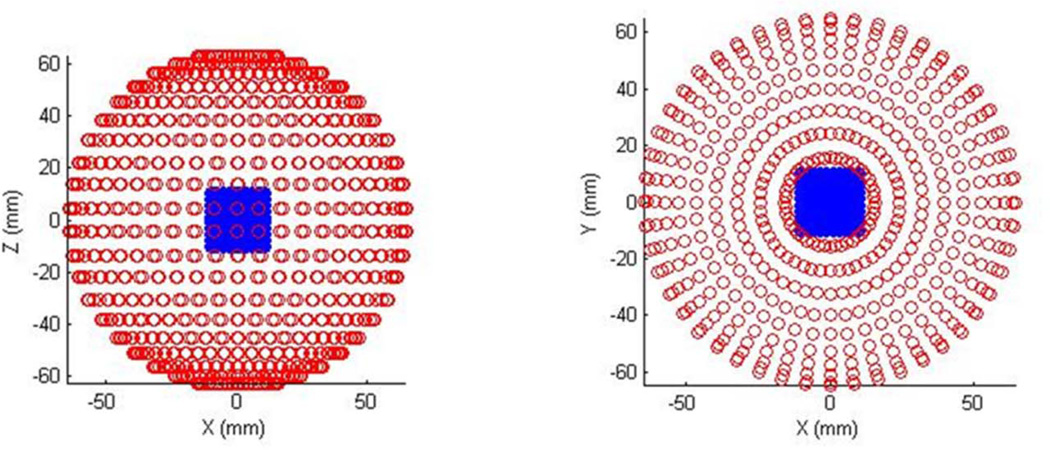
3D OA tomography (OAT) provides high resolution and accurate quantitative imaging of the deposited optical energy. Nowadays, it is primarily used for imaging of the small animal models [3, 16]. The 3D OAT system is using a rotational motor, an arc array of ultrasound transducers, and optical illumination module. The optical illumination can be oriented orthogonally in order to get deeper tissue contrast or conversely placed in backward mode (on the side of the ultrasound detectors) in order to get the skin response. For example, the water-tank based system (Figure 1a) developed by TomoWave Labs (Houston, TX) can employ orthogonal, backward, or quad (orthogonal + backward) illumination. The studied animal is rotated about central axis of the array probe. Its probe consists of 64 transducers equally spaced over an arc of 152° with a focus of 65mm. After a complete scan, it creates a sphere of 9600 transducers spaced with 2.4° intervals (Figure 2). Another implementation of a 3D OAT system involves a rotating acoustically damping bowl (Figure 1b) with a static object of investigation (small animal or breast). Acquired signals are typically processed with a band-pass filter or a multi-scale wavelet. The deconvolution of the acousto-electric impulse response of the system is performed to restore the quantitative information on measured acoustic pressure. 3D filtered radial back-projection is then performed to reconstruct distribution of absorbed optical energy [3, 5, 11].
Figure 1.
Diagrams of the two commercial systems in development for optoacoustic tomography: (a) tank and (b) bowl systems
Figure 2.
Diagram showing distribution of the acoustic transducers after a complete rotational scan of the 3D OAT system.
Small animal imaging
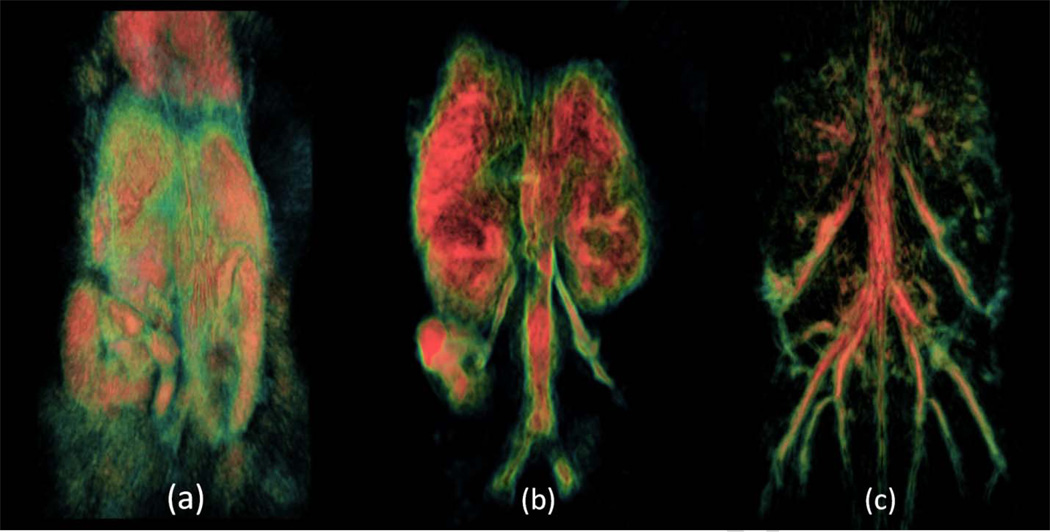
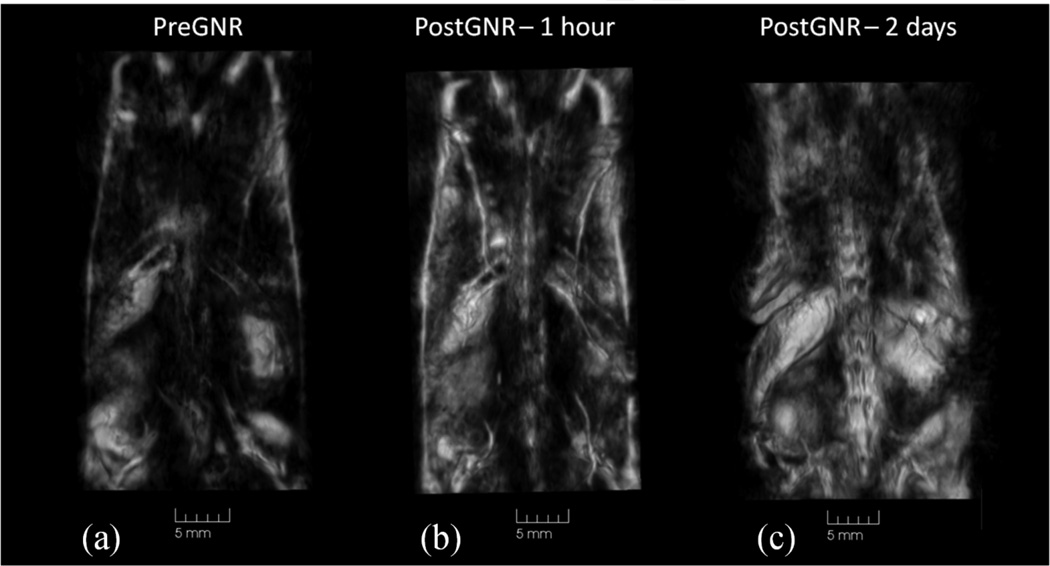
3D OA imaging using near-infrared illumination of mice is being gradually developed for several years [3, 5, 16]. The images of the whole mouse body demonstrated good contrast of major internal organs, like spleen and kidney, as well as vascular network (Figure 3). OA contrasted imaging using gold nanoparticles, carbon nanotubes, etc is being recently heavily researched [9]. Gold nanorods are of the greatest interest due to their narrow absorption band, which can be tweaked within the near-infrared spectrum during synthesis [17, 18]. It has been shown that PEG-GNRs can be tracked within a live mouse over a span of days in order to get the biodistribution of the contrast agent. This technique involves dual-wavelength illumination [3, 11]. One laser wavelength serves as a control where the contrast agent is not absorbing and the second one is absorbed by the contrast agent. Figure 4 shows that we were able to track PEG-GNRs distribution within the mouse body following their intravenous injection. Immediately after the injection, GNRs are seen within the blood circulation and eventually, after two days, they accumulate within certain organs such as kidneys and spleen [3, 15].
Figure 3.
Volumetric representations of mice scans taken at different wavelengths with different processing and visualization in order to see different structures in a live mouse
Figure 4.
Biodistribution of gold nanorods studied in live mouse using 3D optoacoustic imaging. (a) – image prior to GNR injection; (b) – image at 1hr following the injection; (c) – image at 2 days following the injection.
Commercial development
The commercial prototype of a 3D OAT system is being developed by TomoWave Labs in two forms, where the main difference is the imaging area and ability to rotate either the studied object or the detection and illumination modules. The first one represents a more flexible design developed for research purposes and animal studies. The scanning occurs within a water tank that provides easy access and room for additional components such as high frequency ultrasound transducers or additional fibers for photothermal therapy. The second system is a more compact system with rotating probe and illumination module. It is mainly designed for use in optoacoustic tomographic imaging of the breast or simple animal scans.
FUTURE HORIZONS
Our future studies are oriented towards quantitative measurements of blood concentration, oxygenation, and biodistribution of optical contrast agents over a long period of time through the use of multiple laser wavelengths. Optoacoustic imaging has a major preclinical applications include imaging of angiogenesis, tumor microenvironments, brain functions, and metabolic activities. Initial clinical applications include endoscopy of gastrointestinal tract, intravascular catheter imaging, neonatal brain imaging, breast, prostate and lymph node cancer detection, thermal therapy, blood oxygenation imaging, and tissue functional imaging.
Acknowledgements
The studies were supported by NIH Grants 1R43ES021629-01, R44 CA110137-05 and R44 CA110137-05S1.
Abbreviations
- OA
optoacoustic
- GNR
gold nanorods
- PEG-GNR
PEGylated gold nanorods
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oraevsky AA, Karabutov AA. Optoacoustic Tomography. In: Vo-Dinh T, editor. Biomedical Photonics Handbook. Boca Raton: CRC Press; 2003. pp. 34/21–34/34. [Google Scholar]
- 2.Wang LV, Hu S. Photoacoustic Tomography: In Vivo Imaging f rom Organelles to Organs. Science. 2012;23:1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brecht HP, Su R, Fronheiser M, Ermilov SA, Conjusteau A, Oraevsky AA. Whole-body three-dimensional optoacoustic tomography system for small animals. Journal of biomedical optics. 2009;14:064007. doi: 10.1117/1.3259361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao J, Wang LV. Photoacoustic tomography: fundamentals, advances and prospects. Contrast media & molecular imaging. 2011;6:332–345. doi: 10.1002/cmmi.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ermilov SA, Khamapirad T, Conjusteau A, Leonard MH, Lacewell R, Mehta K, Miller T, Oraevsky AA. Laser optoacoustic imaging system for detection of breast cancer. Journal of biomedical optics. 2009;14:024007. doi: 10.1117/1.3086616. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari PM, Vig K, Dennis VA, Singh SR. Functionalized gold nanoparticles and their biomedical applicationsm. Nanomaterials. 2011;1:31–63. doi: 10.3390/nano1010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manohar S, Ungureanu C, Van Leeuwen TG. Gold nanorods as molecular contrast agents in photoacoustic imaging: the promises and the caveats. Contrast media & molecular imaging. 2011;6:389–400. doi: 10.1002/cmmi.454. [DOI] [PubMed] [Google Scholar]
- 8.Liopo A, Conjusteau A, Oraevsky A. PEG-coated gold nanorod monoclonal antibody conjugates in preclinical research with optoacoustic tomography, photothermal therapy, and sensing. Proc. SPIE. 2012;8223:822344. [Google Scholar]
- 9.Oraevsky AA. Gold and silver nanoparticles as contrast agents for optoacoustic imaging. In: Wang L, editor. Photoacoustic imaging and spectroscopy. New York: Taylor and Francis Group; 2009. [Google Scholar]
- 10.Eghtedari M, Liopo AV, Copland JA, Oraevsky AA, Motamedi M. Engineering of Hetero-Functional Gold Nanorods for the in vivo Molecular Targeting of Breast Cancer Cells. Nano letters. 2009;9:287–291. doi: 10.1021/nl802915q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su R, Liopo AV, Brecht HP, Ermilov SA, Oraevsky AA. Gold nanorod distribution in mouse tissues after intravenous injection monitored with optoacoustic tomography. Proceedings SPIE. 2011;7899:78994B. [Google Scholar]
- 12.Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nature biotechnology. 2003;21:803–806. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HF, Maslov K, Stoica G, Wang LV. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nature biotechnology. 2006;24:848–851. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, El-Sayed H, El-Sayed MA. Applications of gold nanorods for cancer imaging and photothermal therapy. Methods in Molecular Biology. 2010;624:343–357. doi: 10.1007/978-1-60761-609-2_23. [DOI] [PubMed] [Google Scholar]
- 15.Liao HW, Nehl CL, Hafner JH. Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine (London, England) 2006;1:201–208. doi: 10.2217/17435889.1.2.201. [DOI] [PubMed] [Google Scholar]
- 16.Su R, Liopo AV, Brecht HP, Ermilov SA, Larin K, Oraevsky AA. Optoacoustic tomography in preclinical research: in vivo distribution of highly purified PEG-coated gold nanorods. Proceedings SPIE. 2011;8089:808902. [Google Scholar]
- 17.Sau TK, Murphy CJ. Seeded High Yield Synthesis of Short Au Nanorods in Aqueous Solution. Langmuir. 2004;20:6414–6420. doi: 10.1021/la049463z. [DOI] [PubMed] [Google Scholar]
- 18.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T. PEG-modified gold nanorods with a stealth character for in vivo applications. Journal of Controlled Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]