Abstract
This study was designed to investigate the effect of surrogate orofacial pain models on the quantitative sensory testing (QST) profile in healthy participants. Capsaicin, menthol or saline (control) were applied topically on the gingiva of 15 healthy subjects for 15 min. During application, the subjects rated pain intensity on a 0–10 electronic visual analogue scale. A standardized intraoral QST protocol was performed before and immediately after application. Data before and after application were compared with Rank sum tests and QST profiles were made after Z-transformation. Application of capsaicin caused moderate levels of pain (VASpeak=6.0±0.7), and menthol produced mild levels of pain (VASpeak=1.8±0.6). Capsaicin induced hypersensitivity to warmth, heat pain, cold pain and hyposensitivity to mechanical stimuli. Menthol induced hypersensitivity to cold and warmth. Saline caused hypersensitivity to heat pain and hyposensitivity to mechanical stimuli. However, somatosensory profiles from Z-scores demonstrated sensory gains regarding warmth detection and heat pain only after capsaicin. In conclusion, a standardized battery of QST showed somatosensory changes after application of capsaicin, menthol and saline to the gingiva. However, the Z-score based profiles may only reflect the most prominent somatosensory changes and thus represent a conservative approach for evaluation of data.
Keywords: Quantitative sensory testing, Capsaicin, Menthol, Sensory profiling
Different clinical signs and symptoms of pain may reflect different underlying pathophysiological mechanisms of pain. Development of diagnosis and treatment of neuropathic pain depends on the insight into underlying mechanism.
Sensory testing is a useful tool to study somatosensory function and can help to study pain mechanisms. In 2006, the ‘German Research Network on Neuropathic Pain (DFNS)’ developed a battery of quantitative sensory testing (QST), which provides an opportunity to characterize the somatosensory profile in a standardized way (1). It can provide parameters for sensory gain (hyperalgesia, allodynia, hyperpathia) and sensory loss (hypoalgesia) and illustrate a comprehensive profile, which may be a “signature” of different pain mechanisms. Previous studies have found good reliability of QST on the face and on upper and lower limbs (2) and also in the orofacial region (3) but a complete battery of standardized intraoral QST according to the recent guidelines has not yet been used to assess somatosensory changes after application of capsaicin and menthol on the gingiva. The standardized QST protocol includes well-established tests for nearly all aspects of somatosensation, while clinical interpretation of the raw QST data is often difficult (1, 4). Reference standard values for some body sites, such as hand, foot and face, have already been established (1), while intraorally, such values remain to be provided.
Experimental pain models play an important role for the study of mechanisms of neuropathic pain (5, 6), and may provide insight into changes in somatosensory sensitivity (1). For example, capsaicin can increase sensitivity to warmth and heat pain (7, 8). Topical application to the skin or intradermal injection of capsaicin is an extensively used model of cutaneous pain and has been shown to provoke thermal hyperalgesia in the primary area and mechanical hyperalgesia in the secondary area (7, 8). Recently, pain models with intraoral application of capsaicin on the tongue mucosa and gingiva showed good reliability and sensitivity (8, 9). The thermal hyperalgesia effect of capsaicin is mediated through a TRPV1 (transient receptor potential cation channel subfamily V member 1) receptor on nociceptive neurons (10). Menthol (C10H20O) is an agonist of the TRPM8 nociceptor (another receptor in the same family) and may induce cold hyperalgesia and mechanical hyperalgesia (11–13). Recently, topical application of menthol has been proposed as a surrogate model of cold hyperalgesia (11, 14, 15).
The aim of this experimental study was to assess the effect of intraoral topical application of capsaicin and menthol on the intraoral quantitative sensory testing (QST) somatosensory profiles on the gingiva in healthy volunteers.
Materials and methods
Subjects
A total of 15 healthy volunteers (8 male and 7 female) with a mean (±SEM) age of 31.5±7.5 yr were recruited by posting an advertisement on ‘www.forsoegsperson.dk’ webpage and at Aarhus University. The inclusion criterion was good health with no orofacial pain complaints or TMD (temporomandibular disorders). The study protocol was approved by the Local Ethics Committee (Central Denmark Region, Denmark) and written informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki. The exclusion criteria were pregnancy, serious mental disorders and hypochondria, allergy to capsaicin/chili/menthol, dental treatment scheduled for the time of study and intake of medication within 48 hours of the investigation (analgesics, antidepressant, or hypnotics).
Study design
The study was performed in a randomized, double-blinded, placebo- controlled crossover manner. The full tests included two sessions with two applications each: capsaicin and control application (session A) and menthol and control application (session B). The sequence of the two different applications in each session was randomized as well as application on the right or left side.
The examiner applied capsaicin, menthol or saline (control) under an oral bandage (Urihesive, ConvaTec, City?, UK) on the buccal aspect of the attached gingiva adjacent to the maxillary first premolar for 15 min (8). Subjects were asked to score their perceived intensity of pain, on a 0–10 electronic visual analog scale (VAS) (16). QST (see below) was performed on the application site (primary zone) before and immediately after (within 1 min) removal of the oral bandage in the same sequence. Then the same procedure was followed on the opposite side. All subjects and the examiner were blinded with respect to the content of the topical applications, which were prepared by a research assistant. QST of each side took ~30 min and testing for one side was performed within 1 h (3, 4). All participants were examined in a quiet room at normal room temperature by the same examiner.
Topical application
The concentration and the applications period were chosen based on earlier studies (8, 14). In the present study, the topical application of capsaicin and menthol was used as a pain-provocation and we wished to obtain mild to moderate levels of pain, lasting throughout the application period. Somatosensory changes after such intraoral applications have been demonstrated earlier, however not with the full QST battery (8). The capsaicin was prepared in 5% concentration (8) and menthol was prepared in 40% concentration (14). A volume of 30 μl of capsaicin, menthol or isotonic saline (control) was applied on a 3 × 3 mm paper disk, which was placed on an oral bandage. The bandage was then carefully applied and fitted to the attached gingiva above the first maxillary premolar. In this way it was possible to prevent the capsaicin/menthol/saline spreading into the entire oral cavity (8). Subjects used an electronic VAS (0 = “no pain”; 10 = “most pain imaginable”) to score their real-time perceived pain intensity during the 15 min of application. The VAS signal was sampled in 1 s intervals. The peak pain, mean and onset of pain were calculated from the VAS signal (16).
Quantitative Sensory Testing
A standardized quantitative somatosensory examination according to the protocol of the DFNS modified for intraoral application (3) was used which comprises 13 test parameters and investigates the following sensory functions in the following sequence:
The tests for thermal sensation were performed using a PATHWAY (MEDOC, Ramat Yishai, Israel) thermal sensory testing device. Cold detection threshold (CDT) and warmth detection threshold (WDT) were measured first. The number of paradoxical heat sensations (PHS) was determined during the thermal sensory limen procedure (TSL), followed by cold pain threshold (CPT), and heat pain threshold (HPT) (1). The mean threshold temperature of three consecutive measurements was calculated. The baseline temperature was 37°C, and all thresholds were obtained with ramped stimuli (1°C/s) that were terminated when the subject pressed a button. Care was taken to minimize the variations from thermode application pressure. Cut-off temperatures were 0 and 50°C and the contact area of the intraoral thermode was 0.81 cm2. During the experiment, the subjects were not able to watch the computer screen.
Mechanical detection thresholds (MDT) were measured using a standardized set of modified von Frey filaments (OptiHair, MARSTOCK Nervtest, Marburg, Germany), which exert forces between 0.25 mN and 512 mN. The contact area of the von Frey hairs were rounded tips, 0.5 mm in diameter, to avoid sharp edges that would facilitate nociceptor activation. The filament was applied perpendicular to the test site, and pressure was slowly increased until the filament began to bend. The time needed to bend was standardized to about 1–2 s and stimulus was maintained for 1–2 s (17). The final threshold was the geometric mean of five series of ascending and descending stimulus intensities (1, 3).
Mechanical pain threshold (MPT) was measured using custom-made weighted pinprick stimulators (flat contact area of 0.2 mm diameter) with fixed stimulus intensities between 8 mN and 512 mN. The final threshold of painful pricking or stinging sensation was the geometric mean of five series of ascending and descending stimulus intensities.
Mechanical pain sensitivity (MPS) was tested using the same weighted pinprick stimuli as for mechanical pain threshold (MPT). In addition, dynamic mechanical allodynia (ALL) were tested by three light tactile stimulators: a cotton wisp (~3 mN), a cotton wool tip (~100 mN), and a brush (~200–400 mN). Each of the 7 intensities of pinpricks and of the 3 intensities of light stroking was applied 6 times in a balanced sequence, and the subjects were asked to give a pain rating for each stimulus on a 0–100 numerical rating scale (0 = “no pain”, 100 = “most pain imaginable”). The mechanical pain sensitivity (MPS) was calculated as the geometric mean of all pain ratings for pinprick stimuli, and allodynia was calculated as the geometric mean of all pain ratings for light touch stimuli.
The perceived magnitude of a single pinprick stimulus was divided by that of a train of 10 pinprick stimuli with the same force, repeated at a rate of 1/s and kept constant using a metronome (MA-30, Korg, City, State?, USA). The instrument that delivered a force, which the subject perceived as “slightly painful”, was chosen for the test (3). Five single pinprick stimuli were alternated with five trains of 10 stimuli. The mean pain rating of the trains was then divided by the mean pain rating of single stimuli (train/single pinprick) to calculate the wind-up ratio (WUR) (1, 3).
Vibration detection threshold (VDT) test was performed with a Rydel-Seiffer tuning fork (64 Hz, 8/8 scale) (18) that was set in motion and placed in contact with the maxillary alveolar process. The vibration detection threshold (VDT) was determined as a disappearance threshold on the 8/8 scale with three stimulus repetitions (1, 3).
The pressure pain threshold (PPT) was measured by a digital pressure algometer (SOMEDIC Algometer, SOMEDIC Sales, City?, Sweden). The probe with a surface area of 0.18 cm2 was used. During the test, pressure was increased at a rate of 50 kPa/s. At the first painful sensation the subjects pressed a button to interrupt stimulation. The pressure pain threshold (PPT) was determined as the mean of three recordings (1, 3).
Statistical analyses
Data are presented as mean values ± SEM (standard error of mean). Log transformation was considered superior when the ratio of raw data to log-transformed data exceeded a factor of 3, so all QST data except paradoxical heat sensation (PHS), cold pain threshold (CPT), heat pain threshold (HPT), and vibration detection threshold (VDT) were transformed logarithmically before statistical analysis (1), and all data were analyzed for their distribution properties. To avoid a loss of zero values, a small constant (0.01) was added to all pain ratings (mechanical pain sensitivity, dynamic mechanical allodynia and wind-up ratio) (4).
To create somatosensory profiles, independent of the different measurement units across QST parameters, mean values of QST parameters of all healthy subjects at baseline (before application) were calculated. These mean values and their standard deviation determined the control Z-values (Z=0) as the reference for the Z-transformed QST parameters assessed after application (1). QST parameters of each subject were transformed into a Z-score using the following equation: (negative Z-score: loss of sensory function, positive Z-score: gain in sensory function) (1). Z-scores higher than 1.96 and lower than −1.96 indicate somatosensory sensitivity outside the 95% confidence interval (CI) of the baseline sensitivity of healthy subjects (1).
In SPSS 11.5, statistical differences in raw QST parameters were tested using Rank sum tests. The mean and SEM of pain from the three applications were calculated by the mean VAS of 15 subjects in each second.
The mean peak VAS pain intensity and SEM from the three applications were calculated from the peak VAS pain of each subject in 15 min. The mean and SEM of the overall mean over time of VAS pain from the three applications from each subject was calculated. Then, VAS values of three applications were tested by one-way ANOVA. For all tests, the significance level at P<0.05 was considered to be significant.
Results
Perceived pain intensity
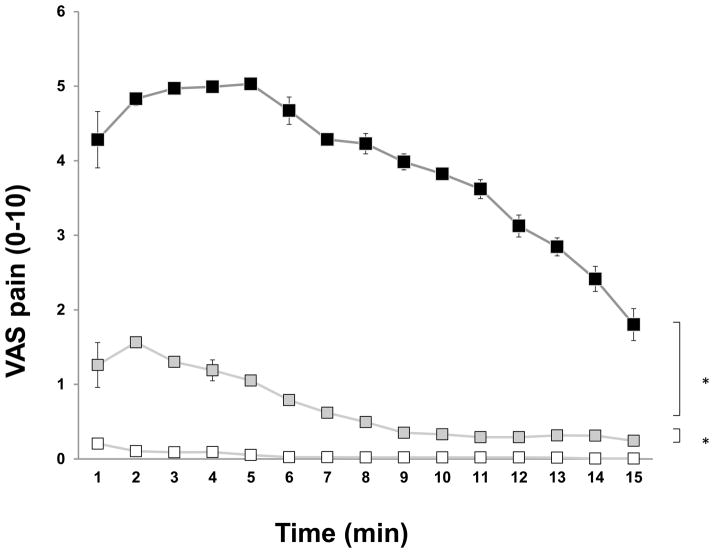
All subjects scored application of capsaicin as moderately painful. The mean peak pain induced by capsaicin was 6.0 ± 0.7 occurring after 228 ± 50 s. The overall mean VAS (4.2 ± 0.7) of the capsaicin-evoked pain was significantly higher than for menthol and control applications (P<0.001) (Fig. 1). For most subjects (13/15), the pain lasted throughout the 15-minute application period. The mean offset of the pain was 850 ± 45 s.
Fig. 1.
Subject-reported visual analog scale (VAS) pain scores (mean values ± SEM) from topical application of capsaicin (black icons), menthol (grey icons) or saline (white icons) on the attached gingiva. Mean values (n=15) during the 900-s (15 min) recording period.
More than half of the subjects (9/15) reported mild levels of pain during menthol application. The mean peak pain induced by menthol was 1.8 ± 0.6 and occurred after 73 ± 32 s. The mean VAS (0.7 ± 0.3) of menthol-evoked pain was significantly higher than control application (0.04 ± 0.02) (P<0.001). For a few subjects (4/15), the pain lasted throughout the 15-min application period, the mean offset of the pain (n=4) was 658 ± 13 s.
A few subjects (6/15) scored very mild pain immediately after application of isotonic saline on the gingiva (0.04 ± 0.02) (Fig. 1). The mild pain lasted throughout the 15-min application period in two subjects, the mean offset of the pain (n=6) was 485 ± 98 s.
Quantitative Sensory Testing findings
Application of capsaicin was associated with significant decreases in the absolute values of warm detection threshold (sensory gain), heat pain threshold (sensory gain) and mechanical pain sensitivity (sensory loss), and increase in mechanical pain threshold (sensory loss) (P<0.05) (Table 1). Application of menthol was associated with a decrease in warm detection threshold (sensory gain), and increase in the temperature of the cold detection threshold (sensory gain) (P<0.05) (Table 1). Application of saline (control) was associated with a decrease in heat pain threshold (sensory gain) and increases in thermal sensory limen and mechanical detection threshold (sensory loss) (P<0.05) (Table 1).
Table 1.
Quantitative sensory testing results before and after application and P values of Ranks sum test (n = 15). Means are displayed with SEM in parenthesis below
| Mean (SEM) | CDT (°C) | WDT (°C) | TSL (°C) | PHS (/3) | CPT (°C) | HPT (°C) | MDT (mN) | MPT (mN) | MPS (NRS) | ALL (NRS) | WUR (ratio) | VDT (/8) | PPT (kPa) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-capsaicin | 17.2 (2.1) | 48.8 (0.3) | 30.9 (2.1) | 0.3 (0.1) | 5.4 (1.6) | 49.5 (0.2) | 35.8 (8.6) | 211.3 (26.6) | 0.4 (0.1) | 0.0 (0.0) | 82.0 (77.5) | 7.4 (0.2) | 80.7 (6.6) |
| Post-capsaicin | 16.0 (2.5) | 45.1 (0.9) | 25.1 (3.2) | 0.7 (0.3) | 11.3 (3.3) | 45.8 (0.8) | 73.1 (18.1) | 315.3 (31.6) | 0.2 (0.0) | 0.0 (0.0) | 42.6 (32.8) | 7.2 (0.3) | 85.3 (7.8) |
| P value | 0.496 | 0.002* | 0.100 | 0.111 | 0.075 | 0.002* | 0.221 | 0.003* | 0.004* | 0.655 | 0.334 | 0.438 | 0.510 |
| Pre-menthol | 16.5 (1.9) | 49.0 (0.3) | 28.7 (2.3) | 0.1 (0.1) | 6.9 (1.2) | 49.1 (0.3) | 42.0 (13.0) | 299.1 (51.4) | 0.3 (0.1) | 0.0 (0.0) | 161.6 (116.1) | 7.6 (0.1) | 80.4 (5.8) |
| Post-menthol | 23.3 (1.6) | 47.4 (0.6) | 28.2 (2.5) | 0.1 (0.1) | 8.9 (1.7) | 48.9 (0.4) | 65.9 (25.7) | 270.4 (35.5) | 0.3 (0.1) | 0.0 (0.0) | 28.5 (19.5) | 7.5 (0.1) | 88.9 (4.9) |
| P value | 0.012* | 0.013* | 0.691 | 0.047* | 0.133 | 1.000 | 0.088 | 0.278 | 0.460 | 0.260 | 0.865 | 0.655 | 0.089 |
| Pre-saline | 17.9 (1.5) | 49.0 (0.2) | 29.6 (1.7) | 0.4 (0.1) | 5.5 (1.2) | 49.7 (0.1) | 30.8 (6.8) | 218.1 (25.5) | 0.5 (0.1) | 0.0 (0.0) | 90.8 (46.7) | 7.4 (0.2) | 79.8 (4.4) |
| Post-saline | 17.4 (1.5) | 48.4 (0.3) | 32.4 (1.7) | 0.2 (0.1) | 6.1 (1.2) | 49.2 (0.2) | 59.4 (19.0) | 239.4 (27.4) | 0.5 (0.2) | 0.0 (0.0) | 34.8 (27.6) | 7.5 (0.1) | 84.8 (4.7) |
| P value | 0.719 | 0.067 | 0.000* | 0.491 | 0.209 | 0.002* | 0.001* | 0.360 | 0.274 | 0.599 | 0.139 | 0.380 | 0.080 |
CDT, cold detection threshold (°C); WDT, warmth detection threshold (°C); TSL, thermal sensory limen (°C); PHS, paradoxical heat sensation (/3); CPT, cold pain threshold (°C); HPT, heat pain threshold (°C); MDT, mechanical detection threshold(mN); MPT, mechanical pain threshold (mN); MPS, mechanical pain sensitivity (mean pain rating,0–100); ALL, dynamic mechanical allodynia (mean pain rating,0–100); WUR, wind-up ratio (ratio of pain rating); VDT, vibration detection threshold (/8); PPT, pressure pain threshold (kPa).
P<0.05.
Somatosensory profiles after application of capsaicin and menthol
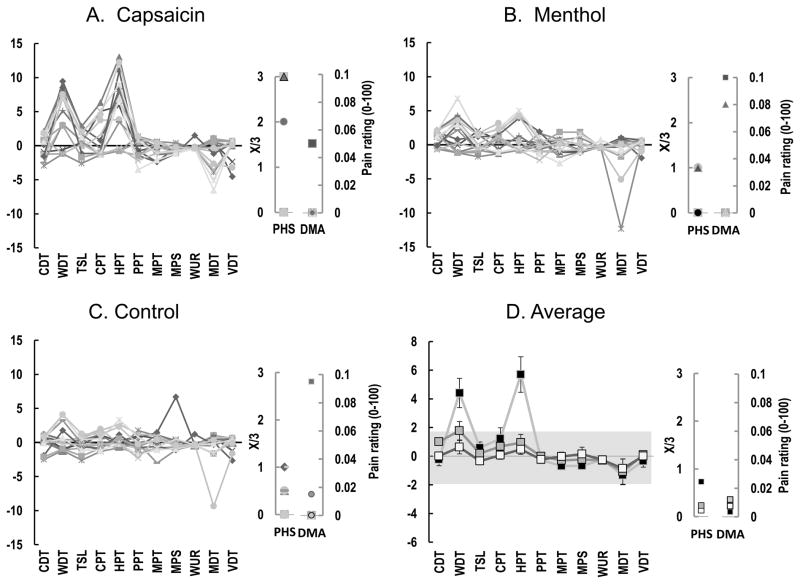
The Z-profiles showed a sensory gain of warmth detection threshold (WDT) (11/15) and heat pain threshold (HPT) (11/15) after application of capsaicin (Fig. 2A–D) whereas there were no robust changes (outside the 95% CI) for menthol or saline applications (Fig. 2B, C).
Fig. 2.
Individual Z-score based quantitative sensory testing (QST) profiles from the attached gingiva after application of capsaicin (A), menthol (B) or saline (C) (n = 15). Part D shows the averaged Z scores. CDT, cold detection threshold (°C); WDT, warmth detection threshold (°C); TSL, thermal sensory limen (°C); CPT, cold pain threshold (°C); HPT, heat pain threshold (°C); PPT, pressure pain threshold (kPa); MPT, mechanical pain threshold (mN); MPS, mechanical pain sensitivity (mean pain rating, 0–100); WUR, wind-up ratio; MDT, mechanical detection threshold (mN); VDT, vibration detection threshold (/8); PHS, paradoxical heat sensation (/3); ALL, dynamic mechanical allodynia. NRS, numerical rating scale (pain intensity from 0–100). The grey zone (Z score between −1.96 and 1.96) represents the 95 % confidence interval of baseline values.
Discussion
Cutaneous capsaicin pain models are well described (7, 19, 20), and cutaneous application of menthol is also frequently used (14, 21), while intraoral capsaicin or menthol pain models are described relatively seldom (8, 22). In the present study, 5% capsaicin and 40% menthol were chosen on the basis of earlier studies (8, 14).
In all 15 subjects, topical application of capsaicin on the gingiva caused moderate level of pain. The pain was similar to our previous study, where capsaicin was also applied on the gingiva (8). Topical application of capsaicin can be considered an effective, safe and reproducible method to elicit intraoral experimental pain.
Few studies have used topically applied menthol on the oral mucosa but so far not on the gingiva (23). In the present study, 40% of menthol provoked mild to moderate levels of pain on the gingiva similar to a previous study, which used 2.5%, 5% and 10% menthol on the mid-volar forearmskin (21). However, the menthol pain duration was shorter than capsaicin and not all subjects experienced pain.
The duration of the QST battery at each test site was approximately 30 minutes, so it is possible that there could have been some time course changes in somatosensory sensitivity after the application of capsaicin and menthol. Such somatosensory changes over time were not determined in the present experiment. However, previous studies have shown that the effect of 1% capsaicin cream topically applied on lip and tongue for 5 min lasted at least 30 min (24) and 40% menthol topically applied on hairy skin for 20 min lasted 225 min (14). Therefore, we speculate that there was sufficient time in the present study to perform the whole standard battery of QST. However, future studies are needed to test the short-term time course of changes in somatosensory sensitivity to each stimulus modality after intraorally applied capsaicin or menthol.
In the present study, reduced warmth detection threshold and heat pain threshold (increased sensitivity) were induced by intraoral application of capsaicin, which is in accordance with a previous intraoral and extraoral study (8, 25). These thermal threshold differences were not only statistically significant in the direct comparison of before and after application (Table 1), but were also reflected in the individual and mean Z-scores as a sensory gain of heat sensitivity (Fig. 2A–D). Thermal tests can reflect C- and A-delta fiber functions (3, 26) and microneurographic investigations in humans have demonstrated that the mechano-heat sensitive part of these fibers is sensitive to capsaicin (27). Peripheral sensitization of C fibers may likely be responsible for the present decrease in warmth detection threshold, and heat pain threshold.
In terms of the mechanical stimuli, previous intraoral QST studies showed no significant difference but a trend toward reduced sensitivity to both non-painful and painful mechanical stimuli when capsaicin was applied topically on gingiva (8). In the present study, mechanical sensitivity decreased (mechanical pain threshold increased and mechanical pain sensitivity decreased) after topical application of capsaicin to the attached gingiva. Intraoral topically applied capsaicin appears to induce changes in the opposite direction than for cutaneous or intradermal applications in terms of mechanical sensitivity (28–31), which normally shows mechanical allodynia and hyperalgesia. This hypoalgesia to mechanical sensitivity is present in the primary zone, which is directly affected by the capsaicin, and it may be explained by a desensitizing effect of capsaicin (31, 32). In the present study, we used a high concentration of capsaicin to induce intraoral experimental pain. Desensitization effects of capsaicin may be due to either higher concentrations or repeated application (33) and several hypotheses have tried to explain the desensitization effect caused by capsaicin. One prevailing hypothesis is an excessive influx of ions (Ca2+, Na+, Cl−) across the neural membranes that block the voltage-gated calcium channels and progressively disables cellular function (34). Other studies have indicated that the desensitization effect is caused by depletion of substance P in capsaicin-sensitive neurons (35).
Finally, it should also be noted that QST was only performed within the primary zone of capsaicin application (36) in this study. Future studies may be able to detect both spatial and temporal aspects of somatosensory changes induced by the application.
Menthol has been found to activate both cold-specific A-delta fibers and nociceptors in humans (13). Our study showed that topical application of menthol on the gingiva elicited cold and warmth hyperesthesia, as the cold detection threshold (CDT) was detected at a significantly higher temperature and the warmth detection threshold was detected at a significantly lower temperature after application compared with before (Table 1). This result is similar to previous studies with topically applied menthol on the hand (14) and volar forearm (37). This cold hyperesthesia may be caused by activation of the TRPM8 receptors, which are located on cold-sensitive C and cold-specific A-delta neurons (13). Topical application of menthol may activate and sensitize these receptors, thereby leading to peripheral sensitization and resulting in cold hyperesthesia (13, 38). In the present study, cold pain threshold (CPT) was not significantly changed by the application of menthol, which was inconsistent with some of the previous studies (11). Although these menthol non-responders have also been reported in other studies (11, 21), the CPT non-responders in this study may be explained by the cut-off temperature, which was used with the purpose of preventing tissue damage. The cut-offs for thermal stimuli were 0 and 50°C, and healthy subjects showed marked variability in response to noxious cold at baseline. In addition, TRPM8 being considered to play a role in the transmission of cold sensation rather than noxious cold, can be another explanation (39).
We found no evidence of mechanical pain threshold (MPT) being modified by application of menthol, which is in accordance with one study (11) but is inconsistent with another (14).
In the present study, some unexpected sensory changes in the control session (saline) occurred. For example, heat pain threshold was statistically significantly lower after application, but the thresholds before (49.7 ± 0.1 °C) and after application (49.2 ± 0.2 °C) only showed marginal absolute difference. Mechanical detection threshold (MDT) was significantly increased after application of saline, which may also be explained by adaptation to mechanical stimuli. These findings nevertheless indicate the importance of a control condition when somatosensory sensitivity is tested.
The present study is the first one to report full QST Z-score profiles after induction of experimental pain. The means and SDs of the QST baseline values (before application) were used as the reference values to compute the Z-scores. Thus, this indicates that it is indeed feasible to create intraoral QST Z-score profiles and it creates a foundation for further intraoral QST studies. In the present study, inspection of the individual and mean Z-scores clearly demonstrated two prominent sensory gains after application of capsaicin, i.e. decreased warmth detection threshold (WDT) and heat pain threshold (HPT) (Fig. 2A–D). The presence of heat hyperalgesia indicated induction of peripheral sensitization. However, the other observed findings from the direct comparison of before and after values (Table 1) could not be identified in the Z-scores and sensory profiles (Fig. 2B–D). This may possibly be due to less stable baseline values, which were used to create the Z-scores. Thus, the Z-score transformation may only be able to illustrate the most robust findings since minor but still significant differences detected in the statistical comparison of absolute values was not represented as Z-scores outside the 95% CI of the baseline values. This may be important to keep in mind when somatosensory function is considered in single individuals versus in groups of individuals.
In conclusion, a standardized QST battery and Z-score based somatosensory profiling is applicable in intraoral somatosensory studies within a reasonable time. Topical application of capsaicin or menthol can be considered effective surrogate models of intraoral pain.
Acknowledgments
The study was in part supported by a NIH grant (R21-DE018768). Research dental nurse Bente Holm Haugsted is thanked for all her assistance to the study.
Footnotes
Conflicts of Interest - The authors declare that we have no conflict of interest.
References
- 1.ROLKE R, BARON R, MAIER C, TOLLE TR, TREEDE RD, BEYER A, BINDER A, BIRBAUMER N, BIRKLEIN F, BOTEFUR IC, BRAUNE S, FLOR H, HUGE V, KLUG R, LANDWEHRMEYER GB, MAGERL W, MAIHOFNER C, ROLKO C, SCHAUB C, SCHERENS A, SPRENGER T, VALET M, WASSERKA B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 2.GEBER C, KLEIN T, AZAD S, BIRKLEIN F, GIERTHMUHLEN J, HUGE V, LAUCHART M, NITZSCHE D, STENGEL M, VALET M, BARON R, MAIER C, TOLLE T, TREEDE RD. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre study. Pain. 2011;152:548–556. doi: 10.1016/j.pain.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 3.PIGG M, BAAD-HANSEN L, SVENSSON P, DRANGSHOLT M, LIST T. Reliability of intraoral quantitative sensory testing (QST) Pain. 2010;148:220–226. doi: 10.1016/j.pain.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 4.ROLKE R, MAGERL W, CAMPBELL KA, SCHALBER C, CASPARI S, BIRKLEIN F, TREEDE RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.SERRA J, CAMPERO M, BOSTOCK H, OCHOA J. Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol. 2004;91:2770–2781. doi: 10.1152/jn.00565.2003. [DOI] [PubMed] [Google Scholar]
- 6.XING H, CHEN M, LING J, TAN W, GU JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PETERSEN KL, ROWBOTHAM MC. A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport. 1999;10:1511–1516. doi: 10.1097/00001756-199905140-00022. [DOI] [PubMed] [Google Scholar]
- 8.BAAD-HANSEN L, JENSEN TS, SVENSSON P. A human model of intraoral pain and heat hyperalgesia. J Orofac Pain. 2003;17:333–340. [PubMed] [Google Scholar]
- 9.NGOM PI, DUBRAY C, WODA A, DALLEL R. A human oral capsaicin pain model to assess topical anesthetic-analgesic drugs. Neurosci Lett. 2001;316:149–152. doi: 10.1016/s0304-3940(01)02401-6. [DOI] [PubMed] [Google Scholar]
- 10.CATERINA MJ, LEFFLER A, MALMBERG AB, MARTIN WJ, TRAFTON J, PETERSEN-ZEITZ KR, KOLTZENBURG M, BASBAUM AI, JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 11.HATEM S, ATTAL N, WILLER JC, BOUHASSIRA D. Psychophysical study of the effects of topical application of menthol in healthy volunteers. Pain. 2006;122:190–196. doi: 10.1016/j.pain.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 12.MCKEMY DD, NEUHAUSSER WM, JULIUS D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 13.WASNER G, SCHATTSCHNEIDER J, BINDER A, BARON R. Topical menthol--a human model for cold pain by activation and sensitization of C nociceptors. Brain. 2004;127:1159–1171. doi: 10.1093/brain/awh134. [DOI] [PubMed] [Google Scholar]
- 14.BINDER A, STENGEL M, KLEBE O, WASNER G, BARON R. Topical high-concentration (40%) menthol-somatosensory profile of a human surrogate pain model. J Pain. 2011;12:764–773. doi: 10.1016/j.jpain.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 15.NAMER B, KLEGGETVEIT IP, HANDWERKER H, SCHMELZ M, JORUM E. Role of TRPM8 and TRPA1 for cold allodynia in patients with cold injury. Pain. 2008;139:63–72. doi: 10.1016/j.pain.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 16.BAAD-HANSEN L, LIST T, JENSEN TS, SVENSSON P. Increased pain sensitivity to intraoral capsaicin in patients with atypical odontalgia. J Orofac Pain. 2006;20:107–114. [PubMed] [Google Scholar]
- 17.KOMIYAMA O, DE LAAT A. Tactile and pain thresholds in the intra- and extra-oral regions of symptom-free subjects. Pain. 2005;115:308–315. doi: 10.1016/j.pain.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 18.WHITTON TL, JOHNSON RW, LOVELL AT. Use of the Rydel-Seiffer graduated tuning fork in the assessment of vibration threshold in postherpetic neuralgia patients and healthy controls. Eur J Pain. 2005;9:167–171. doi: 10.1016/j.ejpain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 19.SIKAND P, SHIMADA SG, GREEN BG, LAMOTTE RH. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain. 2011;152:2485–2494. doi: 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ROWBOTHAM MC, NOTHAFT W, DUAN WR, WANG Y, FALTYNEK C, MCGARAUGHTY S, CHU KL, SVENSSON P. Oral and cutaneous thermosensory profile of selective TRPV1 inhibition by ABT-102 in a randomized healthy volunteer trial. Pain. 2011;152:1192–1200. doi: 10.1016/j.pain.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 21.ROBERTS K, SHENOY R, ANAND P. A novel human volunteer pain model using contact heat evoked potentials (CHEP) following topical skin application of transient receptor potential agonists capsaicin, menthol and cinnamaldehyde. J Clin Neurosci. 2011;18:926–932. doi: 10.1016/j.jocn.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 22.NGOM PI, DUBRAY C, WODA A, DALLEL R. A human oral capsaicin pain model to assess topical anesthetic-analgesic drugs. Neurosci Lett. 2001;316:149–152. doi: 10.1016/s0304-3940(01)02401-6. [DOI] [PubMed] [Google Scholar]
- 23.CLIFF MA, GREEN BG. Sensory irritation and coolness produced by menthol: evidence for selective desensitization of irritation. Physiol Behav. 1994;56:1021–1029. doi: 10.1016/0031-9384(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 24.BOUDREAU SA, WANG K, SVENSSON P, SESSLE BJ, ARENDT-NIELSEN L. Vascular and psychophysical effects of topical capsaicin application to orofacial tissues. J Orofac Pain. 2009;23:253–264. [PMC free article] [PubMed] [Google Scholar]
- 25.CAMPBELL CM, BOUNDS SC, SIMANGO MB, WITMER KR, CAMPBELL JN, EDWARDS RR, HAYTHORNTHWAITE JA, SMITH MT. Self-reported sleep duration associated with distraction analgesia, hyperemia, and secondary hyperalgesia in the heat-capsaicin nociceptive model. Eur J Pain. 2011;15:561–567. doi: 10.1016/j.ejpain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LOSETH S, LINDAL S, STALBERG E, MELLGREN SI. Intraepidermal nerve fibre density, quantitative sensory testing and nerve conduction studies in a patient material with symptoms and signs of sensory polyneuropathy. Eur J Neurol. 2006;13:105–111. doi: 10.1111/j.1468-1331.2006.01232.x. [DOI] [PubMed] [Google Scholar]
- 27.SCHMIDT R, SCHMELZ M, FORSTER C, RINGKAMP M, TOREBJORK E, HANDWERKER H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LIU M, MAX MB, ROBINOVITZ E, GRACELY RH, BENNETT GJ. The human capsaicin model of allodynia and hyperalgesia: sources of variability and methods for reduction. J Pain Symptom Manage. 1998;16:10–20. doi: 10.1016/s0885-3924(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 29.CHEN J, CHEN HS. Pivotal role of capsaicin-sensitive primary afferents in development of both heat and mechanical hyperalgesia induced by intraplantar bee venom injection. Pain. 2001;91:367–376. doi: 10.1016/S0304-3959(00)00458-9. [DOI] [PubMed] [Google Scholar]
- 30.MAGERL W, FUCHS PN, MEYER RA, TREEDE RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124:1754–1764. doi: 10.1093/brain/124.9.1754. [DOI] [PubMed] [Google Scholar]
- 31.FUCHS PN, CAMPBELL JN, MEYER RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84:141–149. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- 32.GREEN BG. Rapid recovery from capsaicin desensitization during recurrent stimulation. Pain. 1996;68:245–253. doi: 10.1016/s0304-3959(96)03211-3. [DOI] [PubMed] [Google Scholar]
- 33.BAUMANN TK, SIMONE DA, SHAIN CN, LAMOTTE RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 34.HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 35.BUCK SH, BURKS TF. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986;38:179–226. [PubMed] [Google Scholar]
- 36.KILO S, SCHMELZ M, KOLTZENBURG M, HANDWERKER HO. Different patterns of hyperalgesia induced by experimental inflammation in human skin. Brain. 1994;117:385–396. doi: 10.1093/brain/117.2.385. [DOI] [PubMed] [Google Scholar]
- 37.FLUHR K, NEDDERMEYER TJ, LOTSCH J. Capsaicin or menthol sensitization induces quantitative but no qualitative changes to thermal and mechanical pain thresholds. Clin J Pain. 2009;25:128–131. doi: 10.1097/AJP.0b013e3181817aa2. [DOI] [PubMed] [Google Scholar]
- 38.BARON R. Neuropathic pain: a clinical perspective. Handb Exp Pharmacol. 2009;194:3–30. doi: 10.1007/978-3-540-79090-7_1. [DOI] [PubMed] [Google Scholar]
- 39.MUNNS C, ALQATARI M, KOLTZENBURG M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–342. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]