Abstract
Understanding of the chronic immune activation, breakdown of immune defense and synergistic effect between HIV and Mycobacterium tuberculosis (Mtb) may provide essential information regarding key factors involved in the pathogenesis of HIV disease. In this study, we aimed to highlight a few of the immunological events that may influence and accelerate the progression of HIV disease in the presence of co-infecting Mtb. A cross-sectional study was performed on cohorts, including anti-tubercular therapy (ATT) naïve active pulmonary tuberculosis (PTB) patients, antiretroviral therapy (ART) naïve HIV-1 infected individuals at different stages of disease, ATT and ART naïve HIV-PTB co-infected individuals and healthy controls. A significantly higher T-regulatory cell (Treg) frequency coupled with the high FoxP3 expression in the CD4 T-cells indicated an immunosuppressive environment in the advance stage of HIV-1 infection. This is further substantiated by high HO-1 expression favoring TB co-infection. Functionally, this change in Treg frequency in HIV-1 infected individuals correlated well with suppression of T-cell proliferation. Mtb infection seems to facilitate the expansion of the Treg pool along with increased expression of FoxP3, specifically the variant-1, as evident from the data in HIV-1 co-infected as well as in patients with only PTB. A significantly lower expression of HO-1 in co-infected individuals compared to patients with only HIV-infection having comparable CD4 count correlated well with increased expression of CCR5 and CxCR4 as well as NF-κB and inflammatory cytokines IL-6 and TNF-α, which collectively may contribute to enhanced viral replication and increased cell death, hence faster disease progression in co-infected individuals.
Introduction
Mycobacterium tuberculosis (Mtb) continues to be one of the most dreaded pathogens around the world and its co-habitation with HIV in a common host fuels both the infections. Of the estimated 39 million people living with HIV, about one-third are estimated to have concomitant latent tuberculosis (Global Tuberculosis Control, WHO, 2010). Depending on the prevalence of HIV in a population, the risk for active tuberculosis becomes 20–37 times higher in individuals living with HIV than in the general population. India has approximately 2.1 million cases of HIV/AIDS predominantly infected with HIV-1 subtype-C [1].
The immune response towards any pathogen is regulated not to cause excessive damage to the host while leading to containment of infectious agent. During HIV-1 infection, this precise balance of the host immune response and its regulation gets disturbed, making the environment favorable for other opportunistic pathogens, especially Mtb. Regulatory T-cells (Tregs) are an important cell population that maintains central tolerance as well as controls the overall response to the invading pathogens, including viruses, however, their role during HIV infection remains controversial. The natural immune-suppressive ability of Tregs may be playing dual role during HIV infection, being harmful to the host for suppressing the viral- specific immune response [2], [3], [4], [5] on one hand and being beneficial at the same time for suppressing the hyperimmune activation state characteristic of this disease [6], [7], [8], [9]. In order to ascertain their behavior in a co-infected host and also if Mtb has any modulatory effect on Tregs that may change the course of HIV infection in the co-infected host, we have investigate the role of Tregs during HIV infection alone or during Mtb co-infection. Overall, we have address a few of the issues related to deadly synergism between HIV-1 and Mtb by focusing on the sensitive balance between immune-suppressive genes like HO-1 and FoxP3 as well as immune-stimulatory genes like NF-κB and how this balance affects the expression of HIV-1 co-receptors, CCR5 and CxCR4 on CD4 T-cell subpopulations. Our findings highlight a pathway in which Mtb down regulate the expression of HO-1 leading to up-regulation of the redox sensitive NF-κB that in turn induces the replication of HIV-1 proviral genome in PTB co-infected individuals, besides increasing the expression of HIV co-receptors CCR5 and CxCR4.
Material and Methods
Ethics statement
The study was approved by the Institutional Ethics Committee (IEC) of PGIMER, Chandigarh, India and 10 ml peripheral blood was obtained from each enrolled subject after an informed written consent. There were no minor/children recruited for this study.
Study subjects
The study was conducted on cohorts, including 27 HIV-1 infected patients, 12 pulmonary tuberculosis patients (PTB), 8 HIV-PTB co-infected patients (HIV-PTB), and 20 healthy controls (HC). HIV-1 infected patients, confirmed positive by three serologic tests as per National AIDS Control Organization (NACO, Govt. of India) guidelines, were enrolled from the Integrated Counselling and Testing Centre (ICTC) in the Department of Immunopathology, PGIMER Chandigarh, India. At the time of recruitment, the patients were interviewed by a counsellor to obtain informed consent and ascertain therapy naïve status. The status of the disease was assessed by absolute CD4 cell count monitored by flow cytometry using BD Tritest containing antibody conjugates CD3-FITC/CD4-PE/CD45-PerCP with BD Trucount tubes (BD Biosciences, San Jose, USA). PTB and HIV-PTB patients, confirmed positive for Mtb infection by chest x-ray and sputum smear positivity were recruited from DOTS (Directly observed therapy-short course) centre at our hospital. The peripheral blood mononuclear cells (PBMCs) were isolated from the heparinized blood by Ficoll-Hypaque density gradient centrifugation (HiMedia, Mumbai, India).
Treg Immunophenotyping
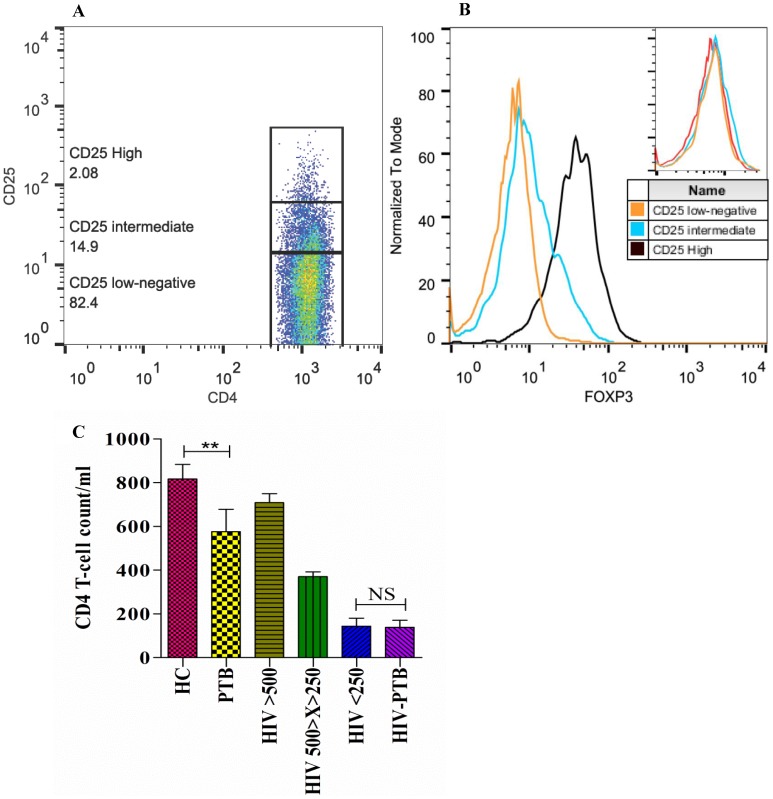
Freshly isolated PBMCs were immunophenotyped for Treg number, FoxP3 expression (MFI) and CCR5/CxCR4 expression using fluorochrome-conjugated monoclonal antibodies (mAb): anti-CD4 PE/FITC, anti-CD25 PE-Cy7, anti-CCR5 PE, and anti-CxCR4 PE (BD Bioscience, San Jose, CA) for cell-surface markers in combination with intracellular Fork-head box protein 3 (FoxP3) mAb conjugated with Alexa488 (eBioscience, San Diego, CA). Samples were acquired into a flow cytometer (FACS Calibur, BD, USA) and analyzed using Cell-Quest (BD Bioscience)/FlowJo (Treestar) softwares. For further analysis, patients were categorized on the basis of level of surface CD25 expression on CD4+ T-cells viz: CD4+CD25high (top 2% with high CD25 expression), CD4+CD25intermediate (middle 15% with intermediate CD25 expression) and CD4+CD25low/negative (lower 83% with very low or no expression of CD25) cells ( Figure 1A & 1B ). Absolute counts for different populations were calculated from the total lymphocyte count in the whole blood.
Figure 1. Lower CD4 T-cell count in PTB individuals as compared to healthy controls.
A. Representative dot plot showing gating strategy for immunophenotyping of the three subpopulations of CD4+ T-cells based on CD25 expression as: CD25high, CD25intermediate and CD25low/negative. B. Representative overlay histogram showing intracellular FoxP3 expression in different CD4+ T-cell subpopulations with isotype control (upper right). C. Bar diagram showing mean±SEM of CD4 counts of each studied group. HC: Healthy control; PTB: pulmonary tuberculosis; HIV >500: HIV-1 infected individuals with CD4 count above 500 cells/µl; HIV 500>X>250: HIV-1 infected individuals with CD4 count between 500 and 250 cells/µl; HIV <250: HIV-1 infected individuals with CD4 count less than 250 cells/µl. Significant differences are represented as the p value <0.01 (**), NS: (non-significant).
T-cell proliferation assay
To examine the functional defects of T-cells in these cohorts, proliferative capability of T cells was assessed on stimulation with mitogen-phytoheamagglutinin [PHA (10 µg/ml] by determining the extent of 3H-thymidine incorporation. Briefly, 1×105 PBMCs/well were plated into 96-well plates in triplicates in complete medium [RPMI 1640 (Sigma) medium supplemented with 10% heat-inactivated FCS, 20 IU/ml penicillin, 20 µg/ml streptomycin, 25 mM HEPES, and 2 mM L-glutamine] incubated for 96 hours at 37°C in 5% CO2 and humidified atmosphere, either in presence or absence of PHA as the stimulant. Eighteen hours prior to termination of the culture, 1 µCi of 3H-thymidine (BARC, India) was added to each well. The cells were then harvested on glass fibre mats (Skatron, Belgium) and radioactivity was counted in a liquid scintillation β-counter (Beckman, USA). All measurements were conducted in triplicate and the results were expressed as the stimulation index (SI), which represented the mean cpm (counts per minute) in the presence of mitogen divided by mean cpm in the absence of the mitogen.
Detection of cytokine in culture supernatants
Patient plasma samples and supernatants were collected from the PHA stimulated PBMC cultures (as above) before addition of 3H-thymidine for estimation of cytokines. The cytokines TNF-α and IL-6 were measured using Th1/Th2 Cytokine CBA Kit II (BD Cytokine Bead Array).
Analysis of Mitochondrial Membrane Potential (Δψm)
Cell death was evaluated flowcytometrically by measuring mitochondrial membrane potential (Δψm) with J-aggregate forming lipophilic action 5, 5′, 6, 6′-tetraethylbenzimidazolocarbocyanine iodide (JC-1) dye (Sigma-Aldrich, USA). Briefly, 2 ml whole blood was lysed with RBC lysing buffer (Himedia, Mumbai, India) and isolated leukocytes were washed with phosphate buffer saline (PBS, Himedia, Mumbai, India) [10]. Finally, 1×106 cells/ml were resuspended in PBS before staining with JC-1 dye (2.5 µM) and incubated at 37°C (5% CO2 humidified atmosphere) for 15 min in dark. Stained cells washed and resuspended, were acquired immediately on flow cytometer (FACSCanto II) and the data was analyzed using software FACSDiva Version 6.1.3 (BD Biosciences, USA). Lymphocytes were gated on forward scatter and side scatter to exclude debris and non-lymphoid cells. JC-1 fluorescence was analyzed on FL1 and FL2 channels for the detection of dye monomers (Green) and J-aggregates (Red), respectively. The dead cell gate was set up by the mitochondrial uncoupling agent protonophore carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) (Sigma-Aldrich, USA). The treatment with the protonophore FCCP resulted in maximum decrease of the JC-1 fluorescence ratio and served as a positive control for disruption of mitochondrial membrane potential. The ratio of red/green fluorescence reflected mitochondrial trans-membrane potential. Δψm was expressed as median percent fluorescence intensity (FL2: FL1/FL2 FCCP: FL1 FCCP) ×100.
Semi-quantitative estimation of gene expression by RT-PCR analysis
Total RNA was extracted from EDTA blood using the QIAmp RNA Blood Mini Kit (Qiagen, Germany). RNA was converted into cDNA using Revert Aid First Strand cDNA Synthesis Kit (MBI Fermentas, Lithuania). The first strand cDNA was used in PCR reaction for measuring the relative expression of various genes normalized against the expression levels of a house keeping gene GAPDH. The primers for FoxP3 splice variant 1 (|NM_014009.3, representing the longer transcript), splice variant 2 (NM_001114377.1; lacking an in-frame segment of the coding region), heme oxygenase 1 (NM_002133.1), CxCR4 (NM_001008540.1) and NF-κB (NM_003998.3) were as follows:
GAPDH forward: 5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse: 5′-GTCCACCACCCTGTTGCTGTAG-3′; FoxP3 forward: 5'-CGGACCATGTTCTGGATGAG-3' and reverse: 5'-TTGTCGGATGATGCCACAG-3'; HO-1 forward: 5'-AGGCCAAGACTGCGTTCCT-3' and reverse: 5'-GCAGAATCTTGCACTTTGTTG -3'; CxCR4 forward: 5′- CTTCTACCCCAATGACTTGTGG-3′ and reverse: 5′- AATGTAGTAAGGCAGCCAACAG-3′; NF-κB forward: 5′-TTCACCAAGCCTGCCCTTGGAC-3′ and reverse: 5′-CTGTCTTGTGGACAACGCAGTGGAATTTTAGG-3′.
The thermal cycler was programmed as: denaturation at 95°C for 5 min, followed by 35 cycles at 95°C for 30 Sec and annealing at 58°C for GAPDH, 64°C for CxCR4, 60°C for FoxP3, 58°C for HO-1 and 62°C for NF-κB for 30 Sec, extension in all was carried at 72°C for 1 min, with a final extension step of 10 min.
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM) and ranges. The difference between groups was tested by one-way student's t-test. Correlation analysis was done using the non-parametric Spearman's rank correlation coefficient. Linear Regression Analysis was carried out and the residuals were calculated. The level of significance was set at p<0.05.
Results
Demographic and Immunological characteristics of study subjects
In this cross-sectional study we recruited a total of 67 subjects including 27 patients infected with HIV-1 only (age range: 21–53 years and CD4+ T-cell count range: 32–854 cells/µl), 12 patients with active PTB (age range: 21–65 years and CD4+ T-cell count range: 326–1368 cells/µl), 8 HIV patients having pulmonary tuberculosis also (HIV-PTB co-infected patients) (age range: 17–34 years and CD4+ T-cell count range: 30–273 cells/µl), and 20 healthy controls (age range: 22–40 years and CD4+ T-cell count range: 444–1375 cells/µl).
There was no significant difference between the mean CD4 count of HIV-1 infected individuals in the advanced stage of disease (CD4 count <250 cells/µl) and HIV-PTB co-infected individuals [CD4 count (mean±SEM cells/µl): 144±36 and 138±32 respectively] ( Figure 1C ). With similar immunological status in terms of CD4 count between the two above mentioned groups, comparisons were made to determine how PTB influences HIV-1 disease in co-infected individuals. The mean CD4 count in individuals with active pulmonary tuberculosis only (HIV-1/2 negative) was significantly lower than healthy controls [CD4 count (mean±SEM cells/µl): 577±102 vs 817±66, p = 0.0064], though well within the normal range.
Increased Treg frequency coincided with poor T-cell proliferation in advanced stage of HIV disease with Mtb co-infection
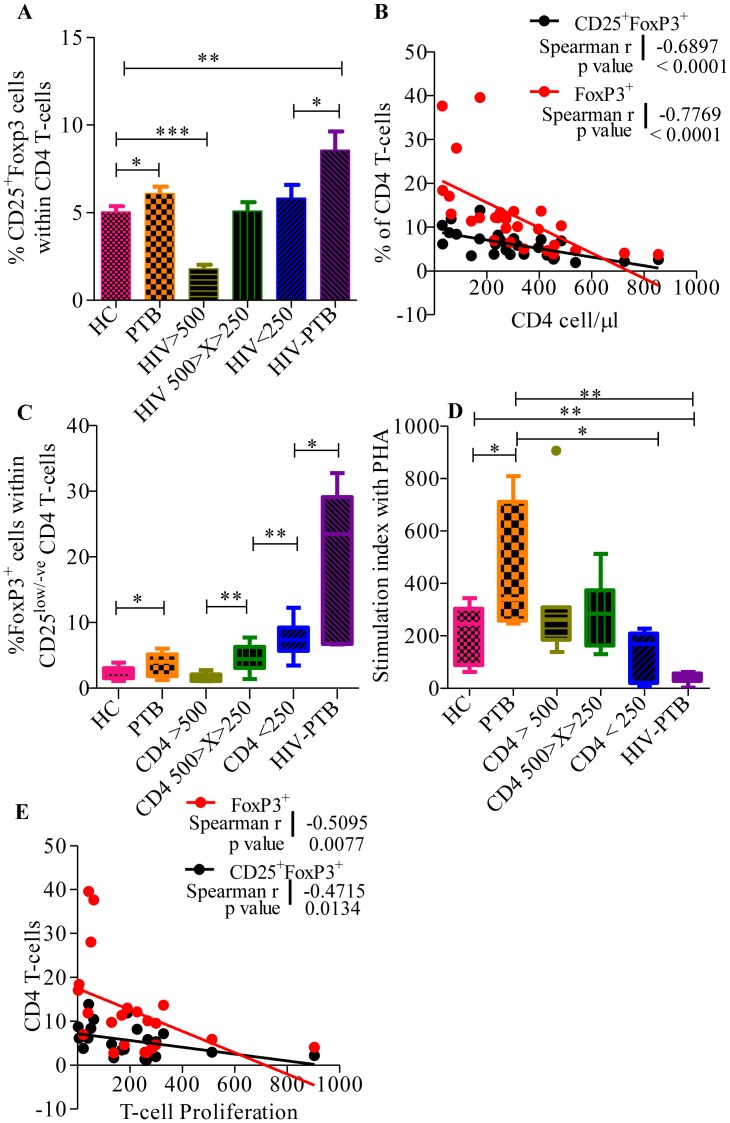
There was a significantly higher frequency of CD25+FoxP3+ CD4 T-cells in PTB infected individuals with (mean±SEM: 8.57±1.07, p = 0.0012) and without (mean±SEM: 6.09±0.39, p = 0.035) HIV-1 co-infection when compared to healthy controls (mean±SEM: 5.04±0.33) ( Figure 2A ). Similar results were observed with significantly higher frequency of FoxP3 expressing CD4 T-cells in HIV-1 infected individuals with CD4 count <250 cells/µl (mean±SEM: 12.48±1.27, p = 0.001), HIV-PTB co-infected individuals (mean±SEM: 24.25±4.36, p<0.0001) and PTB individuals (mean±SEM: 9.45±0.74, p = 0.0146), when compared with healthy controls (mean±SEM: 7.29±0.38). The relationship between HIV-1 disease progression (in terms of CD4 count) with FoxP3+ CD4 T-cells and CD25+FoxP3+ CD4 T-cell was evaluated. Highly significant association was observed in HIV-1 infected individuals with increased FoxP3+ cell frequency as the disease progressed (Spearman r = -0.7769, p value <0.0001) ( Figure 2B ). This was partially contributed by a significant increase in the CD4+CD25low/negative FoxP3+ cells ( Figure 2C ). This trend was also visible in the HIV-PTB co-infected individuals (median = 23.42, mean±SEM: 19.52±4.34, p = 0.049) when compared to only HIV infected individuals with similar CD4 counts (median = 7.37, mean±SEM: 7.63±1.05) ( Figure 2C ).
Figure 2. Higher Tregs frequency linked with PTB in individuals co-infected with or without HIV-1.
A. Cumulative data depicting frequency of CD25+Foxp3+ Tregs within CD4+ T-cell in healthy controls (HC), patients with Pulmonary tuberculosis (PTB) and HIV-1 infected individuals with or without PTB co-infection. B. Association of increased Foxp3+ CD4 T-cell frequency with HIV-1 disease progression (decrease in CD4 count). Dot plot showing significant negative correlation between T-cell proliferation and CD4+Foxp3+ (red dots, Spearman r = −0.7769, p value <0.0001)/CD25+Foxp3+ (black dots, Spearman r = −0.6897, p value <0.0001) frequency in HIV-1 infected individuals. The solid line represents the linear regression of points and dotted lines represents 95% confidence band for their mean values. Rho and p values are from the Spearman rank correlation test. C. Increase in the FoxP3+CD25low/-ve CD4 T-cells in PTB and HIV-PTB co-infected individuals. Whisker box plot showing frequency of FoxP3 positive cells within CD25low/-ve cells in the PBMCs of study subject groups. The horizontal line within the box indicates the median, the boundaries of the box indicate the 25th- and 75th -percentile, and the whiskers indicate the highest and lowest values of the frequency of FoxP3 positive cells. D. Low T-cell proliferation in HIV-PTB co-infected individuals. Whisker box plot showing the proliferative T-cell response profile to PHA [stimulation for 96 hours] in PBMCs from healthy controls, patients with PTB and HIV-1 infected individuals with or without M. tuberculosis co-infection. Stimulation indices were determined by comparison with paired unstimulated cells from each individual. The horizontal line within the box indicates the median, the boundaries of the box indicate the 25th- and 75th -percentile, and the whiskers indicate the highest and lowest values of the stimulation indices. E. Association of increased Foxp3+ CD4 T-cells frequency with decreased T-cell proliferative response. Dot plot showing significant negative correlation between T-cell proliferation and Foxp3+ (red dots, Spearman r = −0.5095, p value <0.0077)/CD25+Foxp3+ (black dots, Spearman r = −0.4715, p value = 0.0134) in HIV-1 infected individuals. The solid line represents the linear regression of points and dotted lines represents 95% confidence band for their mean values. Rho and p values are from the Spearman rank correlation test.
This change in the Tregs frequency correlated well with a significant decrease in the T-cell proliferation in response to PHA stimulation in HIV-PTB co-infected individuals (median = 43.1, mean±SEM: 39.90±10.00, p = 0.0011) when compared to healthy controls (median = 245.7, mean±SEM: 209.7±30.76) ( Figure 2D ). Within the HIV-1 infected group, T-cells from HIV-1 infected individuals in the early stage of disease (CD4 count >500 cells/µl) showed higher T-cell proliferation (mean±SEM: 332.9±97.74, p = 0.1596) as compared to healthy controls or HIV-PTB co-infected subjects, correlating well with a significantly lower frequency of Tregs in these patients (mean±SEM: 3.49±0.31, p = 0.0003). Overall, we observed that the T-cell proliferation was negatively correlated with frequency of CD25+FoxP3+ (Spearman r = −0.4715, p value = 0.0134) and only FoxP3+ (Spearman r = −0.5095, p value <0.0077) CD4 T-cells in HIV infected individuals ( Figure 2E ). The above results indicate higher CD25low/-veFoxP3+ cells and intact suppressive function of expanded Tregs in HIV-PTB co-infected patients.(instead we need a fig showing T-cell proliferation data in different gps)
Higher intracellular FoxP3 protein levels in Tregs from PTB-HIV co-infected individuals is associated with higher FoxP3 variant 1 mRNA levels in PBMCs
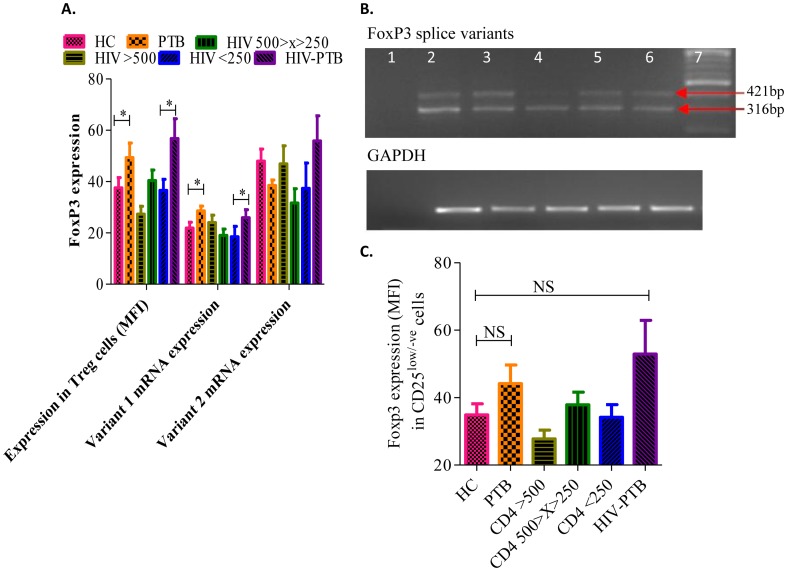
Higher FoxP3 expression has been related to stability of Tregs and their intact suppressive functions. The FoxP3 expression was significantly higher in CD4+ Tregs from PTB patients (mean±SEM: 49.35±5.71, p = 0.0347) and HIV-PTB co-infected individuals (mean±SEM: 56.76±7.75, p = 0.0180) as compared to healthy controls (mean±SEM: 37.48±4.01) ( Figure 3A ). Interestingly, there was significantly higher Foxp3 protein level in Tregs from HIV-PTB co-infected individuals (mean±SEM: 56.76±7.75) in comparison to individuals infected with only HIV having comparable CD4 count of <250 cells/µl (mean±SEM: 36.60±4.30, p = 0.0361) indicating a possible role of Mtb in inducing Foxp3 expression. This significant difference correlates with only FoxP3 variant 1 mRNA expression as evident from its significantly higher expression in PTB (mean±SEM: 28.58±1.85, p = 0.0294) and HIV-PTB co-infected individuals (mean±SEM: 25.92±3.13, p = 0.0476) when compared to healthy controls (mean±SEM: 21.92±2.24) and HIV-1 individuals with similar CD4 count [<250 cells/µl (mean±SEM: 18.51±4.06)]. Though the overall expression level of FoxP3 variant-2 was higher than variant-1, we did not observe any significant difference in the expression levels of variant-2 among any of the studied groups ( Figure 3A & 3B ).
Figure 3. High FoxP3 expression in PTB patients with or without HIV-1 co-infection correlated with FoxP3 variant-1 mRNA expression only.
A. Bar diagram depicting expression of intracellular Foxp3 within CD4+ T-cells, the relative expression of Foxp3 variant-1 and variant 2 mRNA (normalized using GAPDH levels) in all studied groups. Data were expressed as Mean±SEM and p-value < 0.05 (*) was considered significant. B. Representative 2% agarose gel image of RT-PCR products, showing mRNA levels of FoxP3 splice variant 1 (421 bp) & variant 2 (316 bp) and their corresponding housekeeping gene, GAPDH (180 bp) in PBMCs of HIV-1 infected individuals. Lane 1: Negative control, lanes 2-6: HIV-1 infected individuals and lane 7: 100 bp DNA ladder. C. Higher FoxP3 expression in CD25low/-ve cells from PTB patients with or without HIV-1 co-infection. Whisker box plot showing levels of FoxP3 expression (MFI) in CD25low/-ve cells in the PBMCs of study subject groups. The horizontal line within the box indicates the median, the boundaries of the box indicate the 25th- and 75th -percentile, and the whiskers indicate the highest and lowest values of the FoxP3 expression.
Significant increase in the frequency of CD4+CD25low/negative FoxP3+ cells in PTB individuals with and without HIV-1 co-infection had higher FoxP3 expression in these cells (HIV-PTB median = 52.82, mean±SEM: 52.92±9.98 and PTB median = 42.83, mean±SEM: 44.13±5.52) when compared to healthy controls (median = 28.45, mean±SEM: 34.84±3.33) though statistically non-significant ( Figure 3C ).
FoxP3 expression levels positively correlated with HO-1 levels in HIV-1 infected individuals
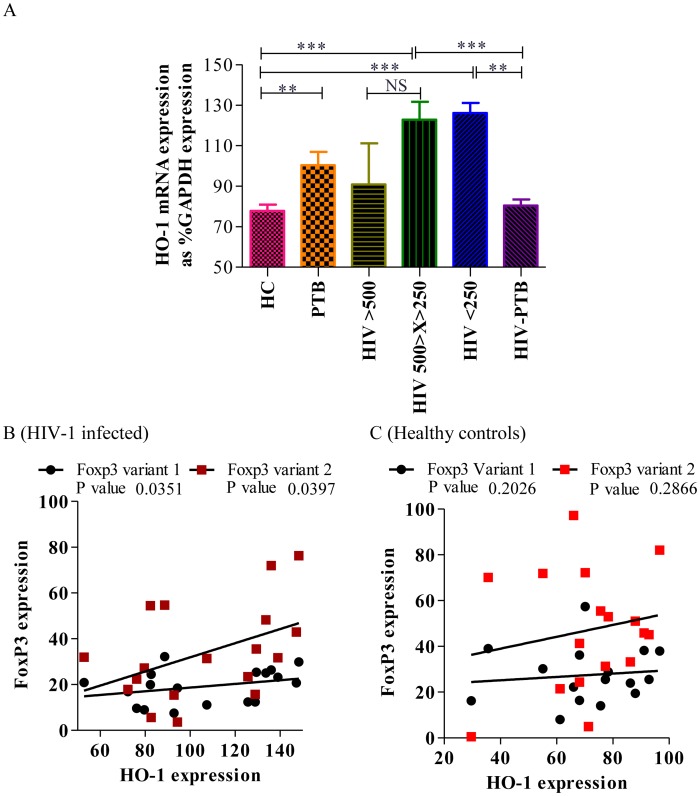
Since HO-1 has been implicated in the activation as well as induction and/or expansion of Tregs with its constitutive expression in human peripheral blood Tregs but not in resting CD4+CD25− non-Tregs, we have also studied the relationship of HO-1 and FoxP3 splice variant levels in our study groups. Though, there was a significantly higher expression of HO-1 in HIV-1 infected individuals as compared to healthy controls, but there was no significant change in HO-1 expression in HIV-1 infected individuals with disease progression ( Figure 4A ). Whereas, HIV-PTB co-infected individuals had significantly lower (mean±SEM: 80.49±2.9) expression of HO-1 in comparison to HIV-1 infected individuals with similar CD4 count (<250 cells/µl (p = 0.0022)). Spearman's correlation analysis revealed a significant positive correlation between HO-1 and both FoxP3 splice variants in HIV-1 infected individuals only [variant-1: r = 0.4365, p = 0.0351, variant-2: r = 0.4241, p = 0.0397 ( Figure 4B )] but not in healthy controls [variant 1: r = 0.2235, p = 0.2026, variant 2: r = 0.4171, p = 0.2866 ( Figure 4C )]. These findings indicate a possible role of HO-1 in FoxP3 mediated suppression of T-cell activation in HIV-1 infected individuals.
Figure 4. High HO-1 expression in the later stage of HIV-1 infection favors TB infection.
A. The relative expression of the HO-1 gene was normalized using GAPDH levels in all study groups. All data are expressed as mean±SEM values. Significant differences are represented as the p value <0.05 (*). B. Represents scatter plot depicting significant positive correlation between HO-1 and FoxP3 gene expression in HIV-1 infected individuals. C. Scatter plot showing no significant correlation between HO-1 and FoxP3 variant 1 & 2 expression in PBMCs of healthy controls. The solid lines represent linear regression of points. Rho and p values are from the Spearman rank correlation test.
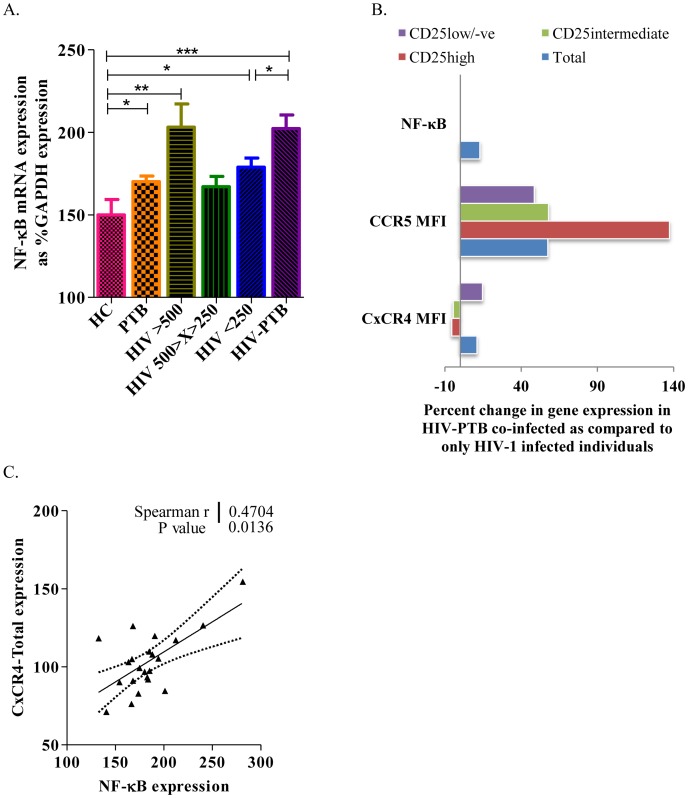
Lower HO-1 levels in HIV-PTB co-infected individuals correlate significantly with higher expression of NF-κB and HIV co-receptors CCR5 and CxCR4 on Tregs
HO-1 induction provides important protection from oxidative stress and cellular damage. Lower levels of HO-1 in PTB co-infected individuals can up-regulate redox sensitive NF-κB, which is known to have a binding site on the Long Terminal Repeats [11] of the integrated HIV-1 provirus and induces its replication. We found significantly higher levels of NF-κB expression in HIV-PTB co-infected individuals (mean±SEM: 202.3±8.3, p = 0.0152) when compared to HIV-1 infected individuals with comparable CD4 counts [<250 cells/µL; mean±SEM: 178.9±5.5 ( Figure 5A )].
Figure 5. Higher NF-κB expression in HIV-PTB co-infected individuals modulate HIV-1 co-receptor expression on CD4 T-cell sub-populations.
A. Represents bar diagram depicting relative expression of NF-κB gene mRNA (normalized using GAPDH levels) in all studied groups. Data were expressed as Mean ± SEM and p-value < 0.05 (*) was considered significant. B. Represents bar diagram depicting relative change in the expression of NF-κB gene mRNA (normalized using GAPDH levels), CCR5 and CxCR4 expression on CD4 T-cell sub-populations in HIV-PTB co-infected individuals as compared to only HIV-1 infected individuals with similar CD4 count. C. Scatter plots depicting the correlation of NF-κB with CxCR4 mRNA expression in PBMCs of HIV-1 infected individuals. The solid line represents the linear regression of points and dotted lines represents 95% confidence band for their mean values. Rho and p values are from the Spearman rank correlation test.
NF-κB is known to promote expression of HIV-1 co-receptors, CCR5 and CxCR4, via binding to the target DNA elements and positively regulating the transcription of these genes. Our results are in concordance with the above observation, showing higher expression of NF-κB in HIV-PTB co-infected individuals (up by 13% as compared to only HIV-1 infected individuals with CD4 count <250 cells/µL, p = 0.0152) correlating with 57% higher expression of CCR5 on CD4 T-cells in these subjects ( Figure 5B ). This increase in the CCR5 expression was observed among all Foxp3+ Tregs, both CD25+ as well as CD25 negative cells, although the expression was more prominent in CD25high cells.
Similarly, expression of CxCR4 (another coreceptor for HIV-1) was also increased in HIV-PTB co-infected individuals by 15%. This increase was seen only in CD25low/negative cells but not in CD25high or CD25intermediate cells ( Figure 5B ), although the mRNA levels of CxCR4 correlated positively with NF-κB in HIV-1 infected individuals [r = 0.4704, p = 0.0136 ( Figure 5C )].
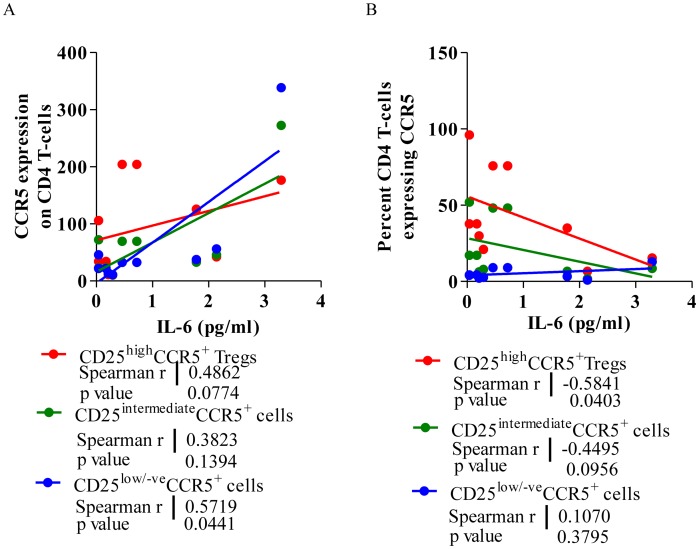
IL-6 levels negatively correlate with CCR5+ Treg frequency in therapy naïve HIV-1 subjects
Interleukin-6 is known to initiate the activation events including phosphorylation of JAK kinases resulting in translocation of NF-κB to the nucleus. With previous observation of NF-κB modulating HIV-1 co-receptors expression, we investigated the role of IL-6 in modulating their expression on CD4 T-cell populations in HIV-1 individuals. Spearmen's correlation analysis revealed a significant positive correlation between CCR5 expression on CD4 T-cells and levels of IL-6 produced on activation (r = 0.5352, p = 0.05). This correlation was also evident with respect to CCR5 expression on CD25high (r = 0.4862, p = 0.0771) as well as CD25low/negative cells (r = 0.5719, p = 0.0421, Figure 6A ). Interestingly, we found a negative correlation between frequency of CCR5+ CD25high Tregs and level of IL-6 (r = −0.5841, p = 0.0381, Figure 6B ), indicating a specific depletion of CD25high cells probably due to enhanced infectivity and faster depletion of these cells expressing higher number of CCR5 receptors in presence of higher levels of IL-6.
Figure 6. IL-6 levels bear a significant negative correlation with higher CCR5 expressing Tregs.
A. A representative scatter diagram depicting the Spearman correlation between TNF-α (pg/ml), CCR5 expression (MFI) on CD4+CD25high Tregs, CD25intermediate and CD25low/negative effector cell in PBMCs from HIV-1 infected individuals. B. A representative scatter diagram depicting the Spearman correlation between IL-6 (pg/ml), percent CCR5+ cell on CD4+CD25high Tregs, CD25intermediate and CD25low/negative effector cell in PBMCs from HIV-1 infected individuals. The solid line represents the linear regression of points. Rho and p-values are from the Spearman rank correlation test.
There was however no correlation between IL-6 levels and CxCR4 expression on the CD4 T-cells (p = 0.1217), Tregs (p = 0.0996), CD25+ cells (p = 0.0631) or CD25low/negative cells (p = 0.1926) in HIV-1 subjects.
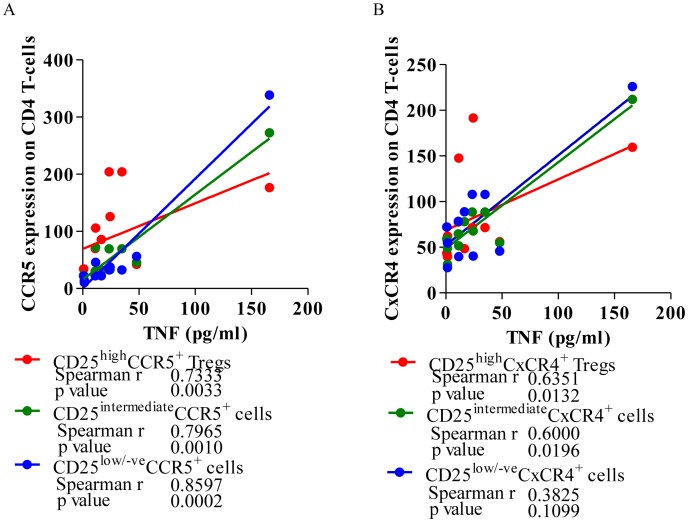
TNF-α level positively correlate with CCR5 and CxCR4 expression on Tregs in therapy naïve HIV-1 subjects
There was highly significant positive correlation between levels of TNF-α production on activation with T-cell mitogen and CCR5 expression on CD4 T-cells [r = 0.8597, p = 0.0002 ( Figure 7A )], suggesting possible involvement of TNF-α in controlling the CCR5 expression on CD4 T-cells, probably because the CCR5 promoter itself has an NF-κB binding site [12], which could potentially respond to TNF-α. This was similar when CD4 T-cells were further divided into Tregs and effector cells based on CD25 expression.
Figure 7. TNF-α level bear a significant correlation with CCR5 and CxCR4 expression on CD4 T-cells.
A. A representative scatter diagram depicting the Spearman correlation between TNF-α (pg/ml), CCR5 expression (MFI) on CD4+CD25high Tregs, CD25intermediate and CD25low/negative effector cell in PBMCs from HIV-1 infected individuals. B. A representative scatter diagram depicting the Spearman correlation between TNF-α (pg/ml), CxCR4 expression (MFI) on CD4+CD25high Tregs, CD25intermediate and CD25low/negative effector cell in PBMCs from HIV-1 infected individuals. The solid line represents linear regression of points. Rho and p-values are from the Spearman rank correlation test.
Overall there was however no such relationship observed between TNF-α levels (pg/ml) and CxCR4 expression on CD4 T-cells in HIV-1 individuals [r = 0.4526, p = 0.0698 ( Figure 7B )]. But when looking into the sub-populations of CD4 T-cells, we found significant positive correlation of TNF-α levels with CxCR4 expression on CD25high Tregs (r = 6351, p = 0.0132) and CD25+ activated T-cells (r = 0.6, p = 0.0196). These findings are further supported by significant positive correlation between NF-κB and CxCR4 mRNA expression in PBMCs of HIV-1 infected individuals (ref to Figure 5C).
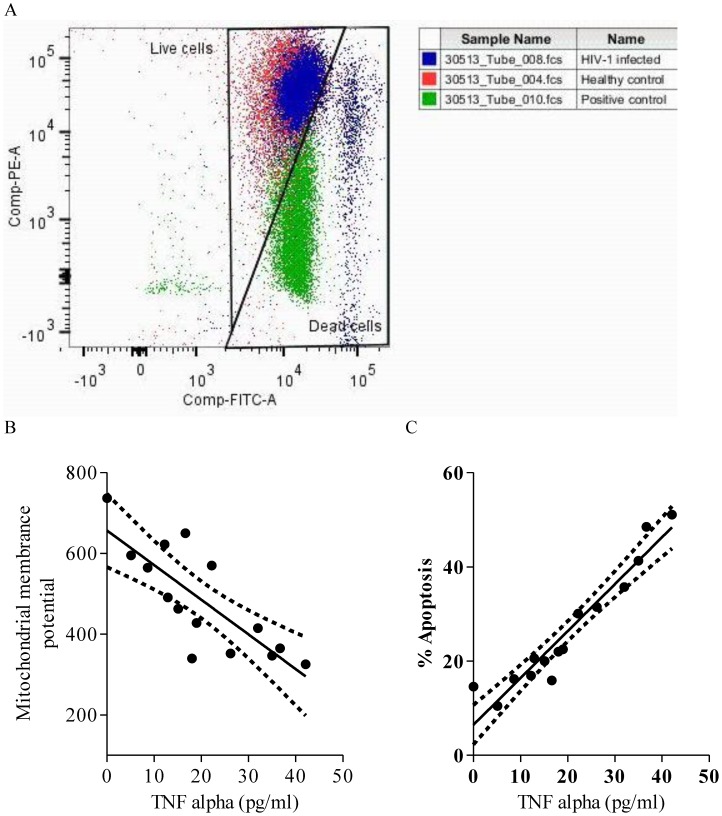
High TNF-α levels associate with up-regulated apoptosis of lymphocytes in HIV-1 infected individuals
As TNF-α showed positive correlation with HIV-1 co-receptors, CxCR4 and CCR5, via NF-κB pathway, its expression could modulate the course of HIV-1 disease progression. We investigated how TNF-α influences the decline of CD4 count, one of the prime cells affected in the lymphocytes in HIV-1 infected individuals in terms of decrease in mitochondrial membrane potential (ΔΨm), which is an indicator of apoptotic cells ( Figure 8A ). We found a highly significant negative correlation between ΔΨm and levels of TNF-α (pg/ml) in HIV-1 infected individuals (r = 0.7821, p = 0.0003) indicating its role in apoptosis ( Figure 8B ). This was supported by a significant positive correlation between TNF-α and the number of -cells undergoing apoptosis (r = 0.9571, p = 0.0001 ( Figure 8C ).
Figure 8. Higher plasma TNF-α levels up-regulate apoptosis of peripheral lymphocytes in HIV-1 infected individuals.
A. Representative overlay dot plot showing JC-1 staining of peripheral blood lymphocytes depicting a shift of fluorescence emission from red (FL2) to green (FL1) indicating loss of mitochondrial membrane potential, which is associated with apoptosis in HIV-1 infected individuals (blue). Negative control (red, healthy control) and positive control (green, protonophore FCCP treated) were used to set the gate for dead cells. B. Representative scatter plots depicting significant negative correlation between TNF-α expression and mitochondrial membrane potential (ΔΨm) in HIV-1 infected individuals. C. Representative scatter plots depicting significant positive correlation between TNF-α expression and cells undergoing apoptosis in HIV-1 infected individuals. Solid line represents linear regression of points and dotted lines represents 95% confidence band for their mean values. Rho and p-values are from the Spearman rank correlation test.
Discussion
Mtb continues to be one of the most dreaded pathogens around the world and its cohabitation with HIV exacerbates its disease progression. However, our current knowledge about the mechanisms of interaction of the two pathogens still has many gaps that need to be bridged in order to develop preventive measures against the two diseases. Much can be learnt about the relationship between HIV and Mtb co-infection by monitoring the immune profile of the host during co-infection and conditions facilitating HIV-1 replication. As CD4+ T-regulatory cells are known to modulate the activity of CD4 T-cells, we have carried out investigations to unravel the possible role of Tregs in HIV-1 infected individuals at different stages of the disease along with its effect on the synergistic relationship of the two pathogens. In order to understand the role of Tregs in HIV-PTB co-infected individuals, we have studied the inter-relationship of these suppressor cells with various factors such as HO-1, NF-κB and HIV-1 co-receptors, highlighting how the virus manipulates the host machinery during Mtb co-infection to its advantage.
The role and frequency of Tregs in HIV-1 infected individuals have long been controversial. Some studies suggest that Tregs get depleted by HIV infection [13], [14], [15], while others indicate that the proportion of these cells may be rather increased [9], [16]. This difference in opinion could be due to differences in the stage of the disease at the time of sampling by the researchers or infection with different HIV-1 strains. In the present study, we have analyzed the frequency and suppressive function of Tregs in ART/ATT naive HIV-1 subtype-C infected subjects at different stages of the disease and shown how the presence of Mtb could affect these cell populations. There was a significant decrease in the frequency of Tregs in the early phase of the HIV-1 infection correlating with significant decrease in Foxp3 expression. These findings indicate that in the initial stage of HIV infection, there is a preferential killing/decrease of Foxp3 expressing CD4 T cells in the peripheral blood. The decreased FoxP3 expression may contribute to higher levels of immune activation thereby facilitating HIV-1 replication that is seen in the early phase of the disease. However, with HIV-1 disease progression, there was an up-regulation of both, the frequency of these Tregs as well as the level of FoxP3 expression. We noticed a significant inverse relationship of CD4 count with respect to the frequency of Tregs and FoxP3 expression, making these subjects more vulnerable to active tuberculosis in this immune-compromised state. These findings correlate and are in agreement with the immune-suppressive nature of Tregs. There was a significant negative correlation between percent Treg frequency and T-cell proliferation, exhibiting intact suppressive activity inherent in these cells. Although, the hallmark of HIV infection is gradual loss of CD4+ cells that results in immunodeficiency state, which further seem to get compounded by the down regulation of CD4 T cell activity with increase in Treg number, making the infected individual severely immune-compromised thereby increasing its susceptibility to opportunistic infections like Mtb.
With the onset of PTB co-infection in the dampened immune environment of HIV-1 infected individuals, we observed further significant increase in Foxp3 expression in CD4 T-cells of co-infected individuals in comparison to individuals infected only with HIV-1 but similar CD4 count (<250 cells/µl). Though statistically non-significant, there was also an increase in the Treg frequency in the PTB co-infected individuals when compared with only HIV-1 infected individuals with compatible CD4 cell count, indicating a possible role of Mtb in inducing Foxp3 expression as well as expansion of Tregs. Quantitatively up-regulated Foxp3 expression per cell (MFI) in Tregs and significantly increased frequency of FoxP3 positive cells among CD4 T-cells of PTB and HIV-PTB co-infected individuals compared to healthy controls, confirmed this observation.
Realizing the controversy in literature regarding the quantitative expression of FoxP3 mRNA in HIV-1 infected individuals [3], [13], [17], we also found no significant difference in relative FoxP3 mRNA expression within the HIV-1 groups at different levels of disease progression, although there was a visible decrease in FoxP3 mRNA expression in PTB subjects in comparison to healthy controls, though statistically non-significant. These results were however in contrast to protein levels assessed by flowcytometry. Absence of correlation between FoxP3 protein and total mRNA levels raised a question and prompted us to look further into the splice variants for a possible answer, as the humans express two main isoforms of FoxP3, variant-1 (NM_104004) and variant-2 (NM_001114377), either of which can confer regulatory function when strongly over expressed [18], [19], [20]. The main deletional isoform of FoxP3, variant-2 however, lacks the proline-rich exon 2, which encodes the Leu-X-X-Leu-Leu motif that is required for the binding to the transcription factor retinoic acid receptor-related orphan receptor-α (RORα) [21], and lacks amino-terminal residues that may mediate the interaction with nuclear factor of activated T cells (NFAT) resulting in transcriptional repression [11], [22]. The role of FoxP3 variant-2 in human Treg biology remains unclear. Interestingly it was FoxP3 variant-1 mRNA expression, which was significantly higher in PTB individuals in comparison to healthy controls. Further, its expression was also significantly higher in the HIV-PTB co-infected individuals when compared to HIV-1 individuals in advanced stage of disease (CD4 count <250 cells/µL). These results were in agreement with the flowcytometry results. On the contrary, there was no significant difference in the expression of FoxP3 variant-2 between the studied groups. These results indicate a direct relationship between FoxP3 mRNA expression and FoxP3 protein expression (MFI) for all the studied groups only with variant-1 and not with variant-2 or whole FoxP3 gene expression.
The FoxP3 expression and Treg function have been linked with hemoxygenase-1 (HO-1), which is implicated in the activation as well as induction and/or expansion of Tregs, as it is constitutively expressed in human peripheral blood Tregs but not in resting CD4+CD25− non-Tregs [23], [24], [25], [26], [27], [28], [29]. Since HO-1 derived carbon monoxide can induce the DosR dormancy regulon in mycobacteria, leading to latency and survival of this organism inside the host granuloma, we hypothesized that such a situation could contribute to 20–30 times higher lifetime risk of developing active tuberculosis in HIV infected individuals. Hence, we studied the HO-1 mRNA expression in patient groups and found a significant increase in the expression of HO-1 in HIV patients. These results are in line with previous findings where HO-1 has been shown to be induced by oxidative stress, and pro-inflammatory cytokines that are characteristic of HIV-1 infection. Thus, the immune-compromised state was further exacerbated by a significant increase in HO-1 expression (via carbon monoxide) in HIV-1 (but PTB negative) group irrespective of the stage of the disease, where HO-1 is also known to play a role in promoting latency and survival of Mycobacteria inside the host granuloma. The increase in HO-1 makes the environment favorable for mycobacterial infection. On the other hand, in HIV-PTB co-infected individuals, we observed significantly lower HO-1 expression when compared to only HIV-1 infected subjects with similar CD4 count (<250 cells/µL). It is evident from this observation and previous findings that low HO-1 levels would lead to decreased levels of DosR gene expression in mycobacterium in co-infected subjects and could be a possible reason why these subjects have active tuberculosis. But what triggers the lowering of HO-1 gene expression in these subjects still needs to be further investigated. Is it the lowering of HO-1 expression that causes active tuberculosis or active tuberculosis leads to lowering of HO-1 is still a question that needs further investigation. Such a decrease in HO-1 expression by Mtb in HIV co-infected subjects results in the increase in redox stress, which has been shown to reactivate latent HIV-1 provirus [30] resulting in high plasma viral load.
The above findings further prompted us to look at the redox sensitive NF-κB expression [31], [32], which on activation translocate into the nucleus [33], where it binds to the Long Terminal Repeats [11] of the integrated HIV-1 provirus and induces its replication [34], [35], [36]. Interestingly, we observed that the presence of Mtb infection in HIV-1 individuals significantly increased NF-κB expression, thereby making the conditions favorable for HIV-1 replication. This is further supported by the fact that corresponding to a significant decrease in HO-1 in HIV-PTB individuals, there was a significant increase in NF-κB in comparison to HIV-1 infected individuals with similar CD4 count (<250 cells/µl). These data suggest that a decrease in HO-1 activity during Mtb co-infection in HIV-1 individuals can reactivate latent HIV-1 which may contribute to high viral load as well as faster disease progression towards AIDS.
Inflammatory cytokines like TNF-α and IL-6, that are known to activate and induce NF-κB expression, can also modulate the co-receptors of HIV. Interestingly, TNF-α showed highly significant positive correlation with CCR5 expression on CD4 T-cells in ART naive HIV-1 infected individuals, where as IL-6, showed moderate association with CCR5 expression on the CD4 T-cells. On the other hand, the TNF-α showed moderate association with CxCR4 expression on Tregs and CD25+ activated CD4 T-cells. Moreover, the NF-κB can also bind to CxCR4 promoter and activate its transcription [37], which was also substantiated by significant positive correlation between NF-κB and CxCR4 mRNA expression in PBMCs of HIV-1 infected individuals.
As TNF-α receptor/ligand pairs initiate a variety of biological responses, primarily through activation of inducible transcription factors such as NF-κB which in turn can affect the expression of HIV-1 co-receptor on the cell surface. We found a significant increase in the expression of NF-κB in HIV-PTB co-infected individuals when compared to HIV-1 infected individuals with comparable CD4 count i.e. <250 cell/µl. This could be one of the mechanisms how Mtb manipulates the host machinery towards rapid HIV-1 replication. Individuals carrying high TNF-α producer haplotype (CAG) have earlier been shown to be associated with faster disease progression due to increased apoptosis of T-cells in HIV-1 infected fast progressors (personal unpublished observation), we also found here that high plasma TNF-α levels were associated with increased apoptosis in lymphocytes.
To summarize, the present study has provided the experimental basis which highlights the interaction of various genes during HIV and Mtb co-infection in human host. This study, to the best of our knowledge, represents the first report reflecting the delicate link between NF-κB, TNF-α, IL-6, FoxP3 splice variants and HO-1 genes and their relationship with HIV disease progression and explains how Mtb co-infection modulates these links to the advantage of HIV. Apparently, HIV-1 makes conditions favorable for Mtb to grow in a co-infected host via increasing Treg numbers, FoxP3 expression and HO-1 expression. On the other hand, Mtb also facilitates HIV-1 replication by increasing redox stress (thereby reducing HO-1), increasing expression of co-receptors on CD4 T-cells via IL-6, TNF-α and NF-κB pathway leading to increased HIV-1 replication and enhanced HIV disease progression.
A close synergistic relationship between HIV-1 and Mtb mediated by interactions between few immune-modulatory genes as depicted in this study, highlights how these gene interactions play an important role in making the environment conducible for each other. These interactions need further attention and may suggest therapeutic intervention to prevent Mtb co-infection and faster HIV-1 progression.
Acknowledgments
We gratefully acknowledge PGIEMR, Chandigarh and Department of Biotechnology (DBT), New Delhi, India for providing research grant and relevant facilities for the completion of the experimental work. We are also thankful to the study participants without whom study would not have been possible.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included in the manuscript.
Funding Statement
This study was supported by Grant No. BT/PR8497/Med/14/1258/2006 from Department of Biotechnology, Govt. of India; (URL: www.dbt.nic.in) Project entitled: 'MECHANISMS INVOLVED IN THE DOWN REGULATION OF EFFECTOR T-CELL ACTIVITY IN TUBERCULOSIS AND HIV CO-INFECTIONS.' The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Getahun H, Gunneberg C, Granich R, Nunn P (2010) HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 50 Suppl 3 S201–207. [DOI] [PubMed] [Google Scholar]
- 2. Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF (2004) Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol 78: 2454–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, et al. (2005) The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol 174: 3143–3147. [DOI] [PubMed] [Google Scholar]
- 4. Cao W, Jamieson BD, Hultin LE, Hultin PM, Detels R (2009) Regulatory T cell expansion and immune activation during untreated HIV type 1 infection are associated with disease progression. AIDS Res Hum Retroviruses 25: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, et al. (2006) HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108: 3808–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, et al. (2009) Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J Infect Dis 199: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 7. Chase AJ, Sedaghat AR, German JR, Gama L, Zink MC, et al. (2007) Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J Virol 81: 12748–12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM (2008) FOXP3 expressing CD127lo CD4+ T cells inversely correlate with CD38+ CD8+ T cell activation levels in primary HIV-1 infection. J Leukoc Biol 83: 254–262. [DOI] [PubMed] [Google Scholar]
- 9. Tsunemi S, Iwasaki T, Imado T, Higasa S, Kakishita E, et al. (2005) Relationship of CD4+CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS 19: 879–886. [DOI] [PubMed] [Google Scholar]
- 10. Sternfeld T, Tischleder A, Schuster M, Bogner JR (2009) Mitochondrial membrane potential and apoptosis of blood mononuclear cells in untreated HIV-1 infected patients. HIV Med 10: 512–519. [DOI] [PubMed] [Google Scholar]
- 11. Lopes JE, Torgerson TR, Schubert LA, Anover SD, Ocheltree EL, et al. (2006) Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J Immunol 177: 3133–3142. [DOI] [PubMed] [Google Scholar]
- 12. Liu R, Zhao X, Gurney TA, Landau NR (1998) Functional analysis of the proximal CCR5 promoter. AIDS Res Hum Retroviruses 14: 1509–1519. [DOI] [PubMed] [Google Scholar]
- 13. Apoil PA, Puissant B, Roubinet F, Abbal M, Massip P, et al. (2005) FOXP3 mRNA levels are decreased in peripheral blood CD4+ lymphocytes from HIV-positive patients. J Acquir Immune Defic Syndr 39: 381–385. [DOI] [PubMed] [Google Scholar]
- 14. Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, et al. (2005) Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol 174: 4407–4414. [DOI] [PubMed] [Google Scholar]
- 15. Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, et al. (2004) CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med 200: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, et al. (2004) Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 104: 3249–3256. [DOI] [PubMed] [Google Scholar]
- 17. Chase AJ, Yang HC, Zhang H, Blankson JN, Siliciano RF (2008) Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J Virol 82: 8307–8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aarts-Riemens T, Emmelot ME, Verdonck LF, Mutis T (2008) Forced overexpression of either of the two common human Foxp3 isoforms can induce regulatory T cells from CD4(+)CD25(−) cells. Eur J Immunol 38: 1381–1390. [DOI] [PubMed] [Google Scholar]
- 19. Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, et al. (2005) The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest 115: 3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, et al. (2004) Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol 16: 1643–1656. [DOI] [PubMed] [Google Scholar]
- 21. Du J, Huang C, Zhou B, Ziegler SF (2008) Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J Immunol 180: 4785–4792. [DOI] [PubMed] [Google Scholar]
- 22. Bettelli E, Dastrange M, Oukka M (2005) Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci U S A 102: 5138–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, et al. (2009) Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 156: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xia ZW, Xu LQ, Zhong WW, Wei JJ, Li NL, et al. (2007) Heme oxygenase-1 attenuates ovalbumin-induced airway inflammation by up-regulation of foxp3 T-regulatory cells, interleukin-10, and membrane-bound transforming growth factor- 1. Am J Pathol 171: 1904–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee SS, Gao W, Mazzola S, Thomas MN, Csizmadia E, et al. (2007) Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB J 21: 3450–3457. [DOI] [PubMed] [Google Scholar]
- 26. Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, et al. (2010) Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus 19: 317–322. [DOI] [PubMed] [Google Scholar]
- 27. Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, et al. (2011) The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood 117: 4826–4835. [DOI] [PubMed] [Google Scholar]
- 28. Burt TD, Seu L, Mold JE, Kappas A, McCune JM (2010) Naive human T cells are activated and proliferate in response to the heme oxygenase-1 inhibitor tin mesoporphyrin. J Immunol 185: 5279–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, et al. (2008) Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol 173: 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pace GW, Leaf CD (1995) The role of oxidative stress in HIV disease. Free Radic Biol Med 19: 523–528. [DOI] [PubMed] [Google Scholar]
- 31. Lander HM, Sehajpal P, Levine DM, Novogrodsky A (1993) Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol 150: 1509–1516. [PubMed] [Google Scholar]
- 32. Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM (2006) Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal 8: 1791–1806. [DOI] [PubMed] [Google Scholar]
- 33. Greene WC (1991) The molecular biology of human immunodeficiency virus type 1 infection. N Engl J Med 324: 308–317. [DOI] [PubMed] [Google Scholar]
- 34. Nabel G, Baltimore D (1987) An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326: 711–713. [DOI] [PubMed] [Google Scholar]
- 35. Pyo CW, Yang YL, Yoo NK, Choi SY (2008) Reactive oxygen species activate HIV long terminal repeat via post-translational control of NF-kappaB. Biochem Biophys Res Commun 376: 180–185. [DOI] [PubMed] [Google Scholar]
- 36. Williams SA, Kwon H, Chen LF, Greene WC (2007) Sustained induction of NF-kappa B is required for efficient expression of latent human immunodeficiency virus type 1. J Virol 81: 6043–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Helbig G, Christopherson KW 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, et al. (2003) NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 278: 21631–21638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included in the manuscript.