Abstract
While the human immunodeficiency virus (HIV)/AIDS pandemic continues, the incidence of HIV infections has fallen because of the deployment of antiretroviral drugs and multiple prevention modalities. To achieve a durable end to the pandemic, a vaccine remains essential. Recent advances in vaccinology offer new promise for an effective HIV vaccine.
Keywords: human immunodeficiency virus (HIV), HIV prevention, HIV vaccine
It is a privilege to contribute to this special issue of Clinical Infectious Diseases in honor of a dear friend and colleague, Dr John Bartlett. One of us (A. S. F. ) worked side by side with Dr Bartlett throughout his career, collaborating with him as cochairs of the Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. In this role and in his work as a clinician, teacher, mentor, and always-compelling speaker and author, Dr Bartlett offered wise counsel to clinicians and patients alike. His thoughtful guidance averted suffering for individuals throughout the United States and indeed around the world. He has been a tireless and compassionate leader in our field, establishing centers for the care and treatment of those infected with human immunodeficiency virus (HIV) when others turned their backs and closed their doors.
In 2012, a diverse group of researchers, caregivers, activists, policy makers, persons living with HIV and others gathered in Washington, DC, for the annual meeting of the International AIDS Society. Considering that >30 million persons were living with HIV around the world and 2.5 million had been infected in the previous year alone [1], the theme of the conference, “Turning the Tide Together” was felt by some to be overly optimistic, and even audacious [1]. However, important scientific and public health advances over the preceding 2–3 years offered the promise of real progress in the fight against HIV/AIDS. That promise is now coming to fruition: 700 000 AIDS deaths were averted in 2012 through the rollout of antiretroviral therapy (ART) and in the period from 2001 to 2012, HIV incidence fell by >30% globally [2]. By December 2013, a total of 13 countries had reached the “tipping point,” empirically defined as that point at which the number of persons starting ART was greater than the number newly infected [3]. Notably, substantial reductions in global HIV incidence and mortality have been achieved in the absence of a vaccine. In the face of these encouraging results, one might reasonably ask whether a vaccine is necessary to reach a durable end of the HIV/AIDS pandemic. We believe that, despite the major advances in HIV treatment and prevention, a safe and effective vaccine is essential to reaching that goal [4]. Our reasons for this conclusion are considered below.
The progress in treating HIV infection and in developing prevention tools has been extraordinary. More than 30 antiretroviral drugs or drug combinations are now licensed. HIV-infected persons can expect to live into their 70s if their infection is diagnosed early in its course and if they receive ART according to accepted guidelines and are provided with other care and support [5]. Indeed, life expectancy at diagnosis of HIV infection, often measured in mere months in the pre-ART era, now approximates that of an uninfected person. Many individuals can be managed with a single combination pill (containing 3 antiretroviral drugs) per day, usually with minor toxic effects. Within the United States, John Bartlett played a significant role in shaping treatment regimens as cochair from 1996 to 2013 of the Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents, and via his many seminal publications. These therapies are now reaching persons in need throughout the world: as of the end of 2012, 9.7 million persons living in low- and middle-income countries had begun ART [2]. Although this is an important accomplishment, this figure represents only 34% of the 28.6 million persons eligible for ART under the 2013 World Health Organization guidelines. Therefore, continued support for the scale-up of ART and the health systems that deliver it will be essential to curbing the epidemic.
The menu of HIV prevention technologies has expanded greatly in recent years. The sources of these advances have ranged from conventional drug development to insights gained from epidemiologic studies. The latter is perhaps best exemplified by voluntary medical male circumcision, an approach that built on the observation that circumcised men were infected at lower rates than uncircumcised men. These findings were confirmed in randomized controlled trials, demonstrating a 60% reduction in HIV acquisition after circumcision [6]. Antiretroviral drugs have been applied in a variety of prevention modalities, including the prevention of mother-to-child transmission. With ART adherence during pregnancy and breastfeeding, along with postexposure prophylaxis for infants, the risk of HIV transmission from mother to infant can be cut from approximately 25%–40% (without ART) to 2% or less. Results have been extraordinary: support for prevention of mother-to-child transmission through the President's Emergency Plan for AIDS Relief (PEPFAR), initiated in 2003, had saved >1 million infants from HIV infection as of July 2013. Although ART has long been used as postexposure prophylaxis to prevent HIV infection, it has recently been employed as preexposure prophylaxis, as a daily oral pill or applied topically to the genital mucosa. With strict adherence, the efficacy of oral preexposure prophylaxis may exceed 90% [7]. Recently, long-acting antiretroviral agents dosed monthly have proved efficacious in animal models and could improve adherence in both prophylactic and treatment regimens [8].
Perhaps most compelling, both observational studies and randomized clinical trials have demonstrated the effectiveness of treating HIV-infected individuals with ART both for their own health and to prevent transmission to uninfected sexual partners. The pivotal HIV Prevention Trials Network 052 study showed a 96% reduction in transmission in serodiscordant couples when ART was started early in the HIV-infected partner (vs later in course of disease) [9]. More recently, the ongoing PARTNER study has indicated that the risk of sexual HIV transmission is exceedingly rare in couples in whom the HIV-infected partner has an undetectable viral load at ART [10].
These advances, together with time-tested prevention modalities such as condoms and the provision of clean needles and syringes for injection drugs users, represent a comprehensive package of prevention tools that could dramatically reduce infections. Although progress has been made, further reductions in incidence have been impeded by programmatic barriers, including inadequate financial and human resources. In addition, limited uptake and adherence have slowed gains. For example, although the international AIDS community is targeting 20 million circumcisions in 14 high-incidence countries in sub-Saharan Africa by 2016, less than one-third of these procedures had been performed by the end of 2013 [11]. A recent study provides some insight into the reason for the delay: despite widespread knowledge of the benefits of circumcision in the Rakai district of Uganda (the site of several circumcision trials), only 27% of surveyed men expressed willingness to undergo the procedure [12]. As already noted, preexposure prophylaxis can be highly efficacious if taken as directed, but some trials have shown disappointing results owing to poor adherence [13]. Finally, the most basic of prevention tools, condoms, remain underused. Improved understanding of the factors affecting uptake and adherence will be essential to progress in prevention. In addition, stigma continues to undermine the fight against HIV/AIDS. Egregious laws that target affected communities, such as those that make homosexuality illegal, endanger both targeted care delivery and individuals seeking care; furthermore, they tend to incite discrimination and violence. In short, the social and cultural barriers to HIV prevention programming have become more daunting, despite the expansion of prevention techniques.
Even if the social and cultural barriers to widespread uptake of prevention technologies were removed, recidivism could pose a constant threat to public health success. Ministries of health, particularly those in low-resource countries, struggle to prioritize expenditures in the face of myriad health threats. Therefore, success in driving down disease incidence too often leads to diversion of attention away from the effort resulting in an eventual resurgence of cases. Attempts at malaria elimination in certain regions teach this lesson. The island of Zanzibar, for example, drove Plasmodium falciparum parasitemia prevalence down from 76% to <5% between 1957 and 1967 [14]. Unfortunately, owing to a premature declaration of victory, the program was defunded in favor of other priorities and the parasite quickly rebounded. Recidivism poses a similar threat to individual behavior. A recent survey in South Africa showed that ART coverage doubled between 2008 and 2012, yet the incidence of HIV infection has remained stable since 2005. This may be explained by the finding that risk-taking behavior, such as forsaking the use of condoms, had increased, demonstrating the danger of complacency [15].
Taken together, inadequate implementation of interventions, whether preventive, therapeutic or both, has limited their effectiveness in the real-world setting. We therefore conclude that a safe and effective vaccine, which (once administered) would provide protection not subject to the daily vicissitudes of human behavior, is essential for the durable control and end of the HIV/AIDS pandemic. However, the pathway to such a vaccine provides a daunting challenge.
Soon after the discovery of the HIV virus in 1983 and the proof that it was the etiologic agent of AIDS in 1984, a US government official projected that a vaccine would be “ready for testing in approximately two years” [16]. Even though she was not far off the mark with regard to the timetable for testing, those early trials and the others that followed have not yielded an effective vaccine. Initial approaches adopted traditional immunization strategies that worked well with other viruses, namely, using a viral subunit to prime the immune system, recapitulating and improving on the protective immunity generated by infection with an actual pathogen. Whole-virus, either killed or attenuated, approaches for HIV vaccines had been deemed unsafe owing to fears of inadequate killing or integration into the host genome and reversion to a virulent form. Initial attempts to create subunit vaccines based on epitopes from the HIV envelope, against which most antibodies are directed, seemed promising. Beginning in 1987, >35 phase I trials were conducted using envelope protein immunogens [16]. Although these immunogens produced antibodies capable of neutralizing HIV grown in immortalized lines of T lymphocytes, the antibodies were ineffective in neutralizing primary HIV isolates taken from patients. Ultimately, 2 phase III human trials of vaccines based on HIV envelope proteins failed to demonstrate protection, confirming the lack of neutralizing capability against primary HIV isolates [17, 18].
Given the difficulty in eliciting protective antibody responses, some scientists turned their attention to T-lymphocyte vaccines [19]. By inducing HIV-specific CD8+ T-lymphocyte responses, vaccinologists hoped to succeed in destroying cells in which HIV was replicating, rather than blocking initial infection. It was speculated that lowering the amount of circulating virus would make individuals less likely to transmit virus and would diminish the destruction of CD4+ T lymphocytes. By 2007, however, 2 major phase III trials of vaccines using adenovirus vectors to express HIV proteins had failed to demonstrate protection, and trial data suggested an increased risk of HIV acquisition in the vaccinated subjects.
With disappointing results from both envelope-based vaccines and CD8+ T-cell–directed vaccines, the future prospects for HIV vaccinology seemed dim. However, an unexpected success surfaced from the RV-144 trial, conducted in Thailand [20]. A 2-part vaccine regimen of a canarypox prime and recombinant gp120 boost yielded a 31% reduction in infections compared with placebo. The protection seemed to be mediated through nonneutralizing or weakly neutralizing antibodies against epitopes in the V1V2 loop of the envelope. Current studies are investigating correlates of protection associated with the RV144 trial, hoping to capitalize on these correlates and improve the modest efficacy of RV144 in future trials. In parallel with these attempts, investigators are exploring the reference standard in vaccine-induced protection, that is, induction of broadly neutralizing antibodies (bNAbs).
Natural infection with HIV produces bNAbs in a minority of individuals, usually after ≥2 years of infection. There are several reasons why neutralization is so difficult. First, to neutralize multiple strains of virus, antibodies must be directed against conserved epitopes, and in HIV, those epitopes tend to be in glycoprotein-rich areas of the envelope that are poorly immunogenic. Second, the virus mutates rapidly and constantly evades the immune response directed against it. Third, the production of BNAbs seems to require high degrees of somatic hypermutation of HIV-specific B cells, meaning that germline B cells do not seem to readily produce such antibodies without extensive adaptation through affinity maturation. In natural infection, the evolution of this high degree of somatic mutation requires continual stimulation over extended periods of time with the virus, a requirement ill suited to replication by a vaccine regimen. A further challenge for HIV vaccinology lies in the autoreactivity of some bNAbs, suggesting that powerful tolerogenic mechanisms may hamper their induction.
Despite these challenges, recent insights have deepened our understanding of bNAb responses, insights that could guide the field toward an effective vaccine. For example, samples from large-cohort studies have revealed >20 bNAbs occurring in natural infection that could possibly be elicited with vaccine immunogen(s). The potential for such bNAbs to protect against HIV infection has been explored by passively providing the antibodies rather than inducing them. In this regard, other viruses have been used as vectors containing relevant immunoglobulin gene inserts to express a bNAb directly in vivo; such an approach has provided protection in animal models and will soon be tested in human trials [21]. In addition, by studying serial blood samples from an HIV-infected individual from the acute infection phase through 3 years of follow-up, scientists have traced the concurrent evolution of both virions and HIV-specific B cells, demonstrating how iterative mutations in the virus led to the induction of B-cell hypermutation that eventually resulted in bNAb production [22]. The work unveiled an essential paradox of HIV B-cell science: as the virus mutates over time to escape the immune system's response, it eventually induces the host to create bNAbs that inhibit a wide range of HIV isolates (with the exception of the virus circulating in the host) [22]. On an encouraging note, another recent study demonstrated that although potent broad neutralization usually required extensive hypermutation, less robust, potentially protective responses were elicited with minor mutations from naive cells [23].
Synthesizing recent investigations in B-cell vaccine science, a developmental pathway toward an effective vaccine has been described. First, the correct target for antibodies on the virion must be defined, and epitopes in regions of the trimeric heterodimer of the HIV envelope have been identified as the most promising sites. Next, the structure of those targets must be delineated, and recent work in cryoelectron microscopy and x-ray crystallography has detailed the conformations of the relevant regions in exquisite detail. Third, the naive B-cell repertoire in bone marrow and secondary lymphoid tissue that is programmed to respond to these epitopes must be engaged, through a process referred to as B-cell lineage immunogen design. Specifically, after identification of bNAbs and their corresponding epitopes, antibody ancestry lineages must be constructed, tracing back to naive B cells. Next, using recombinant antibody technology, members of that lineage must be expressed to identify virion envelope epitopes that bind most avidly. Finally, those epitopes must be converted into immunogens and integrated into a vaccine regimen, probably in a prime-boost fashion, to engage naïve B cells and prod their hypermutation toward bNAb responses [24].
Whereas B-cell vaccinology is generating significant interest, novel T-cell approaches have also been pursued. These could be used in combination with B-cell vaccines to provide broad help, boosting the degree and specificity of antibody responses. Alternatively, the specific actions of T follicular helper cells could be targeted, because their levels seem to correlate with the creation of bNAbs. Finally, the use of a vaccine candidate composed of simian immunodeficiency virus genes inserted into a cytomegalovirus vector seemed to induce CD8+ T-cell responses that resulted in viral clearance in half of nonhuman primates tested [25]. Human trials of this approach are planned.
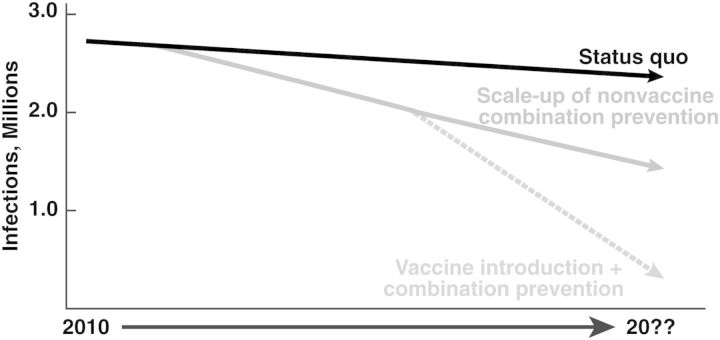
Therefore, recent scientific advances have dramatically altered the HIV science landscape, creating a pathway toward a vaccine that ideally will be safe and sufficiently protective to be part of a larger prevention tool kit. Although nonvaccine approaches to HIV prevention will probably markedly diminish new infections, the challenges impeding optimal deployment of prevention modalities force us to conclude that a safe and effective vaccine (together with vigorous implementation of nonvaccine prevention tools) is essential if we are to realize a timely and sustained end of the HIV/AIDS pandemic (Figure 1).
Figure 1.
Hypothetical model of human immunodeficiency virus (HIV) pandemic evolution. With scaling up of current prevention and treatment modalities, the incidence of HIV infection and associated mortality rates will probably continue to decrease in the absence of a vaccine. However, a safe and at least moderately effective HIV vaccine (combined with other modalities) is essential to achieving a more rapid and sustained end of the HIV/AIDS pandemic.
Notes
Supplement sponsorship. This article was published as part of a supplement titled “The John Bartlett Festschrift: Celebrating a Career in Medicine,” sponsored solely by the Department of Medicine of the Johns Hopkins School of Medicine in recognition of John Bartlett's contributions to medicine.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.International AIDS Society. Turning the tide together: a declaration to end the AIDS epidemic, 2012. Available at: http://www.2endaids.org/wp-content/uploads/dc_declaration_advert_Eng.pdf . Accessed 17 May 2014.
- 2.UN Joint Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic. 2013. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf . Accessed 28 March 2014.
- 3.AIDS Vaccine Advocacy Coalition. The tipping point: understanding a crucial milestone in the AIDS response. 2013 Available at: http://www.thebody.com/content/72903/the-tipping-point-understanding-a-crucial-mileston.html . Accessed 6 June 2014. [Google Scholar]
- 4.Fauci AS, Marston HD. Ending AIDS—is an HIV vaccine necessary? N Engl J Med. 2014;370:495–8. doi: 10.1056/NEJMp1313771. [DOI] [PubMed] [Google Scholar]
- 5.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS. 2012;26:609–15. doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews CD, Spreen WR, Mohri H, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science. 2014;343:1151–4. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodger A, Bruun T, Cambiano V, et al. HIV Transmission risk through condomless sex if HIV+ partner on suppressive ART: PARTNER study. Conference on Retroviruses and Opportunistic Infections (CROI 2014); 3–6 March; Boston, MA. Abstract 153LB. [Google Scholar]
- 11.Sgaier SK, Reed JB, Thomas A, Njeuhmeli E. Achieving the HIV prevention impact of voluntary medical male circumcision: lessons and challenges for managing programs. PLoS Med. 2014;11:e1001641. doi: 10.1371/journal.pmed.1001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong X, Ssekasanvu J, Kigozi G, et al. Male circumcision coverage, knowledge, and attitudes after 4-years of program scale-up in Rakai, Uganda. AIDS Behav. 2014;18:880–4. doi: 10.1007/s10461-014-0740-0. [DOI] [PubMed] [Google Scholar]
- 13.Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J Acquir Immune Defic Syndr. 2013;3(suppl 2):S122–9. doi: 10.1097/QAI.0b013e3182986f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen JM, Smith DL, Cotter C, et al. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:1–17. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Human Sciences Research Council. South African National HIV Prevalence, Incidence & Behaviour Survey. 2012 Available at: http://www.health-e.org.za/wp-content/uploads/2014/04/HRSC-2012.pdf . Accessed 4 April 2014. [Google Scholar]
- 16.Esparza J. A brief history of the global effort to develop a preventive HIV vaccine. Vaccine. 2013;31:3502–18. doi: 10.1016/j.vaccine.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Flynn NM, Forthal DN, Harro CD, et al. Placebo controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 18.Pitisuttithum P, Gilbert P, Gurwith M, et al. Bangkok Vaccine Evaluation Group. Randomized, double-blind placebo controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 19.Korber BT, Letvin NL, Haynes BF. T-cell vaccine strategies for human immunodeficiency virus, the virus with a thousand faces. J Virol. 2009;83:8300–14. doi: 10.1128/JVI.00114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 21.Balazs AB, Ouyang Y, Hong CM, et al. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 2014;20:296–302. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao HX, Lynch R, Zhou T, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–76. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doria-Rose NA, Schramm CA, Gorman J, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol. 2013;13:693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 25.Hansen SG, Piatak M, Jr, Ventura AB, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–4. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]