Highlight text
SlNCED1 and SlCYP707A2 are key genes in the regulation of ABA synthesis and catabolism, and are involved in fruit ripening as positive and negative regulators, respectively.
Key words: Abscisic acid (ABA), SlNCED1, SlCYP707A2, tomato fruit ripening, tobacco rattle virus, virus-induced gene silencing (VIGS).
Abstract
Abscisic acid (ABA) plays an important role in fruit development and ripening. Here, three NCED genes encoding 9-cis-epoxycarotenoid dioxygenase (NCED, a key enzyme in the ABA biosynthetic pathway) and three CYP707A genes encoding ABA 8′-hydroxylase (a key enzyme in the oxidative catabolism of ABA) were identified in tomato fruit by tobacco rattle virus-induced gene silencing (VIGS). Quantitative real-time PCR showed that VIGS-treated tomato fruits had significant reductions in target gene transcripts. In SlNCED1-RNAi-treated fruits, ripening slowed down, and the entire fruit turned to orange instead of red as in the control. In comparison, the downregulation of SlCYP707A2 expression in SlCYP707A2-silenced fruit could promote ripening; for example, colouring was quicker than in the control. Silencing SlNCED2/3 or SlCYP707A1/3 made no significant difference to fruit ripening comparing RNAi-treated fruits with control fruits. ABA accumulation and SlNCED1transcript levels in the SlNCED1-RNAi-treated fruit were downregulated to 21% and 19% of those in control fruit, respectively, but upregulated in SlCYP707A2-RNAi-treated fruit. Silencing SlNCED1 or SlCYP707A2 by VIGS significantly altered the transcripts of a set of both ABA-responsive and ripening-related genes, including ABA-signalling genes (PYL1, PP2C1, and SnRK2.2), lycopene-synthesis genes (SlBcyc, SlPSY1 and SlPDS), and cell wall-degrading genes (SlPG1, SlEXP, and SlXET) during ripening. These data indicate that SlNCED1 and SlCYP707A2 are key genes in the regulation of ABA synthesis and catabolism, and are involved in fruit ripening as positive and negative regulators, respectively.
Introduction
Abscisic acid (ABA) plays an important role in plant growth, stomatal movement, seed dormancy, and germination (Melcher et al., 2009; Nishimura et al., 2009). Moreover, it mediates adaptive responses to abiotic and biotic stresses (Chernys and Zeevaart, 2000; Shang et al., 2010). At present, major progress has been made in research into the role of ABA in the regulation of fleshy fruit ripening (Rodrigo et al., 2006; Zhang et al., 2009a, b; Giribaldi et al., 2010). These physiological processes controlled by ABA are primarily regulated by the bioactive ABA pool size, which is thought to be maintained not only by its biosynthesis, but also by its catabolism (Sawada et al., 2008). The ABA metabolic pathway has been established by genetic approaches. ABA is synthesized de novo from a C40 carotenoid. The carotenoid-biosynthetic pathway begins with the formation of phytoene from two molecules of geranylgeranyl diphosphate (GGPP) in the central isoprenoid pathway. Four desaturation steps give rise to lycopene; cyclizations at both ends of the lycopene molecule produce α-or β-carotene, which undergo hydroxylation at C3 and C3′ to form the xanthophylls lutein and zeaxanthin, respectively. An important phase of ABA biosynthesis is initiated in plastids with the hydroxylation and epoxidation of the β-carotene to produce the all-trans xanthophylls zeaxanthin and violaxanthin. Violaxanthin is then converted into 9-cis-epoxyxanthophylls, which are oxidatively cleaved by 9-cis-epoxycarotenoid dioxygenase (NCED) to yield xanthoxin, the first C15 intermediate of ABA biosynthesis. Xanthoxin exits the plastid into the cytosol where it is oxidized in two further steps to form ABA (Schwartz et al., 2003; Taylor et al., 2005). In addition, the ABA metabolic pathway plays an important role in the regulation of ABA levels (Cutler and Krochko, 1999; Schwartz et al., 2003). The hydroxylation pathway is the main ABA catabolic pathway. In the hydroxylation pathway, among three different methyl groups, C8′ is the predominant position for the hydroxylation reaction, which is mediated in Arabidopsis by proteins encoded by the CYP707A gene family (Saito et al., 2004; Kushiro et al., 2004). Dihydroxyphaseic acid (DPA) may be the major metabolite of ABA (Setha et al., 2005). The 8-hydroxylation is reported to be the major regulatory step in many physiological events controlled by ABA (Kushiro et al., 2004; Saito et al., 2004). Recently, a major breakthrough in the field of ABA signalling was achieved with the identification of the PYR/PYL/RCAR protein family, the type 2C protein phosphatases (PP2Cs), and subfamily 2 of the SNF1-related kinases (SnRK2s) (Ma et al., 2009; Park et al., 2009; Melcher et al., 2009; Santiago et al., 2009). To elucidate the mechanism of ABA action, it is necessary to identify all the components involved in ABA homeostasis, including the functional components in ABA metabolic pathways, signal transduction, and transport.
In tomato (Solanum lycopersicum), three NCED genes have been isolated, and expression analysis indicated that, among them, SlNCED1 may regulate ABA biosynthesis in fruit (Sun et al., 2012a, b). However, the molecular evidence remains to be elucidated. To address this, a key step in ABA biosynthesis, NCED was targeted for inhibition via RNAi in tomato fruit two years previously. Four independent transgenic plants were evaluated (Sun et al., 2012a, b). Our data showed that ABA potentially regulated the development and ripening of tomato fruit. In addition, ABA could control, at least in part, the production and effects of ethylene in climacteric tomato fruit. In recent years, tobacco rattle virus-induced gene silencing (VIGS) has been used as a rapid gene function assay system in molecular biological studies of fleshy fruit (Fu et al., 2006; Hoffmann et al., 2006; Li et al., 2013). In this study, we further identify the function of three NCED and three CYP707A genes by VIGS. Results show that SlNCED1 and SlCYP707A2 are key genes in the regulation of ABA level during development and ripening of tomato fruit.
Materials and methods
Construction of the viral vector and agroinoculation
The pTRV1 and pTRV2 virus-induced gene silencing vectors (described by Liu et al., 2002) were kindly provided by YL Liu (School of Life Science, Tsinghua University, Beijing, China). A 456bp cDNA fragment of an NCED or CYP707A gene was amplified using primers (Table 1). The amplified fragment was cloned into EcoRI/SacI-digested pTRV2. Agrobacterium tumefaciens strain GV3101 containing pTRV1, pTRV2, and the pTRV2-derivative pTRV2-NCED/CYP707A were used for RNAi. Thirty fruits from ten independent plants grown in the greenhouse were selected for inoculation, and each basal pedicel or one side of fruit was injected with the NCED/CYP707A-RNAi TRV vector at the maturation green stage. The fruits were evaluated 3–12 days after treatment.
Table 1.
Specific primers used for VIGS in this study
| Target gene | Oligonucleotides (5′-3′) |
|---|---|
| SlNCED1 F-v | CGGGAATTCTAGTTACGCTTGCCGTTTCACTGAA |
| SlNCED1 R-v | CGGGAGCTCTCAGGAATGACGACGAAGTTCTCAG |
| SlNCED2 F-v | CGGGAATTCACAAGACGACAACTACTTTCACCCT |
| SlNCED2 R-v | CGGGAGCTCTCTAGAGTCATGGCATTTACAATTTG |
| SlNCED3 F-v | CGGGAATTCTCCACGACCCGAATAAAGTATCT |
| SlNCED3 R-v | CGGGAGCTCGTCTTGTTTACTTGTCCCGCTTC |
| SlCYP707A1 F-v | CGGGAATTCCATTTGGATGGTCATGTTAAGGA |
| SlCYP707A1 R-v | CGGGAGCTCCAAATAACTTTCTGTTCAGCCTTGA |
| SlCYP707A2 F-v | CGGGAATTCTCTTTGCAGCTCGAGACACTACTGC |
| SlCYP707A2 R-v | CGGGAGCTCTCCAGCTTGGCTAAGTCATTCCCT |
| SlCYP707A3 F-v | CGGGAATTCTTTCAAGACTCACATATTGGGATG |
| SlCYP707A3 R-v | CGGGAGCTCTAGCACCTCTTTAGATCCTCCCT |
Plant materials
Tomatoes (Solanum lycopersicum L. cv. JiaBao) were grown under standard greenhouse conditions (25±5°C and 70% humidity under a 14h/10h light/dark regime). Fruit ripening stages were divided according to the days after flowering (DAF) and fruit colour: immature green (IM), 15 DAF; mature green (MG), 30 DAF; breaker 0 (B0), 34 DAF; breaker 1 (B1), 35 DAF; breaker 2 (B2), 36 DAF; turning 0 (T0), 37 DAF; turning 3 (T3), 40 DAF; red ripe (R), 42 DAF; and over ripe (OR), 45 DAF. Ten fruits were harvested at each stage and immediately frozen in liquid nitrogen. They were then powdered, mixed, and stored at –80oC until further use. ‘Tom’ tomatoes were also grown in the same greenhouse.
Dehydration treatment of fruits
In order to evaluate the effect of dehydration stress, 60 fruits were harvested at the MG stage and divided evenly into two groups. The first group (control) was stored at 20°C under high relative humidity (RH) (95%) which included 10 control fruits, 10 SlNCED1-RNAi-treated fruits, and 10 SlCYP707A2-RNAi-treated fruits. The second group (dehydration stress) was stored at the same temperature and subjected to the same treatments, but under low RH (55%, dehydrated fruits). The ABA content and expression of related genes in the pulp were determined 0, 3, 5, and 7 d after the treatment of fruits and 0, 1, and 2 d after the treatment of sepals. Every single fruit was weighed immediately after harvest and then weighed again before sampling for calculation of water loss rate. Water loss rate is calculated as the ratio of the decreased fruit weight to the initial fruit weight.
ABA or NDGA treatment
Sixty fruits harvested at the MG stage were divided into two groups (n = 30 for each group), and immediately soaked in 100 µM ABA (Sigma, A1049, USA) (group I) or distilled water (group II, control) for 10min under low vacuum. The fruits were then placed in a tissue culture room at 25°C and 95% RH. After 0, 1, and 3d, the fruits were sampled, frozen with liquid nitrogen, powdered, mixed, and stored at –80oC for further use. Different treatments with nordihydroguaiaretic acid (NDGA) were the same as with ABA; NDGA concentration was 200 µM.
Quantitative real-time PCR analysis
Total RNA was isolated from tomato samples using the hot borate method (Wan and Wilkins, 1994). Genomic DNA was eliminated using an RNase-free DNase I kit (Takara, China) according to the manufacturer’s recommendations. For every RNA sample, quality and quantity were assessed by agarose gel electrophoresis. cDNA was synthesized from total RNA using the PrimeScriptTM RT reagent kit (Takara) according to the manufacturer’s recommendations. Primers used for real-time PCR are listed in Supplementary Table S1 and were designed using Primer 5 software (http://www.premierbiosoft.com/). SAND was used as an internal control gene, and the stability of its expression was tested in preliminary studies (Sun et al., 2011). All primer pairs were tested by PCR. The presence of a single product of the correct size for each gene was confirmed by agarose gel electrophoresis and double-strand sequencing (Invitrogen). The amplified fragment of each gene was subcloned into the pMD18–T vector (Takara), and used to generate standard curves through serial dilution. The real-time PCR was performed using a Rotor-Gene 3000 system (Corbett Research, China) with SYBR Premix Ex TaqTM (Takara). Each 20 µl reaction solution contained 0.8 µl of primer mixer (containing 4 µM of each forward and reverse primer), 1.5 µl cDNA template, 10 µl SYBR Premix Ex TaqTM (2X) mixer, and 7.7 µl water. Reactions were performed under the following conditions: 95°C for 30 s (one cycle), 95°C for 15 s, 60°C for 20 s, and 72°C for 15 s (40 cycles). The changes of relative fold expression were calculated using the relative two standard curves method with Rotor-Gene 6.1.81 software (Invitrogen).
Determination of ABA content
For ABA extraction, 1.0g of pulp was ground in a mortar and homogenized in the extraction solution (80% v/v methanol). Extracts were centrifuged at 10 000g for 20min. The supernatant was eluted through a Sep-Pak C18 cartridge (Waters, www.waters.com) to remove polar compounds, and then were stored at 20°C for ELISA. The stepwise procedure for indirect ELISA of ABA was as follows: each well of a microtitre plate was pre-coated with ABA–BSA conjugater diluted in coating buffer according to the instructions of the manufacturer (ELISA kit for ABA, College of Agronomy and Biotechnology, China Agricultural University). Then, to each well, was added 50 µl standard or sample in assay buffer (8.0g NaCl, 0.2g KH2PO4, 2.96g Na2HPO412 H2O, 1.0ml Tween 20, and 1.0g gelatin, added to 1.000ml water), followed by 50 µl ABA antibody (Invitrogen) diluted 1:2000 in assay buffer. The plates were incubated for 0.5h at 37°C and then washed four times with scrubbing buffer (which contained the same ingredients as assay buffer, but without gelatin). Anti- mouse IgG coupled to alkaline phosphatase (100ml of a 1:1000 dilution) was added to each well, and the plates were incubated for 0.5h at 37°C. The plates were washed as above, and then 100 µl of a 1–2mg ml–1 o-phenylenediamine substrate solution and 0.04% by volume of 30% v/v hydrogen peroxide in substrate buffer (5.10g C6H8O7H2O, 18.43g Na2HPO412 H2O, and 1.0ml Tween 20, added to 1000ml water) were added to each well. After 10–15min, 50 µl of 2.0mol l–1 H2SO4 was added to each well to terminate the reaction. The absorbance was measured at 490nm using a Thermo Electron (Labsystems) Multiskan MK3 (Pioneer, www.pioneerbiomed.com). The concentration of ABA in the sample was calculated from log B/B0-transformed standard curve data, where B and B0 are the absorbance values with or without the competing antigen, respectively.
Determination of ethylene production
The ethylene production of the fruit was measured by enclosing three fruits in 1.0 l airtight containers for 2h at 20°C, withdrawing 1ml of the headspace gas, and injecting it into a gas chromatograph (Agilent model 6890N) fitted with a flame ionization detector and an activated alumina column. Fresh tissues from each fruit were frozen in liquid nitrogen and stored at –80°C until use.
Determination of fruit firmness
Fruits were harvested from all of the plants in each of three replicate plantings at the different ripening stages. Flesh firmness was measured after the removal of fruit skin on three sides of each fruit using a KM-model fruit hardness tester (Fujihara). The strength of flesh firmness was recorded in kg cm–1. Compression of each fruit was measured three times, and the average of the maximum force was used.
Results
Expression patterns of ABA metabolic genes in pulp during fruit development and in response to application of exogenous ABA and dehydration stress
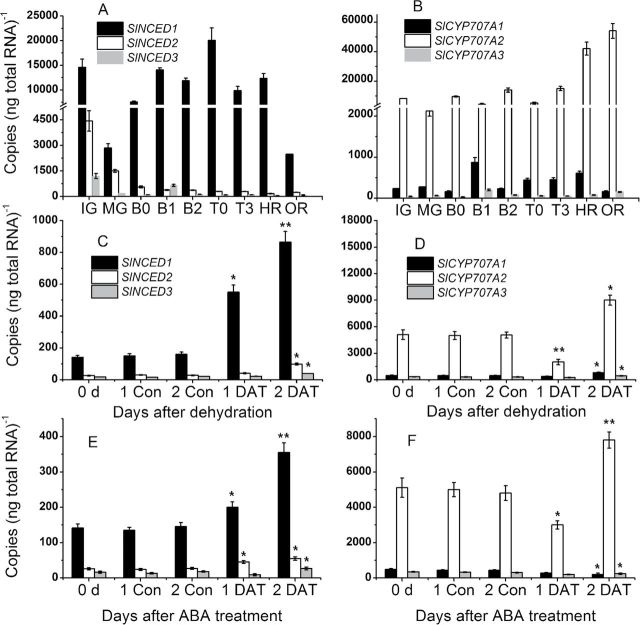
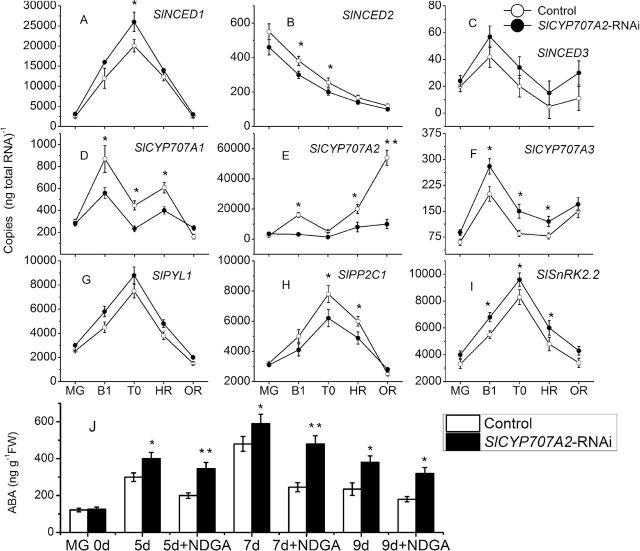
Within the SlNCED gene family, the expression of SlNCED1 was the highest in pulp. SlNCED1 decreases from 10 days after full bloom (DAFB) to the MG stage, then it increased sharply and peaked at the turning stage; after that it declined to a low level at the OR stage (Fig. 1A). The expression variation of SlNCED2 was generally decreased from 10 DAFB to fruit ripening. The expression of SlNCED3 was very low through fruit development and ripening. Among the SlCYP707A gene family, the expression of SlCYP707A2 (Fig. 1B) exhibited a fluctuant expression pattern with four peaks during development, and then it increased rapidly during ripening. Compared to SlCYP707A2, the expression of SlCYP707A1 and SlCYP707A3 (Fig. 1B) was very low during fruit development and ripening. In addition, expression of ABA metabolic genes in response to exogenous ABA treatment and dehydration in tomato fruits was tested. For SlNCED1, expression was significantly increased by exogenous ABA and dehydration at 2 days after treatment (DAT) (Fig. 1C, E). The expression of SlNCED2 and SlNCED3 was also significantly increased in the water stressed and ABA-treated tomato fruits (Fig. 1C, E). For SlCYP707A2, expression was downregulated in the fruits under both ABA treatment and dehydration at 1 DAT (Fig. 1D, F), and then it significantly increased at 2 DAT (Fig. 1D, F). The expression of SlCYP707A1 and SlCYP707A3 was significantly downregulated under both water stress and ABA treatment at 1 DAT, but upregulated at 2 DAT.
Fig. 1.
Changes in the expression of SlNCEDs and SlCYP707As during fruit development, and in response to dehydration stress and the application of exogenous ABA. (A, B) Absolute transcript level profiles for both SlNCEDs and SlCYP707As during fruit development. IG, 15 days after anthesis (DAA); MG, 30 DAA; B0 to B2, 31 to 35 DAA; T0 and T3, 36 to 37 DAA; HR, 38 DAA; and OR, 40 DAA. (C, D) Effect of dehydration stress on the expression of SlNCEDs and SlCYP707As. Fruits were harvested at the MG stage, divided into two groups, and then placed in an incubator at 25oC. The relative humidity for group 1 is 90% as the control, and 45% for group 2 as the dehydration treatment. Each treatment lasted 4 d and the samples were collected at 1 and 2 d after treatment (DAT). (E, F) Effect of exogenous ABA treatment on the expression of SlNCEDs and SlCYP707As. Fruits harvested at the MG stage were divided into two groups and treated with ABA (100mM) or distilled water for 10min. The samples were collected at 1 and 2 DAT. SAND mRNA was used as the internal control. Three biological replicates (n = 3) were used for each analysis. *P value t-test < 0.05; **P value t-test < 0.001. Error bars are SE.
Silencing of the SlNCED1 gene suppresses tomato fruit ripening
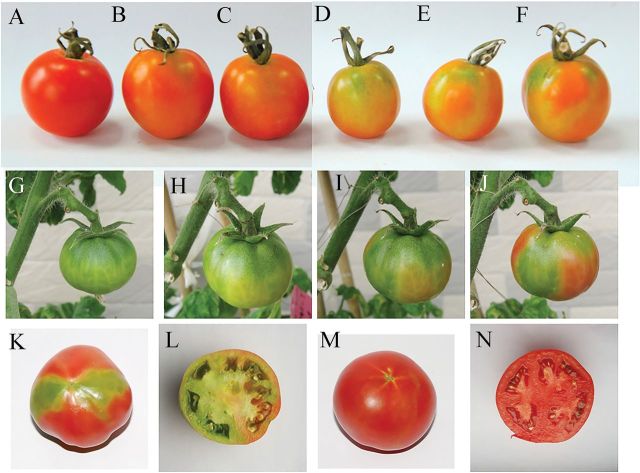
To examine the role of SlNCEDs, the tobacco rattle virus (TRV) vector was used to suppress the expression of SlNCEDs (Fig. 2). When the SlNCED1-RNAi TRV vector was injected into the basal pedicel of 15 fruits (cv. Tom) attached to the plant at the MG stage, fruit ripening slowed down, and the entire fruit turned orange (Fig. 3D, E,F) instead of red as in the control (Fig. 3A). Ten days after the SlNCED1-RNAi TRV vector was injected, the fruits did not show normal ripening (Fig.3D, E, F) as in the control (Fig. 3A). Compared to SlNCED1-RNAi-treated fruits, there were no significant differences in colouring between SlNCED2/3-RNAi-treated fruits and control fruits (Fig. 3B, C) during fruit ripening and in response to dehydration stress. The SlNCED1-RNAi TRV vector was also injected into the attached fruits (cv. JiaBao) at the MG stage. 12 d after injection, control fruits turned red (Fig. 3M, N); however, for SlNCED1-RNAi-treated fruits, parts of the peel and placenta inside the fruit did not turn red as in the control (Fig.3K, L). The water loss of the sepal in the SlNCED1-RNAi-treated fruits was quicker than in the control under the same conditions. Meanwhile, the wilting of the sepals was more serious than that of the control under the same conditions.
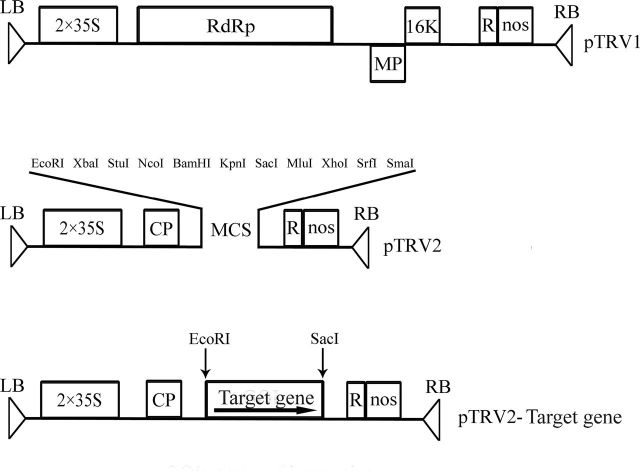
Fig. 2.
Construction of pTRV1, pTRV2, and pTRV2-derivative pTRV2-target gene. The TRV-based virus-induced gene silencing vectors are as described by Liu et al. (2002). TRV cDNA clones were placed between duplicated CaMV 35S promoters and the nopaline synthase terminator in a T-DNA vector. The pTRV2-target gene (sense orientation) was constructed to assess the ability of TRV vectors to suppress expression of the target gene in tomato fruits. RdRp, RNA-dependent RNA polymerase; 16K, 16kDa cysteine-rich protein; MP, movement protein; CP, coat protein; LB and RB, left and right borders of T-DNA, respectively; R, self-cleaving ribozyme; MCS, multiple cloning sites.
Fig. 3.
Phenotype of SlNCED1/2/3-RNAi-treated fruits. At the maturation green stage, 48 tomato fruits (cv. Tom) from 10 independent plants grown in the greenhouse were selected and divided into four groups (each group has 12 fruits for three repetitions; each repetition has four fruits) for VIGS testing. The SlNCED1-RNAi, SlNCED2-RNAi, and SlNCED3 -RNAi TRV vectors were injected from the pedicel into group 1, group 2, and group 3, respectively. Group 4 was injected with TRV vector (no NCED gene) as a control. The fruits were evaluated 3–12 d after inoculation. Panels A to F show Tom tomato fruits 9 d after VIGS treatments: (A) Control; (B) SlNCED2-RNAi-treated fruit; (C) SlNCED3-RNAi-treated fruit; (D–F) SlNCED1-RNAi-treated fruit. Panels G–N show JiaBao tomato fruits treated with SlNCED1-RNAi: (G) MG stage fruit (before treatment); (H) 5 d days after inoculation (DAI); (I) 7 DAI; (J) 9 DAI; (K, L) 12 DAI; (M, N) 12 days after MG stage, control fruit.
SlNCED1-RNAi treatment alters the expression of genes involved in ABA-responsive genes
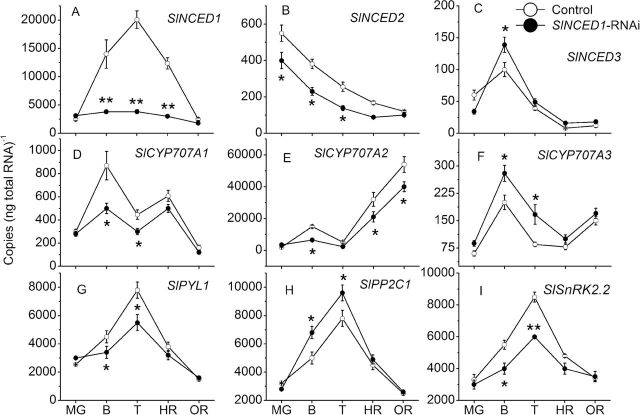
In SlNCED1-RNAi-treated fruits, expression of SlNCED1 was markedly downregulated to 19% of the control while expression of SlNCED2/SlNCED3 was downregulated/upregulated (Fig. 4A, B, C). In fruits with the same treatment, the expression of SlCYP707A1/2 was downregulated, while the expression of SlCYP707A3 was upregulated (Fig.4D, E, F). Among the ABA-signalling genes, including those of the PYR/PYL/RCAR protein family (SlPYL1), type 2C protein phosphatases (SlPP2C1), and subfamily 2 of SNF1-related kinases (SlSnRK2.2) (Ma et al., 2009; Park et al., 2009), SlPYL1and SlSnRK2.2 were downregulated while SlPP2C1 was upregulated (Fig. 4G, H, I). As shown in Fig. 5A, in SlNCED1-RNAi-treated fruits, the ABA content was 21% of the control at the turning stage (7 d after MG); moreover, fruits couldn’t become fully red and this incomplete colouring couldn’t be rescued by the application of exogenous ABA (although ABA contents were increased by application of exogenous ABA) (Fig. 5A). Expression of SlPYL1 and SlSnRK2.2 in SlNCED1-RNAi-treated fruits couldn’t be increased by application of exogenous ABA (Fig. 5B).
Fig. 4.
Expression of ABA-responsive genes in both control and SlNCED1-RNAi-treated fruit during development and ripening of tomato. The JiaBao fruits were injected with SlNCED1-RNAi TRV vectors at THE MG stage. Fruits were sampled 0 d (MG), 5 d (B), 7 d (T), 9 d (HR), and 12 days (OR) after the inoculation, respectively. SAND mRNA was used as the internal control. Three biological replicates (n = 3) were used for each analysis. *P value t-test < 0.05; **P value t-test < 0.001. Error bars are SE.
Fig. 5.
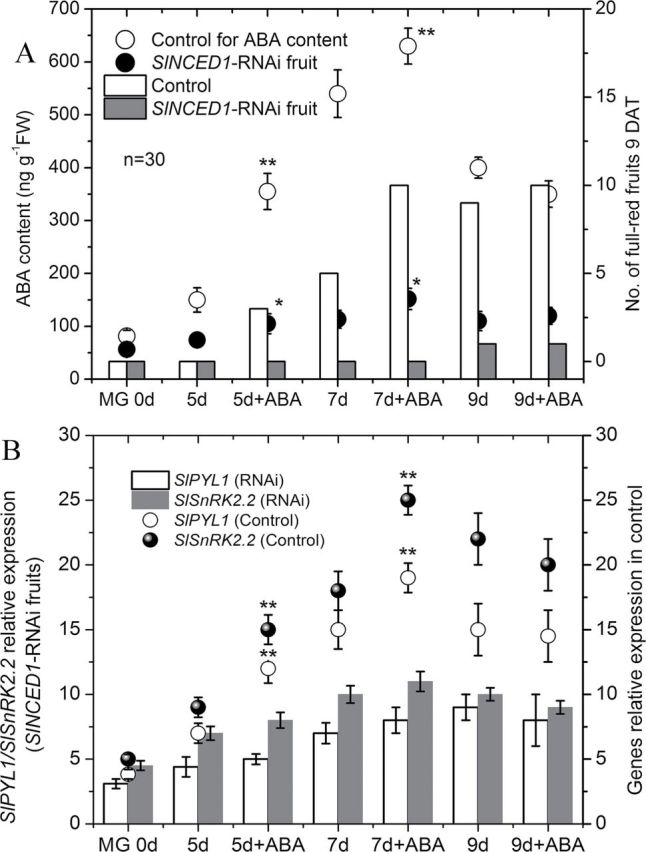
Changes in ABA content, SlPYL1 and SlSnRK2.2 expression, and numbers of fully red fruit 9 d after exogenous ABA treatment in both control and SlNCED1-RNAi-treated fruit. 78 JiaBao fruits were equally divided into two groups at the MG stage and then injected with 1ml ABA (100mM) for group 1, or distilled water for group 2 (control). 30 fruits were used for the investigation of number of fully red fruits and others were sampled at 0, 5, 7, and 9 d after ABA treatment, respectively, for the determination of ABA content and gene expression. SAND mRNA was used as the internal control. Three biological replicates (n = 3) were used for each analysis. *P value t-test < 0.05; **P value t-test < 0.001. Error bars are SE.
SlNCED1-RNAi treatment alters the expression of genes involved in ripening-related genes
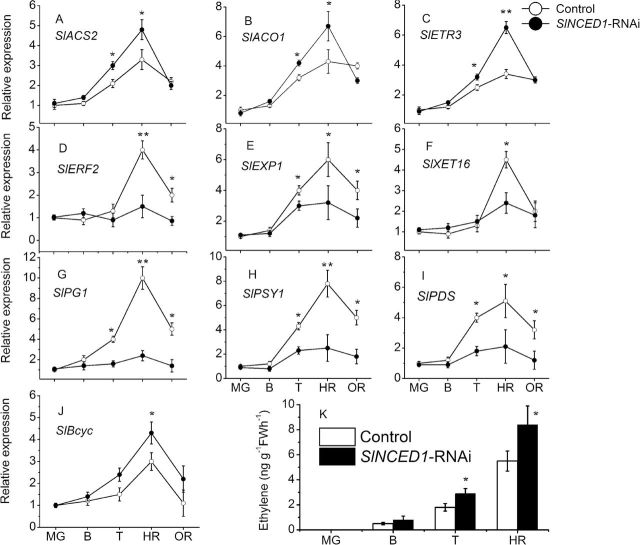
Several ripening-related physiological parameters were measured, including fruit firmness, solid soluble content, and lycopene content. As shown in Fig. 6, in SlNCED1-RNAi fruits, the trends were for a decrease in solid soluble content and lycopene content, but an increase in fruit firmness. To clarify the role of SlNCED1 in the regulation of tomato fruit ripening, several ripening-related genes were examined in both SlNCED1-RNAi-treated fruits and control fruits. SlBcyc, SlPSY1 and SlPDS encode lycopene β-cyclase, phytoene synthetase, and phytoene dehydrogenase, respectively. Relative quantitative real-time PCR analysis showed that the expression of all these genes was downregulated (Fig. 7H, I) except for SlBcyc, which was upregulated (Fig. 7J). In addition, we examined the expression of genes encoding cell-wall hydrolases. In SlNCED1-RNAi fruit, genes encoding polygalacturonase (SlPG1), expansin (SlEXP1), and xyloglucan endotransglycosylase (SlXET16) were all significantly downregulated during fruit ripening compared to the control (Fig. 7E, F, G). Relative expression analysis showed that the expressions of SlACS2 [encoding 1-aminocyclopropane-1-carboxylic acid (ACC) synthase], SlACO1 (encoding ACC oxidase), and SlETR3 (involved in the ethylene response), were upregulated in the SlNCED1-RNAi fruits (Fig. 7A, B, C). The expression of SlERF2 was significantly downregulated in SlNCED1-RNAi fruits (Fig. 7D). Ethylene release was upregulated at turning stage, but downregulated at harvest red stage compared to the control (Fig. 7K).
Fig. 6.
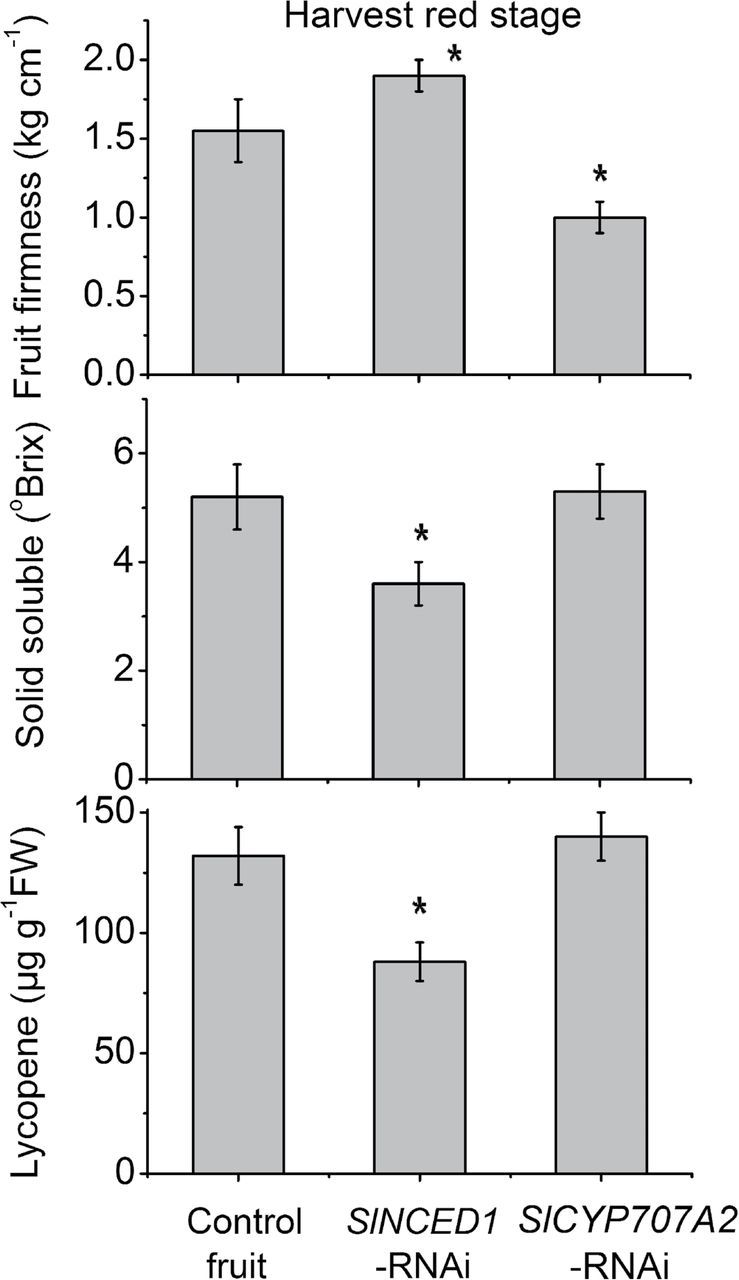
Changes in fruit firmness, solid soluble, and lycopene content in control, SlNCED1-RNAi-treated, and SlCYP707A2-RNAi-treated fruits. Every nine JiaBao fruits from control, SlNCED1-RNAi-treated, and SlCYP707A2-RNAi-treated fruits, respectively, were harvested at the harvest red stage. Three biological replicates (n = 3) were used for each analysis. *P value t-test < 0.05; **P value t-test < 0.001. Error bars are SE.
Fig. 7.
Changes in ethylene release and relative expression of genes involved for ethylene, cell wall catabolism, and lycopene synthesis in both control and SlNCED1-RNAi-treated fruits during development of tomato. The JiaBao fruits were injected with SlNCED1-RNAi TRV vectors at the MG stage. Fruits were sampled 0d (MG), 5d (B), 7d (T), 9d (HR), and 12 d (OR) after the inoculation, respectively. SAND mRNA was used as the internal control. Three biological replicates (n = 3) were used for each analysis. *P value t-test < 0.05; **P-value t-test < 0.001. Error bars are SE.
Silencing of the SlCYP707A2 gene promotes tomato fruit colouring
To clarify the role of SlCYP707A2 in the regulation of ABA levels during fruit ripening, ABA levels and ABA-responsive genes were examined in both RNAi-treated and control fruits. When the SlCYP707A2 -RNAi TRV vector was injected into the 15 fruits attached to the plant at the MG stage, the fruits could become red quicker (Fig. 8F), and ripening was also faster than in the control (Fig. 8G). In SlCYP707A1/2/3-RNAi-treated Tom fruits (Fig. 8B, C, D), fruit ripening (Fig. 8B, C, D) was the same as with control fruit (Fig. 8A) at harvest stage. In SlCYP707A2-RNAi-treated fruits, the ABA content was higher than the control at 5 and 7 d after MG, and fruit ripening couldn’t be inhibited by the application of NDGA, which delayed the control fruit ripening (Fig. 9J).
Fig. 8.
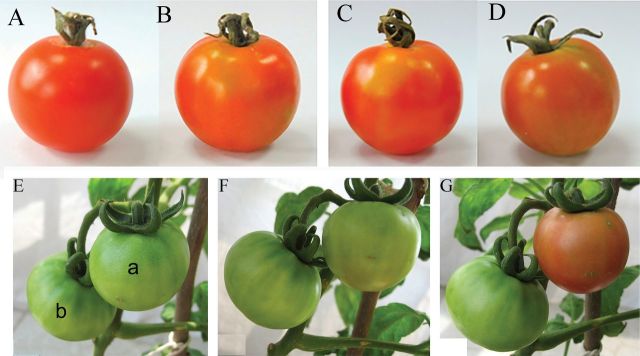
Phenotype of SlCYP707A1/2/3-RNAi-treated fruits. At the MG stage, 48 tomato fruits (cv. Tom) from 10 independent plants grown in the greenhouse were selected and divided into four groups (each group, 12 fruits for three repetitions; each repetition, four fruits) for the VIGS test. The SlCYP707A1-RNAi, SlCYP707A2-RNAi, and SlCYP707A3-RNAi TRV vectors were injected from the pedicel into group 1, group 2, and group 3, respectively. Group 4 was injected with TRV vector (no CYP707A gene) as a control. The fruits were evaluated 3–12 d after inoculation. Panels A–D are Tom tomato fruits. (A) Control; (B) SlCYP707A1-RNAi-treated fruit; (C) SlCYP707A3-RNAi-treated fruit; (D) SlCYP707A2-RNAi-treated fruit. Panels E to G are JiaBao tomato fruits. (E) 30 days after anthesis (before treatment; a, SlCYP707A2-RNAi fruit; b, control fruit); (F) 3 d after SlCYP707A2-RNAi treatment; G, 6 d after SlCYP707A2-RNAi treatment.
Fig. 9.
Changes in the expression of genes related to ABA metabolism and signalling in both control and SlCYP707A2-RNAi-treated fruits during development, and the ABA contents in fruits under the application of NDGA. For panels A to I, JiaBao fruits were injected with SlCYP707A2-RNAi TRV vector at the MG stage. Fruits were sampled 0 d (MG), 5 d (B), 7 d (T), 9 d (HR), and 12 d (OR) after the inoculation, respectively. (J) Fruits on plants were divided into two groups and then injected with 1ml NDGA (200mM) or distilled water at the MG stage. The samples were collected at 0, 5, 7, and 9 d after treatment. Three biological replicates (n = 3) were used for each analysis. *P value t-test < 0.05; **P value t-test < 0.001. Error bars are SE.
SlCYP707A2-RNAi treatment alters the expression of genes involved in ABA-responsive genes
In the SlCYP707A2-RNAi-treated fruits, expression of SlCYP707A1 was downregulated, but SlCYP707A3 was upregulated; however, SlCYP707A2 was markedly downregulated to 18% of the control (Fig. 9D, E, F). The expression of SlNCED1/3 was upregulated, while SlNCED2 was downregulated in SlCYP707A2-RNAi fruits (Fig. 9A, B, C). The expression of SlPYL1 and SlSnRK2.2 in ABA signalling was upregulated, while SlPP2C1 expression was downregulated in SlCYP707A2-RNAi fruit (Fig. 9G, H, I).
SlCYP707A2-RNAi treatment alters the expression of genes involved in ripening-related genes
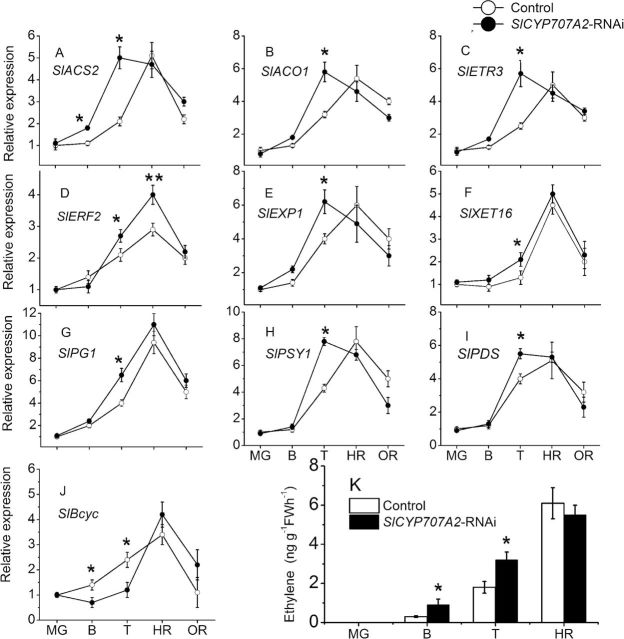
As shown in Fig. 6, fruit firmness was lower than that of the control in SlCYP707A2-RNAi fruits, while the soluble solid content and lycopene content did not show significant differences compared to the control fruits. In SlCYP707A2-RNAi fruits, genes encoding polygalacturonase (SlPG), expansin (SlEXP) and xyloglucan endotransglycosylase (SlXET16) were upregulated at breaker and turning stages; however, there were no significant changes compared to the control fruits at the harvest stage (Fig. 10E, F, G). In the lycopene synthesis pathway, compared with the control, the relative expression levels of SlPSY1 and SlPDS were higher, but SlBcyc was lower, at breaker and turning stages (Fig. 10H, I, J). With respect to ethylene, relative expression analysis showed that the expression of SlACS2 [encoding (ACC) synthase], SlACO1 (encoding ACC oxidase), and SlETR3 (involved in the ethylene response), which were consistent with ethylene release (Fig. 10K) in the SlCYP707A2-RNAi fruits, were upregulated at breaker and turning stages; however, there was no significant difference at harvest stage compared to control fruits (Fig. 10J). The expression of the SlERF2 was upregulated at the T and HR stages. SlCYP707A2-RNAi-treated fruits during the harvest stage were unusual, with uneven colouring in pulp compared to control fruits.
Fig. 10.
Changes in the relative expression of genes involved for ethylene, cell wall catabolism and lycopene synthesis, and ethylene release in both control and SlCYP707A2-RNAi-treated fruits. JiaBao fruits were injected with SlCYP707A2-RNAi TRV vectors at the MG stage. Fruits were sampled 0 d (MG), 5 d (B), 7 d (T), 9 d (HR), and 12 d (OR) after the inoculation, respectively. SAND mRNA was used as the internal control. Three biological replicates (n = 3) were used for each analysis. *P value t-test < 0.05; **P value t-test < 0.001. Error bars are SE.
Dehydration of SlNCED1/SlCYP707A2-RNAi-treated fruits
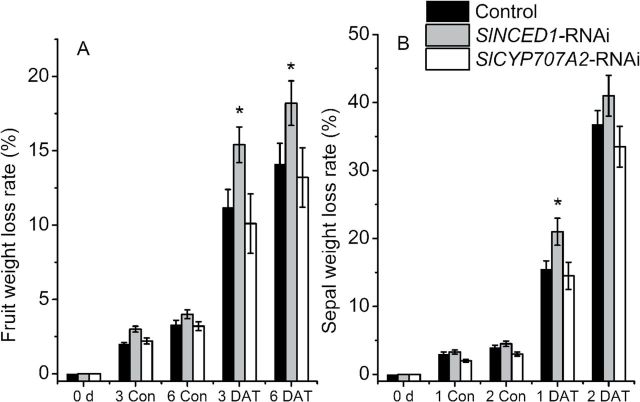
Both control and SlNCED1/SlCYP707A2-RNAi-treated fruits were harvested 6 d after the SlNCED1/SlCYP707A2-RNAi treatments (Fig. 11). The fruits were then incubated in the laboratory (20°C, 50% relative humidity). As shown in Fig. 11, the weight loss rate in SlNCED1-RNAi fruits was higher than the control fruit 3–6 d after dehydration. However, there was not a large difference in the rate of water loss comparing control and SlCYP707A2-RNAi fruit (Fig. 11A). The water loss of the sepal in the SlNCED1/SlCYP707A2-RNAi treated fruit was also similar to that of fruit under the same conditions (Fig. 11B).
Fig. 11.
Effects of dehydration stress on control, SlNCED1-RNAi-treated, and SlCYP707A2-RNAi-treated fruits. Fruits at the MG stage were placed into an incubator: control, 25oC and 90% relative humidity; dehydration, 25oC and 45% relative humidity. Fruits were sampled 0, 3, and 6 d after dehydration treatment (DADT), respectively. The sepal samples were collected at 1 and 2 DADT. Three biological replicates (n = 3) were used for each analysis. *P value t-test < 0.05; **P value t-test < 0.001. Error bars are SE.
Discussion
NCED and CYP707A are generally encoded by a small gene families, respectively (Krochko et al., 1998; Burbidge et al., 1999; Zhang et al., 2009a). Among the three NCED genes in tomato, SlNCED1 may play a primary role in regulating ABA biosynthesis during fruit ripening (Fig. 1A) in response to ABA application (Fig. 1E) and dehydration (Fig. 1C). Besides biosynthesis, catabolism of ABA is also an important way of regulating ABA levels (Kushiro et al., 2004; Li et al., 2011; Ren et al., 2011). A similar result to the reports of Ren et al. (2010) and Wang et al. (2013) was obtained in this work: the expression of SlCYP707A2 was higher than SlCYP707A1 and SlCYP707A3, and was opposite to the change of ABA content during development (Fig. 1B) in response to ABA treatment (Fig. 1F) and dehydration (Fig. 1D). Previously, to suppress SlNCED1 specifically in tomato fruits, we used an RNA interference construct driven by the fruit-specific E8 promoter. ABA accumulation and SlNCED1 transcript levels in transgenic fruit were downregulated to between 20 and 50% of the levels measured in control fruit. This significant reduction in NCED activity led to a downregulation in the transcription of genes encoding major cell wall-degrading enzymes, specifically polygalacturonase (SlPG), pectin methyl esterase (SlPME), and expansin (SlExp). This led to a significant extension of the shelf life to 15 to 29 d compared with a shelf life of only 7 d for the control fruit (Sun et al., 2012a). Fruits of RNAi lines displayed deep red colouration compared with the pink colour of control fruit. The decrease in endogenous ABA in these transgenic lines resulted in an increase in ethylene. These results indicate that, at least in part, ABA potentially regulated fruit ripening, and ethylene production and action, in climacteric tomato fruit (Sun et al., 2012b).
In the present study, we used VIGS to further verify the function of genes related to ABA metabolism in tomato fruit. The SlNCED1-RNAi-treated fruits showed various chimeric symptoms similar to those reported by Fu et al. (2006) and Hoffmann et al. (2006), and fruit ripening was inhibited such that fruit couldn’t become fully red or softened. Transcript levels of the SlNCED1 gene decreased after the inoculation (RNAi), concomitant with the decrease of ABA concentration, indicating that SlNCED1 directly affects ABA biosynthesis in tomato fruit. In addition to the SlNCED1 gene (downregulated by 81%), most of the ABA-responsive genes were downregulated in SlNCED1-RNAi fruits (Fig. 4). Moreover, exogenous ABA could not rescue the uncoloured phenotype of these SlNCED1-RNAi-treated fruits (Fig. 5A). The ABA content was significantly upregulated in SlNCED1-RNAi fruit treated by exogenously applied ABA at 5 and 7 DAT (Fig. 5A). As high as the ABA content is in RNAi fruit, they still cannot complete full reddening, suggesting that may be attributed to the repressed effect on the numbers of ABA signal molecules when the SlNCED1 is repressed by RNAi (Fig. 4). Real-time PCR analysis of the mRNA expression levels of PYL1 and SnRK2.2 showed that transcripts of these genes were significantly downregulated while PP2C1 was upregulated in RNAi fruits compared with control fruits (Fig. 4). A recent report shows that in the presence of ABA, the PYR1 receptor proteins can disrupt the interaction between the SnRK2s and PP2Cs, thus preventing the PP2C-mediated dephosphorylation of SnRK2s. This results in the activation of the SnRK2 kinases, turning on downstream factors of ABA signals, such as AREB/ABF, and finally leads to ABA-responsive physiological events (Fujii et al., 2009). In this study, PYL1 and SnRK2.2 genes were downregulated in RNAi fruit (Fig. 4) and could not be rescued by exogenous ABA treatment (Fig. 5B), which may lead to the suppression of ABA responses and, in turn, destroy ABA-responsive physiological events, such as fruit reddening and ripening.
In contrast, CYP707A, encoding 8′-hydroxylase, can alter the dynamic balance of ABA level (Saito et al., 2004; Ren et al.,2011). The role of three CYP707A genes (SlCYP707A1-3) was verified by VIGS, and the ABA level in fruit was mainly regulated by SlCYP707A2 at the transcriptional level (Fig. 1B). SlCYP707A2-RNAi caused an increase of ABA content at breaker and turning stages, and accelerated fruit colouring and softening compared to control fruits (Figs 8 and 9). This result is similar to the overexpression of NCED genes, which could cause the increase of ABA level (Qin and Zeevaart 2002; Wan and Li, 2006; Zhang et al. 2008; Hwang et al., 2010). Thus, SlCYP707A2 is a key gene in the ABA catabolic pathway in tomato fruit. SlCYP707A1/2/3 can be upregulated by the application of exogenous ABA, suggesting that ABA might regulate its own accumulation in fruits (Sun et al., 2012a; Wang et al., 2013). Our results also suggest that appropriate ABA contents are required for fruit ripening; too much or too little could lead to abnormal ripening.
Water deficit in fruit is a direct response caused by osmotic stress, including drought and salinity. In this study, SlNCED1-RNAi treatment could decrease the tolerance of fruit under dehydration stress, concurrent with the decrease of ABA accumulation and increased water loss (Fig. 11). Interestingly, the effect of silencing SlCYP707A2 in tomato fruits was different from previous findings in Arabidopsis. Loss of function of CYP707A was reported to enhance drought tolerance in Arabidopsis (Umezawa et al., 2006). In contrast, SlCYP707A2-silenced tomato fruits did not show any improvements in terms of rate of weight loss of silenced fruits compared to control fruits under water stress, and this result was similar in wheat (Manmathan et al., 2013). The differences in response to dehydration stress may be due to differences in stress adaptation pathways in tomato and Arabidopsis. Another possible reason for the observed difference may have to do with the structure of the CYP707A gene family in different species (Kushiro et al., 2004; Manmathan et al., 2013). In tomato fruits, the expression of SlERF2, encoding an ERF (ethylene response factor) protein and belonging to the ERF gene family, increases during fruit ripening (Fig. 7D). Individual members of the ERF family have been shown to be either positive or negative regulators of transcription (Fujimoto et al., 2000; Ohta et al., 2000, 2001). In SiNCED1-RNAi-treated fruits, SlERF2 expression was markedly downregulated: therefore, a decrease of ethylene release at the HR stage may be influenced by SlERF2 transcript (Fig. 7D,K).
In conclusion, six major genes including three SlNCEDs and three SlCYP707As involved in ABA biosynthesis and catabolism were identified by VIGS. VIGS-treated tomato fruits had significant reductions in target gene transcripts. The SlNCED1-RNAi-treated fruits did not undergo normal ripening compared to control fruits. In comparison, the SlCYP707A2-RNAi treatment could promote ripening; for example, colouring was quicker than the control. Silencing SlNCED1 or SlCYP707A2 by VIGS significantly altered the transcripts of a set of both ABA-responsive and ripening-related genes during ripening. These data indicate that SlNCED1 and SlCYP707A2 are key genes in the regulation of ABA synthesis and catabolism, and are involved in fruit ripening as a positive and a negative regulator, respectively.
Supplementary material
Supplementary data can be found at JXB online.
Supplementary Table S1. Specific primer sequences used for real-time quantitative PCR.
Funding
This work was partly supported by the 973 Programme 2012CB113900 to Yang-Dong Guo.
Supplementary Material
References
- Burbidge A, Grieve TM, Jackson A, Thompson A, McCarty DR, Taylor IB. 1999. Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maize Vp14. The Plant Journal 17, 427–431. [DOI] [PubMed] [Google Scholar]
- Chernys JT, Zeevaart JAD. 2000. Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abcisic acid biosynthesis in avocado. Plant Physiology 124, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler AJ, Krochko JE. 1999. Formation and breakdown of ABA. Trends in Plant Science 4, 472–478. [DOI] [PubMed] [Google Scholar]
- Fu DQ, Zhu BZ, Zhu HL, Zhang HX, Xie YH, Jiang WB, Zhao XD, Luo KB. 2006. Enhancement of virus-induced gene silencing in tomato by low temperature and low humidity. Molecules and Cells 21, 153–160. [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK. 2009. In vitro reconstitution of an abscisic acid signaling pathway. Nature 462, 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. 2000. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. The Plant Cell 12, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giribaldi M, Gény L, Delrot S, Schubert A. 2010. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. Journal of Experimental Botany 61, 2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann T, Kalinowski G, Schwab W. 2006. RNAi-induced silencing of gene expression in strawberry fruit (Fragaria × ananassa) by agroinfiltration: a rapid assay for gene function analysis. The Plant Journal 48, 818–826. [DOI] [PubMed] [Google Scholar]
- Hwang SG, Chen HC, Huang WY, Chu YC, Shii CT, Cheng WH. 2010. Ectopic expression of rice OsNCED3 in Arabidopsis increases ABA level and alters leaf morphology. Plant Science 178, 12–22. [Google Scholar]
- Krochko JE, Abrams GD, Loewen MK, Abrams SR, Cutler AJ. 1998. (+)-Abscisic acid 8′-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiology 118, 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E. 2004. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. The EMBO Journal 23, 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li P, Sun L, Wang YP, Ji K, Sun YF, Dai SJ, Chen P, Duan CR, Leng P. 2011. Expression analysis of β-glucosidase genes that regulate abscisic acid homeostasis during watermelon (Citrullus lanatus) development and under stress conditions. Journal of Plant Physiology 169, 78–85. [DOI] [PubMed] [Google Scholar]
- Li Q, Ji K, Sun YF, Luo H, Wang HQ, Leng P. 2013. The role of FaBG3 in fruit ripening and B. cinerea fungal infection of strawberry. The Plant Journal 76, 24–35. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. 2002. Virus-induced gene silencing in tomato. The Plant Journal 31, 777–786. [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. 2009. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324, 1064–1068. [DOI] [PubMed] [Google Scholar]
- Manmathan H, Shaner D, Snelling J, Tisserat N, Lapitan N. 2013. Virus-induced gene silencing of Arabidopsis thaliana gene homologues in wheat indentifies genes conferring improved drought tolerance. Journal of Experimental Botany 64, 1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher K, Ng LM, Zhou XE, et al. 2009. A gate-latch-lock mechanism for hormone signaling by abscisic acid receptors. Nature 462, 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Hitomi K, Arvai AS, Rambo RP, Hitomi C, Cutler SR, Schroeder JI, Getzoff ED. 2009. Structural mechanism of abscisic acid binging and signaling by dimeric PYR1. Science 326, 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Matsui K, Hiratsu K, Shinshi H, and Ohme-Takagi M. 2001. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. The Plant Cell 13, 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Ohme-Takagi M, Shinshi H. 2000. Three ethyleneresponsive transcription factors in tobacco with distinct transactivation functions. The Plant Journal 22, 29–38. [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, et al. 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324, 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XQ, Zeevaart JAD. 2002. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiology 128, 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Chen P, Dai SJ, Li P, Li Q, Ji K, Wang YP, Leng P. 2011. Role of abscisic acid and ethylene in sweet cherry fruit maturation: molecular aspects. New Zealand Journal of Crop and Horticultural Science 39, 1–14. [Google Scholar]
- Ren J, Sun L, Wu JF, Zhao SL, Wang CL, Wang YP, Ji K, Leng P. 2010. Cloning and expression analysis of cDNAs for ABA 8′-hydroxylase during sweet cherry fruit maturation and under stress conditions. Journal of Plant Physiology 167, 1486–1493. [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Alquezar B, Zacarias L. 2006. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). Journal of Experimental Botany 57, 633–643. [DOI] [PubMed] [Google Scholar]
- Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M. 2004. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiology 134, 1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Rodrigues A, Saez A, Rubio S, Antoni R, Dupeux F, Park SY, Marquez JA, Cutler SR, Rodriguez PL. 2009. Modulation of drought resistance by the abscisic acid receptor PYL5 throught inhibition of clade A PP2Cs. The Plant Journal 60, 575–588. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Aoki M, Nakaminami K, et al. 2008. Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiology 146, 1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Qin XQ, Zeevaart JAD. 2003. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiology 131, 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setha S, Kondo S, Hirai N, Ohigashi H. 2005. Quantification of ABA and its metabolites in sweet cherries using deuterium-labeled internal standards. Plant Growth Regulation 45, 183–188. [Google Scholar]
- Shang Y, Yan L, Liu ZQ, et al. 2010. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. The Plant Cell 22, 1909–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Sun Y, Zhang M, et al. 2012a. Suppression of 9-cis-epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomato. Plant Physiology 158, 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang YP, Chen P, Ren J, Ji K, Li Q, Li P, Dai SJ, Leng P. 2011. Transcriptional regulation of SlPYL, SlPP2C, and SlSnRK2 gene families encoding ABA signal core components during tomato fruit development and drought stress. Journal of Experimental Botany 62, 5659–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yuan B, Zhang M, Wang L, Cui MM, Wang Q, Leng P. 2012b. Fruit-specific RNAi-mediated suppression of SlNCED1 increases both lycopene and β-carotene contents in tomato fruit. Journal of Experimental Botany 63, 3097–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor IB, Sonneveld T, Bugg TDH, Thompson AJ. 2005. Regulation and manipulation of the biosynthesis of abscisic acid, including the supply of xanthophyll precursors. Journal of Plant Growth Regulation 24, 253–273. [Google Scholar]
- Umezawa T, Okamoto M, Kushiro T, Nambara E, Oono Y, Seki M, Kobayashi M, Koshiba T, Kamiya Y, Shinozaki K. 2006. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana . The Plant Journal 46, 171–182. [DOI] [PubMed] [Google Scholar]
- Wan CY, Wilkins TA. 1994. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Analytical Biochemistry 223, –7–12. [DOI] [PubMed] [Google Scholar]
- Wan XR, Li L. 2006. Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cis-epoxycarotenoid dioxygenase gene. Biochemical and Biophysical Research Communications 347, 1030–1038. [DOI] [PubMed] [Google Scholar]
- Wang YP, Wang Y, Ji K, et al. 2013. The role of abscisic acid in regulating cucumber fruit development and ripening and its transcriptional regulation. Plant Physiology and Biochemistry 64, 70–79. [DOI] [PubMed] [Google Scholar]
- Zhang M, Leng P, Zhang GL, Li XX. 2009a. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. Journal of Plant Physiology 166, 1241–1252. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan B, Leng P. 2009b. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. Journal of Experimental Botany 60, 1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Yang JF, Lu SY, Cai JL, Guo ZF. 2008. Overexpressing SgNCED1 in tobacco increases ABA level, antioxidant enzyme activities, and stress tolerance. Journal of Plant Growth Regulation 27, 151–158. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.