Abstract
Brain inflammation is a primary pathological driving force of many neurodegenerative disorders. In the destructive process, pro-inflammatory cytokines (IL-1β and TNF-α), are robustly released, affecting normal neural progenitor cell (NPC) differentiation, and resulting in a vast number of astrocytes and a diminished neural population. A counteractive mechanism is still unknown. In this study, we have identified a link between brain inflammation and the signal transducer and activator of transcription 3 (STAT3) pathway: IL-1β and TNF-α induce STAT3 activation in NPCs. Then to investigate STAT3’s effects on NPC fate, we observed that an inhibition of STAT3 expression by siRNA inhibited astrocytic differentiation and increased neuronal differentiation of human NPCs in fetal bovine serum (FBS)-induced astrocyte differentiation condition. Furthermore, STAT3-targeting siRNA abrogated IL-1β and TNF-α-induced astrocyte differentiation and partially restored neuronal differentiation. Elimination of STAT3 expression also countered IL-1β and TNF-α-induced inhibition of proneural bHLH genes, mammalian achaete-schute homologue-1 (Mash1), Neurogenin1 (Ngn1), and Neurogenin 2 (Ngn2). These data suggest that a suppression of STAT3 during brain inflammation would inhibit astrogliogenesis and promote neurogenesis. Thus, STAT3 could be a potential target of drug therapy for neurodegenerative disorders.
Keywords: cytokine, differentiation, inflammation, neural progenitor cell, neurogenesis, STAT3
Introduction
In the adult brain, active neurogenesis occurs constitutively in two defined regions: the subgranular zone of the hippocampus and the subventricular zone (SVZ) lining the lateral ventricles (1, 2). Neural stem/progenitor cells (NSPCs) within these neurogenic regions can self-renew, proliferate, and differentiate into neurons or glia, that allows them to generate a reservoir of neurons, astrocytes, and oligodendrocytes as back-up in times of normal cell turnover or brain injury (3, 4).
Recent findings have revealed that uncontrolled, chronic brain inflammation is an underlying driving force to intensifying the rapid deterioration of the central nervous system in neurodegenerative diseases such as Alzheimer’s disease (AD), HIV-1 Associated dementia (HAD), multiple sclerosis, and Parkinson’s disease (PD) (5). Following brain injury or exposure to pathogens, an inflammatory response is driven by the activation of resident microglia, astrocytes, and local invasion of circulating immune cells (4, 6). Activated microglia, usually dormant, contribute to neuronal loss by enhancing oxidative stress and mediating cell apoptosis pathways (7). Contrary to a normally functioning CNS where neurons and astrocytes share a symbiotic relationship, chronic inflammation transforms astrocytes from a basal to a reactive state, forcing the neuron “helper” cells to abandon their supportive roles, and eventually damage neurons (8, 9). Brain inflammation also contributes to neural stem cell dysfunction and plays a detrimental role for neurogenesis in adult brain (10-13). In the destructive process, pro-inflammatory cytokines (IL-1β and TNF-α) are robustly released, affecting normal neural progenitor cell (NPC) differentiation, and resulting in a vast number of astrocytes and a diminished neural population (14).
Signal transducer and activator of transcription (STAT) 3, a member of the STAT family, is a transcription factor that primarily mediates cytokine- and growth factor-induced signals that culminate in diverse biological responses including cell proliferation, differentiation, and apoptosis (15). In addition, the STAT3 signaling pathway plays a critical role in the determination of NPC fate, especially in promoting astrogliogenesis. Mouse knockout studies have demonstrated that genetic deficiency in major components of this pathway leads to impaired astroglial differentiation (16-18). The STAT3 pathway has also been shown to contribute to inflammation-mediated astrogliogenesis (12, 19, 20). Ramified microglia and activated microglia promote astrogliogenesis through the activation of STAT3 (12, 19).
In this study, we used a human embryonic cortical neural progenitor cell (NPC) culture. NPCs were isolated and expanded as neurospheres in the presence of bFGF, EGF and LIF. These cells continue self-renewal and once deprived from GF, they give rise to neurons and astrocytes. This in vitro system allows the further analysis of molecular mechanisms during differentiation under defined conditions. We first investigated the potential role of STAT3 on human NPC differentiation under normal conditions. An inhibition of STAT3 expression with siRNA transfection consequently inhibits astrocytic differentiation and increases neuronal differentiation of human NPCs. Next, we treated human NPCs with two common pro-inflammatory cytokines, IL-1β and TNF-α, resembling a brain inflammation environment, and analyzed the STAT3 effects on cytokine-induced NPC differentiation. Finally, we examined the potential downstream effect of STAT3 by observing the mRNA expressions of proneural basic helix-loop-helix (bHLH) transcription factors mammalian achaete-schute homologue-1 (Mash1), Neurogenin1 (Ngn1), and Neurogenin2 (Ngn2), demonstrating that a deletion of STAT3 expression in NPCs promoted neurogenesis even with cytokine pressure. Harnessing the capability of STAT3 and delving into its underlying, novel implications in both brain inflammation and NPC fate regulation may be instrumental in suppressing the detrimental effects of brain inflammation and finding a potential target of drug therapy for neurodegenerative disorders.
Materials and Methods
Human Neural Progenitor Cell culture
Human cortical NPCs were isolated from human brain tissue (12-16 weeks post-conception). NPCs were seeded at a concentration of 200,000 cells/ml into substrate-free tissue culture flasks and grown as spheres in neurosphere initiation medium (NPIM), which included X-Vivo 15 (BioWhittaker, Walkersville, ME) with N2 supplement (GIBCO Invitrogen, Carlsbad, CA), neural cell survival factor-1 (NSF-1, BioWhittaker), basic fibroblast growth factor (bFGF, 20 ng/mL, Sigma-Aldrich, St. Louis, MO), epidermal growth factor (EGF, 20 ng/mL, Sigma-Aldrich), leukemia inhibitory factor (LIF, 10 ng/mL, Chemicon, Temecula, CA), and 60 ng/mL N-acetylcysteine (Sigma-Aldrich). Cells were passaged every two weeks to ensure greatest medium availability with 15 minutes Trypsin, which dissociated cell clusters, along with gentle mechanical dissociation (21).
NPC Differentiation
Single-cell suspension NPCs were cultured in poly-D-lysine-coated coverslips or 6-well plates (Sigma-Aldrich) in NPIM for 24 hours and then induced to differentiate into neurons or astrocytes by serum-free neurobasal medium (GIBCO) supplemented with B27 (NB27; GIBCO) or by Dulbecco’s modified Eagle’s medium (DMEM)/F12 (GIBCO) with 1% FBS, respectively. For siRNA transfection, pre-designed siRNA duplexes targeted against STAT3 mRNA (siSTAT3) were synthesized by Ambion Inc. (Austin, Texas). NPCs were transfected with 100 nM nonspecific control siRNA (sicon) or siSTAT3 in the presence of siImporter (Upstate Cell Signaling Solutions, Charlottesville, VA) according to the manufacturer’s instructions. Cells were grown in differentiation media for 6 days before analysis.
Immunocytochemical Staining
NPCs were fixed with ice-cold methanol/acetone (1:1) and then washed 3× with phosphate buffered saline (PBS). The cells were then blocked in 2% bovine serum albumin (BSA) with 0.1% Triton X-100 in PBS for 1 hour. Cells were then incubated overnight in 4° C with primary antibodies against Nestin (1:400; Chemicon), SRY (sex determining region Y)-box 2 (Sox2; 1:400; Abcam Cambridge, MA), glialfibrillary acidic protein (GFAP; 1:2000; Dako, Carpinteria, CA), and/or neuron-specific class III β-Tubulin (Tuj-1; 1:1500; Sigma-Aldrich). Cells were washed 3x with PBS and then incubated with fluorophore-labeled 2nd antibodies, Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 goat anti-rabbit (1:800; Molecular Probes, Eugene, OR) for 1 hour in room temperature. Hoechst 33342 (1:3000 with PBS; Sigma-Aldrich)was used to identify cell nuclei. The coverslips were then mounted onto a slide with Fluoromount (Sigma-Aldrich) to be examined under a Nikon Eclipse E800 microscope with a 20× objective. Ten to fifteen pictures per condition were captured randomly with the microscope digital imaging system, and then imported onto Image-ProPlus 4.0 (Media Cybernetics, Silver Spring, MD) for quantification. The numbers of neurons and astrocytes for each condition (total 300-500 cells per condition) were manually counted by a blinded investigator. Percentage of Tuj-1/ GFAP-positive cells was calculated based on the total count of cell nuclei, marked by Hoechst (blue). Percentages were then normalized as fold of control.
RNA extraction and real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA from NPC cultures was extracted using TRIzol reagent during the lysing process followed by the RNeasy Mini Kit Assay (QIAGEN, Valencia, CA). RNA concentration was determined by the NanoDrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE). Real-time RT-PCR was conducted with Assays-on-Demand primers, using the one-step quantitative TaqMan real-time RT-PCR system (Applied Biosystems Inc.; Foster City, CA) following manufacturing protocol. STAT3, Mash1, Ngn1, and Ngn2 mRNA levels were determined and standardized by comparison to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Applied Biosystems Inc.).
Western Blot
Cells were lysed with Protein Extraction Buffer (Pierce, Rockford, IL) containing 1× protease inhibitor cocktail (50:1 dilution; Roche Diagnostics, Indianapolis, IN). Protein concentration was determined using the Bicinchoninic Acid (BCA) Protein Assay Kit (Pierce). Protein samples (10-20 ng) were then separated by a 10% Sodium Dodecyl Sulfate (SDS) - polyacrylamide gel electrophoresis (PAGE) and transferred onto an Immuno-Blot polyvinylidene fluoride polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Hercules, CA). The membrane was subsequently blocked with a Tris-Buffered Saline Tween 20 (TBST) and 5% nonfat milk solution for 1 hour. Next, primary antibodies (diluted in 5% BSA and TBST) against Phosphorylated-STAT3 (P-STAT3), Total-STAT3 (T-STAT3), and b-actin, were added and incubated overnight at 4° C, followed by a 1 hr incubation with horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies diluted with 5% milk buffer (1:10,000; Cell Signaling Technologies). The membrane was developed using Enhanced Chemiluminescent (ECL) solution (Pierce). The film was then scanned with a CanonScan 9950F scanner, and the band intensities were quantified with the ImageJ computer program.
Statistical analyses
Data were expressed as means ± S.D. The data were evaluated statistically by analysis of One-way variance (ANOVA) followed by the Tukey-test for paired observations or an unpaired, two-tail T-test when comparing only two sets of data. Significance was considered to be either p ≤ 0.01 (**/##) or p ≤ 0.05 (*/#). To account for any donor-specific differences, all experiments were performed with human NPCs from at least three donors. All assays were performed at least three with triplicate samples in each assay.
Results
Suppression of STAT3 in human NPCs inhibits astrogliogenesis and enhances neurogenesis
To establish the specific function of STAT3 signaling in differentiating human NPCs, we used our well-established human cortical NPC neurosphere culture system (21). In undifferentiated conditions (in the presence of only bFGF, EGF, and LIF), neurosphere-dissociated cells maintained stem cell properties, as evidenced by the strong immunoreactivity to Nestin and Sox2. Under these conditions, over 80% of NPCs were immunopositive for the cytoskeletric protein, Nestin, a marker for multi-potent precursors (Figure 1A, C), and the neural stem cell-specific transcription factor, Sox2 (Figure 1A, D). This suggests that the majority of the cells were in an undifferentiated state in NPIM culture condition. To analyze the effect of STAT3 on NPC differentiation, cells were differentiated into a neuronal-enriched population by changing to NB27 medium. Alternatively, they were differentiated in 1% FBS to induce more astrocyte differentiation.
Figure 1. Characterization of human cortical NPCs.
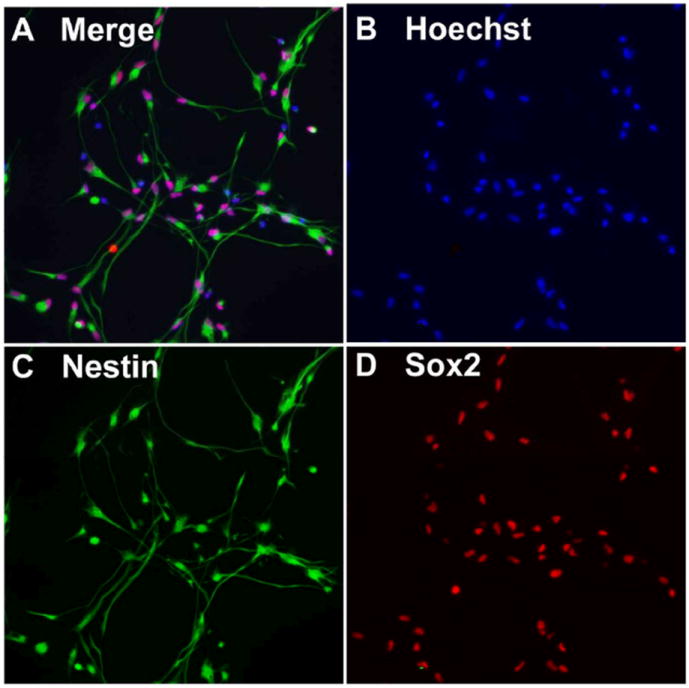
Neurospheres were dissociated to single cell suspension and plated in poly-D-lysine-coated cover slips for 24 h. Cells were fixed and stained for Nestin (green, C) and Sox2 (red, D). Nuclei were stained using Hoechst 33342 (blue, B). Figure 1A shows a merged image of B, C, and D. The original magnification is × 20. Results are representative of three donors.
The Jak-STAT3 signaling is a critical part of the astrogliogenic machinery in mouse brain development (22, 23), but its roles in human NPC differentiation have been unclear. We initially examined whether human NPCs expressed STAT3 after siRNA transfection. We transfected human NPCs with a STAT3-targeting siRNA (siSTAT3) or a negative control siRNA (siCon), which has no targeting sequence. To examine the effectiveness of our transfection assay, we first observed the effect of siSTAT3 on STAT3 mRNA expression in NPCs by real-time RT-PCR. Seven days after transfection, quantitative results revealed that the mRNA level of STAT3 in NPCs transfected with siSTAT3 was, indeed, significantly decreased (by 60.67%), compared with that in NPC transfected with siCon (Figure 2A). The STAT3 protein expression in human NPCs was tested through utilizing Western blotting (Figure 2B). P-STAT3 was little expressed in control and siCon-treated NPCs, whereas siSTAT3 significantly decreased both the protein levels of T-STAT3 and P-STAT3 in NPCs (by 59.27% and 93.60%, respectively), compared with those in untransfected NPCs (control) (Figure 2C, D). These two experiments verified that transfection with siSTAT3 eliminated STAT3 expression and phosphorylation of STAT3 in NPC, to ensure the reliability of the assay in later experiments.
Figure 2. SiSTAT3 blocks STAT3 expression.
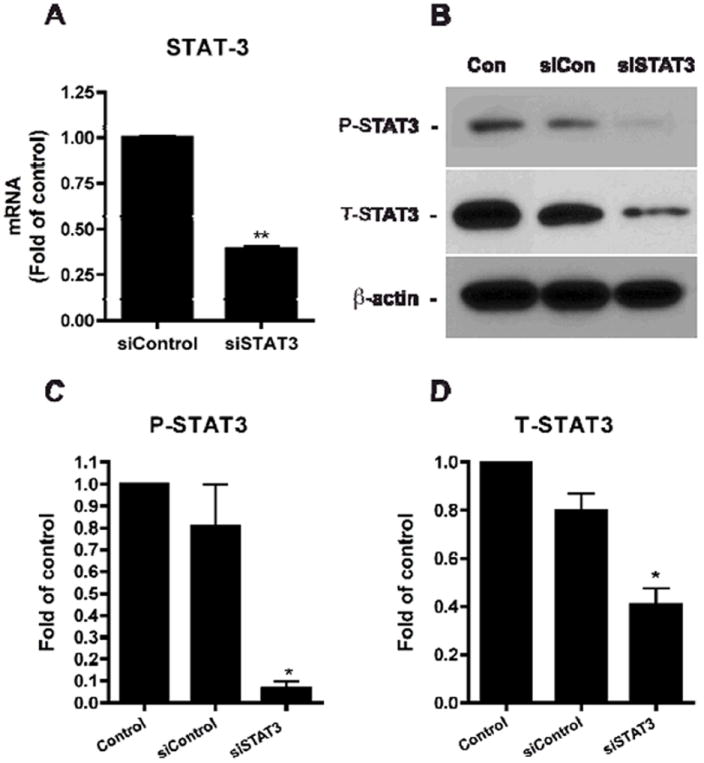
A. Human NPCs were transfected with siCon or siSTAT3. Six days later, total mRNA was extracted and real-time RT-PCR was conducted to examine the mRNA expression levels of STAT3. STAT3 expression was normalized to GAPDH as an internal gene expression control and data is presented as fold of siCon. Data were obtained from three independent donors. ** differs significantly compared to siCon (p < 0.01). B-D. Expressions of STAT3 were detected by Western blotting. The films were scanned and the acquired images were analyzed using the public domain NIH image program for data quantification. Expression of P-STAT3 (C) and T-STAT3 (D) were normalized to β-actin. Data is presented as fold of control (NB27). Results are an average of three independent donors. * differs significantly compared to control (p < 0.05).
We next investigated whether inhibition of STAT3 expression could influence human NPC differentiation. To address this issue, human NPCs were transfected with siCon or siSTAT3 for 24 hours and then were differentiated in NB27 with or without 1% FBS for an additional 6 days. Then we performed immunocytochemical staining with antibodies against Tuj-1 (a specific marker of neurons) and GFAP (a specific marker of astrocytes) (Figure 3A-F). In an NB27 culture condition, NPCs differentiated into 47.14%Tuj-1-positive cells and 33.16% GFAP-positive cells; with 1% FBS, NPCs differentiated into 17.26% Tuj-1-positive cells and 73.55% GFAP-positive cells. In both conditions, introduction of siSTAT3 into NPC culture showed a trend of increase of Tuj-1-positive cells and decrease of GFAP-positive cells as compared to control or siCon, although in NB27 neuronal differentiation condition, changes were negligible (Figure 3G, H). However, with 1% FBS medium, siSTAT3 induced a significant increase of neurons (3.08 fold of control, Figure 3I) and a significant decrease of astrocytes (0.68 fold of control, Figure 3J). Taken together, these data suggest that elimination of STAT3 in NPC by siSTAT3 inhibits astrogliogenesis and promotes neurogenesis of human NPCs.
Figure 3. Suppression of STAT3 reduces astrocytic and increases neuronal proportions.
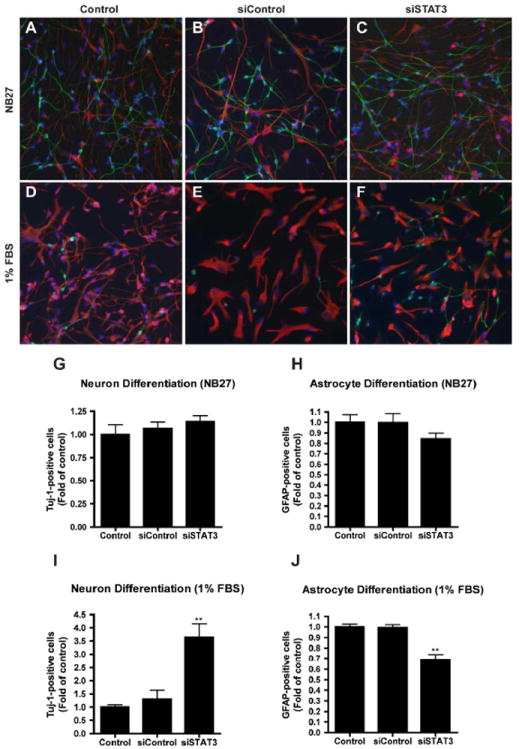
A-F. Human NPCs were transfected with either siCon (B, E) or siSTAT3 (C, F) and differentiated in NB27 (A-C) or 1% FBS (D-F). Six days later, neuron and astrocyte differentiation were evaluated by immunocytochemical staining with antibodies against Tuj-1 (green) and GFAP (red). Representative fluorescence overlay-micrographs display the morphology of neurons (green) and astrocytes (red). G-J. Percentage of Tuj-1/ GFAP-positive cells was calculated based on the total count of cell nuclei, marked by Hoechst (blue). Percentages were then normalized as fold of control (G-J). Data were collected from four independent measurements. */** differs significantly compared to Control and siControl (* p < 0.05; ** p < 0.01). Scale = 200 μm.
IL-1β and TNF-α induce STAT3 activation and astrogliogenesis
In order to detect if STAT3 is also involved in inflammation-induced astrogliogenesis, NPCs were treated with pro-inflammatory cytokines, IL-1β (1 ng/ml) and TNF-α (20 ng/ml) for 24 hours. In a parallel experiment, NPCs were differentiated in NB27 media with or without IL-1β and TNF-α for 6 days. Expressions of P-STAT3 and T-STAT3 were detected by Western blotting of total cell lysates. Our results demonstrated that IL-1β and TNF-α induce significant increases of both P-STAT3 and T-STAT3 expressions (Figure 4). This is correlated to the increase of astrocyte differentiation in IL-1β and TNF-α treatments from previous observations (14).
Figure 4. Pro-inflammatory cytokines activate STAT3.
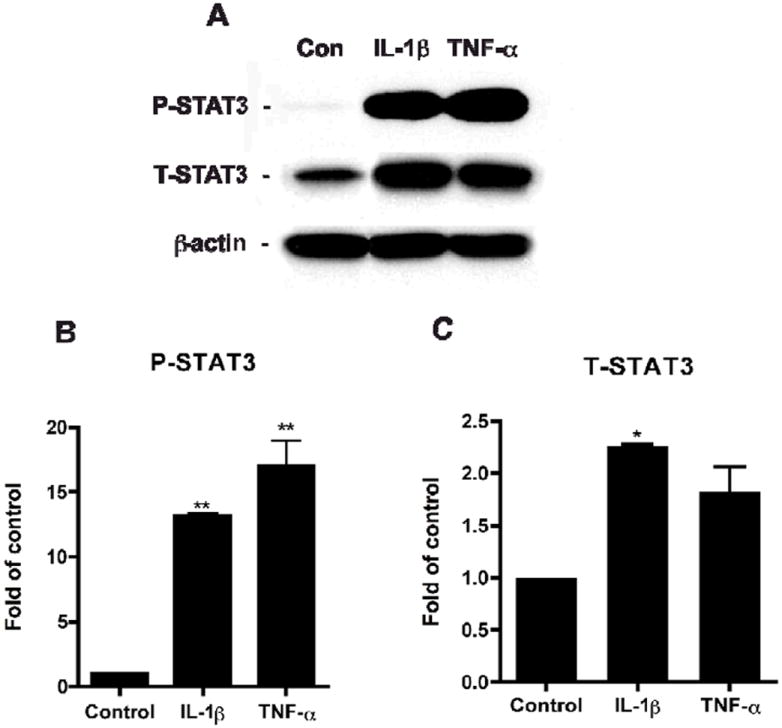
Human NPCs were treated with IL-1β or TNF-α. Cells were subsequently lysed, and proteins were collected 6 days after treatment for Western blotting (A). P-STAT3 (B) and T-STAT3 (C) expressions were detected, and the films with the acquired images were later scanned and analyzed for data quantification. Expression was normalized to β-actin as a loading control, and data is presented as fold of Control. Results were representative of three independent experiments. */** differs significantly compared to Control (* p < 0.05; ** p < 0.01).
IL-1β and TNF-α affect NPC differentiation through STAT3
To fully comprehend STAT3’s role during brain inflammation, we investigated cytokines’ effects on NPC differentiation after blocking STAT3 expression. NPCs were transfected with siCon or siSTAT3 and subsequently, treated with or without IL-1 β or TNF-α for 24 hours. Expressions of T-STAT3 and P-STAT3 were detected by Western blotting (Figure 5A). The results showed siSTAT3 decreased total STAT3 expression and inhibited both IL-1β- and TNF-α-induced STAT3 activation (Figure 5B, C). In parallel, NPCs were transfected with siCon and siSTAT3 and differentiated with or without IL-1β and TNF-α for 6 days. Immunocytochemical staining was performed to check if an inhibition of STAT3 could affect NPC differentiation with inflammatory pressure (Figure 6A-F). Our quantitative results revealed that, consistent with other well-established studies, IL-1β and TNF-α significantly inhibit neurogenesis (0.59 fold and 0.34 fold, respectively) and induce astrogliogenesis (1.51 fold and 1.89 fold, respectively) (Figure 6G, I). However, after STAT3 expression was eliminated, the cytokine effects were greatly diminished. The fold of Tuj-1-postive cells showed trends of upregulation (siCon + cytokine vs. siSTAT3 + cytokine) for both IL-1β and TNF-α treatment (0.59 ± 0.24 to 0.85 ±0.15; 0.34 ± 0.22 to 0.72 ± 0.11, respectively). The fold of GFAP-positive cells significantly decreased (1.51 ± 0.22 to 1.11 ± 0.15; 1.89 ± 0.40 to 1.34 ± 0.23, respectively). This result suggests that the cytokines, IL-1β and TNF-α, key components in brain inflammation, affect neurogenesis through the STAT3 pathway. Elimination of STAT3 in NPC by siSTAT3 inhibits cytokine-induced astrogliogenesis and promotes neurogenesis of human NPCs.
Figure 5. SiSTAT3 blocks STAT3 expression under cytokine treatment.
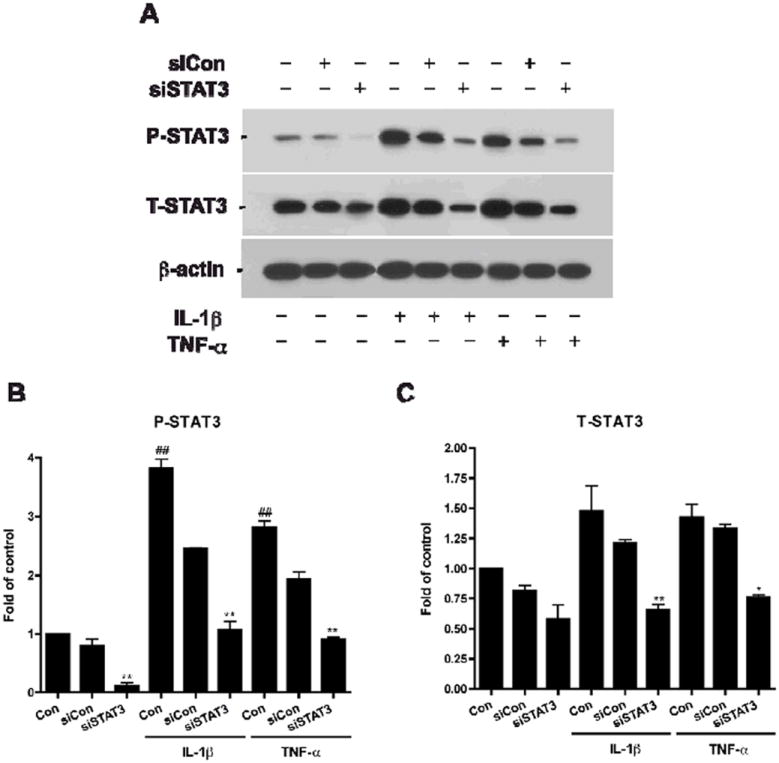
Human NPCs were transfected with siCon or siSTAT3 and then were treated with IL-1β or TNF-α for 24 h. Cells were subsequently lysed, and proteins were collected for Western blotting (A). P-STAT3 (B) and T-STAT3 (C) expressions were detected, and the films with the acquired images were later scanned and analyzed for data quantification. Expression was normalized to β-actin as a loading control, and data is presented as fold of control (NB27). Results were representative of two independent experiments. */** differs significantly compared to respective control (NB27 + IL-1β or NB27 + TNF-α, * p < 0.05; ** p < 0.01)), ## differs significantly compared to Control (## p < 0.01).
Figure 6. Suppression of STAT3 reduces cytokine-induced increase of astrocytes and decrease of neurons.
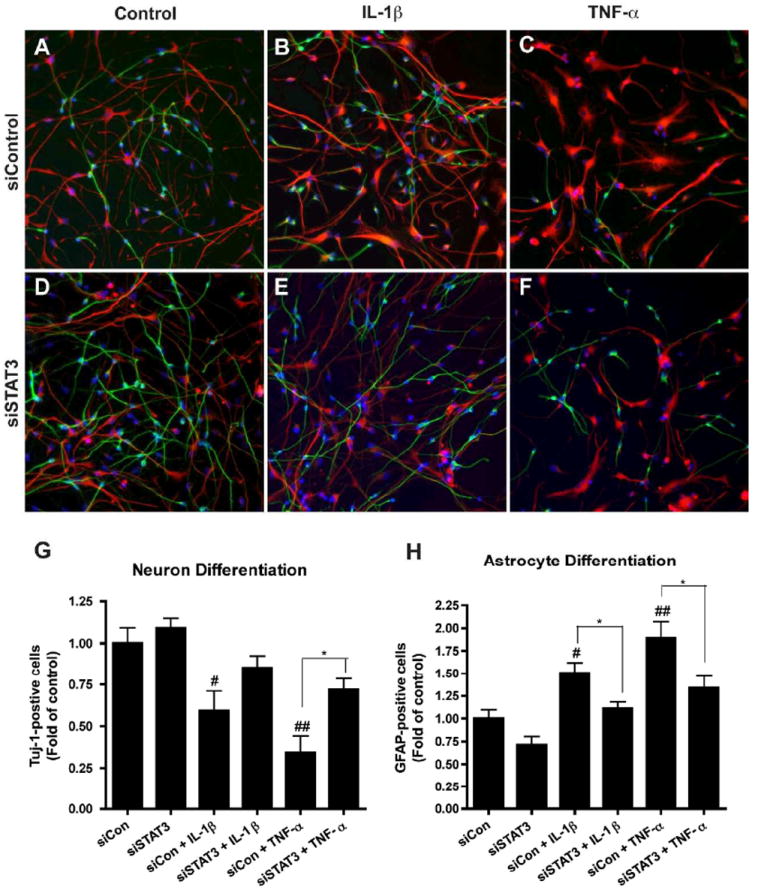
A-F. Human NPCs were transfected with siCon (A-C) or siSTAT3 (D-F) and were differentiated with or without IL-1β (B, E) or TNF-α (C, F) for 6 days. Neuron and astrocyte differentiation expressions were evaluated by immunocytochemical staining with antibodies against Tuj-1 (green) and GFAP (red). Representative fluorescence overlay-micrographs display the morphology of neurons (green) and astrocytes (red). G-H. Percentage of Tuj-1/GFAP-positive cells was calculated based on the total count of cell nuclei, marked by Hoechst (blue). Percentages were then normalized as fold of siCon. Data were collected from four independent measurements. */** differs significantly compared to respective siCon ((siCon + IL-1β or siCon + TNF-α, * p < 0.05; ** p < 0.01), and #/## differs significantly compared to siCon, # p < 0.05; ## p < 0.01). Scale = 200 μm.
STAT3 affects neurogenesis through the bHLH family transcription factors
It is well known that bHLH transcription factors play an important role in the development of the mammalian neocortex as well as the timing of NPC differentiation and proliferation (24, 25). Our earlier results suggested that eliminating STAT3 shows promise for a promotion of neurogenesis, so for an advancement of this study, we chose to focus on three proneural bHLH factors, Mash1, Ngn1, and Ngn2. NPCs were transfected with siCon or siSTAT3 and then treated with cytokines for 24 h. Total mRNA was extracted and quantitative real-time RT-PCR was conducted. Results showed mRNA levels of Mash1, Ngn1, and Ngn2 were upregulated with siSTAT3 transfection (Figure 7). IL-1β and TNF-α significantly decreased Mash1 (0.38 and 0.42 fold, respectively), Ngn1 (0.27 and 0.46 fold, respectively), and Ngn2 (0.34 fold, IL-1β only) compared to siCon. However, after suppression of STAT3, cytokine effects were consequently reduced, and mRNA levels of all three bHLH factors, reversely, significantly increased again as compared to siCon + IL-1β or TNF-α. Mash1 mRNA level after transfection exhibited significant increases with IL-1β treatment (by 0.31). Ngn1 mRNA levels after transfection significantly increased with both IL-1β and TNF-α treatments (0.27 ± 0.04 to 1.44 ± 0.05; 0.46 ± 0.05 to 0.89 ± 0.13, respectively), as did Ngn2 mRNA levels (0.34 ± 0.01 to 2.19 ± 0.36; 0.63 ± 0.02 to 1.11 ± 0.06, respectively). Based on these data, there is strong evidence that even under brain inflammation pressure, suppression of STAT3 may overcome cytokine stimulus and promote neurogenesis.
Figure 7. Suppression of STAT3 regulates cytokine-mediated bHLH factor expression.
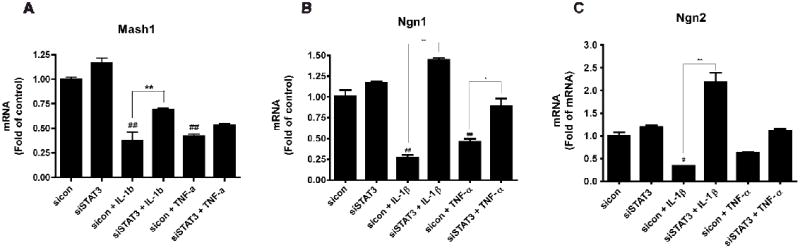
Human NPCs were transfected with siCon or siSTAT3 and were then treated with IL-1β or TNF-α for 24 h. Total mRNA was extracted and real-time RT-PCR were conducted to detect transcription factors (Mash1, Ngn 1, and Ngn2) expressions. A-C. Data were obtained from three independent measurements. Data were presented as fold of siCon. */** differs significantly compared to respective siCon (siCon + IL-1β or siCon + TNF-α, * p < 0.05; ** p < 0.01), and #/## differs significantly compared to siCon (# p < 0.05; ## p < 0.01).
Discussion
In this study, we used human NPCs to study the potential role of STAT3 on NPC differentiation. We demonstrated that STAT3 is critical for astrogliogenesis in both a neuronal differentiation condition (NB27) and an astrocytic differentiation condition (1% FBS) (Figure 3). Further, pro-inflammatory cytokines, IL-1β and TNF-α induce STAT3 activation and both increase astrogliogenesis and decrease neurogenesis of human NPCs (Figure 4). Cytokine-mediated astrogliogenesis is mediated through the STAT3 pathway, since the siRNA-mediated knockdown of STAT3 expression results in a reduction of cytokine-induced STAT3 activation and subsequent astrocytic differentiation (Figure 5-6). The STAT3 pathway also contributes to cytokine-mediated inhibition of neurogenesis through regulation of proneural bHLH factors (Ngn1, Ngn2, and Mash1) (Figure 7). Thus, we report a dual role of the STAT3 pathway in brain inflammation-mediated NPC differentiation.
The Jak-STAT3 signaling is a critical part of the astrogliogenic machinery during brain development (22, 26). Knockout of the major components of this pathway, including LIF, its receptors LIFRβ and gp130, or the signaling molecules STAT1/3, leads to impaired astroglial differentiation (16-18, 27). Activation of this pathway can lead to the association of P-STAT3 with the transcriptional coactivator CREB binding protein (CBP/p300) to activate expression of astrocyte-specific genes and promote astrocytic differentiation (18, 22, 28). In our study, we further demonstrated that STAT3 is critical for astrocytic differentiation in human NPCs. In neuronal differentiation condition, STAT3 is only slightly activated, while a majority of cells differentiate into neurons (Figure 2). In an astrocytic differentiation condition induced by FBS, introduction of siSTAT3 into NPC culture decreases astrocytic differentiation and increases neurogenesis. Furthermore, pro-inflammatory cytokines, IL-1β and TNF-α, induce STAT3 activation and increase astrocytic differentiation; siSTAT3 decreases cytokine-induced STAT3 activation (Figure 5) and astrocytic differentiation (Figure 6). Thus, the STAT3 pathway plays an important role in astrocytic differentiation of human NPCs under both normal and inflammation conditions.
Many cell fate-specifying transcription factors can both positively regulate one fate and negatively regulate alternative fates. Besides its effect on promoting astrogliogenesis by functioning as a transcriptional activator, the STAT3 pathway has been shown to inhibit neurogenesis through regulating other cell fate-determining transcription factors. Previous studies demonstrated that over-expression of a dominant negative form of STAT3 (STAT3F) results in promotion of neurogenesis and inhibition of astrogliogenesis (29). In that report, mRNA levels of proneural bHLH transcription factors, such as Math1, Ngn3 and NeuroD, were significantly increased, while Notch family members (Notch1, 2, and 3) and inhibitory bHLH transcriptional factors (Hes5, Id2, and Id3) for gliogenesis were significantly decreased after an overexpression of STAT3F. In another study, Cao et al. used the STAT3flox/flox mouse embryos and demonstrated that a conditional deletion of STAT3 in NSC induced neurogenesis and inhibited astrogliogenesis through a reduction in Notch1 and Notch2 mRNA expression (30). In our study, we also demonstrated that elimination of STAT3 expression by siRNA promoted neurogenesis and inhibited astrogliogenesis, but with human NPCs. siSTAT3 slightly increased the expression of proneural bHLH factors, such as Mash1, Ngn1 and Ngn2. Interestingly, while cytokines inhibit proneural bHLH factor expression, siSTAT3 reversed cytokine-induced inhibition of these factors and partially restore neurogenesis. Therefore, STAT3 signaling plays a dual role in determining the neural cell lineage fate. The mechanism by which STAT3 reduces the level of proneural bHLH factors is unknown. A more detailed characterization of the various mechanism by which STAT3 inhibits proneural bHLH factors may need further investigation.
Cell fate specification involves the reciprocal activation of genes related to a particular cell fate and the suppression of genes of alternative fates. In our study, pro-inflammatory cytokines, IL-1β and TNF-α, promote astrocytic differentiation and inhibit neurogenesis. This effect may not be only through activation of the STAT3 pathway, but also through down-regulation of the proneural bHLH factor expression (Figure 7).
Proneural bHLH proteins, such as Ngn1, Ngn2, Mash1, and NeuroD, are key regulators of neurogenesis (24, 25). During brain development, after early stages of NSC expansion and self-renewal, NPCs differentiate and mature into neurons, astrocytes, and oligodendrocytes in a lineage-restricted and sequential manner. During the neurogenic period, proneural bHLH factors are highly expressed and induce neurogenesis by functioning as a transcriptional activator. Meanwhile, proneural bHLH genes such as Ngn1 are also potent inhibitors of the Jak-STAT pathway for astrogliogenesis machinery. Ngn1 inhibits the differentiation of neural stem cells into astrocytes by sequestering the CBP-Smad1 transcription complex away from astrocyte differentiation genes or inhibiting the activation of STAT transcription factors that are necessary for gliogenesis (18, 31). In our study, STAT3 is only slightly activated in neuronal differentiation condition, while a majority of cells differentiated into Tuj-1-positive neurons, suggesting the inhibition effect of proneural bHLH factors on the STAT3 pathway in a neuronal differentiation condition. However, during brain inflammation, cytokine (IL-1β and TNF-α) down-regulated proneural bHLH factor expression. Considering the critical role of proneural bHLH factors on neurogenesis, the downregulation of these factors may contribute to cytokine-mediated inhibition of neurogenesis. Furthermore, the downregulation of proneural bHLH factors may also release the inhibition on astrocytic differentiation machinery, which results in an activation of STAT3 and a subsequent promotion of astrogliogenesis.
NSPC-based therapy is a promising treatment for many intractable CNS disorders, such as Alzheimer’s disease and Parkinson’s disease. Transplantation of embryonic dopaminergic neurons into the brains of patients with Parkinson’s disease has demonstrated the clinical benefits (32). Thus, it is important to clarify the molecular mechanisms regulating the cell fate of NPCs in order to increase the number of neurons. During brain inflammation, neural stem cells rarely give rise to neurons, possibly because inflammatory factors inhibit expression levels of neurogenic bHLH factors. In this study, we have provided evidence that IL-1β and TNF-α promote astrocytic differentiation and inhibit neuronal differentiation of NPCs through activation of STAT3 and inhibition of proneural bHLH factors. Inhibition of STAT3 rescues a damaged CNS from cytokine-induced astrogliogenesis and promotes neurogenesis. This pathway could become a drug target for the purpose of increasing the number of neurons in NPC-based therapy. We propose that the manipulation of neurogenic bHLH factors with neural stem cells may provide a means of enhancing the ability of stem cells to generate large numbers of neurons that might be useful for treating neurodegenerative disorders or repairing the injured nervous system.
Acknowledgments
This work was supported by research grants from the National Institutes of Health: R01 NS 41858-01 (JCZ), R01 NS 061642-01 (JCZ), P20 RR15635-01 (JCZ), and R21 NS 066841 (HP), the State of Nebraska (DHHS-LB606 Stem Cell 2009-10 to JCZ), the China National Science and Technology major project (2014CB965001), and the National Natural Science Foundation of China (81028007 and 81329002 to JCZ, and 81271419 to CT). We kindly acknowledge Dr. Tsuneya Ikezu and Ms. Li Wu who provided technical support for this work. Dr. Charles Kuszynski and Ms. Victoria Smith performed the flow cytometry support.
References
- 1.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.McKay R. Stem cells in the central nervous system. Science. 1997 Apr 4;276(5309):66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 3.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 4.Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009 Jan 19; doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002 Oct 15;202(1-2):13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 6.Russo I, Barlati S, Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem. 2011 Mar;116(6):947–56. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009 Apr;30(4):174–81. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller S, Steele M, Munch G. Activated astroglia during chronic inflammation in Alzheimer’s disease--do they neglect their neurosupportive roles? Mutat Res. 2010 Aug 7;690(1-2):40–9. doi: 10.1016/j.mrfmmm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001 Sep 1;21(17):6480–91. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003 Nov 11;100(23):13632–7. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003 Dec 5;302(5651):1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007 Feb;25(3):649–58. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- 13.Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008 Mar;56(4):412–25. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- 14.Peng H, Whitney N, Wu Y, Tian C, Dou H, Zhou Y, et al. HIV-1-infected and/or immune-activated macrophage-secreted TNF-alpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia. 2008 Jun;56(8):903–16. doi: 10.1002/glia.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol. 2001 Apr;13(2):211–7. doi: 10.1016/s0955-0674(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 16.Bugga L, Gadient RA, Kwan K, Stewart CL, Patterson PH. Analysis of neuronal and glial phenotypes in brains of mice deficient in leukemia inhibitory factor. J Neurobiol. 1998 Sep 15;36(4):509–24. doi: 10.1002/(sici)1097-4695(19980915)36:4<509::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Koblar SA, Turnley AM, Classon BJ, Reid KL, Ware CB, Cheema SS, et al. Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3178–81. doi: 10.1073/pnas.95.6.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, et al. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci. 1999 Jul 1;19(13):5429–34. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu P, Hata R, Cao F, Gu F, Hanakawa Y, Hashimoto K, et al. Ramified microglial cells promote astrogliogenesis and maintenance of neural stem cells through activation of Stat3 function. Faseb J. 2008 Nov;22(11):3866–77. doi: 10.1096/fj.08-105908. [DOI] [PubMed] [Google Scholar]
- 20.Peng H, Sun L, Jia B, Lan X, Zhu B, Wu Y, et al. HIV-1-infected and immune-activated macrophages induce astrocytic differentiation of human cortical neural progenitor cells via the STAT3 pathway. PLoS ONE. 2011;6(5):e19439. doi: 10.1371/journal.pone.0019439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, et al. Stromal cell-derived factor 1 mediated CXCR4 signaling in rat and human cortical neural progenitor cells. Journal of Neuroscience Research. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- 22.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997 Oct 17;278(5337):477–83. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 23.He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, et al. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci. 2005 May;8(5):616–25. doi: 10.1038/nn1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito H, Nakajima A, Nomoto H, Furukawa S. Neurotrophins facilitate neuronal differentiation of cultured neural stem cells via induction of mRNA expression of basic helix-loop-helix transcription factors Mash1 and Math1. J Neurosci Res. 2003 Mar 1;71(5):648–58. doi: 10.1002/jnr.10532. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda S, Taga T. Cell fate determination regulated by a transcriptional signal network in the developing mouse brain. Anat Sci Int. 2005 Mar;80(1):12–8. doi: 10.1111/j.1447-073x.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 26.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996 Dec 15;10(24):3129–40. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 27.Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999 Apr 16;284(5413):479–82. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 28.Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998 May 15;18(10):3620–9. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu F, Hata R, Ma YJ, Tanaka J, Mitsuda N, Kumon Y, et al. Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J Neurosci Res. 2005 Jul 15;81(2):163–71. doi: 10.1002/jnr.20561. [DOI] [PubMed] [Google Scholar]
- 30.Cao F, Hata R, Zhu P, Nakashiro K, Sakanaka M. Conditional deletion of Stat3 promotes neurogenesis and inhibits astrogliogenesis in neural stem cells. Biochem Biophys Res Commun. Apr 9;394(3):843–7. doi: 10.1016/j.bbrc.2010.03.092. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001 Feb 9;104(3):365–76. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 32.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001 Mar 8;344(10):710–9. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]


