Abstract
Pluripotent stem cells exist in naive and primed states, epitomized by mouse embryonic stem cells (ESCs) and the developmentally more advanced epiblast stem cells (EpiSCs; ref. 1). In the naive state of ESCs, the genome has an unusual open conformation and possesses a minimum of repressive epigenetic marks2. In contrast, EpiSCs have activated the epigenetic machinery that supports differentiation towards the embryonic cell types3–6. The transition from naive to primed pluripotency therefore represents a pivotal event in cellular differentiation. But the signals that control this fundamental differentiation step remain unclear. We show here that paracrine and autocrine Wnt signals are essential self-renewal factors for ESCs, and are required to inhibit their differentiation into EpiSCs. Moreover, we find that Wnt proteins in combination with the cytokine LIF are sufficient to support ESC self-renewal in the absence of any undefined factors, and support the derivation of new ESC lines, including ones from non-permissive mouse strains. Our results not only demonstrate that Wnt signals regulate the naive-to-primed pluripotency transition, but also identify Wnt as an essential and limiting ESC self-renewal factor.
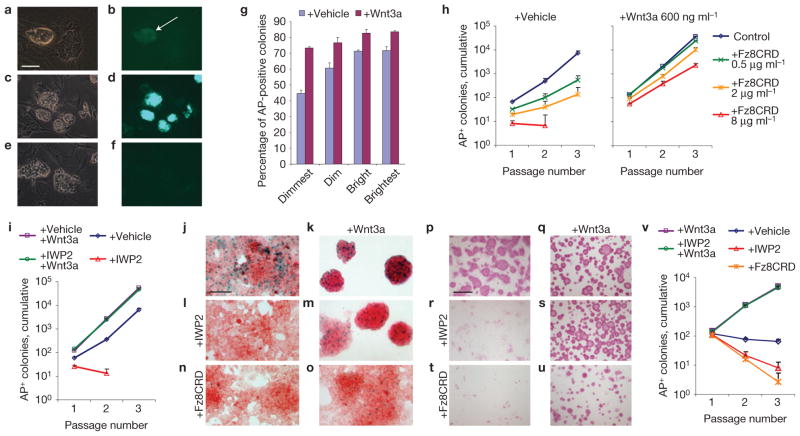
We visualized activation of the Wnt pathway in ESCs using R1 cells carrying the Wnt reporter 7xTcf–eGFP (enhanced green fluorescent protein; ref. 7), cultured on mouse embryo fibroblast (MEF) feeder layers. ESC colonies with sharp boundaries and hard-to-distinguish individual cells—characteristics of undifferentiated colonies—showed higher levels of reporter activity than flattened colonies with distinct individual cells (Fig. 1a,b). We verified the Wnt responsiveness of the reporter by its induction by purified Wnt3a protein (Fig. 1c,d), and by its extinction by the Wnt antagonist Fz8CRD, a soluble domain of the Wnt receptor that binds and sequesters Wnt proteins (Fig. 1e,f). These data demonstrate that R1 ESCs grown on MEFs experience paracrine or autocrine stimulation by Wnt ligands. Indeed, Wnts are expressed by ESCs themselves (Supplementary Fig. S1a) and by MEFs (ref. 8).
Figure 1.
ESC self-renewal requires Wnt signals. (a–f) The 7xTcf–eGFP reporter is active in a subset (arrow) of ESCs cultured for 2 days on MEFs (a,b); Wnt3a protein activates the reporter in all cells (c,d), whereas Fz8CRD extinguishes it (e,f). (a,c,e) Phase-contrast microscopy; (b,d,f) eGFP. (g) The ability of 7xTcf–eGFP cells to form alkaline phosphatase-positive (AP+) colonies in the absence of MEFs correlated with the level of eGFP, and was enhanced by the presence of Wnt3a protein (mean ± s.e.m., n = 3). (h) The expansion of R1 ESCs able to form alkaline phosphatase-positive colonies on MEFs was progressively repressed by increasing concentrations of the Wnt antagonist Fz8CRD. This effect was counteracted by simultaneous addition of Wnt3a protein (mean + s.e.m., n = 3). (i) The expansion of R1 ESCs able to establish alkaline phosphatase-positive colonies on MEFs was repressed by IWP2. This repression was relieved by simultaneous addition of Wnt3a protein (240 ng ml−1) (mean+ s.e.m., n = 3). (j–o) Axin2LacZ ESCs cultured in the absence of MEFs, untreated (j) or treated for 3 days with IWP2 (l,m), 2 μg ml−1 Fz8CRD (n,o) and/or 200 ng ml−1 Wnt3a (k,m,o) and stained with X-gal and Nuclear Red. (p–u) CGR8 ESCs cultured in the absence of MEFs, untreated (p) or treated for three passages with IWP2 (r,s), 2 μg ml−1 Fz8CRD (t,u) and/or 200 ng ml−1 Wnt3a (q,s,u) and stained for alkaline phosphatase. (v) The expansion of CGR8 ESCs able to form alkaline phosphatase-positive colonies in the absence of MEFs was repressed by IWP2 or 500 ng ml−1 Fz8CRD, and promoted by 200 ng ml−1 Wnt3a protein. Scale bars, 100 μm (a–f, j–o), 500 μm (p–u).
To determine whether these endogenous Wnt ligands aid in self-renewal, we FACS-sorted the 7xTcf–eGFP cells into four populations, on the basis of eGFP level. Cells with less eGFP were less likely to establish colonies positive for the ESC marker alkaline phosphatase (Fig. 1g). Moreover, a higher percentage of cells formed colonies when plated in the presence of Wnt3a protein (Fig. 1g), demonstrating that endogenous Wnt ligands support ESC self-renewal.
To quantify to what extent ESC self-renewal depends on Wnt signals, we measured the expansion of cells able to establish alkaline phosphatase-positive colonies in the presence of Fz8CRD over three passages at clonal density. The Wnt antagonist reduced, and at high concentration completely suppressed, self-renewal (Fig. 1h and Supplementary Fig. S1b). This effect was countered by addition of Wnt3a protein (Fig. 1h and Supplementary Fig. S1b), demonstrating that it relied on the Wnt-binding ability of Fz8CRD. Furthermore, ESC self-renewal was also suppressed by the small-molecule inhibitor IWP2, which interferes with the ability of cells to produce active Wnt proteins by blocking Porcupine, an enzyme essential for acylating Wnt proteins9 (Fig. 1i). Importantly, this effect was rescued by addition of Wnt3a protein (Fig. 1i), demonstrating that the inhibition is specific for Wnt signal production. Thus, endogenous Wnt ligands are essential for self-renewal of R1 ESCs.
ESC lines such as E14 and CGR8 self-renew in the absence of MEFs, indicating that they produce sufficient Wnt proteins themselves. Using E14 ESCs carrying a LacZ reporter targeted into the Wnt target gene Axin2 (ref. 10; also known as Conductin), we indeed detected widespread reporter activation in the absence of MEFs (Fig. 1j). This was due to endogenous Wnt proteins as the reporter was extinguished by IWP2 and by Fz8CRD, which was counteracted by the addition of Wnt3a protein (Fig. 1j–o). E14 ESCs therefore produce active Wnt proteins. In addition, we found that CGR8 ESCs also express Wnt-encoding genes in the absence of MEFs (Supplementary Fig. S1a).
Inhibition of the endogenous Wnt signals by Fz8CRD or IWP2 resulted in differentiation of both E14tg2a and CGR8 cells, as indicated by the loss of alkaline phosphatase expression (Fig. 1p,r,t and Supplementary Fig. S2a). Again, addition of Wnt3a protein rescued these effects (Fig. 1p–u and Supplementary Fig. S2a). We next quantified the effect of Wnt inhibition on the self-renewal on CGR8 cells and the E14 sublines E14tg2a and IB10. At clonal density, self-renewal of these ESC lines was very limited, indicating the importance of paracrine signalling (Fig. 1v and Supplementary Fig. S2b). The remaining self-renewal ability was eliminated by Fz8CRD or IWP2, indicating that it depended on endogenous Wnt signals (Fig. 1v and Supplementary Fig. S2b). Furthermore, addition of Wnt3a protein strongly promoted self-renewal at clonal density (Fig. 1v and Supplementary Fig. S2b), further confirming that Wnt proteins are the paracrine factors necessary for ESC self-renewal.
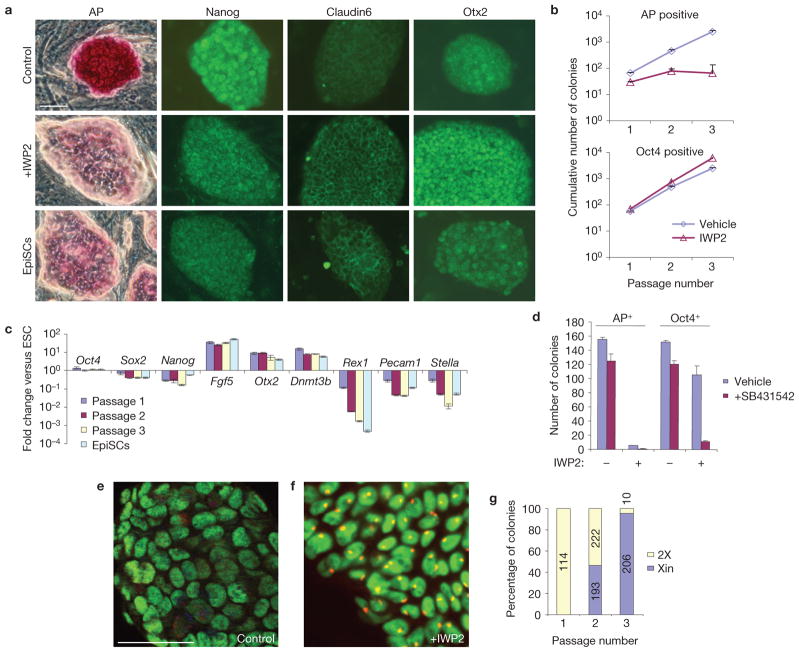
Interestingly, when R1 ESCs were cultured on MEFs in the presence of IWP2, they started to resemble EpiSCs, losing alkaline phosphatase expression and acquiring a flattened morphology (Fig. 2a). The colonies remained positive for the ESC and EpiSC markers Oct4 and Nanog, and induced the EpiSC markers Claudin6 and Otx2 (ref. 4; Fig. 2a and Supplementary Fig. S3a). To confirm that the ESCs differentiated towards EpiSCs, we passaged the cells as small clumps using collagenase IV, as is required to preserve EpiSCs (refs 3,4), and evaluated their marker profile, growth-factor dependence and epigenetic state. In the presence of IWP2, only Oct4-positive colonies negative for alkaline phosphatase expanded (Fig. 2b and Supplementary Fig. S3a), and the cells acquired a marker-gene expression profile similar to that of EpiSCs (Fig. 2c). Furthermore, similarly to EpiSCs, IWP2-treated ESCs became dependent on Activin/Nodal signalling through the ALK receptors (ActivinA receptors, type 1), as colony formation was severely repressed by the ALK inhibitor SB431542 (Fig. 2d). Finally, EpiSCs differ from ESCs in their epigenetic state, most markedly shown by the presence of an inactive X chromosome in female EpiSCs. Indeed, IWP2 treatment of female ESCs induced the formation of trimethyl histone H3 Lys 27 (H3K27me3) foci, which is diagnostic for the inactive X chromosome (Fig. 2e–g). In addition, the treated cells activated the DNA methylation machinery as shown by the induction of the de novo DNA methylase gene Dnmt3b (Fig. 2c). Combined, these data demonstrate that Wnt signals are required to inhibit the differentiation of ESCs into EpiSCs. This is in agreement with a report that ESCs mutant for the Wnt signal transducer β-catenin (also known as Ctnnb1) show a morphology and marker pattern similar to that of EpiSCs11.
Figure 2.
Wnt signals are required to inhibit the differentiation of ESCs into EpiSCs. (a) R1 ESCs cultured for 3 days on MEFs in the presence of IWP2 form flattened colonies that reduce alkaline phosphatase (AP) and Nanog expression, and increase Claudin6 and Otx2 expression. (b) On passaging of R1 ESCs in the presence of IWP2, only alkaline phosphatase-low but Oct4-positive colonies expand (mean+ s.e.m., n = 3). (c) Gene expression profiles of R1 ESCs cultured on MEFs in the presence of IWP2, and of EpiSCs, relative to untreated ESCs (mean± s.e.m., n = 3). (d) After two passages in the presence of IWP2 or vehicle on MEFs, the cells were passaged in the presence of 2 μM SB431542, and the number of alkaline phosphatase- and Oct4-positive colonies determined (mean± s.e.m., n = 3). (e,f) Female ESCs were cultured on MEFs for two passages in the presence of vehicle (e) or IWP2 (f) and immunostained for H3K27me3 (red) and Oct4 (green). A yellow focus indicates the presence of an inactive X chromosome. (g) In the presence of IWP2, either no or virtually all cells of a colony showed the inactive X focus. The number of colonies showing no focus (2X) or X inactivation (Xin) was determined over multiple passages. The absolute numbers are plotted within the bars. Scale bars, 50 μm (a,e,f).
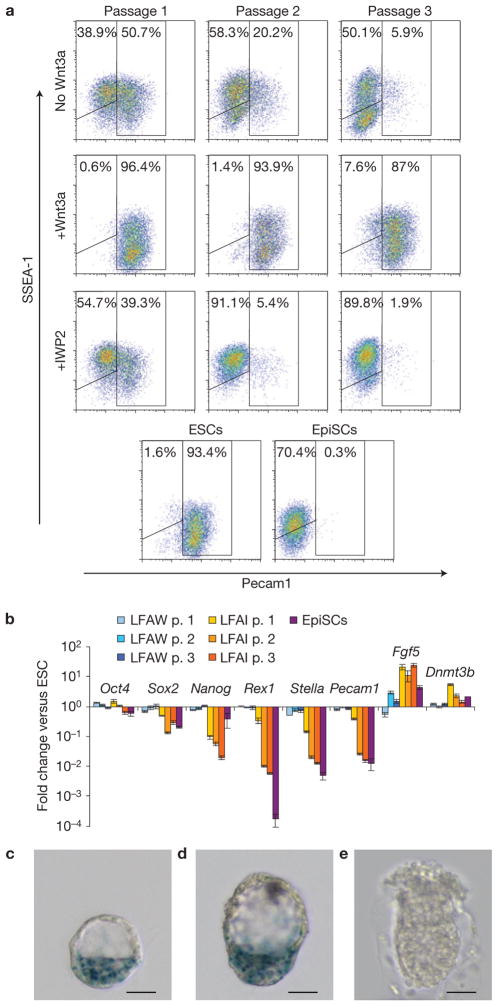
ESCs readily differentiate into EpiSCs on transfer to EpiSC medium6, and we tested whether Wnt3a protein would be sufficient to block this transition. R1 ESCs plated in serum-free N2B27 medium supplemented with basic fibroblast growth factor (bFGF), ActivinA and LIF rapidly acquired EpiSC character, as indicated by FACS analysis for the markers Pecam1 (for ESCs only, also known as CD31) and SSEA1 (for both ESCs and EpiSCs; ref. 5; Fig. 3a). In contrast, differentiation was inhibited in the presence of Wnt3a (Fig. 3a). Remarkably, the differentiation was even quicker and more efficient in the presence of IWP2 (Fig. 3a), indicating that endogenous Wnt signals interfered with EpiSC differentiation. Gene expression analysis showed profiles consistent with ESCs or EpiSCs in the presence of Wnt3a or IWP2, respectively (Fig. 3b). Withdrawal of LIF in the presence of Wnt3a caused loss of the cells, indicating that, although sufficient to inhibit EpiSC differentiation, Wnt3a is insufficient to maintain ESCs on its own. Combined, the data show that Wnt proteins are necessary and sufficient to inhibit the differentiation of ESCs towards EpiSCs.
Figure 3.
Wnt3a protein is sufficient to inhibit the differentiation of ESCs into EpiSCs. (a) FACS plots of EpiSCs and of R1 cells passaged every 3 days in N2B27 supplemented with LIF, bFGF and ActivinA, in the presence of Wnt3a protein (240 ng ml−1) or IWP2 (2 μM) as indicated, and stained with anti-SSEA1-PE (phycoerythrin) and anti-Pecam1-FITC (fluorescein isothiocyanate) antibodies (10,000 cells/plot). (b) Gene expression profiles of EpiSCs and R1 ESCs cultured in N2B27 supplemented with LIF, bFGF, ActivinA and either Wnt3a (LFAW) or IWP2 (LFAI), relative to untreated ESCs (mean± s.e.m., n = 3). (c–e) The Axin2LacZ/+ reporter indicates Wnt activity in the E3.5 (c) and E4.5 (d) ICM, but is inactive in E5.5 implanted embryos (e). Scale bars, 25 μm.
ESCs originate from the inner cell mass (ICM) of blastocyst-stage embryos, and interestingly we found activity of the Wnt signalling reporter Axin2LacZ in this tissue (Fig. 3c,d). This is consistent with the expression of several Wnts and nuclear β-catenin in the blastocyst12,13. However, the reporter was extinguished on implantation (Fig. 3e and Supplementary Fig. S3b,c), when the ICM differentiates towards post-implantation epiblast, the source of EpiSCs. These observations are consistent with a possible physiological role for Wnt in regulating this differentiation step during normal development. Although β-catenin knockout embryos develop through the blastocyst stage14, this may be explained by the presence of maternal β-catenin15,16. Alternatively, transient abnormalities, such as premature differentiation of ICM into epiblast, might have gone unnoticed, or Wnt signals might be required for maintenance of the ICM during delayed implantation, as observed for LIF/gp130 signalling17.
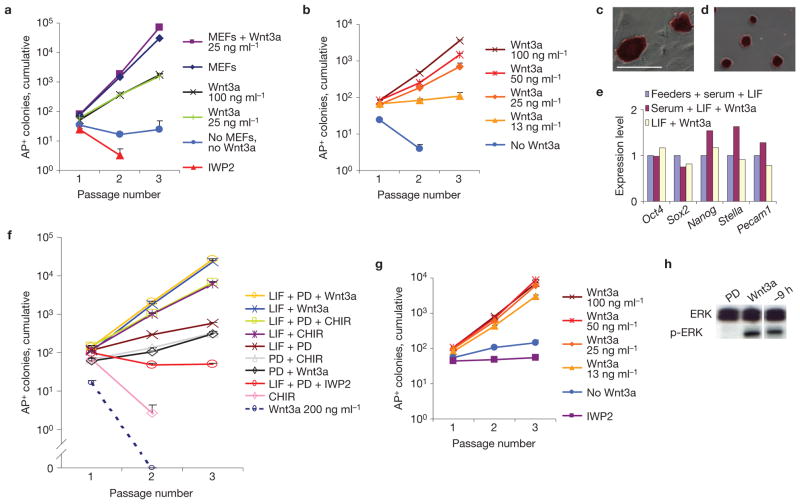
Our results demonstrate that the combination of Wnt and LIF signals is essential for ESC self-renewal, and we next tested whether this combination was also sufficient. Indeed, addition of Wnt3a protein supported the expansion of R1 ESCs at clonal density in the absence of MEFs (Fig. 4a), and even in serum-free N2B27 medium supplemented with LIF (Fig. 4b and Supplementary Fig. S4a,b). In N2B27 + LIF + Wnt3a, the cells adhered poorly to gelatin-coated surfaces but plating efficiency was restored by further fibronectin coating, and the expansion was then similar to that on MEFs (compare Fig. 4a,f). No adaptation period was necessary for culture in N2B27+ LIF+ Wnt3a. The cells formed colonies with a distinct, dome-shaped morphology that stained intensely for alkaline phosphatase (compare Fig. 4c,d), and real-time PCR analysis demonstrated normal expression levels of ESC markers (Fig. 4e). 5-bromodeoxyuridine analysis revealed no role of Wnt3a in proliferation, indicating that inhibition of differentiation is its main function in self-renewal (Supplementary Fig. S4c). Using single-cell deposition in N2B27 + LIF + Wnt3a, R1 cells formed colonies in 55 out of 95 wells. Several clones were further expanded over the course of eight passages (24 days) and functionally tested by blastocyst injections, resulting in chimaeras with high coat-colour chimaerism, a strong sex distortion in favour of males and a high proportion of germline transmission (Supplementary Table S1 and Fig. S4d). These results demonstrate that Wnt3a protein in combination with LIF is sufficient to support self-renewal of germline-competent ESCs at similar efficiencies to serum and MEFs, even at clonal density.
Figure 4.
LIF and Wnt3a are sufficient to support ESC self-renewal. (a,b) Expansion over multiple passages of alkaline phosphatase-positive (AP+) R1 ESC colonies in medium containing serum and LIF (a) or in N2B27 containing LIF (b) (mean + s.e.m., n = 3). (c,d) Alkaline phosphatase-stained R1 ESCs cultured on MEFs (c) or maintained for six passages in N2B27 medium supplemented with LIF and Wnt3a (d). (e) Gene expression profile of R1 ESCs following four passages in the indicated conditions. (f) Expansion of alkaline phosphatase-positive R1 ESC colonies in N2B27 medium. Where indicated, Wnt3a was added at 200 ng ml−1. (g) Expansion of alkaline phosphatase-positive R1 ESC colonies in N2B27 medium containing LIF and PD0325901. (h) MEK activity (indicated by the presence of phospho-ERK (p-ERK; extracellular signal-regulated kinase) on western blot) in R1 ESCs cultured in N2B27 with LIF, was repressed by PD0325901 (PD) but not by Wnt3a, or by withdrawal of Wnt3a for 9 h (−9 h). Uncropped images of blots are shown in Supplementary Fig. S4g. Scale bar, 200 μm (c,d).
An alternative way to activate the Wnt pathway is by inhibiting the glycogen synthase kinase 3 (GSK3) kinases, which stabilizes β-catenin18 (Supplementary Fig. S4e). However, the GSK3 inhibitor CHIR99021 was less effective than Wnt3a in supporting clonal expansion of ESCs (Fig. 4f), indicating that the other effects of GSK3 inhibition19 are disadvantageous. Further concerns about GSK3 inhibition arise from its interference with chromosomal alignment at mitosis and from its induction of chromosome instability20,21.
Recently, the combined inhibition of the GSK3 and MAP-kinase kinase (MEK) kinases was reported to support ESC self-renewal in the absence of LIF (ref. 22). We found that, in combination with the MEK inhibitor PD0325901, Wnt3a protein was as effective as CHIR99021 in supporting limited self-renewal (Fig. 4f). The self-renewal effect of GSK3 inhibition can therefore be ascribed to its ability to activate the Wnt pathway, consistent with a recent report23. Interestingly, in the presence of LIF, MEK inhibitor lowered the requirement for Wnt protein in ESC self-renewal (compare Fig. 4g and b), but provided no benefit when sufficient Wnt3a protein was provided (Fig. 4f and Supplementary Fig. S4f). MEK inhibition did not activate the Wnt pathway, as neither the 7xTcf–eGFP reporter nor the Axin2LacZ reporter responded to PD0325901 in ESCs (not shown), nor did Wnt3a inhibit the MEK pathway (Fig. 4h), suggesting that the interaction between these pathways is further downstream.
We next investigated the effect of Wnt on ESC derivation, using N2B27 medium in the absence of MEFs. ESC derivation from whole blastocysts of the mouse strain 129Sv was 96% efficient when Wnt3a and LIF were combined with the MEK inhibitor PD0325901, which aids ESC derivation by preventing the differentiation of ICM into primitive endoderm in whole-blastocyst culture24 (Supplementary Table S2). Following derivation, Wnt3a and LIF were sufficient to support expansion of the new lines. The newly derived cells stained positive for alkaline phosphatase, Oct4 and Nanog (Supplementary Fig. S5a), and several lines contributed to germline chimaeras following blastocyst injections (Supplementary Table S3 and Fig. S5b).
Most mouse strains are non-permissive for ESC derivation; that is, no ESCs develop from embryos cultured on MEFs in the presence of LIF (ref. 25). However, we readily derived ESCs from non-permissive FVB/N embryos on addition of Wnt3a protein to these conditions, and also in the absence of MEFs, using N2B27 supplemented with LIF, Wnt3a and PD0325901 (Supplementary Table S2). The new lines stained positive for alkaline phosphatase, Oct4 and Nanog (Fig. 5a–c and Supplementary Fig. S5a), several lines contributed to high-percentage chimaeras (Supplementary Table S4) and germline transmission was observed from line FN3 (Fig. 5d). Following derivation, Wnt3a and LIF were sufficient to support expansion of FVB/N ESCs (Fig. 5e). We found that FVB/N ESCs required Wnt3a supplementation for expansion on MEFs (Fig. 5f), indicating that MEFs provide insufficient Wnt ligands to support them. Non-permissive ESCs therefore differ from their permissive counterparts by an increased requirement for Wnt ligands.
Figure 5.
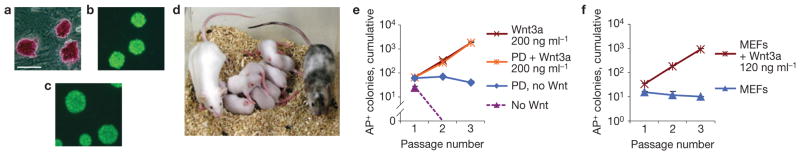
Wnt3a supports derivation of non-permissive ESCs. (a–c) Alkaline phosphatase, Oct4 and Nanog stainings, respectively, of the newly derived FVB/N ESC line FN3. Scale bar, 200 μm. (d) Chimaera (black–white spotted) obtained from injection of C57Bl/6 blastocysts with passage 7 ESC line FN3 together with FVB mate and pups showing germline transmission (white pups). (e) Expansion of alkaline phosphatase-positive (AP+) colonies from newly derived FVB/N ESCs in N2B27 medium with LIF (mean + s.e.m., n = 3). PD, PD0325901. (f) Expansion of alkaline phosphatase-positive colonies from FVB/N ESCs on MEFs in medium containing serum and LIF (mean+ s.e.m., n = 3).
This work shows that ESC self-renewal depends on extrinsic Wnt signals, and identifies the Wnt pathway as a regulatory signal between ESCs and EpiSCs. Moreover, we show that the combination of Wnt3a protein with LIF is sufficient to support the expansion of germline-competent ESCs, at clonal efficiencies similar to those obtained with feeders. Wnt signals may inhibit differentiation to EpiSCs through the transcription factor TCF3, which regulates the expression of multiple-pluripotency genes26–28.
A function of Wnt signals in ESCs has been suspected since it was reported that Apc modulates the differentiation of ESCs through the β-catenin pathway29. Although subsequent reports demonstrated that Wnt ligands can improve ESC self-renewal30–32, no requirement or specific function for Wnt signals in ESC self-renewal had been established. In fact, for some time it has been unclear whether the self-renewing state of ESCs requires extrinsic signals or is autonomous. Although the cytokine LIF had been identified as an essential ESC self-renewal factor33,34, a recent report shows that combined inhibition of the MEK signal transducers and the GSK3 kinases (2i condition) is sufficient to support ESC self-renewal22, suggesting that extracellular factors are not required. However, GSK3 inhibition has many effects19, among which is activation of the Wnt signalling pathway18. Our demonstration that Wnt3a can substitute for the GSK3 inhibitor in the 2i condition indicates that the effect of GSK3 inhibition can be ascribed to its ability to activate the Wnt pathway.
The hitherto unappreciated requirement for Wnt signals for ESC self-renewal may have implications for the establishment of naive pluripotent cells from other species, including humans. This is underscored by our demonstration that Wnt3a protein supports the derivation of germline-competent ESCs from non-permissive FVB mice. The Wnt requirement also explains findings of an instable naive state in reprogrammed non-permissive NOD cells35 as a consequence of insufficient paracrine support by Wnt proteins. Finally, the dependence of ESC self-renewal on (heterogeneous) paracrine Wnt signals may explain the heterogeneity that is observed in ESC cultures, with significant subpopulations showing a partial differentiation towards EpiSCs (ref. 5).
METHODS
Recombinant proteins and small molecules
Recombinant ActivinA and human bFGF proteins were purchased from Peprotech. Fz8CRD–Fc fusion protein (Supplementary Fig. S5c) and control Fc were produced as described36. Recombinant mouse Wnt3a protein was produced in Drosophila S2 cells grown in suspension culture, and purified by Blue Sepharose affinity and gel filtration chromatography as described37 (Supplementary Fig. S5c). Wnt3a activity was determined in a luciferase reporter assay using L cells stably transfected with the SuperTOPFlash reporter as described38 (Supplementary Fig. S4e). Some experiments were reproduced using recombinant human Wnt3a protein generously donated by R&D Systems.
IWP2, CHIR99021, SB431542 (all purchased from Stemgent) and PD0325901 (Merck) were diluted from 2 mM (IWP2) or 10 mM (all others) stocks in dimethylsulphoxide, and used at 2, 3, 6 and 0.9 μM, respectively. Media, recombinant proteins and small molecules were changed daily in all experiments except when indicated otherwise.
Cell and embryo culture
R1 and CGR8 ESCs were obtained from the Stanford Transgenic Facility. E14tg2a ESCs were purchased from ATCC. Axin2LacZ ESCs10 were donated by W. Birchmeier (Berlin). Female C57Bl6/Mus musculus castaneus E8 ESCs were donated by J. Gribnau (Rotterdam).
Routine culture of R1 and E8 ESCs was carried out on a feeder layer of irradiated primary MEFs (GlobalStem) in mESC medium (DMEM plus 15% fetal bovine serum (Hyclone), 1 mM sodium pyruvate, MEM non-essential amino acids, 50 μMβ-mercaptoethanol, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin (all from Invitrogen) and 1,000 U ml−1 LIF (Chemicon)) on gelatin-coated plates.
N2B27 medium39 consisted of one volume DMEM/F12 combined with one volume Neurobasal medium, supplemented with 0.5% N2 Supplement, 1% B27 Supplement, 0.033% BSA 7.5% solution, 50 μMβ-mercaptoethanol, 2 mM Glutamax, 100 U/ml penicillin and 100 μg ml−1 streptomycin (all from Invitrogen).
CGR8, Axin2LacZ and E14tg2a ESCs were maintained on gelatin-coated plates in mESC medium.
Cells were passaged as a single-cell suspension using 0.25% trypsin–EDTA. For serum-free culture, trypsin was quenched using soybean trypsin inhibitor (Sigma).
129S2 C1a EpiSCs were donated by L. Vallier, and maintained on plates coated with gelatin and FCS in N2B27 medium supplemented with 12 ng ml−1 bFGF and 20 ng ml−1 ActivinA. The cells were passaged every 3–4 days as clumps using 0.5 mg ml−1 collagenase IV (Sigma). For culture on MEFs, EpiSCs were maintained in mEpiSC medium (DMEM/F12 plus 15% knockout serum replacement (KOSR), MEM non-essential amino acids, 100 μMβ-mercaptoethanol (all Invitrogen) and 10 ng ml−1 bFGF).
E5.5 Axin2LacZ/+ embryos10 were obtained from matings of homozygous Axin2LacZ/LacZ males with wild-type females. Embryos were placed in mESC medium without LIF, supplemented with 3 μM CHIR99021 or vehicle and cultured overnight at 37 °C in 5% CO2 atmosphere. Cultured embryos were fixed for 2 min in 1% paraformaldehyde, washed in PBS and stained with 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal). Axin2LacZ EpiSCs were derived from E5.5 Axin2LacZ/+ embryos as described4.
Correlation between Wnt reporter and ability to form ESC colonies
For 7xTcf–eGFP FACS, cells were plated on MEFs and cultured for 2 days, dissociated with 0.25% trypsin–EDTA and resuspended in PBS with 1% serum and 0.05% propidium iodide, and live cells sorted into four categories on the basis of eGFP intensity using a BD FACSAria II cell sorter. Gates were set such that the dimmest and brightest categories each contained 13% of the total population, whereas the intermediate categories each contained 33% (the remainder were lost in the gaps between the gates). Sorted cells were plated on gelatin-coated plates in mESC medium, containing 300 ng ml−1 Wnt3a as indicated, at a density of 250 cells/cm−2, and stained for alkaline phosphatase after 4 days. The stained plates were dried and imaged using a Cellavista automated imager equipped with a ×4 objective and bright-field illumination (Roche Diagnostics). The total number of colonies in each well (alkaline phosphatase-positive and negative) was counted manually, whereas the number of alkaline phosphatase-positive colonies was determined by using CellProfiler biological image analysis software40 to ensure consistency. The percentage of alkaline phosphatase-positive colonies was calculated and plotted ±s.e.m. (n = 3).
Clonal self-renewal assays
To quantify self-renewal over multiple passages, single cells were plated at a density of 100 cells cm−2 in gelatin-coated six-well plates and in gelatin-coated 24-well plates in triplicate, containing an MEF layer when indicated. For assays in N2B27, the plates were additionally coated with 15 μg ml−1 human plasma fibronectin (Sigma). Every 3 days, six-well plates were trypsinized to single cells, and passaged to a new set of plates at a dilution that would lead to a density not higher than but as close as possible to 100 cells cm−2. At the same time, the 24-well plates were stained for alkaline phosphatase, or for Oct4 using an Oct4 antibody and 3,3′-diaminobenzidine (DAB) staining (see Marker staining). Stained plates were rinsed with water, dried and scanned using a CellCelector automated imager (Aviso) equipped with a ×4 objective and bright-field illumination. The number of positive colonies was determined using CellProfiler biological image analysis software40. The cumulative number of colonies was determined by multiplying the colony counts by the dilution factor used for passaging. Results are plotted as the mean of three wells ±s.e.m.
ESC to EpiSC differentiation assays
A single-cell suspension of R1 ESCs was plated onto MEFs at a density of 100 cells cm−2 in mESC medium in six-well plates, and in 24-well plates in triplicate. EpiSCs were plated as small clumps onto MEFs in mEpiSC medium. One day after plating, IWP2 was added as indicated. After 3 more days, the 24-well plates were processed for marker staining, and the six-well plates passaged 1:10 using 0.5 mg ml−1 collagenase IV every 3 days. At the second passage, further 24-well plates were prepared with 6 μM SB431542 included in the media for the ALK-inhibitor assay. Stained plates were manually counted. The cumulative number of colonies was determined by multiplying the colony counts by the dilution factor used for passaging. Results are plotted as the mean± s.e.m. of three wells.
For serum-free conditions, a single-cell suspension of R1 ESCs was plated onto gelatin/serum-coated plates at a density of 10,000 cells cm−2 in N2B27 medium supplemented with LIF and 120 ng ml−1 Wnt3a. The next day, the media were replaced with N2B27/LIF supplemented with 12 ng ml−1 bFGF, 20 ng ml−1 ActivinA and 120 ng ml−1 Wnt3a or 1 μM IWP2 as indicated. The cells were then passaged 1:4–1:10 every 3 days as small clumps using 0.5 mg ml−1 collagenase IV. At every passage, a portion of the cells was triturated to single-cell suspension, stained with anti-SSEA1–PE (eBioscience 12-8813) and anti-Pecam1–FITC (BD Biosciences 553372) antibodies, and analysed using a Facscan flow cytometer.
ESC derivation
E3.5 blastocysts were obtained from natural matings of 129Sv or FVB/N mice and plated intact in 96-well plates on MEFs or on plates coated first with gelatin, followed by 15 μg ml−1 human plasma fibronectin (Sigma) in PBS. An equal volume of fresh medium was added on days 2 and 3, followed by daily medium changes. The medium used for derivation on MEFs consisted of DMEM plus 15% KOSR (Invitrogen), MEM non-essential amino acids (Invitrogen), 50 μMβ-mercaptoethanol and 1,000 U ml−1 LIF (Chemicon). In the absence of MEFs, derivation was carried out in N2B27 medium containing 1,000 U ml−1 LIF. Wnt3a protein or the MEK inhibitor PD0325901 was added as indicated to a concentration of 300 ng ml−1 or 1 μM, respectively. When both Wnt3a and MEK inhibitor were added, the MEK inhibitor was removed after the outgrowths were passaged. After 6 days, outgrowths were trypsinized and transferred to fresh 96-well plates. Newly formed cell lines were expanded for a minimum of seven passages, and analysed by alkaline phosphatase, Nanog and Oct4 staining and blastocyst injections using C57Bl/6 host blastocysts. The percentage of coat colour chimaerism of the chimaeras was estimated visually. To determine germline transmission, chimaeras from 129Sv-derived ESCs were mated with C57Bl/6 mice, and chimaeras from FVB/N-derived ESCs were mated with FVB/N mice.
Marker staining and immunohistochemistry
Alkaline phosphatase activity was detected using the SCR004 kit (Millipore). For immunostaining, cells were fixed with 4% paraformaldehyde for 15 min, washed thrice with PBS/0.5% Triton X-100 (PBT), permeabilized for 20 min with ice-cold methanol and blocked with 10% normal donkey serum (NDS)/PBT for 30 min. Samples were then incubated with primary antibody in NDS/PBT overnight at 4 °C, washed three times with PBT and either stained using a Vector ABC Elite kit and DAB (Vector), or primary antibodies detected by DyLight 488-labelled secondary antibodies (Jackson ImmunoResearch), followed by imaging. Antibodies and concentrations: anti-Oct4 (Santa Cruz sc-8628, 0.8 μg ml−1), anti-Nanog (Cosmo Bio REC-RCAB0002P-F, 1:250), anti-Claudin6 (Santa Cruz sc-17669, 2 μg ml−1), anti-Otx2 (Abcam ab21990, 1 μg ml−1), anti-H3K27me3 (Abcam ab6002, 1:100), anti-ERK and anti p-ERK (Cell Signalling 9102 and 4376S, respectively, both 1:1,000).
Gene expression analysis
Total RNA was prepared using a QIAGEN RNeasy mini kit with on-column DNase digestion, followed by reverse transcription using Superscript II (Invitrogen). When MEFs were present, cells were first plated for 30 min on gelatin-coated plates to enable MEFs to attach and ESCs or EpiSCs gently flushed off the plate. Quantitative PCR with reverse transcription was carried out on a Roche Lightcycler 480 using Lightcycler 480 SYBR Green Master mix (Roche). Relative quantification was carried out using glyceraldehyde-3-phosphate dehydrogenase as a reference gene. All PCRs were carried out in triplicate, and the mean crossing point was used for quantification. Primer sequences were designed using Lightcycler Probe Design Software 2.0 (Roche) such that they spanned splice junctions when possible.
For detection of Wnt gene expression in R1 and CGR8 cells, PCR reactions were carried out in quadruplicate. Primers were tested on cDNA derived from E12.5 whole-embryo RNA, and product length was verified on gels. Primer sequences are provided in Supplementary Table S5.
Acknowledgments
These studies were supported by the Howard Hughes Medical Institute, the Erasmus MC Stem Cell Institute and grants from the California Institute of Regenerative Medicine (RC1-00133-1), the National Institutes of Health (DK67834-01) and the European Union (FP7-PEOPLE-2009-RG-256560). We thank H. Zeng for technical advice, J. Kong-A-San for assistance with blastocyst injections and R. van der Linden for assistance with FACS. We are grateful for the use of the Cellavista imager and the assistance of V. Vincent and Roche Diagnostics.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website
AUTHOR CONTRIBUTIONS
D.t.B., D.K. and T.B. designed and carried out experiments, analysed data and wrote the paper. W.K., A.M., R.S. and E.E. designed and carried out experiments and analysed data. R.N. designed experiments and wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints
References
- 1.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Niwa H. Open conformation chromatin and pluripotency. Genes Dev. 2007;21:2671–2676. doi: 10.1101/gad.1615707. [DOI] [PubMed] [Google Scholar]
- 3.Brons IG, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 4.Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi K, Lopes SM, Tang F, Surani MA. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell. 2008;3:391–401. doi: 10.1016/j.stem.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo G, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ten Berge D, et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 9.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anton R, Kestler HA, Kuhl M. β-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 2007;581:5247–5254. doi: 10.1016/j.febslet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- 13.Wang QT, et al. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell. 2004;6:133–144. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- 14.Haegel H, et al. Lack of β-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 15.Ohsugi M, et al. Expression and cell membrane localization of catenins during mouse preimplantation development. Dev Dyn. 1996;206:391–402. doi: 10.1002/(SICI)1097-0177(199608)206:4<391::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 16.De Vries WN, et al. Maternal β-catenin and E-cadherin in mouse development. Development. 2004;131:4435–4445. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- 17.Nichols J, Chambers I, Taga T, Smith A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development. 2001;128:2333–2339. doi: 10.1242/dev.128.12.2333. [DOI] [PubMed] [Google Scholar]
- 18.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 19.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tighe A, Ray-Sinha A, Staples OD, Taylor SS. GSK-3 inhibitors induce chromosome instability. BMC Cell Biol. 2007;8:34. doi: 10.1186/1471-2121-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acevedo N, Wang X, Dunn RL, Smith GD. Glycogen synthase kinase-3 regulation of chromatin segregation and cytokinesis in mouse preimplantation embryos. Mol Reprod Dev. 2007;74:178–188. doi: 10.1002/mrd.20495. [DOI] [PubMed] [Google Scholar]
- 22.Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly KF, et al. β-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214–227. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner RL, Brook FA. Reflections on the biology of embryonic stem (ES) cells. Int J Dev Biol. 1997;41:235–243. [PubMed] [Google Scholar]
- 26.Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam WL, et al. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells. 2008;26:2019–2031. doi: 10.1634/stemcells.2007-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi F, Pereira L, Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–1960. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kielman MF, et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of β-catenin signaling. Nat Genet. 2002;32:594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- 30.Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/β-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- 32.Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- 33.Williams RL, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 34.Smith AG, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 35.Hanna J, et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell. 2009;4:513–524. doi: 10.1016/j.stem.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt–frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci USA. 1999;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 38.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin–TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 40.Lamprecht MR, Sabatini DM, Carpenter AE. CellProfiler: free, versatile software for automated biological image analysis. Biotechniques. 2007;42:71–75. doi: 10.2144/000112257. [DOI] [PubMed] [Google Scholar]