Abstract
Continuous generation of visual chromophore through the visual (retinoid) cycle is essential to maintain eyesight and retinal heath. Impairments in this cycle and related pathways adversely affect vision. In this review, we summarize the chemical reactions of vitamin A metabolites involved in the retinoid cycle and describe animal models of associated human diseases. Development of potential therapies for retinal disorders in these animal models is also introduced.
INTRODUCTION
Activation of rhodopsin in the retina is the initial step for light perception in vertebrates. Continuous vision depends on recycling of all-trans-retinal, the product of light dependent photoisomerization of the visual chromophore, back to 11-cis-retinal [1]. This process is enabled by the visual (retinoid) cycle, a series of biochemical reactions in photoreceptor and adjacent RPE cells [1]. Impairments in the retinoid cycle can cause retinal degeneration, and animal models exhibiting disruption of the retinoid cycle have been instrumental for testing of gene transfer therapies and pharmacological interventions aimed at restoring the function of this cyclic pathway [2]. Notably, promising gene transfer therapies for human patients with RPE65 mutations were initially developed in knockout mice [3, 4]. An in-depth mechanistic understanding of the retinoid cycle in combination with relevant animal models promises to result in the establishment of successful interventions for human retinal disorders [1, 5–8].
1. Visual chromophore is regenerated by the retinoid cycle
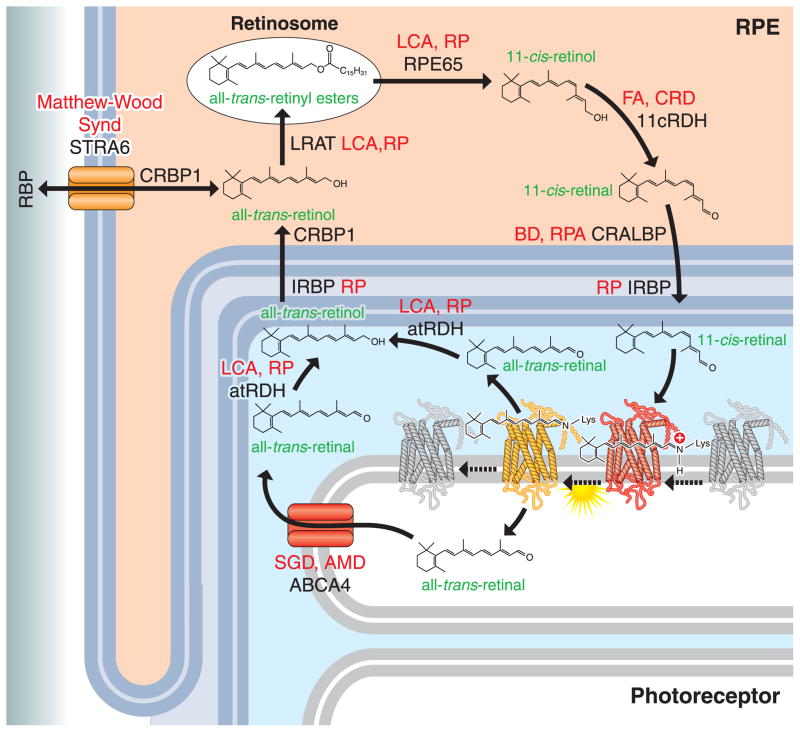
Regeneration of the visual chromophore, 11-cis-retinal, through the retinoid cycle consists of several biochemical reactions taking place in photoreceptors and adjacent RPE cells (Fig. 1). When photons are absorbed by visual pigments, the protein bound chromophore is photo-isomerized from an 11-cis to an all-trans conformation, inducing a conformational change in the complex that initiates phototransduction. This process mediates the conversion of light into electrical signals that are eventually conducted to the visual and frontal cortex of the brain. All-trans-retinal is hydrolyzed from bleached rhodopsin generating apo-opsin. Most of all-trans-retinal is released from opsin into the cytoplasm of rod outer segments (ROS) of the photoreceptor cell, but a fraction is released to the intra-discal lumen. This fraction is transported to the cytosolic space of photoreceptors by the ATP-binding cassette transporter (ABCA4), to join the rest of released all-trans-retinal that is then reduced to all-trans-retinol by all-trans-retinol dehydrogenases (RDHs, including RDH8 and RDH12). All-trans-retinol is then transported to RPE cells where lecithin:retinol acyltransferase (LRAT) esterifies it to generate all-trans-retinyl esters which can be stored in lipid droplets called ‘retinosomes’. All-trans-retinol is also supplied to the retina from the blood circulation through the activities of several protein factors, including serum retinol-binding protein (RBP), a cellular RBP receptor known as Stimulated by Retinoic Acid 6 (STRA6), cellular retinol-binding protein type 1 (CRBP1) and LRAT. All-trans-retinyl esters can be isomerized and hydrolyzed into to 11-cis-retinal by the retinoid isomerase RPE65. 11-cis-retinol is subsequently oxidized into 11-cis-retinol by 11-cis-RDHs such as RDH5 and RDH11 in RPE cells. The regenerated 11-cis-retinal is transported back to photoreceptors by carriers including the cellular retinaldehyde-binding protein (CRALBP) and interphotoreceptor retinoid-binding protein (IRBP). After replenishing photoreceptors, 11-cis-retinal covalently binds to the opsin moiety of visual pigments via a Schiff-base linkage to regenerate ground-state rhodopsin, which completes the retinoid cycle (recently reviewed [1]. Impairment of any reaction in this cycle can lead to human visual disorders.
Figure 1. Vitamin A metabolism in the eye and retinal disorders.
Vitamin A (all-trans-retinol) is supplied from blood circulation by the concerted action of serum retinol-binding protein (RBP), stimulated by retinoic acid 6 (STRA6), cellular retinol-binding protein type 1 (CRBP1) and lecithin:retinol acyl transferase (LRAT). Vitamin A is stored as all-trans-retinyl ester in the retinosomes of RPE cells. With all-trans-retinyl ester as substrate, RPE65 isomerizes the ester to 11-cis-retinol and then 11-cis-retinol dehydrogenases (RDHs) such as RDH5 and RDH11 oxidize 11-cis-retinol to 11-cis-retinal. 11-cis-Retinal binds to retinoid binding proteins including cellular retinaldehyde-binding protein (CRALBP) and inter-photoreceptor retinoid-binding protein (IRBP), and is then transported to photoreceptors to regenerate light-sensitive rhodopsin. When light hits the light-sensitive form of rhodopsin with 11-cis-retinal, 11-cis-retinal is photo-isomerized to all-trans-retinal. This conformational change initiates rhodopsin activation. All-trans-retinal is largely released from rhodopsin into the cytosolic lumen of rod photoreceptor cell outer segments, but a fraction remains in the intra-discal lumen. This portion of the aldehyde is flipped into the cytosolic lumen of photoreceptors by the ATP-binding cassette transporter (ABCA4), to join the rest of released all-trans-retinal that then becomes reduced by all-trans-retinol dehydrogenases (RDHs, including RDH8 and RDH12) to all-trans-retinol. All-trans-retinol is transported back to RPE cells by binding with IRBP and CRBP1. Impairment of any reaction in this cycle is closely associated with human retinal disorders (red). LCA, Leber congenital amaurosis; RP, retinitis pigmentosa; CRD, cone-rod dystrophy; FA, fundus albipunctatus; BD, Bothnia dystrophy; RPA, retinitis punctata albescens; SGD, Stargardt disease; AMD, age-related macular degeneration; Matthew-Wood Synd, Matthew-Wood Syndrome. This current work focuses mainly on models in mouse, which is rod dominant species, but identification of supplementary retinoid transformations in Müller cells could open the possibility to study the cone retinoid cycle that, in addition to the canonical retinoid cycle pathway, supports cone pigment regeneration [1, 5–8].
Note that Stecher et. al. [91] showed two pools of retinyl esters in the RPE, only one of which were substrates for retinoid isomerase. This discovery was followed by findings made by 1) Imanishi et al. who showed that a large amount of the esters are stored in retinosomes [23, 92]; and 2) Orban et al. and others showed that RPE65 does not localize retinosomes [93]. Thus, the fraction of retinyl esters embedded in the smooth ER are like the source of substrate for RPE65 (not distinct from retinosomes on the figures).
2. Animal models of human retinal diseases caused by retinoid cycle impairments
The successful application of modern scientific technology has led to breakthrough discoveries of key components for vitamin A production, transport and visual chromophore generation and recycling. Various animal models with genetic defects in these genes have been generated (Table 1). Many of these animal models essentially recapitulate blinding human retinal diseases.
Table 1.
Animal models with impaired retinoid cycle.
| Animal models | Species | Localization | Photoreceptor changes | RPE changes | Retinoids | Other changes | Ref. |
|---|---|---|---|---|---|---|---|
| Bcmo1−/− | mouse | RPE | pro-vitamin in adipose tissues, fatty liver | [9] | |||
| Bcdo2−/− | mouse | RPE | carotenoids acml7, mitochondrial dysfunction | [11] | |||
| Rbp4−/− | mouse | Plasma, RPE | low atROL2 in the eye | [12] | |||
| Stra6−/− | mouse | RPE | rapid cone deg.1, slow progressive rod deg. | no retinosomes | no 11cRAL3, 95% loss of RE4 | [18] | |
| Crbp1−/− | mouse | RPE | low RE | [22] | |||
| Lrat−/− | mouse | RPE | rapid cone deg., slow progressive rod deg. | no retinosomes | no 11cRAL, no RE | [25, 26] | |
| Rpe65−/− | mouse | RPE | rapid cone deg., slow progressive rod deg. | large retinosomes | atRE4 acml, no 11cRAL | [32] | |
| Rpe65 (R91W/ R91W) | mouse | RPE | rapid cone deg., slow progressive rod deg. | atRE acml, no 11cRAL | [35] | ||
| Rpe65- rd12 | mouse | RPE | rapid cone deg., slow progressive rod deg. | atRE acml, no 11cRAL | [33] | ||
| RPE65−/− (4bp- deletion) | dog | RPE | rapid cone deg., slow progressive rod deg. | atRE acml, no 11cRAL | [34] | ||
| C57BL/6 (Leu450 Met) | mouse | RPE | slow 11cRAL reg. 8 | [36] | |||
| Rdh5−/− | mouse | RPE | 13cRE5 acml, slow 11cRAL reg. | delayed DA9 | [41] | ||
| Rdh11−/− | mouse | RPE and IS11 | [42, 90] | ||||
| Rdh5−/− Rdh11−/− | mouse | slow cone deg. | 13cRE acml, slow 11cRAL reg. | delayed DA | [42, 45] | ||
| Rdh10−/− | mouse | RPE and Müller cells | Embryonic lethality | [43] | |||
| Rlbp1−/− | mouse | RPE and Müller cells | slow 11cRAL reg. | LD10, delayed DA | [50] | ||
| Abca4−/− | mouse | OS12 | slow rod deg. in albino | lipofuscin | A2E6 acml, delayed atRAL clearance | LD, delayed DA | [55, 56] |
| Rdh12−/− | mouse | IS | lipofuscin | A2E acml | LD, delayed DA | [64, 65] | |
| Rdh8−/− | mouse | OS | lipofuscin | A2E acml, delayed atRAL clearance | LD, delayed DA | [63] | |
| Rdh8−/− Rdh12−/− | mouse | slow cone and rod deg. | lipofuscin | A2E acml, delayed atRAL clearance | LD, delayed DA | [66] | |
| Abca4−/− Rdh8−/− | mouse | rod and cone deg. | lipofuscin, RPE atrophgy | A2E acml, delayed atRAL clearance | LD, delayed DA | [68] | |
| Irbp−/− | mouse | extracellular matrix of photoreceptors | slow rod and cone deg. | [70, 71] | |||
| Rgr−/− | mouse | RPE and Müller cells | low 11cRAL under light adapted eyes | [73] | |||
|
Rgr−/− Rpe65−/− |
mouse | [74, 75] | |||||
| Des1−/− | mouse | Müller cells and RPE (widely expressed) | Small size, scaly skin, liver dysfunctions, die at 8–10 weeks old | [80] |
deg1, degeneration; atROL2, all-trans-retinol; 11cRAL3, 11-cis-retinal; RE4, retinyl esters; atRE4, all-trans-retinyl ester; 13cRE5, 13-cis-retinyl ester; A2E6, di-retinoid-pyridinium-ethanolamine; acml7, accumulation; reg8, regeneration; DA9, dark adaptation; LD10, light-induced damage; IS11, photoreceptor inner segment; OS12, photoreceptor outer segment.
2.1. Impaired transport of retinoids from the systemic circulation to the eyes is associated with retinal diseases
In humans, β-carotene is a precursor of vitamin A (all-trans-retinol). All-trans-retinal is produced after cleavage of β-carotene supplied from the diet by the enzymes β-carotene-15,15′-monooxygenase (BCMO1). A second related enzyme β-carotene-9′,10′-dioxygenase (BCDO2) helps maintain carotenoid homeostasis more generally. Once generated and reduced in enterocytes of the intestine, all-trans-retinol is esterified to form retinyl esters (REs) and packaged into chylomicrons. Some RE from chylomicrons is taken up into peripheral cells in process that involves hydrolysis by lipoprotein lipase. The vast majority is delivered to the liver for storage. The liver secretes all-trans-retinol bound to RBP that travels through the blood circulation. From there it is transported into the eye through a process that is facilitated by STRA6, CRBP1 and LRAT in the RPE (Fig. 1).
Bcmo1−/− and Bcdo2−/− mice have been generated and previously characterized [9, 10]. Neither of these mouse models display a pathological eye phenotype when maintained on diets that contain preformed vitamin A. Moreover, no human retinal diseases attributed to these gene deficiencies have been reported. Bcmo1−/− mice dietary supplemented with carotene accumulate large quantities of this pro-vitamin in adipose tissues. Besides defects in carotene metabolism, Bcmo1−/− mice display general abnormalities in lipid homeostasis. Even when provided with vitamin A-sufficient chow, Bcmo1−/− mice develop fatty livers and display altered serum lipid levels with elevated unesterified fatty acids [9, 10]. This gene knockout mouse is also more susceptible to high fat diet-induced disruptions in fatty acid metabolism. In Bcdo2-deficient mice, carotenoid homeostasis is disrupted and carotenoids accumulate in several tissues, especially in hepatic tissue. These accumulated carotenoids are associated with mitochondrial dysfunction that can cause oxidative stress and disease [11].
Characterization of Rbp4−/− mice, reported in 1999 [12], revealed undetectable levels of serum all-trans-retinol. Amounts of all-trans-retinol in the eye are reduced to 25% at 3 weeks of age, but retinoid levels surprisingly increase with age. However, compared with WT eyes, retinoid levels are still lower in aged Rbp4−/− mice. Retinal function is impaired due to insufficient levels of retinoids, and ERG recordings from 4-week-old Rbp4−/− mice reveal a 100-fold lowered sensitivity to light. As levels of retinoids in the eye increase with age, 24 weeks-old Rbp4−/− mice manifest apparently normal ERGs with deep a-waves, high b-waves and a rapid falling phase. RBP4 mutations have been found in patients with RPE and retinal atrophy [13, 14]. Additionally, elevation of serum RBP4 in patients has also been linked to type 2 diabetes, cardiovascular disease and diabetic retinopathy [15–17].
STRA6 is essential for facilitating vitamin A uptake into to the eye [18] and Stra6−/− mice have drastically diminished levels of retinoids in their eyes [18, 19]. Due to the lack of 11-cis-retinal, their retinas are not functional. The consequences of STRA6 mutations are manifested in a variety of human disorders [20, 21], which include pulmonary dysgenesis, cardiac malformations, mental retardation, and microphthalmia/anophthalmia, collectively referred to as Matthew-Wood syndrome. In contrast, Stra6−/− mice only display an abnormal eye phenotype.
Crbp1−/− mice display reduced all-trans-retinyl esters to 33% of WT levels, however the amount of 11-cis-retinal is similar to WT mice [22]. This phenotype indicates that CRBP1 plays a major role in diffusing all-trans-retinol into the RPE cells. Mutations in CRBP1 have not yet been reported in human retinal disorders.
2.2. Mutations in LRAT cause LCA and RP
LRAT esterifies all-trans-retinol to all-trans-retinyl esters which are stored in retinosomes [23] of RPE cells. Mutations in LRAT cause Leber congenital amaurosis (LCA) and retinitis pigmentosa (RP) [24] (Fig. 1). Retinoid analyses of Lrat−/− mouse eyes reveal only all-trans-retinol [25]. Due to the absence of 11-cis-retinal, Lrat−/− retinas lack visual function and exhibit nearly non-detectable ERG responses and no pupillary response to light illumination. In 2005, Lin et. al. described Lrat−/− mice that do not carry a neo-cassette in the knockout target construct [26]. Similar to the previously reported Lrat−/− mice [25], nearly no retinoids are detected in the eyes, demonstrating the neo-cassette insertion did not affect the phenotype in the original report. Notably, visual function loss in Lrat−/− mice can be reversed by either supplementation with artificial visual chromophores or Lrat gene transfer [27].
2.3. Defects in RPE65 are associated with LCA and RP
RPE65 is an RPE-specific isomerase which generates 11-cis-retinol from an all-trans-retinyl ester substrate [28–30]. RPE65 mutations are associated with human retinal disorders including LCA and RP [31]. Redmond et. al. generated an Rpe65−/− mouse and reported its visual phenotype in 1998 [32]. Since then, this mouse model has been widely used for understanding the pathogenesis of human RPE65-associated diseases and testing potential interventions. In 2005, a causal gene for retinal degeneration 12 (rd12) mice with naturally occurring retinal degeneration was identified as a RPE65 mutation [33]. Sequence analysis of Rpe65 cDNA from rd12 mice shows a single base substitution at position 130 (C to T) of exon 3, which leads to a stop codon at amino acid position 44 (CGA to TGA). Rpe65−/− and rd12 mice show very similar phenotypes with early cone degeneration, slow progressive rod degeneration, lipid inclusions in RPE cells, retinyl ester accumulation, leading to exacerbated retinosome formation [23], and no endogenous production of 11-cis-retinal. Due to lack of 11-cis-retinal, Rpe65−/− mice and rd12 mice display only barely detectable ERG responses to high intensity light impulses at various wavelengths. It was also found that an RPE65 mutation spontaneously occurs in blind dogs [34]. Samardzija et. al. generated a mouse with the R91W mutation in RPE65 to study LCA phenotypes observed in humans with the same mutation [35]. C57BL/6 mice naturally carry the Rpe65 mutation that results in a Leu to Met substitution at position 450 [36], resulting in reduced enzymatic activity. Decreased production of 11-cis-retinal results in a milder light-induced retinal degeneration phenotype in mice expressing this RPE65 variant.. In 2001, the first gene transfer therapy was conducted with Swedish Briard dogs carrying an RPE65 mutation [37]. To date, many gene transfer studies involving RPE65 dogs, Rpe65−/− mice and rd12 mice have been performed. In addition to gene transfer therapies, these RPE65 animals have been used for developing pharmacological treatments, including supplementation with the visual chromophores 9-cis-retinal and 11-cis-retinal [38].
2.4. RDH5 mutations cause fundus albipunctatus and late-onset cone dystrophy
Oxidation of 11-cis-retinol to 11-cis-retinal is catalyzed by 11-cis-retinol dehydrogenases in the RPE. Currently RDH5, RDH11 and RDH10 have detectable RPE expression and 11-cis-RDH activity (Fig. 1). RDH5 (one of the 11cis-RDHs of the RPE) mutations are found in patients with fundus albipunctatus (FA) which is characterized by white dots in fundus images and night blindness [39]. Some patients with FA have been reported to develop cone dystrophy after their 4th decade of life [40]. Unexpectedly, Rdh5−/− mice do not recapitulate any human visual phenotype except a mild delay in dark adaptation [41]. Rdh5−/−Rdh11−/− mice show slightly slowed dark adaptation, and late-onset cone dysfunction at 12 months of age [42]. Because Rdh5−/−Rdh11−/− mice manifest only a slightly abnormal retinal phenotype, a contribution of other RDHs, most likely RDH10, to generation of 11-cis-retinal from 11-cis-retinol has been suspected. However, because knockout of Rdh10 gene produces mice that are not viable [43], this hypothesis can only be tested by conditional knockout of Rdh10. Cone dysfunction of Rdh5−/−Rdh11−/− mice can be prevented by supplementation with 9-cis-retinal [44], indicating that retinal degeneration associated with 11-cis-RDH deficiency is characterized by lower levels of visual chromophore. Defects in RDH function in mice is also associated with accumulation of 13-cis-retinyl ester in RPE cells [45].
2.5. RLBP1 defects are associated with RP
CRALBP, encoded by the retinaldehyde-binding protein 1 (RLBP1) gene, transports 11-cis-retinal to photoreceptor cells. A complex consisting of RPE65, RDH5 and CRALBP can form in vitro and CRALBP increases the production of 11-cis-retinol by RPE65 in biochemical assays [46]. Mutations in RLBP1 are associated with RP, Bothnia dystrophy and retinitis punctata albescens (RPA) [47–49]. Although Rlbp1−/− mice do not show retinal degeneration under room light conditions, acute retinal degeneration can be induced in this mouse model by intense light illumination [50]. Moreover, delayed 11-cis-retinal regeneration also occurs after intense light exposure.
2.6. ABCA4 mutations are associated with human macular degeneration
ABCA4 mutations are associated with Stargardt disease, a juvenile form of macular dystrophy [51]. Higher risk of AMD has been also reported in individuals with ABCA4 mutations [52, 53]. ABCA4 translocates all-trans-retinal from the intra-discal lumen of photoreceptor discs to the cytosolic space of outer segments [54]. Abca4−/− mice show accelerated age-related di-retinoid-pyridinium-ethanolamine (A2E) accumulation in their RPE cells [55]. A2E is an important component of autofluorescent lipofuscin granules. Mild rod photoreceptor degeneration was reported in albino Abca4−/− mice [56]. Unexpectedly, Abca4−/− mice do not mimic the retinal degeneration observed in humans with ABCA4 defects until crossbred with Rdh8−/− mice (described in section 2.8). Pharmacological interventions by retinoid cycle modulators and gene transfer therapies have been tested in both Abca4−/− and the latter double knockout models [57–62].
2.7. Mutations in RDH12 cause LCA and severe types of RP
Delayed clearance of all-trans-retinal plays a leading role in the pathogenesis of retinal degeneration because this retinoid is a reactive aldehyde. All-trans-retinal can also form condensation products such as A2E and all-trans-retinal dimers which also are closely associated with retinal disease pathology. Conversion of all-trans-retinal to all-trans-retinol is catalyzed by all-trans-RDHs including RDH8 and RDH12. RDH8 is found in the outer segments of rod and cone photoreceptors [63], whereas RDH12 is located in the inner segments of rod and cone cells [64, 65]. In mice, RDH8 is responsible for ~70% of the all-trans-RDH activity and RDH12 carries out ~30% [66]. However, mice with a double deletion of RDH8 and RDH12 still display residual all-trans-RDH activity in vivo. Both Rdh8−/− and Rdh12−/− mice accumulate large amounts of A2E in their RPE cells but don’t display obvious degenerative changes of the eyes under room lighting conditions. But these mice do exhibit light-induced retinal degeneration after exposure to intense illumination. Rdh8−/−Rdh12−/− mice display slowly progressive cone and rod photoreceptor degeneration with A2E over-accumulation in the RPE cells. Currently no causal mutations of RDH8 are known in patients with retinal disorders but mutations in RDH12 are found in patients with LCA and RP [67].
2.8. Double knockout of ABCA4 and RDH8 in mice recapitulates human retinal pathology
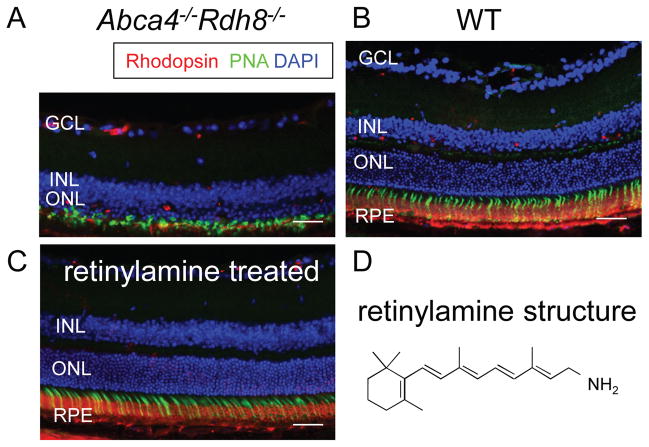
Abca4−/−Rdh8−/− mice, deficient in the clearance of all-trans-retinal from photoreceptor cells, display progressive rod-cone degeneration with A2E over-accumulation, RPE atrophy and complement deposition at Bruch’s membrane [68]. Additionally, some Abca4−/−Rdh8−/− mice develop choroidal neovascularization. The phenotype of this mouse shows many similarities to human AMD and Stargardt disease. Notably, these mice develop age-related retinal degeneration as well as acutely accelerated degeneration in response to intense light illumination (Fig. 2). Most photoreceptors undergo apoptosis with TUNEL-positive staining observed 24 h after bright light exposure [69]. Importantly, retinal degeneration in this mouse can be prevented by pre-treatment with a retinoid cycle inhibitor, retinylamine (Fig. 2). Thus, this mouse has already proven valuable for testing the efficacy of drug candidates that ameliorate retinal dystrophies [61, 62].
Figure 2. Acute bright light-induced retinal degeneration could be prevented by treatment of retinylamine in Abca4−/− Rdh8−/− mice.
Light exposure at 10,000 lux for 30 min can cause light-induced retinal degeneration in Abca4−/− Rdh8−/− mice (A), whereas the stimulus has virtually no impact on wild-type (WT) mice (B). Light-induced retinal degeneration in Abca4−/− Rdh8−/− mice can be prevented by pre-treatment with retinylamine at 1 mg/ml given by oral gavage (C). The chemical structure of retinylamine is presented (D). Retinal sections were prepared 7 days after light exposure. Rod photoreceptors, cone photoreceptors and nuclei were stained by anti-rhodopsin antibody (red), peanut agglutinin (PNA, green) and DAPI (blue), respectively. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigmented epithelium. Scale bars indicate 30 μm.
2.9. IRBP defects are associated with RP
Interphotoreceptor retinoid-binding protein (IRBP), or interstitial retinol-binding protein 3 (RBP3), is proposed to be important for the intercellular exchange of retinoids, serving to prevent the potentially cytotoxic effects of these compounds. As early as postnatal day (PND) 11, histological examination of the retinas of Irbp−/− mice reveals a loss of photoreceptor nuclei and changes in the structural integrity of the outer segments [70]. At PND30, the photoreceptor abnormalities become more severe and electroretinographic recordings reveal a marked loss in photosensitivity. Other studies with Irbp−/− mice suggest a role for IRBP in the rapid regeneration of cone pigments. Under photopic ERG conditions with 10 Hz flickering light (a condition used to isolate cone responses), the Irbp−/− mouse does not regenerate cone pigments as quickly as C57BL/6J (WT) mice [71]. These observations indicate that IRBP plays roles in both photoreceptor development and function and knockout of IRBP can result in a slowly progressive degeneration of retinal photoreceptors.
2.10. RGR mutations are associated with human RP
Retinal G protein-coupled receptor (RGR) was first cloned from a retinal epithelium enriched-cDNA library as an opsin homolog bound with 11-cis-retinal [72]. RGR localizes specifically to intracellular membranous compartments of RPE and Müller cells. Rgr−/− mice were characterized in 2001, as displaying less efficient light-dependent 11-cis-retinal production from all-trans-retinal. From this finding it was concluded that RGR is involved in an alternative pathway of 11-cis-retinal regeneration by harnessing light energy in an RPE65-independent manner [73]. However, other groups failed to confirm the contribution of RGR to rhodopsin regeneration, suggesting that RGR contributes only slightly to the retinoid cycle, potentially as an enhancer of isomerization or a retinal flow mediator [74, 75]. RGR mutations have been detected in a sub-population of RP patients [76].
2.11. DES1 is associated with cone pigment regeneration
Based on biochemical results, cone retinoid cycle was proposed to supplement chromophore for cone pigment regeneration in additional to the canonical retinoid cycle [77, 78]. Dihydroceramide desaturase-1 (DES1) is a potential isomerase that supports the cone retinoid cycle [79]. Des1−/− mice reveal an incompletely penetrant lethality and surviving animals are small in size with a complex phenotype [80]. This dysfunction includes decreased body weight, scaly skin, hematological abnormalities, and abnormal liver function. The Des1−/− animals die 8 to 10 weeks after birth. The eye phenotype has not been examined. No DES1 mutations are known to be causal for retinal disorders in patients.
3. Therapies for animal models of retinal degeneration pertaining to defective retinoid cycle
Two major pharmacological concepts underlie the treatment of retinal disorders that are associated with a defective retinoid cycle, namely the supplementation of visual chromophore or drug analogs thereof and the prevention of the formation of toxic of retinoid cycle biproducts including all-trans-retinal and A2E. Gene replacement therapy has been also tested in animal models as a method to restore the healthy state and thus vision in degenerative diseases of the retina.
3.1. Pharmacological therapies
Supplementation of visual chromophore has been performed with both 11-cis-retinal and 9-cis-retinoids [38, 81]. Impaired production of the 11-cis-retinal chromophore is found in Rpe65−/−, Lrat−/−, Rlbp1−/− and Rdh5−/−Rdh11−/− mice [27, 38, 44, 71]. Supplementation with the visual chromophore successfully reverses retinal dysfunction and degeneration in these mice [82].
Preventive approaches against toxic side effects of components of the retinoid cycle, all-trans-retinal and its condensation product A2E, have been implemented in animals which show delayed clearance of all-trans-retinal after light exposure, including Abca4−/− and Abca4−/−Rdh8−/− mice. The first approach to reduce toxicity of retinoid cycle byproducts relied on slowing down the retinoid cycle. Such an approach could theoretically reduce production of all-trans-retinal and subsequently A2E. Isotretinoin (Accutane) (which can decrease RDH5 activity [57]), N-(4-hydroxyphenyl) retinamide (HPR) and A1120 (which reversibly reduce serum retinol [58, 59]) and retinylamine which inhibits RPE65 activity [83] have all been tested. All of these compounds can mitigate retinal pathology; however, they all also cause delayed dark adaptation due to 11-cis-retinal deficiency. In 2011, all-trans-retinal-associated retinal degeneration was prevented using FDA-approved drugs containing primary amino groups. It was demonstrated that these drugs form a transient Schiff-base intermediate with all-trans-retinal, thereby reducing the toxicity by this reactive aldehyde [61]. In addition to drugs with primary amino groups, inhibition of downstream pathways involved in all-trans-retinal-induced toxicity, including phospholipase C and NADPH oxidase, also successfully prevents retinal degeneration [62]. Unlike inhibition of the retinoid cycle, such indirect approaches do not grossly affect the retinoid cycle and can avoid drug-induced delayed dark adaptation.
3.2. Gene transfer therapies
Gene transfer therapies for RPE65-deficient dogs [37], as well as Rpe65−/− [84], rd12 [85] and RPE65-R91W/R91W [86] mice have been intensely pursued based on adeno-associated and Lenti viruses. Over the years more than two dozen studies have been reported [37, 84, 87]. These efforts have contributed to successful treatment of human patients with RPE65 mutations [3, 4]. Gene transfer therapies with Lenti virus in Lrat−/− mice also rescued visual functions [27]. In Abca4−/− mice, the use of non-viral nanoparticles to deliver a 6.8 kb ABCA4 cDNA ameliorates the visual phenotype of this mouse mutant [60].
3.3 Other approaches
Retinal cell death is the final consequence of retinal degeneration associated with a defective retinoid cycle. Pharmacological interventions have been employed to target the mechanisms of cellular death, as has been done for other types of neural degeneration. These interventions include inhibition of apoptosis pathways and suppression of oxidative stress [88, 89]. Thus, several parallel approaches are currently being employed to develop therapies.
CONCLUSIONS
Continuous regeneration of the visual chromophore through the retinoid cycle is essential to vision. Impairment of this pathway can lead to human retinal disorders. The reactions of these pathways have been well-documented, especially in rod photoreceptor cells. Transgenic and knockout mice lacking retinoid metabolic enzymes, such as Rpe65−/− and Lrat−/− animals, recapitulate many features of the human diseases. These animal models allow testing of novel therapeutics for retinal diseases. Effective gene transfer and pharmacologic therapies developed in these animal models have successfully been applied to humans. Progress from the test tube to the bed-side has become a reality for retinal diseases involving the retinoid cycle.
Highlights.
The visual (retinoid) cycle is a critical enzymatic pathway that sustains vision
Mutations in genes encoding visual cycle proteins cause retinal degeneration
Animal models were generated with faulty visual cycles that mimic human pathologies
These animals are instrumental for testing new therapies to prevent blindness
Acknowledgments
We thank Dr. L. T. Webster Jr. (Case Western Reserve University, CWRU), Dr. Johannes von Lintig, Dr. T. Maeda (CWRU) and members of the Palczewski and Maeda laboratories for their comments. This work was supported by funding from the National Eye Institute of the National Institutes of Health (grants R24EY021126 (KP), R01EY009339 (KP), R01EY022658 (AM)), Research to Prevent Blindness Foundation, Foundation Fighting Blindness, and Ohio Lions Eye Research Foundation. K.P. is John H. Hord Professor of Pharmacology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kiser PD, et al. Chemistry of the Retinoid (Visual) Cycle. Chem Rev. 2013 doi: 10.1021/cr400107q. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Lintig J, et al. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem Sci. 2010;35:400–410. doi: 10.1016/j.tibs.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maguire AM, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bainbridge JW, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 5.McBee JK, et al. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retina Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 6.Travis GH, et al. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang PH, et al. New insights into retinoid metabolism and cycling within the retina. Prog Retina Eye Res. 2013;32:48–63. doi: 10.1016/j.preteyeres.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retina Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Hessel S, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–33561. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 10.Amengual J, et al. Beta-Carotene Reduces Body Adiposity of Mice via BCMO1. PLoS One. 2011;6:e20644. doi: 10.1371/journal.pone.0020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amengual J, et al. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011;25:948–959. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quadro L, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeliger MW, et al. Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis. Invest Ophthalmol Vis Sci. 1999;40:3–11. [PubMed] [Google Scholar]
- 14.Cukras C, et al. Exome analysis identified a novel mutation in the RBP4 gene in a consanguineous pedigree with retinal dystrophy and developmental abnormalities. PLoS One. 2012;7:e50205. doi: 10.1371/journal.pone.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li ZZ, et al. Serum retinol-binding protein 4 levels in patients with diabetic retinopathy. J Int Med Res. 2010;38:95–99. doi: 10.1177/147323001003800111. [DOI] [PubMed] [Google Scholar]
- 16.Graham TE, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, et al. Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: a prospective analysis among women in the nurses’ health study. Circulation. 2013;127:1938–1947. doi: 10.1161/CIRCULATIONAHA.113.002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz A, et al. Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci. 2012;53:3027–3039. doi: 10.1167/iovs.11-8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawaguchi R, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 20.Pasutto F, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golzio C, et al. Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am J Hum Genet. 2007;80:1179–1187. doi: 10.1086/518177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saari JC, et al. Analysis of the visual cycle in cellular retinol-binding protein type I (CRBPI) knockout mice. Invest Ophthalmol Vis Sci. 2002;43:1730–1735. [PubMed] [Google Scholar]
- 23.Imanishi Y, et al. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson DA, et al. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet. 2001;28:123–124. doi: 10.1038/88828. [DOI] [PubMed] [Google Scholar]
- 25.Batten ML, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- 27.Batten ML, et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moiseyev G, et al. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redmond TM, et al. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin M, et al. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marlhens F, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 32.Redmond TM, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 33.Pang JJ, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA) Mol Vis. 2005;11:152–162. [PubMed] [Google Scholar]
- 34.Aguirre GD, et al. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998;4:23. [PubMed] [Google Scholar]
- 35.Samardzija M, et al. R91W mutation in Rpe65 leads to milder early-onset retinal dystrophy due to the generation of low levels of 11-cis-retinal. Hum Mol Genet. 2008;17:281–292. doi: 10.1093/hmg/ddm304. [DOI] [PubMed] [Google Scholar]
- 36.Wenzel A, et al. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acland GM, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 38.Van Hooser JP, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci U S A. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto H, et al. Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat Genet. 1999;22:188–191. doi: 10.1038/9707. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura M, et al. A high association with cone dystrophy in Fundus albipunctatus caused by mutations of the RDH5 gene. Invest Ophthalmol Vis Sci. 2000;41:3925–3932. [PubMed] [Google Scholar]
- 41.Driessen CA, et al. Disruption of the 11-cis-retinol dehydrogenase gene leads to accumulation of cis-retinols and cis-retinyl esters. Mol Cell Biol. 2000;20:4275–4287. doi: 10.1128/mcb.20.12.4275-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim TS, et al. Delayed dark adaptation in 11-cis-retinol dehydrogenase-deficient mice: a role of RDH11 in visual processes in vivo. J Biol Chem. 2005;280:8694–8704. doi: 10.1074/jbc.M413172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandell LL, et al. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeda A, et al. Improvement in rod and cone function in mouse model of Fundus albipunctatus after pharmacologic treatment with 9-cis-retinal. Invest Ophthalmol Vis Sci. 2006;47:4540–4546. doi: 10.1167/iovs.06-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeda A, et al. Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters. Biochemistry. 2006;45:4210–4219. doi: 10.1021/bi052382x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golczak M, et al. Importance of membrane structural integrity for RPE65 retinoid isomerization activity. J Biol Chem. 2010;285:9667–9682. doi: 10.1074/jbc.M109.063941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maw MA, et al. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat Genet. 1997;17:198–200. doi: 10.1038/ng1097-198. [DOI] [PubMed] [Google Scholar]
- 48.Burstedt MS, et al. Bothnia dystrophy caused by mutations in the cellular retinaldehyde-binding protein gene (RLBP1) on chromosome 15q26. Invest Ophthalmol Vis Sci. 1999;40:995–1000. [PubMed] [Google Scholar]
- 49.Morimura H, et al. Recessive mutations in the RLBP1 gene encoding cellular retinaldehyde-binding protein in a form of retinitis punctata albescens. Invest Ophthalmol Vis Sci. 1999;40:1000–1004. [PubMed] [Google Scholar]
- 50.Saari JC, et al. Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron. 2001;29:739–748. doi: 10.1016/s0896-6273(01)00248-3. [DOI] [PubMed] [Google Scholar]
- 51.Allikmets R, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 52.Allikmets R, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 53.Fritsche LG, et al. A subgroup of age-related macular degeneration is associated with mono-allelic sequence variants in the ABCA4 gene. Invest Ophthalmol Vis Sci. 2012;53:2112–2118. doi: 10.1167/iovs.11-8785. [DOI] [PubMed] [Google Scholar]
- 54.Quazi F, et al. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Commun. 2012;3:925. doi: 10.1038/ncomms1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weng J, et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 56.Wu L, et al. Photoreceptor cell degeneration in Abcr (−/−) mice. Adv Exp Med Biol. 2010;664:533–539. doi: 10.1007/978-1-4419-1399-9_61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radu RA, et al. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Proc Natl Acad Sci U S A. 2003;100:4742–4747. doi: 10.1073/pnas.0737855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radu RA, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 59.Dobri N, et al. A1120, a nonretinoid RBP4 antagonist, inhibits formation of cytotoxic bisretinoids in the animal model of enhanced retinal lipofuscinogenesis. Invest Ophthalmol Vis Sci. 2013;54:85–95. doi: 10.1167/iovs.12-10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Z, et al. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J Clin Invest. 2012;122:3221–3226. doi: 10.1172/JCI64833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maeda A, et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol. 2012;8:170–178. doi: 10.1038/nchembio.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, et al. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J Biol Chem. 2012;287:5059–5069. doi: 10.1074/jbc.M111.315432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maeda A, et al. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maeda A, et al. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurth I, et al. Targeted disruption of the murine retinal dehydrogenase gene Rdh12 does not limit visual cycle function. Mol Cell Biol. 2007;27:1370–1379. doi: 10.1128/MCB.01486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maeda A, et al. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci U S A. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Janecke AR, et al. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36:850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- 68.Maeda A, et al. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maeda A, et al. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liou GI, et al. Early onset photoreceptor abnormalities induced by targeted disruption of the interphotoreceptor retinoid-binding protein gene. J Neurosci. 1998;18:4511–4520. doi: 10.1523/JNEUROSCI.18-12-04511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parker RO, et al. Normal cone function requires the interphotoreceptor retinoid binding protein. J Neurosci. 2009;29:4616–4621. doi: 10.1523/JNEUROSCI.0063-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang M, et al. An opsin homologue in the retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1993;34:3669–3678. [PubMed] [Google Scholar]
- 73.Chen P, et al. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet. 2001;28:256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- 74.Maeda T, et al. Evaluation of the role of the retinal G protein-coupled receptor (RGR) in the vertebrate retina in vivo. J Neurochem. 2003;85:944–956. doi: 10.1046/j.1471-4159.2003.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wenzel A, et al. The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J Biol Chem. 2005;280:29874–29884. doi: 10.1074/jbc.M503603200. [DOI] [PubMed] [Google Scholar]
- 76.Morimura H, et al. Mutations in RGR, encoding a light-sensitive opsin homologue, in patients with retinitis pigmentosa. Nat Genet. 1999;23:393–394. doi: 10.1038/70496. [DOI] [PubMed] [Google Scholar]
- 77.Berson EL, Goldstein EB. The early receptor potential in sex-linked retinitis pigmentosa. Invest Ophthalmol. 1970;9:58–63. [PubMed] [Google Scholar]
- 78.Hood DC, Hock PA. Recovery of cone receptor activity in the frog’s isolated retina. Vision Res. 1973;13:1943–1951. doi: 10.1016/0042-6989(73)90065-5. [DOI] [PubMed] [Google Scholar]
- 79.Kaylor JJ, et al. Identification of DES1 as a vitamin A isomerase in Muller glial cells of the retina. Nat Chem Biol. 2013;9:30–36. doi: 10.1038/nchembio.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Rohrer B, et al. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46:3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- 82.Palczewski K. Retinoids for treatment of retinal diseases. Trends Pharmacol Sci. 2010;31:284–295. doi: 10.1016/j.tips.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Golczak M, et al. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc Natl Acad Sci U S A. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rakoczy PE, et al. Assessment of rAAV-mediated gene therapy in the Rpe65−/− mouse. Adv Exp Med Biol. 2003;533:431–438. doi: 10.1007/978-1-4615-0067-4_55. [DOI] [PubMed] [Google Scholar]
- 85.Nusinowitz S, et al. Cortical visual function in the rd12 mouse model of Leber Congenital Amarousis (LCA) after gene replacement therapy to restore retinal function. Vision Res. 2006;46:3926–3934. doi: 10.1016/j.visres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Kostic C, et al. Gene therapy regenerates protein expression in cone photoreceptors in Rpe65(R91W/R91W) mice. PLoS One. 2011;6:e16588. doi: 10.1371/journal.pone.0016588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Narfstrom K, et al. Functional and structural evaluation after AAV. RPE65 gene transfer in the canine model of Leber’s congenital amaurosis. Adv Exp Med Biol. 2003;533:423–430. doi: 10.1007/978-1-4615-0067-4_54. [DOI] [PubMed] [Google Scholar]
- 88.Zhang T, et al. Chemical chaperone TUDCA preserves cone photoreceptors in a mouse model of Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2012;53:3349–3356. doi: 10.1167/iovs.12-9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maeda T, et al. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Invest Ophthalmol Vis Sci. 2009;50:4917–4925. doi: 10.1167/iovs.09-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kasus-Jacobi A, et al. Functional characterization of mouse RDH11 as a retinol dehydrogenase involved in dark adaptation in vivo. J Biol Chem. 2005;280:20413–20420. doi: 10.1074/jbc.M413789200. [DOI] [PubMed] [Google Scholar]
- 91.Stecher H, et al. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J Biol Chem. 1999;274:8577–8585. doi: 10.1074/jbc.274.13.8577. [DOI] [PubMed] [Google Scholar]
- 92.Imanishi Y, et al. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J Cell Biol. 2004;166:447–453. doi: 10.1083/jcb.200405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Orban T, et al. Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J Biol Chem. 2011;286:17248–17258. doi: 10.1074/jbc.M110.195198. [DOI] [PMC free article] [PubMed] [Google Scholar]