Abstract
The endophytic bacterium, Sphingomonas SaMR12, isolated from Sedum alfredii Hance, appears to increase plant biomass and zinc-extraction from contaminated soil; however, the mechanism by which this occurs is not clear. Here, the ability of SaMR12 to promote zinc extraction and its effects on root morphology and exudation were examined in hydroponics. Zinc treatment increased shoot biomass by 30 to 45%, and by a further 10 to 19% when combined with SaMR12 inoculation. Zinc treatment also increased zinc accumulation modestly and this too was enhanced with SaMR12. Both biomass and zinc levels increased in a dose-dependent manner with significant effects seen at 50 µM zinc and apparent saturation at 500 µM. Zinc and the endophyte also increased levels of auxin but not at 50 µM and zinc increased levels of superoxide and hydrogen peroxide but mainly at 500 µM. As for root morphology, SaMR12 increased root branching, the number of root tips, and surface area. Zinc and SaMR12 also increased the exudation of oxalic acid. For most assays the effects of the endophyte and zinc were additive, with the notable exception of SaMR12 strongly reducing the production of reactive oxygen species at 500 µM zinc. Taken together, these results suggest that the promotion of growth and zinc uptake by exposure to zinc and to SaMR12 are independent of reactive oxygen and do not involve increases in auxin.
Introduction
Zinc is an essential trace element that can have toxic effects on organisms at millimolar levels through soil or water contamination. Excessive zinc is toxic to plants and negatively affects human health [1], [2]. Phytoremediation represents a thorough, economical, and environmentally friendly method compared to conventional technology, that can absorb heavy metals from soil and transport them from the root to shoot, decreasing the soil concentration of metals [3]. According to Barceló et al. [4], two important aspect of phytoremediation are phyto-extraction, which denotes reducing the concentration of heavy metal in soil by a hyperaccumulator taking up the metal, and phyto-stabilization, which denotes stabilizing pollutants in soil by a plant that converts them to less available forms.
For successful phytoremediation, to overcome the shortcomings of low biomass and low growth rate, various approaches have been examined, such as identifying hyperaccumulators, fertilizing soil to improve plant biomass, and applying organic acid or chelators to increase metal bioavailability [5]–[8]. Plant-derived chelators, known as phytochelatins, are produced by roots (and include organic and amino acids as well as their derivatives) and often in quantities that are linearly correlated with the ambient level of heavy metal.
A member of the Crassulaceae, Sedum alfredii is a plant found in an ancient, Chinese mining region near Quzhou (Zhejiang province) and has a strong ability to extract heavy metals from soil. In previous studies [5], [9], [10], S. alfredii was found to show heavy metal resistance and accumulation, as well as the ability to efficiently transport heavy metals from the root to the shoot. Roots of S. alfredii mainly produce malic acid, oxalic acid, and tartaric acid, presumably contributing to enhanced bioavailability of heavy metals [11].
However, root exudates not only improve access to metals they also significantly affect microbial growth and community structure within the rhizoshpere [12]. Microbes in the rhizosphere facilitate plant growth, enhance nitrogen absorption through the biological fixation of nitrogen, and promote root elongation and the formation of root hairs [13]. Taghavi et al. [14] found that endophytic bacteria can promote plant root development and significantly improve shoot growth. Meharg and Killham [15] found that soil microorganisms can increase or decrease root exudation modestly but, arguably, enough to affect rhizosphere microbial processes.
According to a previous study, S. alfredii roots are host to at least four species of endophytic bacteria, and when this plant is inoculated with one of them, Sphingomonas SaMR12, plant biomass and heavy metal absorption are both significantly increased [16]. However, the mechanisms of this enhancement are not clear. Particularly unclear are the effects of SaMR12 on heavy metal bioavailability and root exudation. According to Přikryl and Vančura [17], inoculation of Pseudomonas putida enhanced the release of root exudates. Therefore, here, we hypothesize that, in Sedum alfredii, SaMR12 affects root morphology and organic acid composition of root exudates, thereby altering zinc bioavailability and rhizosphere conditions, and leading to larger plant biomass and higher zinc extraction.
Materials and Methods
Experiment design and materials
Sedum alfredii Hance, a zinc/cadmium hyperaccumulator identified previously [9], was obtained from an old lead and zinc mining area in Zhejiang Province, China. The work described here did not involve endangered or protected species. Specific permissions were not required for collection of samples in this location.
The endophytic bacterium Sphingomonas SaMR12, previously isolated from S. alfredii [16], was grown on solid Luria-Bertani (LB) medium at 37°C overnight. A single bacterial clone was cultured in LB liquid medium overnight. The cells was collected by centrifugation and washed three times with sterile water.
Healthy shoots of S. alfredii were collected in the field and cultured in Hoagland nutrient solution for 2 months in 2.5 L (10.2-cm top diameter) standard round green plastic pots, aerated continuously and renewed every week. Single shoot tips were excised and grown as described above for another month to remove heavy metals. Plants showing uniform growth were treated with the indicated concentrations of zinc, with 6 plants per pot for hydroponic experiments. Supplemental zinc was supplied as ZnSO4·7H2O and added to maintain the zinc concentration every three days. Unsupplemented Hoagland's contains 5 µM zinc (considered here as the ‘control’ level of zinc). A second group of plants was subjected to the same treatment and also inoculated with SaMR12 at densities of 104 to 105 CFU/mL. Each treatment was repeated three times and the experiment lasted for one month.
Measurement of root morphological parameters
At the end of the one-month experiment, roots were washed with distilled water three times, photographed by a digital camera (D3000, Nikon, Tokyo, Japan), scanned using an automatic root scanner (STD1600, Seiko Epson Corp., Japan), and analyzed using WinRHIZO software (Version 3.9, Regents Instruments Inc., 2001, Quebec, Canada). Additionally, 3 cm apical root segments were imaged through a light microscope (Eclipse E600W, Nikon), from which root hair morphology was assessed.
Measurement of biomass and zinc concentration
At the end of the experiment, shoots and roots were separated and weighed. The roots were washed in several changes of 5 mM Tris-HCl pH 6.0 containing 5 mM EDTA, and were then washed with distilled water to remove non-specifically bound zinc. Shoots and roots were oven-dried at 80°C to a consistent weight. For subsequent analysis, oven-dried samples were ground using a stainless steel mill (MM400, Retsch, Haan, Germany) to an average particle size of 0.5 mm. Next, the ground material was digested using acid HNO3-HClO4 (v/v, 5∶1) for 8 h at 180°C before subjecting the samples to inductively coupled plasma-mass spectroscopy (7500a, Agilent, Santa Clara, CA, USA). To ensure accuracy of determination, a standard zinc solution was run first to calibrate the instrument.
Root exudates collection and examination
Root exudates were collected according to the method of Ma et al. [18]. Briefly, the root systems of intact plants were placed in 100 mL of 0.5 mM CaCl2 for 4 h. The solution was passed through a resin column [Amberlite IR–120 (H+ form)] and then through another resin column [Dowex 1 × 8 100–200 (Cl− form)]. Exudates were dried using a rotary evaporator and resolved in methanol before high-pressure liquid chromatography (HPLC) analysis (Agilent 1200 series, Waldbronn, Germany). Organic acids were detected at 210 nm by UV detection by comparing retention times and absorption spectra with organic acid standards.
Assay for indole-3-acetic acid (IAA) and active oxygen generation
IAA concentration in the plant was determined according to the method described by Hou et al. [19] with modifications. First, 5 g of young leaves were ground to a fine powder with liquid nitrogen and IAA was extracted with 20 mL 80% methanol at 4°C overnight. The liquid phase was obtained by centrifugation at 10,000×g for 10 min and extracted two additional times with a 10 mL extraction solution for 1 h. After concentration, liquid partition, column separation, and elution, the eluent was passed through a sterile 0.2 µm filter prior to injection into the HPLC.
The generation of intracellular reactive oxygen species (ROS) was analyzed by measuring the superoxide anion as described by Nag et al. [20] and H2O2 as described by Tang et al. [21], using an ultraviolet spectrophotometer (Lambda 35, PerkinElmer, Waltham, MA, USA).
Statistical analyses
Data were compared by least significant difference (LSD) tests at a 5% significance level. Two-way analysis of variance was performed to determine the effects of SaMR12 inoculation (S), zinc concentration (Zn), and inoculation × zinc concentration (S × Zn) on root morphology using the SPSS software (version 20.0, SPSS Inc., Chicago, IL, USA). All figures were produced using Origin (version 8.5, OriginLab, Northampton, MA, USA).
Results
Effect of SaMR12 on S. alfredii growth and zinc uptake
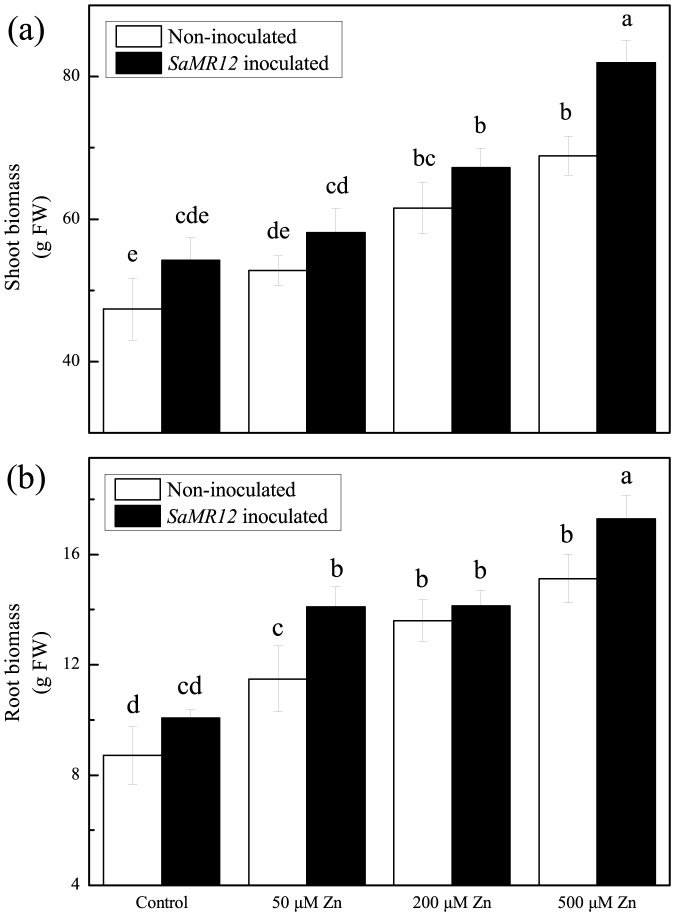
Both zinc concentration and SaMR12 inoculation significantly stimulated plant growth (Fig. 1). Shoot biomass significantly increased with zinc concentration, increasing by 30% on 200 µM zinc and by 45% on 500 µM zinc. At all Zinc concentrations, inoculation with SaMR12 also promoted shoot growth significantly, and the effect appeared to be additive with that of zinc. Similar results were observed for root biomass (Fig. 1b).
Figure 1. Shoot and root biomass of S. alfredii affected by zinc and SaMR12 treatment.
Bars plot mean ± SD of three replicate experiments. The different letters above the bars indicate significant differences among treatments at the P<0.05 level.
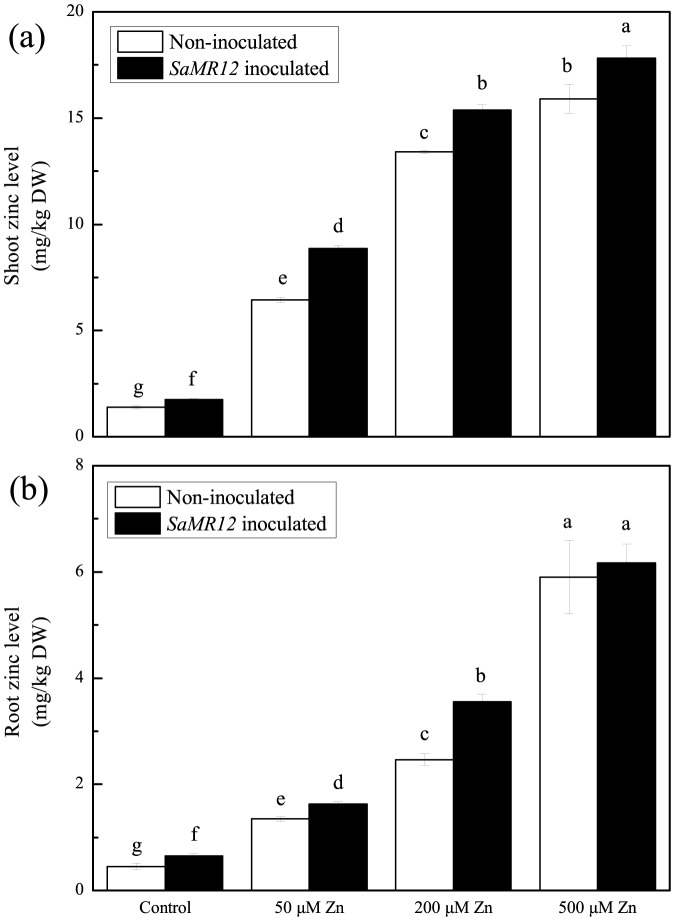
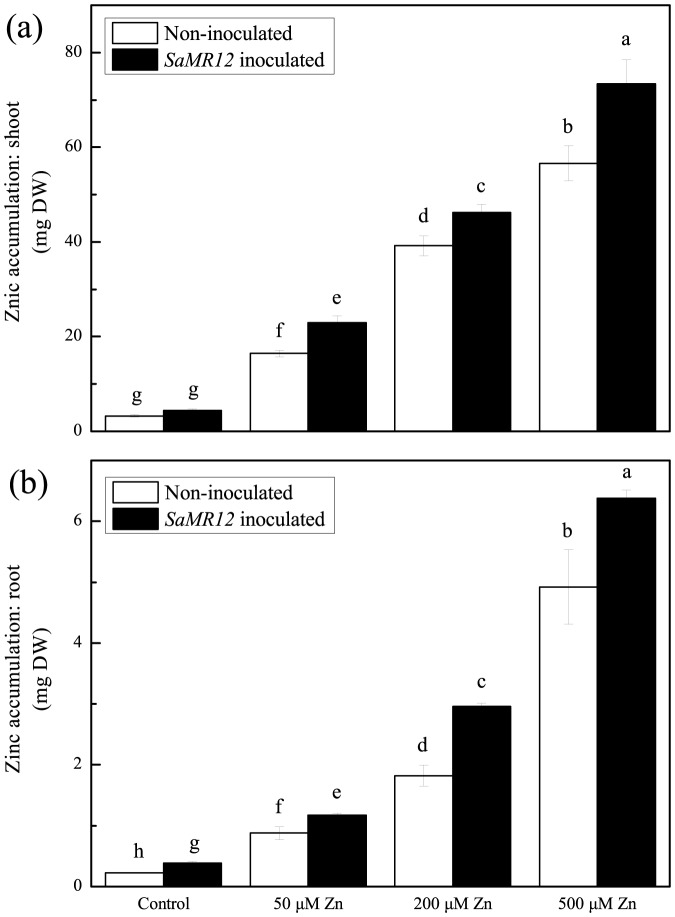
Zinc supply also significantly affected the concentration and accumulation of zinc within the plant (Figs. 2 and 3). Over the range of tested levels, the zinc concentration increased ∼11-fold for shoots (Fig. 2a) and ∼13-fold for roots (Fig. 2b). For treatments that included SaMR12, zinc concentration increased modestly but significantly at all treatment levels for the shoot (Fig. 2a) and at all levels except for the highest for the root (Fig. 2b). Reflecting the increased biomass, these patterns were closely reflected by absolute zinc amount in both shoots and roots (Fig. 3). In fact, on 500 µM zinc, total zinc amount was increased 17 times at least that of the control in both organ system, and the presence of the bacterium the increase was closer to 23 fold.
Figure 2. Zinc concentration in the plant affected by SaMR12 and the zinc treatment.
Bars plot mean ± SD of three replicate experiments. Letters show significance as for Figure 1.
Figure 3. Zinc accumulation affected by SaMR12 and zinc treatment.
Bars plot mean ± SD of three replicate experiments. Letters show significance as for Figure 1.
Plant IAA concentration as affected by SaMR12 and zinc treatment levels
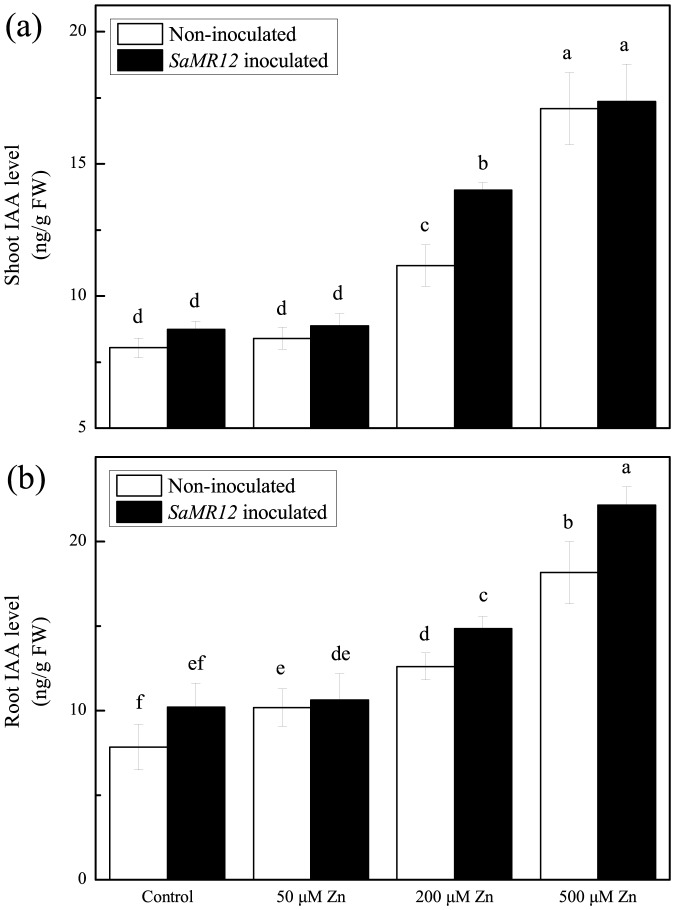
Zinc did not alter the concentration of IAA in the shoot up to 50 µM, but shoot IAA concentration significantly increased by 33% on 200 µM zinc, and by 104% on 500 µM (Fig. 4a). These levels were increased further by SaMR12 inoculation only at 200 µM, where the increase was 26% more than the non-inoculated. In contrast, roots appeared slightly more sensitive, increasing IAA level at all tested zinc concentrations, and increasing even more with inoculation, except at 50 µM (Fig. 4b). The relative increase in IAA was similar in the two organ systems although the magnitude of the increases in the shoot seemed to be somewhat larger.
Figure 4. IAA concentration as affected by SaMR12 and zinc treatment.
Bars plot mean ± SD of three replicate experiments. Letters show significance as for Figure 1.
Effect of zinc and SaMR12 treatment on oxidative stress
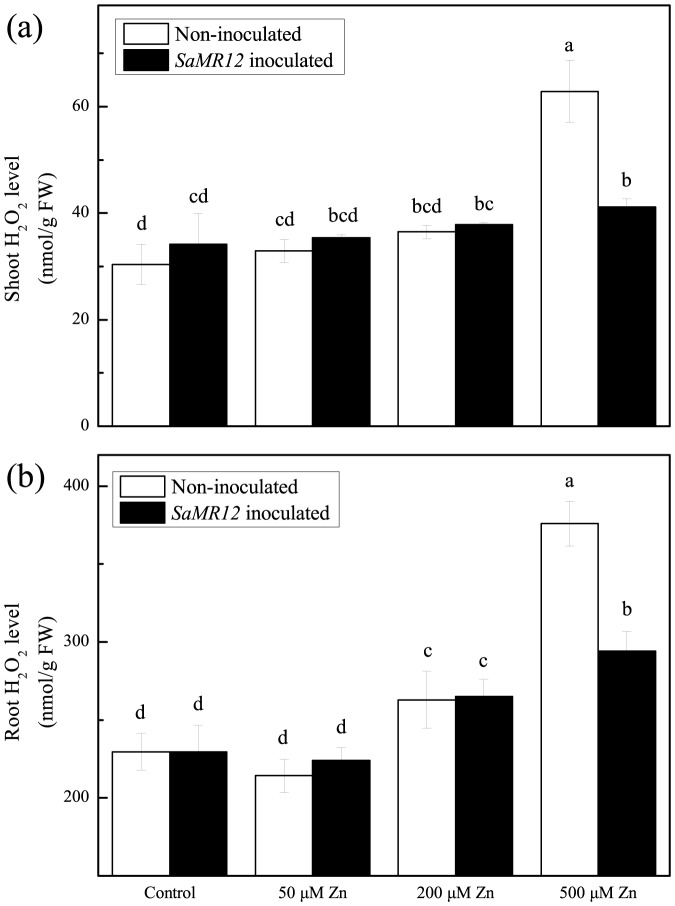
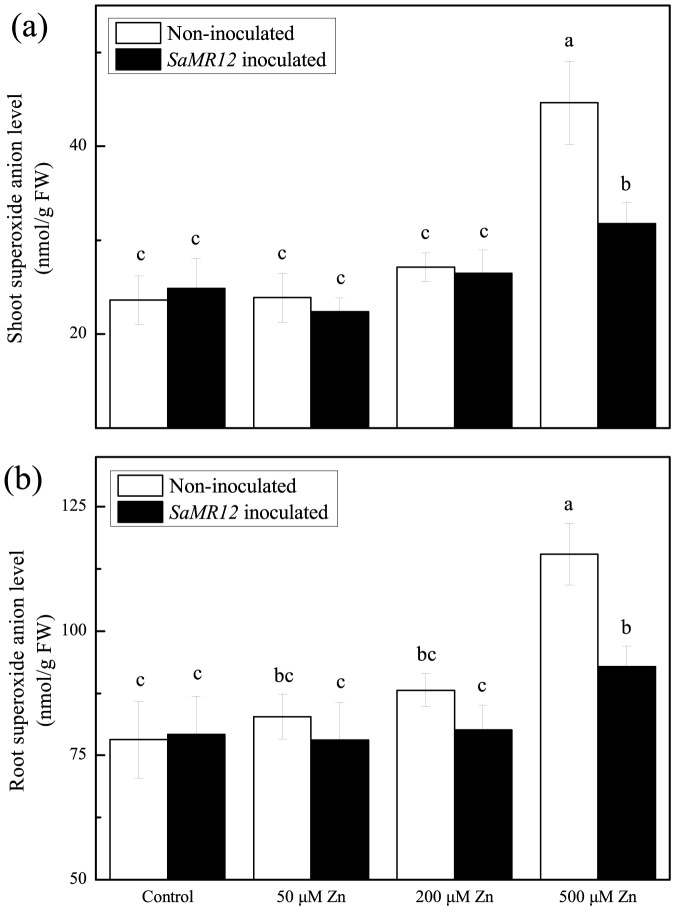
Zinc is an important component of the reactive oxygen scavenging system as a part of copper/zinc-superoxide dismutase; in addition, the element can also aggravate oxidative stress through increased production of reactive oxygen. Here, to examine the role of reactive oxygen in this system, we quantified the levels of hydrogen peroxide and superoxide in shoots and root (Figs. 5 and 6). Up to and including 200 µM, neither species changed appreciably (not at all in the shoot and only slightly in the root) and there was no alteration in level caused by the bacterium. However, at 500 µM zinc, both anions more or less doubled in level in both organs and in all cases inoculation with the bacterium reduced these increases to almost control levels. These results suggest that reactive oxygen species have little or nothing to do with the enhanced growth and metal accumulation seen here.
Figure 5. H2O2 concentration as affected by SaMR12 and the zinc treatment.
Bars plot mean ± SD of three replicate experiments. Letters show significance as for Figure 1.
Figure 6. Superoxide anion concentration as affected by SaMR12 and the zinc treatment.
Bars plot mean ± SD of three replicate experiments. Letters show significance as for Figure 1.
Effect of zinc and SaMR12 on root system morphology
Both zinc and SaMR12 treatments enhanced root growth (Fig. 7). When the zinc concentration was 200 or 500 µM, the roots appeared more healthly, with longer roots, larger biomass, and lighter color. The total root length, number of root tips, and root surface area were significantly promoted with increasing zinc treatment concentration (Table 1). After inoculation with SaMR12, the root biomass was further increased compared to non-inoculated treatment at the same zinc levels (Fig. 1), and total root length was increased by 12 to 26%, root tip number by 24 to 33%, and surface area by 17 to 32% (Table 1).
Figure 7. Root architecture as affected by SaMR12 and the zinc treatment.
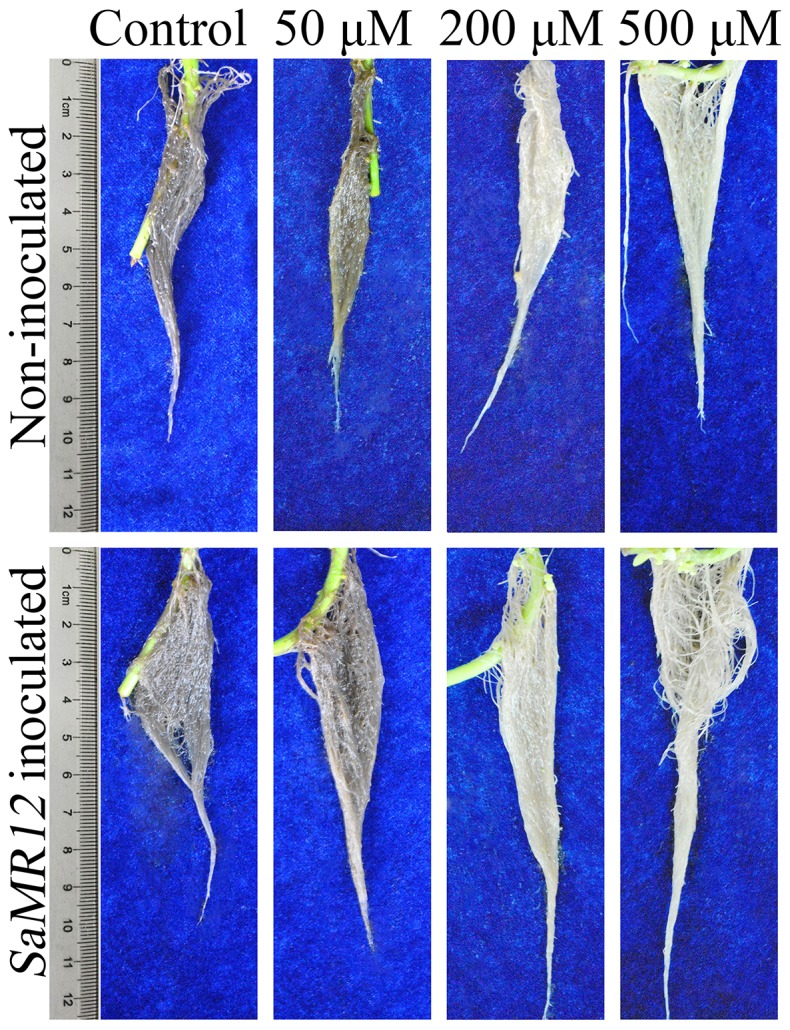
Representative images.
Table 1. Root parameters and main and interactive effects of zinc and SaMR12 on root tips, root total length, and root surface area of S. alfredii.
| Treatments | Root tips (tips plant−1) | Total root length (cm plant−1) |
 Surface area (cm2 plant−1) Surface area (cm2 plant−1) |
||||
| Control | Non-inoculated | 647±53 f | 785±88 d | 166±9 e | |||
| SaMR12 inoculated | 809±31 e | 986±30 c | 203±16 d | ||||
| 50 µM | Non-inoculated | 647±48 f | 785±36 d | 191±11 d | |||
| SaMR12 inoculated | 857±51 e | 964±90 c | 224±11 c | ||||
| 200 µM | Non-inoculated | 940±32 d | 1069±63 c | 208±7 cd | |||
| SaMR12 inoculated | 1210±24 b | 1202±70 b | 257±10 b | ||||
| 500 µM | Non-inoculated | 1113±13 c | 1233±54 b | 222±3 c | |||
| SaMR12 inoculated | 1377±44 a | 1440±54 a | 292±6 a | ||||
| Two-way analysis of variance | |||||||
| Source of variation | F | P | F | P | F | P | |
| SaMR12 (S) | 199.1 | 0.000** | 47.6 | 0.000** | 140.3 | 0.000** | |
| Zn level (Zn) | 247.5 | 0.000** | 71.9 | 0.000** | 61.7 | 0.000** | |
| Interaction (S × Zn) | 2.465 | 0.100 | 0.418 | 0.743 | 4.158 | 0.023* | |
Values represent the mean ± standard deviation of three replicates.
*Significant at the P<0.05 level,
**Significant at the P<0.01 level.
The different letters following the values in the same column indicate significant differences between the treatments at P<0.05 (Duncan's test). Main and interactive effects of zinc and SaMR12 on the root tips, root total length and root surface area of S. alfredii were examined by two-way analysis of variance. Both SaMR12 and zinc treatment significantly affected root tips (F = 199.2, P<0.01), total length (F = 47.6, P<0.01), and total surface area (F = 140.3, P<0.01) of S. alfredii (P<0.01). The total surface area of the root was also significantly affected by the interactive effects of (S × Zn) at the P<0.05 level.

The surface areas did not include root hairs which cannot be detected using the root automatic scan apparatus.
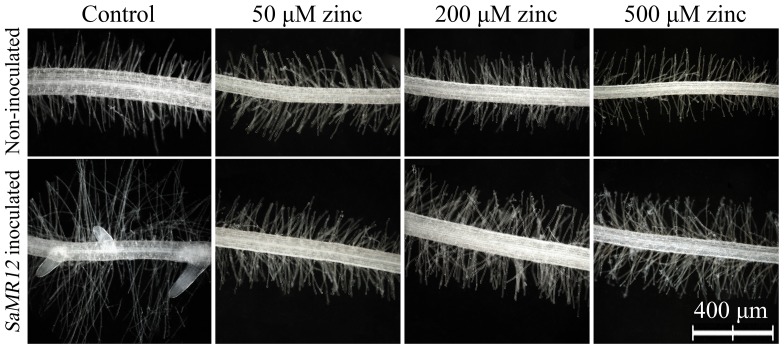
Regarding root hairs, zinc treatment alone caused little conspicuous alteration except that on 500 µM zinc they appeared to be less numerous (Fig. 8). After inoculation with SaMR12, root hair length and lateral root formation both appeared to be strongly increased under control conditions (i.e., 5 µM zinc) but at the higher concentrations of zinc, lateral root formation was not stimulated and root hairs were more numerous and only slightly longer than that of the respective non-inoculated treatment control.
Figure 8. Root hairs affected by SaMR12 and zinc treatment.
Representative images.
Effect of zinc and SaMR12 on root exudates
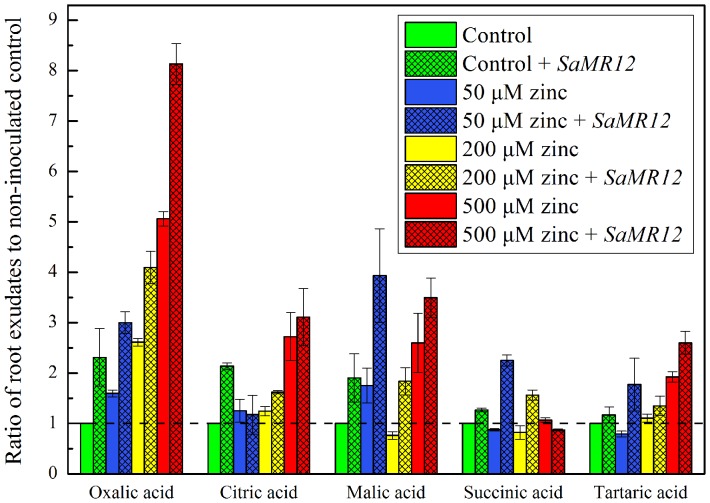
Organic acids in root exudates were detected by HPLC. Absolute values and significance are shown in Table 2 but for ease of understanding, the data are shown graphically in Fig. 9, normalized against the values for non-inoculated control. Succinate exudation was scarcely affected at any treatment, as were tartarate and citrate, except at 500 µM zinc when they were roughly doubled, regardless of the presence of inoculum. The pattern of malate exudation was more complex, being stimulated to about the same extent (2 to 4 fold) on 50 and 500 µM zinc but being little affected on 200 µM. In contrast, oxalate exudation showed a clear response to zinc, increasing in a dose-dependent way and being substantially larger with inoculation. The pattern of oxalic acid exudation resembles that of biomass increase as well as zinc accumulation and suggests that the exudation of this acid plays a pivotal role in zinc acquisition.
Table 2. Root exudates affected by zinc and SaMR12 inoculation.
| Treatment | Organic acid (mg/kg h, FW) | |||||
| Oxalic acid | Citric acid | Malic acid | Succinic acid | Tartaric acid | ||
| Control | Non-inoculated | 15.9±1.06 f | 15.1±0.96 bc | 53.2±8.72 d | 61.2±5.76 b | 31.2±3.45 bc |
| SaMR12 inoculated | 32.3±6.99 cd | 27.7±0.68 a | 88.9±4.78 bc | 66.2±1.56 b | 30.5±1.13 bc | |
| 50 µM | Non-inoculated | 19.5±2.6 ef | 14.6±2.79 bc | 67.6±6.79 cd | 40.3±2.36 c | 22.4±1.02 d |
| SaMR12 inoculated | 29.3±1.62 de | 18.5±0.64 b | 123.1±5.77 a | 84.1±4.28 a | 28.6±2.46 bcd | |
| 200 µM | Non-inoculated | 26.5±1.31 de | 12.0±1.01 c | 57.9±5.15 d | 32.2±4.42 c | 23±0.76 d |
| SaMR12 inoculated | 39.9±2.48 bc | 15.9±0.87 bc | 61.1±7.49 d | 58.2±1.92 b | 25.9±1.89 cd | |
| 500 µM | Non-inoculated | 46.3±3.02 b | 23.6±2.89 a | 75.9±3.32 bcd | 37.4±2.95 c | 35.3±2.29 b |
| SaMR12 inoculated | 64.9±3.71 a | 12.3±0.98 c | 91.6±11.52 b | 35.4±4.55 c | 45.4±2.3 a | |
Values represent the mean ± standard deviation of three replicates. *Significant at the P<0.05 level, **Significant at the P<0.01 level.
The different letters following the values in the same column indicate significant differences between the treatments at P<0.05 (Duncan's test).
Figure 9. Organic acid exudation as affected by SaMR12 and zinc treatment.
Bars plot mean ± SD of three replicate experiments. The level of each acid is normalized to the value obtained for the non-inoculated and un-supplemented treatment. Raw data are shown in table 2.
Discussion
During phytoremediation, it is an important for plants to continually absorb heavy metals from the soil and transport them from the root to the shoot. Three factors are necessary for this process to be effective: (1) the plants must be resistant to heavy metal; (2) the plants must absorb metals from soil; (3) the metal should be transported from the root to the shoot [22]. In aiding phytoremediation, endophytic bacteria can produce IAA, 1-aminocyclopropane-1-carboxylate deaminase, siderophore, or promote phosphate solubilization and nitrogen fixation [23], [24]. These are important mechanisms for alleviating metal stress, promoting nutrient absorption, and providing growth-promoting substances [25]–[28]. For instance, Chen et al. [29] found that plant growth-promoting endophytes significantly increased Solanum nigrum biomass and enhanced cadmium extraction from the soil. In a previous study, the endophytic bacterium SaMR12 was shown to be effective for promoting growth of the host plant [16]. Here, we found that SaMR12 affects plant growth, root morphology, oxidative stress, and root exudates.
The biomass of a plant is a primary factor limiting successful remediation of heavy metal contamination. In sugar beet, above-ground, endophytic bacteria increased photosynthetic efficiency by increasing chlorophyll content, leading to increased carbohydrate synthesis [30]. In red pepper, Islam et al. [31] found a significant increase in chlorophyll content after inoculation with a nitrogen-fixing bacterium. And in micropropagated sugarcane, Oliveira et al. [32] proved that inoculation endophytic diazotrophic species resulted in 30% increasing of total plant nitrogen content. Underground, a large area of contact between the root and soil is necessary to increase absorption of the metals from the soil; as such, remediation efficiency can be increased through an enlarged root system [33], [34].
Here, following inoculation with the endophytic bacterium, root length, number, and surface area increased significantly (Table 1), which agrees with the results of a previous study [35]. There has been speculation that these changes are driven by auxin, produced either directly by the endophyte or indirectly by the plant in response. For example, the roots of canola were more than 35% longer following treatment with wild-type P. putida GR12-2 compared with non-inoculated control, while the IAA-deficient mutant did not show this increase [36]. Various studies have demonstrated that IAA-producing bacteria promoted plant growth to some extent [37]. The black truffle fungus, Tuber melanopsorum, induces alterations in both host (Cistus incanus) and non-host (Arabidopsis thaliana) roots, such as promoting root hair elongation, that are reminiscent of altered auxin levels [38], [39]. Nevertheless, although we find that zinc and the endophyte SaMR12 increase auxin levels, the concentration dependence differed from the promotion of biomass and zinc accumulation. Both of the latter were clearly stimulated on 50 µM zinc whereas there was little increase in IAA. Furthermore, root hairs responded vigorously to the presence of endophyte but this response seemed more or less independent of the level of zinc (Fig. 8). Taken together, these results imply that IAA increases are secondary or even inconsequential for the action of zinc and endophyte in promoting biomass and metal accumulation in S. alfredii.
Zinc is typically not considered to induce reactive oxygen in plants, however, various studies have demonstrated that zinc can activate copper/zinc superoxide dismutase and proline production to alleviate metal-induced oxidative stress or enhance the activities of antioxidant enzymes [40], [41]. In this study, the concentrations of H2O2 and superoxide were little changed until the zinc treatment concentration reached 500 µM, and at that concentration, the oxidative stress of the inoculated plants was found to be ameliorated compared with non-inoculated plants (Figs. 5 and 6). Similar effects of endophytes on ryegrass and Achnatherum inebrians (drunken horse grass) have been reported [42], [43]. However in S. alfredii, it remains unclear whether the observed alleviation is a consequence of better nutrition or a direct action of the endophyte, such as the production of anti-oxidative enzymes or siderophore.
Root exudates comprise a complex set of compounds, which help mobilize mineral nutrients and condition the rhizosphere [44]. According to a review by Hinsinger et al. [45], root exudates improve metal element availability by decreasing rhizosphere pH, potentially by making absorption by the plant easier [46]. However, distinct organic acids have distinct efficacy. Wu et al. [47] found that the sequence of organic acids that solublize heavy metals is: malate ≥ citrate ≈ oxalate for zinc; and malate ≥ citrate > oxalate for cadmium. For various plants, Cieśliński et al. [48] found that the high cadmium accumulator secreted significantly more low-molecular-weight organic acids than the low cadmium accumulator in three soil types, and the extractable soil cadmium and cadmium accumulation in plants gradually increased with higher low-molecular-weight organic acid production.
In a previous study, the exogenous addition of citric or tartaric acid significantly increased the availability and absorption of cadmium [46]. Here, the root secreted oxalic acid as a function of zinc concentration, and this secretion rate was further increased when the plant was inoculated with SaMR12. Higher zinc (500 µM) treatment stimulated citric acid secretion, which agreed with the results of Saber et al. [49], who found for sunflower a marked increase in the excretion of malate and citrate with exposure to zinc or aluminum, and high concentrations of malic and citric acids alleviated the toxicity effect of these metals on plant growth. In Pinus sylvestris, oxalic acid was stimulated by exposure to elevated aluminum and was found to be significantly higher in mycorrhizal-treated plants than in non-mycorrhizal controls [50].
The activating effect of organic acid on heavy metal uptake is commonly related to pH. Succinate enhanced the mobilization of arsenic, copper, lead, and zinc under neutral pH, but inhibited this mobilization under acidic conditions [51]. Cadmium-resistant tomato plants were not susceptible to cadmium stress because of oxalate secretion by the root apex, which can form a precipitate with cadmium to efficiently ameliorate toxicity [52]. In contrast, Wu et al. [53] found that oxalate, citrate, or malate in the soil at the same concentrations had virtually no effect on metal uptake by Indian mustard (Brassica juncea).
Conclusion
This is apparently the first study to investigate the effects of inoculation with the endophytic bacterium, SaMR12, on plant growth, root morphology, and root exudates, a study that might facilitate the understanding of mechanisms of microbe-assisted phytoremediation. Through hydroponic experiments, SaMR12 inoculation significantly enhanced the efficiency of zinc phyto-extraction by increasing S. alfredii biomass, promoting zinc absorption, improving root morphology, and enhancing root exudates. Further we could show that although IAA levels and reactive oxygen species rose, both could be plausibly disconnected from the promoting action of both zinc itself and the endophyte. The application of endophytes to phytoremediation not only strengthens the ability of phyto-accumulators to acclimate to the contaminated environment, but also expands the application range of phytoremediation.
Acknowledgments
The authors would like to thank Dr. Tobias Isaac Baskin (Academic Editor, PLoS One) for his contribution in the compilation of this paper.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the projects from the “863” project from the Ministry of Science and Technology of China (No. 2012AA100605), Natural Science Foundation of China (No. 21177107), and the Ministry of Education of China (No. 310003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Smit C, Van Gestel C (1996) Comparison of the toxicity of zinc for the springtail Folsomia candida in artificially contaminated and polluted field soils. Appl Soil Ecol 3: 127–136. [Google Scholar]
- 2.Kochian LV (2000) Molecular physiology of mineral nutrient acquisition, transport, and utilization. In: Buchanan BB, Gruissem W, Jones RL, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp. 1204–1249. [Google Scholar]
- 3. Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56: 15–39. [DOI] [PubMed] [Google Scholar]
- 4. Barceló J, Poschenrieder C (2003) Phytoremediation: principles and perspectives. Contributions to Science 2: 333–344. [Google Scholar]
- 5. Li TQ, Yang XE, He ZL, Yang JY (2005) Root morphology and Zn2+ uptake kinetics of the Zn hyperaccumulator of Sedum alfredii Hance. J Integr Plant Biol 47: 927–934. [Google Scholar]
- 6. Yang XE, Peng HY, Jiang LY, He ZL (2005) Phytoextraction of copper from contaminated soil by Elsholtzia splendens as affected by EDTA, citric acid, and compost. Intern J Phytoremediat 7: 69–83. [DOI] [PubMed] [Google Scholar]
- 7. Yang J, Yang X, He Z, Li T, Shentu J, et al. (2006) Effects of pH, organic acids, and inorganic ions on lead desorption from soils. Environ Pollut 143: 9–15. [DOI] [PubMed] [Google Scholar]
- 8. Liu D, Li T, Yang X, Islam E, Jin X, et al. (2007) Enhancement of lead uptake by hyperaccumulator plant species Sedum alfredii Hance using EDTA and IAA. B Environ Contam Tox 78: 280–283. [DOI] [PubMed] [Google Scholar]
- 9. Yang X, Long X, Ye H, He Z, Calvert D, et al. (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259: 181–189. [Google Scholar]
- 10. Yang X, Li T, Yang J, He Z, Lu L, et al. (2006) Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta 224: 185–195. [DOI] [PubMed] [Google Scholar]
- 11. Li T, Tao Q, Liang C, Shohag M, Yang X, et al. (2013) Complexation with dissolved organic matter and mobility control of heavy metals in the rhizosphere of hyperaccumulator Sedum alfredii . Environ Pollut 182: 248–255. [DOI] [PubMed] [Google Scholar]
- 12. el Zahar Haichar F, Marol C, Berge O, Rangel-Castro JI, Prosser JI, et al. (2008) Plant host habitat and root exudates shape soil bacterial community structure. The ISME Journal 2: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 13. Kothari S, Marschner H, George E (1990) Effect of VA mycorrhizal fungi and rhizosphere microorganisms on root and shoot morphology, growth and water relations in maize. New Phytol 116: 303–311. [Google Scholar]
- 14. Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, et al. (2009) Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microb 75: 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meharg A, Killham K (1995) Loss of exudates from the roots of perennial ryegrass inoculated with a range of micro-organisms. Plant Soil 170: 345–349. [Google Scholar]
- 16. Zhang X, Lin L, Zhu Z, Yang X, Wang Y, et al. (2013) Colonization and modulation of host growth and metal uptake by endophytic bacteria of Sedum alfredii . Intern J Phytoremediat 15: 51–64. [DOI] [PubMed] [Google Scholar]
- 17. Přikryl Z, Vančura V (1980) Root exudates of plants. Plant Soil 57: 69–83. [Google Scholar]
- 18. Ma JF, Zheng SJ, Matsumoto H (1997) Specific secretion of citric acid induced by Al stress in Cassia tora L. Plant Cell Physiol. 38: 1019–1025. [Google Scholar]
- 19. Hou S, Zhu J, Ding M, Lv G (2008) Simultaneous determination of gibberellic acid, indole-3-acetic acid and abscisic acid in wheat extracts by solid-phase extraction and liquid chromatography–electrospray tandem mass spectrometry. Talanta 76: 798–802. [DOI] [PubMed] [Google Scholar]
- 20. Nag S, Saha K, Choudhuri MA (2000) A rapid and sensitive assay method for measuring amine oxidase based on hydrogen peroxide–titanium complex formation. Plant Sci 157: 157–163. [DOI] [PubMed] [Google Scholar]
- 21. Tang B, Zhang L, Hu JX, Li P, Zhang H, et al. (2004) Indirect determination of superoxide anion radical in the plant of red sage based on vanillin-8-aminoquinoline with fluorescence. Anal Chim Acta 502: 125–131. [Google Scholar]
- 22. Rajkumar M, Ae N, Freitas H (2009) Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 77: 153–160. [DOI] [PubMed] [Google Scholar]
- 23. Jha P, Kumar A (2009) Characterization of novel plant growth promoting endophytic bacterium Achromobacter xylosoxidans from wheat plant. Microb Ecol 58: 179–188. [DOI] [PubMed] [Google Scholar]
- 24. Zhang YF, He LY, Chen ZJ, Zhang WH, Wang QY, et al. (2011) Characterization of lead-resistant and ACC deaminase-producing endophytic bacteria and their potential in promoting lead accumulation of rape. J Hazard Mater 186: 1720–1725. [DOI] [PubMed] [Google Scholar]
- 25. Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46: 237–245. [DOI] [PubMed] [Google Scholar]
- 26. Chen Y, Ren CG, Yang B, Peng Y, Dai CC (2013) Priming effects of the endophytic fungus Phomopsis liquidambari on soil mineral N transformations. Microb Ecol 65: 161–170. [DOI] [PubMed] [Google Scholar]
- 27. Harish S, Kavino M, Kumar N, Balasubramanian P, Samiyappan R (2009) Induction of defense-related proteins by mixtures of plant growth promoting endophytic bacteria against Banana bunchy top virus. Biol Control 51: 16–25. [Google Scholar]
- 28. Marques AP, Pires C, Moreira H, Rangel AO, Castro PM (2010) Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol Biochem 42: 1229–1235. [Google Scholar]
- 29. Chen L, Luo S, Xiao X, Guo H, Chen J, et al. (2010) Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl Soil Ecol 46: 383–389. [Google Scholar]
- 30. Shi Y, Lou K, Li C (2010) Growth and photosynthetic efficiency promotion of sugar beet (Beta vulgaris L.) by endophytic bacteria. Photosynth Res 105: 5–13. [DOI] [PubMed] [Google Scholar]
- 31. Islam M, Sultana T, Joe MM, Yim W, Cho JC, et al. (2013) Nitrogen-fixing bacteria with multiple plant growth-promoting activities enhance growth of tomato and red pepper. J Basic Microb 53: 1004–1015. [DOI] [PubMed] [Google Scholar]
- 32. Oliveira A, Urquiaga S, Döbereiner J, Baldani J (2002) The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 242: 205–215. [Google Scholar]
- 33. Berkelaar E, Hale B (2000) The relationship between root morphology and cadmium accumulation in seedlings of two durum wheat cultivars. Can J Botany 78: 381–387. [Google Scholar]
- 34. Li T, Yang X, Lu L, Islam E, He Z (2009) Effects of zinc and cadmium interactions on root morphology and metal translocation in a hyperaccumulating species under hydroponic conditions. J Hazard Mater 169: 734–741. [DOI] [PubMed] [Google Scholar]
- 35. Malinowski D, Brauer D, Belesky D (1999) The endophyte Neotyphodium coenophialum affects root morphology of tall fescue grown under phosphorus deficiency. J Agron Crop Sci 183: 53–60. [Google Scholar]
- 36. Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68: 3795–3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuttavanichakul W, Lawongsa P, Wongkaew S, Teaumroong N, Boonkerd N, et al. (2012) Improvement of peanut rhizobial inoculant by incorporation of plant growth promoting rhizobacteria (PGPR) as biocontrol against the seed borne fungus, Aspergillus niger . Biol Control 63: 87–97. [Google Scholar]
- 38. Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in arabidopsis. Plant Physiol 149: 1579–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Splivallo R, Fischer U, Göbel C, Feussner I, Karlovsky P (2009) Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol 150: 2018–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, et al. (2007) Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 85: 837–844. [DOI] [PubMed] [Google Scholar]
- 41. Tripathi BN, Gaur J (2004) Relationship between copper-and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 219: 397–404. [DOI] [PubMed] [Google Scholar]
- 42. Bonnet M, Camares O, Veisseire P (2000) Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv Apollo). J Exp Bot 51: 945–953. [PubMed] [Google Scholar]
- 43. Zhang X, Li C, Nan Z (2010) Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense . J Hazard Mater 175: 703–709. [DOI] [PubMed] [Google Scholar]
- 44. Jones DL, Darah PR, Kochian LV (1996) Critical evaluation of organic acid mediated iron dissolution in the rhizosphere and its potential role in root iron uptake. Plant Soil 180: 57–66. [Google Scholar]
- 45. Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248: 43–59. [Google Scholar]
- 46. Lu LL, Tian SK, Yang XE, Peng HY, Li TQ (2013) Improved cadmium uptake and accumulation in the hyperaccumulator Sedum alfredii: the impact of citric acid and tartaric acid. J Zhejiang Univ-Sc B 14: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu L, Luo Y, Christie P, Wong M (2003) Effects of EDTA and low molecular weight organic acids on soil solution properties of a heavy metal polluted soil. Chemosphere 50: 819–822. [DOI] [PubMed] [Google Scholar]
- 48. Cieśliński G, Van Rees K, Szmigielska A, Krishnamurti G, Huang P (1998) Low-molecular-weight organic acids in rhizosphere soils of durum wheat and their effect on cadmium bioaccumulation. Plant Soil 203: 109–117. [Google Scholar]
- 49. Saber N, Abdel-Moneim A, Barakat S (1999) Role of organic acids in sunflower tolerance to heavy metals. Biol Plantarum 42: 65–73. [Google Scholar]
- 50. Ahonen Jonnarth U, Van Hees PA, Lundström US, Finlay RD (2000) Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations. New Phytol 146: 557–567. [Google Scholar]
- 51. Wang S, Mulligan CN (2013) Effects of three low-molecular-weight organic acids (LMWOAs) and pH on the mobilization of arsenic and heavy metals (Cu, Pb, and Zn) from mine tailings. Environ Geochem Hlth 35: 111–118. [DOI] [PubMed] [Google Scholar]
- 52. Zhu XF, Zheng C, Hu YT, Jiang T, Liu Y, et al. (2011) Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esulentum . PlantCell Environ 34: 1055–1064. [DOI] [PubMed] [Google Scholar]
- 53. Wu L, Luo Y, Xing X, Christie P (2004) EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk. Agr Ecosyst Environ 102: 307–318. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.