Abstract
Background
Renal dysfunction associated with acute decompensated heart failure (ADHF) is associated with impaired outcomes. Its mechanism is attributed to renal arterial hypoperfusion or venous congestion, but its prognostic impact based on each of these clinical profiles requires elucidation.
Methods and Results
ADHF syndromes registry subjects were evaluated (N = 4,321). Logistic regression modeling calculated adjusted odds ratios (OR) for in-hospital mortality for patients with and without renal dysfunction. Renal dysfunction risk was calculated for subgroups with hypoperfusion-dominant (eg. cold extremities, a low mean blood pressure or a low proportional pulse pressure) or congestion-dominant clinical profiles (eg. peripheral edema, jugular venous distension, or elevated brain natriuretic peptide) to evaluate renal dysfunction's prognostic impact in the context of the two underlying mechanisms. On admission, 2,150 (49.8%) patients aged 73.3±13.6 years had renal dysfunction. Compared with patients without renal dysfunction, those with renal dysfunction were older and had dominant ischemic etiology jugular venous distension, more frequent cold extremities, and higher brain natriuretic peptide levels. Renal dysfunction was associated with in-hospital mortality (OR 2.36; 95% confidence interval 1.75–3.18, p<0.001), and the prognostic impact of renal dysfunction was similar in subgroup of patients with hypoperfusion- or congestion-dominant clinical profiles (p-value for the interaction ranged from 0.104–0.924, and was always >0.05).
Conclusions
Baseline renal dysfunction was significantly associated with in-hospital mortality in ADHF patients. The prognostic impact of renal dysfunction was the same, regardless of its underlying etiologic mechanism.
Introduction
Despite advances in pharmacological and mechanical therapies, acute decompensated heart failure (ADHF) remains one of the most frequently encountered and life-threatening cardiovascular conditions [1]. The EuroHeart Failure Survey, which included 11,327 patients with ADHF, showed that post-discharge mortality rates reached 8.1% and 20.5% within 3 months and 1 year, respectively [2].
Baseline renal dysfunction is one of the most important predictors of short- and long-term cardiovascular outcomes in patients with ADHF [3]–[5]. Although several mechanisms coexist in the deterioration of renal function among ADHF patients [6], [7], two hemodynamic mechanisms, renal arterial hypoperfusion and renal venous congestion, broadly describe the processes underlying renal dysfunction. Traditionally, renal dysfunction associated with ADHF has been attributed to hypoperfusion of the kidney caused by the progressive impairment of cardiac output [8]. However, recent studies have demonstrated that hypotension is rarely observed in patients with renal dysfunction [9], and that the elevation of central venous pressure (CVP) is more closely associated with worsening renal function than the cardiac index [10]. This suggests that in patients with ADHF admitted to hospital, renal dysfunction is more dependent on venous congestion than on the impairment of cardiac output.
The contributions of renal hypoperfusion and congestion to renal dysfunction have not been thoroughly investigated. Hemodynamic profiles can be assessed by measuring blood pressure, performing physical examinations, and by measuring laboratory markers [11], [12], and these parameters are used to assess the mechanisms underlying renal dysfunction. This study aimed to clarify differences in the prognostic impact of renal dysfunction on in-hospital mortality in patients admitted with ADHF, based on the underlying hemodynamic mechanisms.
Methods
Data sources
The study was conducted in accordance with the Declaration of Helsinki and the Japanese ethical guidelines for clinical studies. The study protocol was registered to the University Hospital Medical Information Network (UMIN 000000736), and approved by the ethics committee at each site.
The Acute Decompensated Heart Failure Syndromes (ATTEND) registry is a nationwide, multicenter, prospective cohort study that focuses on ADHF in Japan. The details of this cohort study have been reported previously [13]. In brief, patients hospitalized for ADHF who met the modified Framingham criteria, were eligible for the study. The ATTEND registry enrolled patients from April 2007 to December 2011 in 52 hospitals throughout Japan. Approximately 200 variables were collected on admission for each patient, and clinical variables included the patient's history, physical examination results, echocardiographic data, and laboratory data. Patients aged <20 years and those not considered suitable for the study by attending physicians were excluded. The present study also ruled out acute coronary syndrome. In-hospital mortality was defined as (1) death from any cause, (2) death from cardiac causes, including sudden cardiac death and heart failure death, and (3) death from cerebral or vascular causes. Death was considered cardiac-related (defined as heart failure death, sudden death, or other cardiac death), unless a specific non-cardiac cause was identified by the primary physicians. The end-point classification committee, comprising two experienced cardiologists who were not study investigators, reviewed the data and, if any problems were encountered, they asked the primary physician to confirm the cause of death. Finally, the committee categorized each event for use in the present analysis. All data are managed at an independent biostatistics and data center (STATZ Institute, Inc., Tokyo, Japan). In this study, the data was collected from multiple institutions in Japan, and the IRB approval was obtained individually from each sites. Therefore, the full set of data cannot be made available to public. The reader may contact the corresponding author to request the data.
Study population
After excluding patients who were on hemodialysis or who had stage 5 chronic kidney disease (defined as an estimated glomerular filtration rate [eGFR] <15 mL/min/1.73 m2) and were supported by intracardiac balloon pumping or percutaneous cardiopulmonary support, the remaining 4,321 subjects were analyzed in this study.
Evaluation of renal function
The National Kidney Foundation advocates estimating the GFR by using the Modification of Diet in Renal Disease (MDRD) formula to detect the early stages of renal dysfunction [14]. On the basis of this recommendation, renal function in this study was evaluated by estimating the GFR, which was calculated using the abbreviated MDRD study equation:
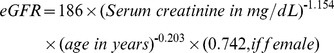 |
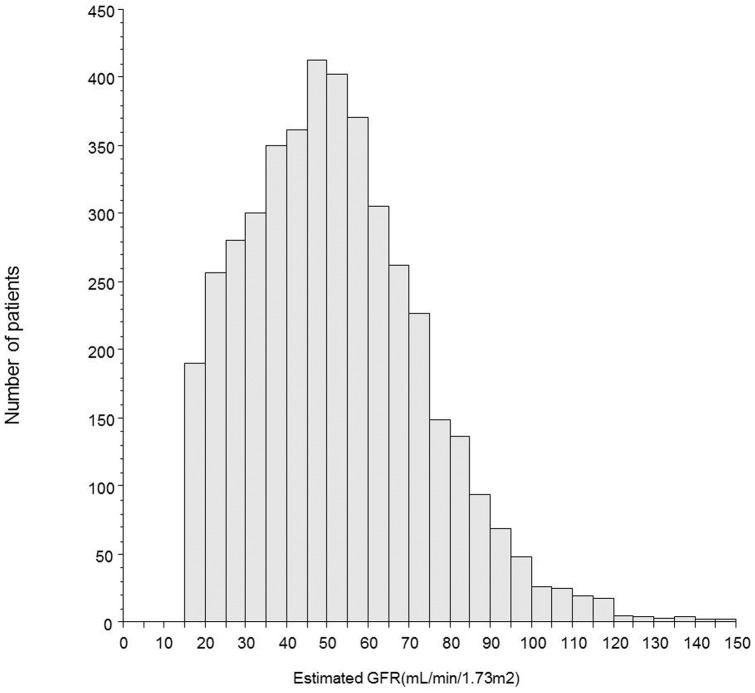
The distribution of eGFRs is shown in Figure 1. An evaluation of the receiver operating characteristic curve determined that the optimal cut-off value for renal dysfunction was estimated as a GFR ≤50 mL/min/1.73 m2 (Figure 2), and the area under the curve was 0.63 (95% confidence interval [CI] = 0.61–0.64, p<0.001).
Figure 1. Distribution of estimated glomerular filtration rates levels on admission to hospital.
GFR, glomerular filtration rate
Figure 2. Evaluation of the receiver operating characteristic curve for renal dysfunction.
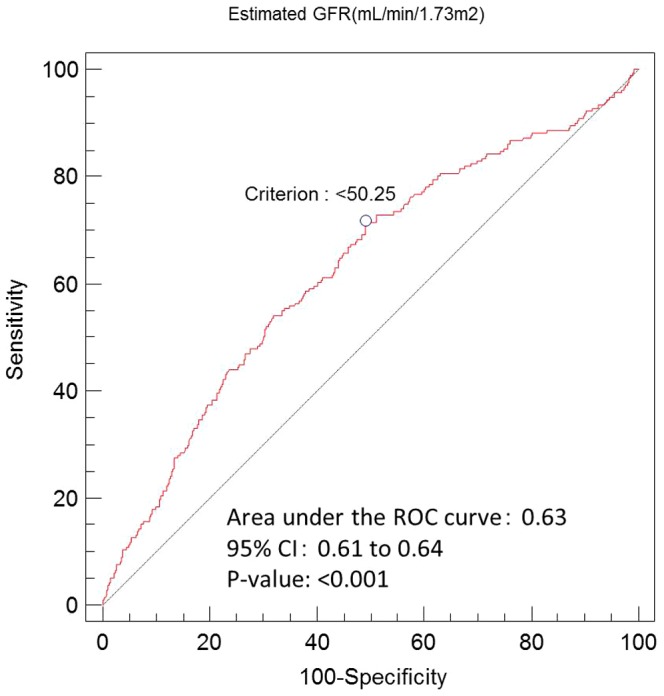
The area under the curve was 0.63 (95% confidence interval = 0.61–0.64, p<0.001), and the cut-off value for the greatest sensitivity and specificity was 50.25 mL/min/1.73 m2. GFR, glomerular filtration rate; CI, confidence interval; ROC, receiver operating characteristic.
Assessing renal dysfunction mechanisms
Renal dysfunction as it relates to hypoperfusion, which is usually caused by a low-output status, was defined as the presence of cold extremities, a low left ventricular ejection fraction (LVEF) of ≤40%, a low mean blood pressure (mBP) of ≤100 mmHg [15], or a low proportional pulse pressure (PPP) of ≤40% [16]. In contrast, renal dysfunction as it relates to congestion was defined as the presence of peripheral edema or jugular venous distension (JVD), or elevated brain natriuretic peptide (BNP) levels of >677 pg/mL [17]. The cutoff values of mBP, PPP, and BNP were determined according to the respective median values.
Statistical analysis
All data are expressed as means ± standard deviations or medians with the interquartile ranges. The receiver operating characteristic curve for renal dysfunction was used to evaluate the optimal cut-off value. Differences in each variable between patients with and without renal dysfunction were evaluated using the chi-square test or Fisher's exact test for categorical variables, and using Student's unpaired t-test or Mann-Whitney U test for continuous variables. A logistic regression model was used to evaluate the influence of renal dysfunction on in-hospital mortality. In the logistic regression models, the covariates were age, gender, etiology (ischemic or non-ischemic), systolic blood pressure, and heart rate. The covariates incorporated into these models were clinically associated with in-hospital mortality in patients with ADHF.
Data analyses were performed using SAS, software version 9.1 (SAS Institute Inc., Cary, North Carolina). All p-values were two-sided, and significance was defined as p<0.05. All analyses were performed at an independent biostatistics and data center (STATZ Institute, Inc., Tokyo, Japan).
Results
Of the 4,321 patients hospitalized with ADHF, renal dysfunction was present in 2,150 (49.8%) patients and was determined on the basis of a GFR cut-off value of ≤50 mL/min/1.73 m2. Table 1 presents a comparison of the demographic and baseline characteristics of patients with and without renal dysfunction. In comparison with those patients without renal dysfunction, patients with renal dysfunction were older, they were more likely to have an ischemic etiology and to have histories of hospitalization for heart failure, and they were more likely to have risk factors for cardiovascular disease, which included hypertension, dyslipidemia, and diabetes mellitus. On admission to hospital, physical findings, including JVD and cold extremities, were more frequently observed in patients with renal dysfunction than in patients without renal dysfunction. Patients with renal dysfunction had significantly lower blood pressures and heart rates, and significantly higher plasma BNP levels, compared with those without renal dysfunction.
Table 1. Baseline characteristics of patients with and without renal dysfunction.
| Total | eGFR >50 mL/min/1.73 m2 | eGFR ≤50 mL/min/1.73 m2 | ||
| (N = 4,321) | (n = 2,171) | (n = 2,150) | p-value | |
| Mean age (years) | 73.3±13.6 | 70.2±14.4 | 76.5±11.9 | <0.001 |
| Men, n (%) | 2,501 (57.9) | 1,300 (59.9) | 1,201 (55.9) | 0.007 |
| Ischemic cause of HF, n (%) | 1,283 (29.7) | 564 (26.0) | 719 (33.4) | <0.001 |
| Medical history | ||||
| Prior hospitalization for HF, n (%) | 1,521 (35.2) | 576 (26.5) | 945 (44.0) | <0.001 |
| Hypertension, n (%) | 2,980 (69.0) | 1,417 (65.3) | 1,563 (72.7) | <0.001 |
| Dyslipidemia, n (%) | 1,558 (36.1) | 736 (33.9) | 822 (38.2) | 0.003 |
| Diabetes mellitus, n (%) | 1,391 (32.2) | 667 (30.7) | 724 (33.7) | 0.036 |
| Smoking, n (%) | 1,840 (42.6) | 990 (45.6) | 850 (39.5) | <0.001 |
| Atrial flutter or fibrillation, n (%) | 1,745 (40.4) | 849 (39.1) | 896 (41.7) | 0.096 |
| Chronic respiratory disease, n (%) | 538 (12.5) | 263(12.1) | 275 (12.8) | 0.501 |
| Stroke/transient ischemic attack, n (%) | 611 (14.1) | 261 (12.0) | 350 (16.3) | <0.001 |
| Pacemaker/ICD, n (%) | 380 (8.8) | 142 (6.5) | 238 (11.1) | <0.001 |
| Cardiac resynchronization therapy, n (%) | 86 (2.0) | 24 (1.1) | 62 (2.9) | <0.001 |
| Clinical profile on admission | ||||
| Paroxysmal nocturnal dyspnea, n (%) | 2,288 (53.0) | 1,161 (53.5) | 1,127 (52.4) | 0.609 |
| Orthopnea, n (%) | 2,717 (62.9) | 1,368 (63.0) | 1,349 (62.7) | 0.795 |
| Rales, n (%) | 3,075 (71.2) | 1,548 (71.3) | 1,527 (71.0) | 0.938 |
| Third heart sound, n (%) | 1,518 (35.1) | 745 (34.3) | 773 (36.0) | 0.35 |
| Jugular venous distension, n (%) | 2,246 (52.0) | 1,088 (50.1) | 1,158 (53.9) | 0.005 |
| Peripheral edema, n (%) | 2,887 (66.8) | 1,423 (65.5) | 1,464 (68.1) | 0.075 |
| Cold extremities, n (%) | 917 (21.2) | 409 (18.8) | 508 (23.6) | <0.001 |
| EF≤40%, n (%) | 2,301 (53.3) | 1,181 (54.4) | 1,120 (52.1) | 0.158 |
| NYHA functional class | ||||
| I, n (%) | 74 (1.7) | 40 (1.8) | 34 (1.6% | 0.458 |
| II, n (%) | 706 (16.3) | 372 (17.1) | 334 (15.5) | |
| III, n (%) | 1,657 (38.3) | 825 (38.0) | 832 (38.7) | |
| IV, n (%) | 1,834 (42.4) | 909 (41.9) | 925 (43.0) | |
| Mean heart rate (beats/min) | 99.0±29.3 | 102.9±29.4 | 95.0±28.7 | <0.001 |
| Mean systolic blood pressure (mmHg) | 146.1±35.8 | 147.4±34.8 | 144.8±36.7 | 0.016 |
| Mean diastolic blood pressure (mmHg) | 83.1±22.4 | 85.4±21.7 | 80.9±23.0 | <0.001 |
| Median B-type natriuretic peptide (pg/mL) | 677 (350–1,220) | 562 (298–981) | 848 (439–1,490) | <0.001 |
| Mean blood urea nitrogen (mg/dL) | 25.2±18.6 | 18.7±15.5 | 31.8±19.2 | <0.001 |
| Mean serum creatinine (mg/dL) | 1.15±0.52 | 0.80±0.18 | 1.50±0.51 | <0.001 |
| Mean eGFR (mL/min/1.73 m2) | 51.9±21.6 | 68.9±15.9 | 34.8±9.7 | <0.001 |
| Mean serum sodium (mEq/L) | 139.4±4.3 | 139.6±4.2 | 139.3±4.3 | 0.023 |
| Mean hemoglobin (g/dL) | 12.2±2.6 | 12.7±2.4 | 11.6±2.7 | <0.001 |
| Median total bilirubin (mg/dL) | 0.8 (0.5–1.1) | 0.8 (0.6–1.2) | 0.7 (0.5–1.1) | <0.001 |
Data are expressed as mean ± standard deviation, as number (percentage), or as median (interquartile range).
eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; EF, ejection fraction; NYHA, New York Heart Association.
Before admission to the hospital and with the exception of digitalis, most types of medication, including diuretics, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, calcium-channel blockers, beta blockers, nitrate, and statins, were more frequently used by patients with renal dysfunction than those without renal dysfunction. Although vasodilator use was similar in both groups during hospitalization, the use of intravenous diuretics and inotropes was significantly higher in patients with renal dysfunction. Non-pharmacologic management, including non-invasive or invasive positive-pressure ventilation, was similar for both groups, except for the application of revascularization therapy, which was more commonly used in patients without renal dysfunction (Table 2).
Table 2. Management of patients with and without renal dysfunction.
| Total | eGFR>50 mL/min/1.73 m2 | eGFR≤50 mL/min/1.73 m2 | ||
| (N = 4,321) | (n = 2,171) | (n = 2,150) | p-value | |
| Intravenous therapy | ||||
| Diuretics, n (%) | 3,306 (76.5) | 1,622 (74.7) | 1,684 (78.3) | 0.005 |
| Vasodilators, n (%) | 3,392 (78.5) | 1,708 (78.7) | 1,684 (78.3) | 0.781 |
| Inotropes, n (%) | 676 (15.6) | 290 (13.4) | 386 (18.0) | <0.001 |
| In-hospital management | ||||
| Oxygen supplementation, n (%) | 2,736 (63.3) | 1,361 (62.7) | 1,375 (64.0) | 0.355 |
| NIPPV, n (%) | 1,012 (23.4) | 501 (23.1) | 511 (23.8) | 0.592 |
| Intubation, n (%) | 259 (6.0) | 117 (5.4) | 142 (6.6) | 0.094 |
| Revascularization, n (%) therapy | 348 (8.1) | 202 (9.3) | 146 (6.8) | 0.002 |
| Valve replacement, n (%) | 98 (2.3) | 66 (3.0) | 32 (1.5) | <0.001 |
| Outpatient medications before admission | ||||
| Loop or thiazide diuretics, n (%) | 2,037 (47.1) | 779 (35.9) | 1,258 (58.5) | <0.001 |
| ACE-I or ARB, n (%) | 2,043 (47.3) | 854 (39.3) | 1,189 (55.3) | <0.001 |
| Calcium-channel blockers, n (%) | 1,192 (27.6) | 528 (24.3) | 664 (30.9) | <0.001 |
| Beta blockers, n (%) | 1,428 (33.0) | 571 (26.3) | 857 (39.9) | <0.001 |
| Digitalis, n (%) | 556 (12.9) | 288 (13.3) | 268 (12.5) | 0.432 |
| Nitrate, n (%) | 726 (16.8) | 286 (13.2) | 440 (20.5) | <0.001 |
| Amiodarone, n (%) | 188 (4.4) | 53 (2.4) | 135 (6.3) | <0.001 |
| Statins, n (%) | 993 (23.0) | 430 (19.8) | 563 (26.2) | <0.001 |
| Length of hospital stay (days) | ||||
| Median (interquartile range) | 20 (13–30) | 19 (13–28) | 21 (13–33) | <0.001 |
| Mean ± SD | 27±34 | 25±28 | 29±39 | <0.001 |
Data are expressed as mean ± standard deviation (SD), as number (percentage), or as median (interquartile range).
eGFR, estimated glomerular filtration rate; NIPPV, non-invasive positive-pressure ventilation; ACE-I, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker.
The all-cause death rate was significantly higher in patients with renal dysfunction at 6.8% compared with 3.0% for those without renal dysfunction. Furthermore, cardiac death rates were significantly higher in patients with renal dysfunction compared with those without renal dysfunction (4.8% vs. 2.1%, respectively, p<0.001) (Figure 3). Logistic regression analysis demonstrated that the presence of renal dysfunction was an independent predictor of all-cause death after adjustment for associated factors (OR: 2.36, 95% CI: 1.75–3.18, p<0.001).
Figure 3. Relationship between the baseline estimated glomerular filtration rates and in-hospital mortality.
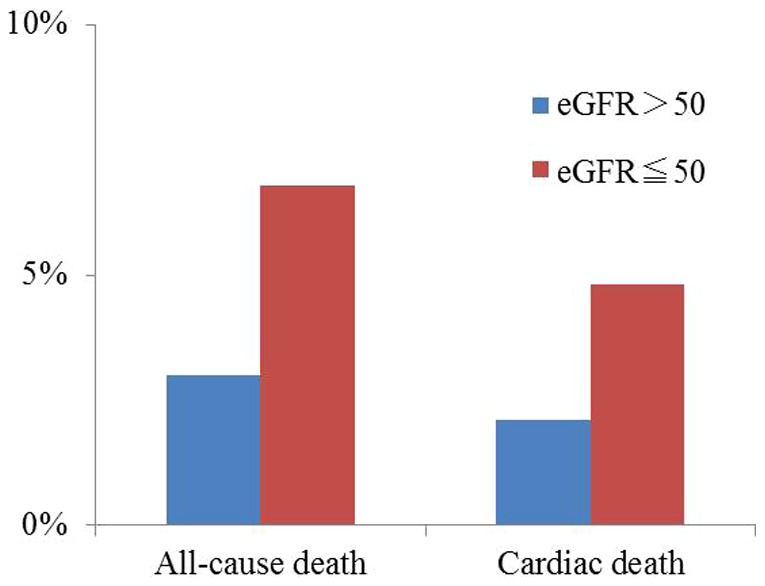
eGFR, estimated glomerular filtration rate.
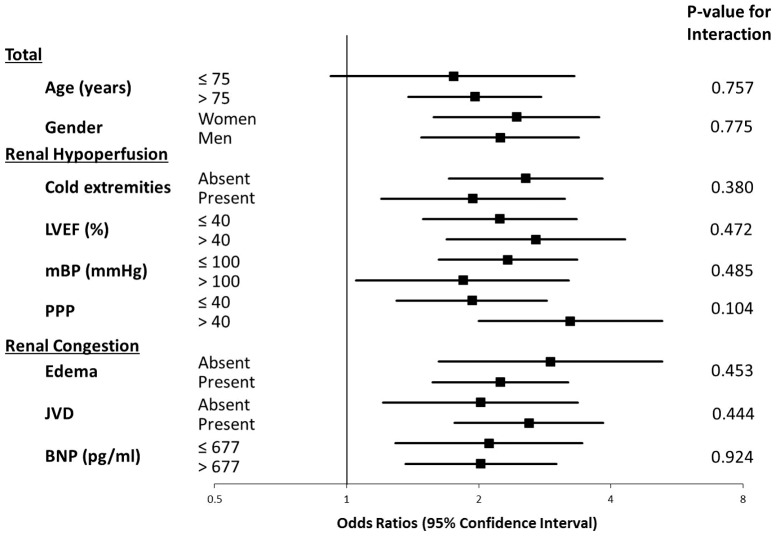
To evaluate the prognostic impact of renal dysfunction in the context of the two underlying hemodynamic mechanisms, we performed logistic regression analyses on subgroups of patients with or without hypoperfusion-dominant characteristics (e.g., patients with cold extremities, low LVEFs, low mBPs, or low PPPs) and on subgroups of patients with or without congestion-dominant characteristics (e.g., edema, JVD or high BNP levels). As shown in Table 3, all-cause mortality was consistently higher in patients with renal dysfunction. The prognostic impact of renal dysfunction quantified using ORs, was similar across all of the subgroups, regardless of whether the clinical signs of hypoperfusion or congestion were present (Figure 4). The p-value for the interaction ranged from 0.104–0.924 and was always >0.05.
Table 3. All-cause mortality in different patient subgroups.
| Normal Renal Function | Renal Dysfunction | ||||||
| eGFR>50 mL/min/1.73 m2 | eGFR≦50 mL/min/1.73 m2 | ||||||
| No. of patients | No. of Events | No. of Events (%) | No. of Patients | No. of Events | No. of Events (%) | ||
| Total | 2171 | 65 | 3.0% | 2150 | 146 | 6.8% | |
| Age (years) | ≤75 | 1259 | 18 | 1.4% | 850 | 21 | 2.5% |
| >75 | 912 | 47 | 5.2% | 1300 | 125 | 9.6% | |
| Gender | Women | 871 | 30 | 3.4% | 949 | 76 | 8.0% |
| Men | 1300 | 35 | 2.7% | 1201 | 70 | 5.8% | |
| mBP (mmHg) | ≤100 | 940 | 43 | 4.6% | 1115 | 112 | 10.0% |
| >100 | 1218 | 21 | 1.7% | 1024 | 32 | 3.1% | |
| PPP | ≤42 | 1114 | 42 | 3.8% | 955 | 67 | 7.0% |
| >42 | 11044 | 22 | 2.1% | 1184 | 77 | 6.5% | |
| JVD | Absent | 917 | 24 | 2.6% | 815 | 42 | 5.2% |
| Present | 1088 | 37 | 3.4% | 1158 | 97 | 8.4% | |
| Edema | Absent | 724 | 16 | 2.2% | 663 | 41 | 6.2% |
| Present | 1423 | 46 | 3.2% | 1464 | 102 | 7.0% | |
| Cold extremities | Absent | 1663 | 35 | 2.1% | 1551 | 81 | 5.2% |
| Present | 409 | 26 | 6.4% | 508 | 59 | 11.6% | |
| BNP (pg/ml) | ≤677 | 1182 | 28 | 2.4% | 822 | 40 | 4.9% |
| >677 | 830 | 35 | 4.2% | 1175 | 97 | 8.3% | |
| LVEF (%) | ≤40 | 1181 | 39 | 3.3% | 1120 | 77 | 6.9% |
| >40 | 960 | 25 | 2.6% | 993 | 67 | 6.7% | |
Abbreviation; eGFR, estimated glomerular filtration rate; mBP, mean blood pressure; PPP, proportional pulse pressure; JVP, juglur venous distension; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction.
Figure 4. The prognostic impact of renal dysfunction in the prediction of all-cause mortality in relation to the underlying etiologic mechanisms.
LVEF, left ventricular ejection fraction; mBP, mean blood pressure; PPP, proportional pulse pressure; JVD, jugular venous distension; BNP, brain natriuretic peptide.
Discussion
The major finding from this study was that renal dysfunction was significantly associated with an increased risk of in-hospital mortality in patients admitted with ADHF. Furthermore, this adverse effect of renal dysfunction on short-term outcomes remained the same, regardless of the underlying hemodynamic mechanism. The present study confirms previous findings from studies performed in Western countries that reported an association between baseline renal dysfunction and an increased risk of short-term mortality in patients admitted with ADHF [3], [4]
While various mechanisms have been proposed for renal dysfunction in patients admitted with ADHF, these mechanisms fall into two broad categories from the perspective of hemodynamics, namely renal hypoperfusion and renal congestion. A scientific statement to assess and grade congestion in acute heart failure has been proposed by the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology [17]. Thus, if peripheral edema, JVD, and elevated BNP levels are the variables associated with congestion, then cold extremities and low LVEFs, mBPs, and PPPs could be the variables associated with hypoperfusion, because this type of renal dysfunction is attributed to reduced systemic perfusion. Using these definitions for each clinical profile, we demonstrated that, contrary to common belief, the typical physical findings indicative of renal hypoperfusion and renal congestion, including cold extremities and JVD, were more frequently observed in patients with renal dysfunction on hospitalization.
Traditionally, a reduction in renal blood flow, namely renal hypoperfusion, has been considered the main cause of renal dysfunction associated with ADHF. Although the precise mechanism that connects cardiac output with renal blood flow remains unclear in the context of ADHF, it is hypothesized that neurohormonal activation, for example via the renin-angiotensin system, results in afferent vasoconstriction, thereby reducing renal blood flow and hence the effective volume of circulating fluid, as is expected in patients with ADHF [7]. In contrast, recent studies have highlighted the association between an increased CVP and renal dysfunction or renal congestion. According to this hypothesis, elevated CVP is directly transmitted to the renal vein and increases renal perfusion pressure, which raises the interstitial intrarenal pressure and causes tubule collapse, leading to a decrease in GFR [18]. The association between a higher CVP and decreasing GFR has been demonstrated in several studies [10], [19]–[21]. Our study suggests that the resulting renal dysfunction could impact on patient outcomes, regardless of the etiology underlying the renal dysfunction.
In our study, patients' clinical presentation parameters and vital signs were primarily used to differentiate the underlying etiologic mechanisms of renal dysfunction; however, novel biomarkers could differentiate these mechanisms in more objective and reproducible fashion. Several novel biomarkers are emerging, and we evaluated their potential in the clinical settings. Among these biomarkers, soluble suppression of tumorigenicity 2 (sST2) could be a leading candidate. sST2, a member of the interleukin (IL)-1 receptor family, has been established as a predictor of mortality in the long-term follow-up of ADHF patients [22], [23]. As sST2 is a biomarker for cardiac remodeling and fibrosis, it may be more prominent in patients with hypoperfusion than in those with congestion.
Hypoperfusion has traditionally been considered the predominant cause of renal dysfunction in patients with ADHF [8]. However, a recent study reported that venous congestion may also be an important hemodynamic factor in this condition [10], and its impact has received strong attention. In turn, our study found the adverse impact of renal dysfunction on in-hospital outcomes to be consistent regardless of etiology. This finding has established the prognostic importance of renal dysfunction complicated with ADHF under any circumstances. Furthermore, our study also reconfirmed the adverse impact of renal dysfunction on in-hospital outcomes in the Asian population who have completely different clinical characteristics compared with the Western population. Previously, we demonstrated the key differentiating characteristics of heart failure patients in Western countries as compared with those in Asian countries [13]. Notably, we found an increased prevalence of patients with de novo heart failure and non-ischemic etiology in Japan versus in Western countries. Additionally, the length of hospital stay for this category of patients was much longer in Japan than in Western countries, probably owing to the differences in health insurance systems. All these complicating factors could potentially have mitigated the effect of eGFR.
Study Limitations
Our study has several limitations. Firstly, the calculation of the GFR was originally developed for use in patients with chronic kidney disease whose renal functions are relatively stable; the applicability of this calculation for patients with ADHF has not been sufficiently validated. However, previous studies have demonstrated an association between reduced GFRs and adverse outcomes in patients with ADHF [24]–[26]. Our intent was to estimate the level of renal dysfunction in our study population, rather than to determine the precise renal function levels of these patients. Secondly, it could be argued that an invasive approach, such as right heart catheterization, should have been used to evaluate patients' hemodynamic profiles more precisely. However, we believe that evaluations based on accessible and non-invasive clinical measures, including vital signs, physical findings, laboratory markers, and echocardiograms, are relevant to clinical decision making. Furthermore, these non-invasive parameters reflect values assessed with an invasive modality, and they are considered sufficient substitutes for a more invasive approach [15]–[17]. Moreover, analyses based on these clinical measures may be more practical for patient assessments and more applicable in routine practice. Third, we could not evaluate the associations between renal dysfunction and long-term outcomes, because long-term follow-up data were not available for this study. Further study is needed regarding long-term assessments. Finally, hospital stays were much longer in the ATTEND registry than those reported from Western countries, which is associated with Japan's health insurance system [13], and in-hospital mortality in the data within the ATTEND registry might differ from its counterparts in other countries. However, a previous analysis of data from the ATTEND registry has shown that most sudden cardiac deaths occurred within 14 days of admission [27], therefore a hospital stay of less than 7 days might be too short to accurately evaluate short-term outcomes. From this perspective, our results may reflect short-term mortality more precisely.
Conclusions
In conclusion, baseline renal dysfunction was significantly associated with in-hospital mortality in patients admitted with ADHF. The prognostic impact of renal dysfunction was the same, regardless of its underlying etiologic mechanism.
Supporting Information
ATTEND Study Investigators.
(DOC)
Acknowledgments
We wish to extend our appreciation to the investigators of the ATTEND registry, who are listed in Appendix S1.
Disclaimer: All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data are from the ATTEND registry. In this study, the data was collected from multiple institutions in Japan, and the IRB approval was obtained individually from each site. Therefore, the full set of data cannot be made available to public. The reader may contact the corresponding author to request the data.
Funding Statement
This study was supported by the Japan Heart Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gheorghiade M, Pang PS (2009) Acute heart failure syndromes. J Am Coll Cardiol 53: 557–573. [DOI] [PubMed] [Google Scholar]
- 2. Harjola VP, Follath F, Nieminen MS, Brutsaert D, Dickstein K, et al. (2010) Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail 12: 239–248. [DOI] [PubMed] [Google Scholar]
- 3. Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, et al. (2008) Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol 52: 347–356. [DOI] [PubMed] [Google Scholar]
- 4. O'Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, et al. (2008) Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 156: 662–673. [DOI] [PubMed] [Google Scholar]
- 5. Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ (2005) Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 293: 572–580. [DOI] [PubMed] [Google Scholar]
- 6. Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, et al. (2010) Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 31: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haase M, Muller C, Damman K, Murray PT, Kellum JA, et al. (2013) Pathogenesis of cardiorenal syndrome type 1 in acute decompensated heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 182: 99–116. [DOI] [PubMed] [Google Scholar]
- 8. Schrier RW, Abraham WT (1999) Hormones and hemodynamics in heart failure. N Engl J Med 341: 577–585. [DOI] [PubMed] [Google Scholar]
- 9. Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, et al. (2004) Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43: 61–67. [DOI] [PubMed] [Google Scholar]
- 10. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, et al. (2009) Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 53: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, et al. (2003) Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 41: 1797–1804. [DOI] [PubMed] [Google Scholar]
- 12. Mebazaa A, Gheorghiade M, Pina IL, Harjola VP, Hollenberg SM, et al. (2008) Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med 36: S129–139. [DOI] [PubMed] [Google Scholar]
- 13. Sato N, Kajimoto K, Asai K, Mizuno M, Minami Y, et al. (2010) Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: rationale, design, and preliminary data. Am Heart J 159: 949–955. [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, et al. (2003) National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139: 137–147. [DOI] [PubMed] [Google Scholar]
- 15. Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, et al. (2007) Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27–28 April 2006. Intensive Care Med 33: 575–590. [DOI] [PubMed] [Google Scholar]
- 16. Stevenson LW, Perloff JK (1989) The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 261: 884–888. [PubMed] [Google Scholar]
- 17. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, et al. (2010) Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 18. Braam B, Cupples WA, Joles JA, Gaillard C (2012) Systemic arterial and venous determinants of renal hemodynamics in congestive heart failure. Heart Fail Rev 17: 161–175. [DOI] [PubMed] [Google Scholar]
- 19. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, et al. (2009) Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 53: 582–588. [DOI] [PubMed] [Google Scholar]
- 20. Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, et al. (2008) Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 51: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 21. Uthoff H, Breidthardt T, Klima T, Aschwanden M, Arenja N, et al. (2011) Central venous pressure and impaired renal function in patients with acute heart failure. Eur J Heart Fail 13: 432–439. [DOI] [PubMed] [Google Scholar]
- 22. Rehman SU, Mueller T, Januzzi JL Jr (2008) Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol 52: 1458–1465. [DOI] [PubMed] [Google Scholar]
- 23. Pascual-Figal DA, Manzano-Fernandez S, Boronat M, Casas T, Garrido IP, et al. (2011) Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail 13: 718–725. [DOI] [PubMed] [Google Scholar]
- 24. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, et al. (2007) High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 13: 422–430. [DOI] [PubMed] [Google Scholar]
- 25. Takagi A, Iwama Y, Yamada A, Aihara K, Daida H (2010) Estimated glomerular filtration rate is an independent predictor for mortality of patients with acute heart failure. J Cardiol 55: 317–321. [DOI] [PubMed] [Google Scholar]
- 26. Cioffi G, Mortara A, Di Lenarda A, Oliva F, Lucci D, et al. (2013) Clinical features, and in-hospital and 1-year mortalities of patients with acute heart failure and severe renal dysfunction. Data from the Italian Registry IN-HF Outcome. Int J Cardiol 168: 3691–3697. [DOI] [PubMed] [Google Scholar]
- 27. Kajimoto K, Sato N, Keida T, Mizuno M, Sakata Y, et al. (2013) Association between length of stay, frequency of in-hospital death, and causes of death in Japanese patients with acute heart failure syndromes. Int J Cardiol. 168: 554–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ATTEND Study Investigators.
(DOC)
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data are from the ATTEND registry. In this study, the data was collected from multiple institutions in Japan, and the IRB approval was obtained individually from each site. Therefore, the full set of data cannot be made available to public. The reader may contact the corresponding author to request the data.