Abstract
Effective therapies are needed to control excessive bleeding in a range of clinical conditions. We describe a surprisingly useful approach to improve hemostasis in vivo using a variant of coagulation factor Xa (FXaI16L). This conformationally pliant derivative is partially inactive due to a defect in transitioning from zymogen to protease 1,2. Using mouse models of hemophilia, we show that FXaI16L has a prolonged half-life, relative to wild-type FXa and does not cause excessive activation of coagulation. Once clotting mechanisms are activated to produce its cofactor FVa, FXaI16L is driven to the protease state and restores hemostasis in hemophilic animals upon vascular injury. Moreover, using human or murine analogs, we show that FXaI16L is more efficacious than FVIIa which is used to treat bleeding in hemophilia inhibitor patients3. Because of its underlying mechanism of action, FXaI16L may provide an effective strategy to enhance blood clot formation and act as a rapid pan-hemostatic agent for the treatment of bleeding conditions.
The formation of a blood clot following vascular injury is a vital host defense mechanism. This process is compromised in patients with congenital or acquired bleeding disorders including hemophilia A (HA; factor VIII (FVIII) deficiency) and hemophilia B (HB; factor IX (FIX) deficiency)4. FVIII and FIX function in the intrinsic pathway of coagulation to maintain normal hemostasis by affecting the conversion of FX to FXa. Membrane-bound FXa in the presence of its cofactor FVa (prothrombinase complex) converts prothrombin to thrombin, which activates platelets and converts fibrinogen to fibrin to form the thrombus5. Management of patients with HA and HB has dramatically improved over the past three decades6. However, development of inhibitory neutralizing alloantibodies (inhibitors) in response to infusion of FVIII or FIX protein remains a serious threat to disease related morbidity and mortality7,8. Protein-based biopharmaceuticals that bypass the intrinsic pathway such as activated prothrombin complex concentrates (aPCCs; FEIBA) and recombinant FVIIa (FVIIa; NovoSeven) are viable strategies to re-establish hemostasis in these patients3,9. However, neither is universally effective nor can completely normalize thrombin generation with some patients experiencing a variable response to either bypassing agent10. Furthermore, frequent high-dose infusions to stop bleeding result in costly treatment11.
Existing bypassing strategies are directed towards enhancing FXa production to accelerate the formation of thrombin12,13. FVIIa at pharmacological doses (90 µg/kg; 1.8 nmol/kg; ~25 nM) activates FX at sites of vascular injury where tissue-factor (TF) is exposed and activated platelets are abundant. In principle, infusion of FXa to increase the concentration of cell surface prothrombinase at the injury site would represent a more direct approach to enhance thrombin production. However, FXa is rapidly inactivated by circulating protease inhibitors resulting in a short half-life (<1–2 min) and can activate a range of procoagulant clotting factors possibly leading to pathological activation of coagulation14. These seemingly insurmountable limitations associated with the infusion of FXa have been borne out by findings in hemophilic animals 15,16.
Our approach to the problem is based on knowledge of the biochemistry of zymogen activation and protease formation in the S1 peptidase clan of chymotrypsin-like serine proteases17. Rather than attempting to enhance enzyme function, we recently developed FXa variants (e.g. FXaI16L and FXaV17A; numbered after chymotrypsinogen 18) which have an impaired conformational transition from the zymogen to protease. In this family, the zymogen precursor is cleaved between Arg15-Ile16 liberating a new N-terminus (typically, Ile16-Val-Gly-Gly…)17. After cleavage, the intermediate is in a zymogen-like state, which equilibrates to the protease state following insertion of the nascent N-terminus into a binding pocket forming a salt-bridge between Ile16 and Asp194. Completion of this transition is critical for full expression of enzyme activity. For FXa, modification at Ile16 or Val17 perturbs the zymogen to protease transition1. As a result, FXaI16L and FXaV17A have zymogen-like properties including an immature active site, decreased sensitivity towards plasma inhibitors and poor reactivity towards other physiological ligands1. Notably, binding to FVa facilitates the transition to the active conformation and rescues the procoagulant activity of FXaI16L and FXaV17A resulting in normalized thrombin generation even in the context of hemophilic plasma1,2. The current report examines whether zymogen-like FXa variants correct the hemostatic defect in vivo using murine models of HA and HB.
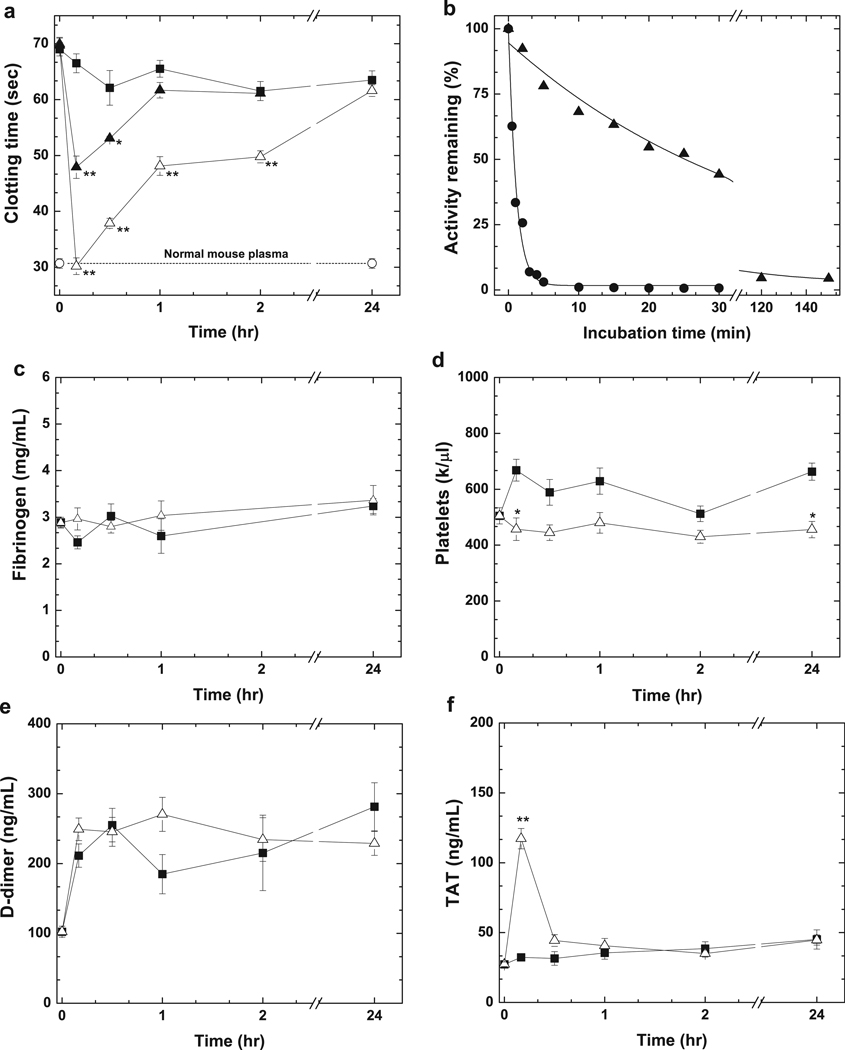
Hemophilic mice are useful models to probe the effectiveness of procoagulant therapeutics as they recapitulate aspects of the human disease. In a modified aPTT clotting assay (Fig. 1a), untreated HB mice have a prolonged clotting time compared to hemostatically normal wild-type (wt)-mice. Intravascular administration of human FXaI16L (hFXaI16L; 450 µg/kg or 9.8 nmol/kg, ~100 nM) corrects the prolonged aPTT in HB mice 10 min post infusion whereas PBS has no effect. The aPTT was shortened at 2 hrs and returned to baseline at 24 hrs post-infusion. Shortening of the aPTT with hFXaI16L was dose-dependent as 225 µg/kg gave an intermediate result. In contrast to hFXaI16L, administration of wt-hFXa in HB mice (450 µg/kg; n = 4) did not shorten the aPTT at any time point (not shown) likely due to its extremely short half-life (<0.5 min) in mouse plasma (Fig. 1b). The zymogen-like conformation protects hFXaI16L in mouse plasma from inhibitors and extends the half-life by a factor of 30–60-fold (Fig. 1b). Estimated ex vivo half-lives in mouse plasma are approximately 5–10-fold shorter compared to human hemophilic plasma likely reflecting the increased activity of protease inhibitors as previously suggested19. Like HB mice, there is a dose-dependent shortening of the aPTT in HA mice treated with hFXaI16L compared to PBS treated mice (Supplemental Fig. 1). Taken together, these data show that hFXaI16L has an extended half-life compared to wt-hFXa and corrects the prolonged clotting time in both HA and HB mice. These latter findings indicate that sufficient amounts of FVa were generated during the coagulation assays to rescue the activity of hFXaI16L.
Figure 1.
Effect of hFXaI16L on coagulation parameters. In a, HB mice (Balb/c) were injected with PBS (-■-; n = 7) or hFXaI16L (-▲-, 225 µg/kg, n = 4; -△-, 450 µg/kg, n = 9). At the indicated time intervals a modified one-stage aPTT was performed on processed mouse plasma. Clotting times for wt-mice (-○-, n = 7) is indicated. In b, wt-hFXa (-●-, 20 nM) or hFXaI16L (-▲-, 20 nM) were incubated in diluted HB (Balb/c) mouse plasma and residual activity was assessed by clotting assay. The solid lines were drawn following analysis of data sets to a single exponential decay with fitted half-lives of: wt-hFXa, 0.15 ± 0.01 min; hFXaI16L, 5.3 ± 0.46 min. These values have been appropriately adjusted for sample dilution. The data are representative of two to three similar experiments. In c–f, markers of coagulation activation were measured. HB mice (Balb/c) were injected with PBS (-■-; n = 3–10) or hFXaI16L (-△-, 450 µg/kg, n = 3–8). At the indicated time intervals, levels of c) fibrinogen, d) platelets, e); D-dimer and f) TAT complex were measured. In panels a and c–f, all measurements are presented as mean ± SEM. For statistical comparisons, treated animals are compared to HB-PBS controls at the same time point; ** p<0.001; * p<0.05.
Safety and the possibility of uncontrolled and systemic activation of coagulation is a major concern with any procoagulant protein therapeutic. Compared to PBS-treated animals, there was no major change in fibrinogen, platelets, or D-dimer levels over an extended time course with hFXaI16L at 450 µg/kg (Fig. 1c–e). There was a transient increase in thrombin-antithrombin (TAT) 10 min post-infusion which returned to baseline at 30–60 min (Fig. 1f). Histopathological evaluation of lung, liver, kidney, and spleen from HB mice infused with hFXaI16L revealed no evidence of treatment-related increases in fibrin deposition (not shown). To further evaluate the safety of hFXaI16L, multi-day infusion experiments were performed in wt-mice (Balb/c). Animals were injected with hFXaI16L at 450 µg/kg either once (n = 5) or twice (n = 5; injections spaced 4 hrs apart) daily for three consecutive days. Compared to pre-infusion controls or PBS-infused mice (n = 5), there were no statistically significant difference in platelets, fibrinogen, D-dimer, or TAT levels in mice treated with hFXaI16L (not shown). Furthermore, hFXaI16L was well tolerated as all mice survived the multi-dose infusion. A contributing factor to this favorable safety stem from its relatively poor reactivity towards a range of physiological ligands in the absence of circulating FVa1,2.
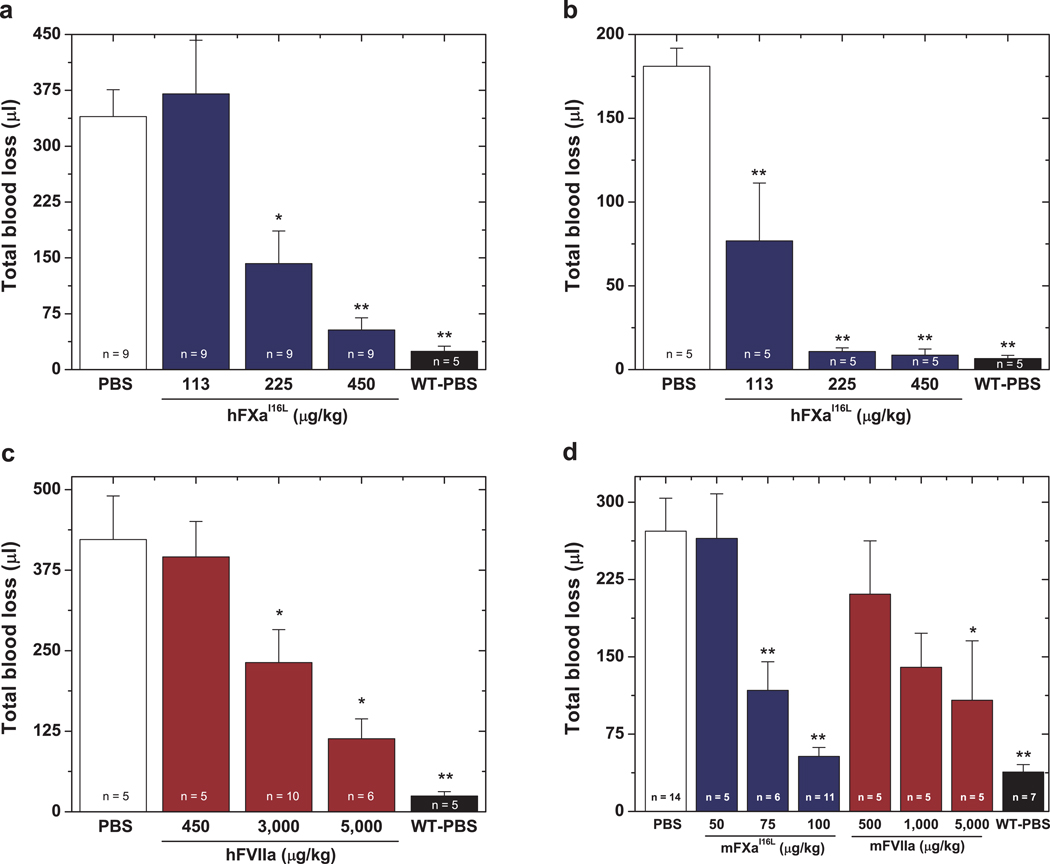
To evaluate the efficacy of hFXaI16L in vivo, blood loss was monitored following a hemostatic challenge. HB mice on the C57BL/6 (Fig. 2a) or Balb/c (Supplemental Fig. 2) background exhibit substantial blood loss following sectioning of the distal portion of the tail compared to normal mice. Infusion of hFXaI16L in HB mice 5 min prior to injury reduced bleeding following tail clip in a dose-dependent manner. Treatment with the highest dose (450 µg/kg) reduced total blood loss to that seen with wt-mice whereas 225 µg/kg of hFXaI16L was intermediate; there was no significant reduction in total blood loss at 113 µg/kg (Fig. 2a). Similar dose response results with hFXaI16L were obtained in HA mice (Supplemental Fig. 3). Infusion of a second variant (hFXaV17A; 450 µg/kg) with similar biochemical properties as hFXaI16L into HB mice also decreased total blood loss to that obtained with normal mice (Supplemental Fig. 2). In contrast, HB mice infused with wt-hFXa (450 µg/kg) showed no improvement in blood loss compared to HB controls (Supplemental Fig. 2). These data show that infusion of hFXaI16L prior to injury is effective in reducing blood loss in hemophilic animals following a severe hemostatic challenge.
Figure 2.
Blood loss following tail-clipping. In a, five minutes prior to injury, PBS (white column) or hFXaI16L (blue columns) was administered to HB mice (C57Bl/6) via tail vein at the indicated dosage. Total blood loss (µl) was then measured following tail transection. In b, tail clipping was performed on wt-mice or HB mice (Balb/c) prior to protein infusion and blood was collected for 2 min (total blood loss at 2 min: wt-mice: 17 ± 5.4 µl; HB: 49 ± 7.7 µl). Subsequently, PBS (white column) or hFXaI16L (blue columns) was administered to HB mice via a pre-inserted jugular vein cannulus at the indicated dosage; wt-mice also received PBS (black column). Blood was collected for an additional 10 min in a fresh tube of saline and total blood loss (µl) was measured. Data in (b) represent total blood loss subsequent to protein infusion. In c, five minutes prior to injury, PBS (white column) or hFVIIa (red columns) was administered to HB mice (C57Bl/6) via tail vein at the indicated dosage. Total blood loss (µl) was then measured following tail transection. In d, five minutes prior to injury, PBS (white column), mFXaI16L (blue columns), or mFVIIa (red columns) was administered to HB mice (Balb/c) via tail vein at the indicated dosage. Total blood loss (µl) was then measured following tail transection. In a–d, hemostatically normal mice on the appropriate strain infused with PBS (WT-PBS, black column) served as a control. In each panel, the number of animals per group is indicated and all measurements are presented as mean ± SEM. For statistical comparisons, treated animals are compared to HB-PBS controls ** p<0.001; * p<0.05.
Since pro-hemostatic agents are generally administered following a bleeding episode, experiments examined whether hFXaI16L is capable of reducing bleeding when infused after an injury (Fig. 2b). Following tail injury and an observation period of 2 min, hFXaI16L or PBS were injected into mice via a pre-inserted jugular vein cannulus and total blood loss was monitored for an additional 10 min. Using this strategy, hFXaI16L was very effective at decreasing bleeding in HB mice: 225 µg/kg and 450 µg/kg reduced total blood loss to that seen with wt-mice and 113 µg/kg showed partial reduction in blood loss. The increased effectiveness of hFXaI16L when administered after tail injury (Fig. 2b) versus before (Fig. 2a) is likely due to inactivation of a proportion of the hFXaI16L by circulating inhibitors prior to the injury.
Another useful test of therapeutic procoagulants is injury to the carotid artery using FeCl3 as hemophilic animals do not generally form occlusive thrombi in this model20. Following application of FeCl3 (15%) to the surface of the carotid artery for 2 min, wt-mice or HB mice infused with hFIX after injury present with full occlusion characterized by interruption of blood flow (Table 1 and Supplemental Fig. 4). In contrast, no evidence of clot formation was detected in PBS-treated HB mice. To test hFXaI16L in HB mice, FeCl3-induced injury was monitored for 10 min to ensure that blood flow remained stable; subsequently hFXaI16L was administered via a jugular vein cannulus. Using this model, hFXaI16L rapidly induced complete vessel occlusion in all mice (n = 13) at 180–450 µg/kg (Table 1 and Supplemental Fig. 4). At a lower dose (90 µg/kg) transient and occlusive thrombi were observed. In a second method, hFXaI16L (n = 5; 450 µg/kg) was administered to HB mice 10 min prior to the application of FeCl3. Subsequent injury to the carotid artery resulted in complete occlusion in 5 out of 5 mice at 2.5 ± 1.1 min following injury (not shown). In these experiments, hFXaI16L induced vessel occlusion more rapidly (~2 min) than untreated control mice (~10–14 min). We speculate that since infusion of hFXaI16L bypasses both the intrinsic and extrinsic pathways the rate of thrombin formation would be expected to be much faster than in wt-mice. Consistent with this, introduction of hFXaI16L into a thrombin generation assay using human hemophilic plasma substantially shortens the lag time or onset of thrombin generation compared to normal plasma without added FXa2. Similarly, FVIIa and FEIBA also shorten the lag time in a thrombin generation assay21 and the time to occlusion following FVIIa administration in the FeCl3 model is comparable to FXaI16L (see Table 1).
Table 1.
Time to carotid artery occlusion following FeCl3-induced injury.
| Genotype | Sample | Dose | Total # of mice |
No Occlusion |
Transient Occlusion |
Complete Occlusion |
Time to Occlusion |
|---|---|---|---|---|---|---|---|
| µg/kg | # of mice | # of mice | # of mice | # of mice | min | ||
| Controls | |||||||
| WT (Balb/c) | PBS | -- | 7 | 0 | 0 | 7 | 10.1 ± 1.0 |
| HB (Balb/c) | PBS | -- | 7 | 7 | 0 | 0 | -- |
| WT (C57BL/6) | PBS | -- | 4 | 0 | 0 | 4 | 11.9 ± 1.0 |
| HB (C57BL/6) | PBS | -- | 4 | 4 | 0 | 0 | -- |
| HB (Balb/c) | hFIX | 500 | 5 | 0 | 0 | 5 | 14.6 ± 1.1 |
| Experimental—Human Proteins | |||||||
| HB (Balb/c) | hFXaI16L | 90 | 5 | 0 | 3 | 2 | 3.2 ± 0.6 |
| HB (Balb/c) | hFXaI16L | 180 | 3 | 0 | 0 | 3 | 2.4 ± 0.6 |
| HB (Balb/c) | hFXaI16L | 450 | 10 | 0 | 0 | 10 | 2.2 ± 0.2 |
| HB (C57BL/6) | hFXaI16L | 450 | 4 | 0 | 0 | 4 | 1.2 ± 0.1 |
| HB (Balb/c) | hFVIIa | 1,000 | 4 | 2 | 1 | 1 | 3.4 ± 0.1 |
| HB (Balb/c) | hFVIIa | 3,000 | 3 | 0 | 2 | 1 | 3.3 ± 0.3 |
| HB (Balb/c) | hFVIIa | 5,000 | 4 | 0 | 0 | 4 | 2.4 ± 0.2 |
| Experimental—Murine Proteins | |||||||
| HB (Balb/c) | mFXaI16L | 50 | 3 | 0 | 2 | 1 | 2.6 ± 0.4 |
| HB (Balb/c) | mFXaI16L | 100 | 5 | 0 | 1 | 4 | 2.3 ± 0.4 |
| HB (Balb/c) | mFVIIa | 500 | 3 | 3 | 0 | 0 | -- |
| HB (Balb/c) | mFVIIa | 3,000 | 7 | 5 | 0 | 2 | 2.4 ± 0.1 |
| HB (Balb/c) | mFVIIa | 5,000 | 4 | 0 | 0 | 4 | 2.5 ± 0.2 |
WT, hemostatically normal mice; HB, hemophilia B mice; PBS, phosphate buffer saline, FIX, factor IX.
The total number of mice per experiment is indicated. In HB mice, protein was infused 10 min after FeCl3 exposure. Mice presenting with no occlusion, transient occlusion or complete occlusion of the carotid artery following FeCl3 exposure is tabulated. Occlusion time measurements are presented as mean ± SEM and are derived from both transient and complete events. Representative Doppler blood flow versus time tracings can be found in Supplemental Fig. 4.
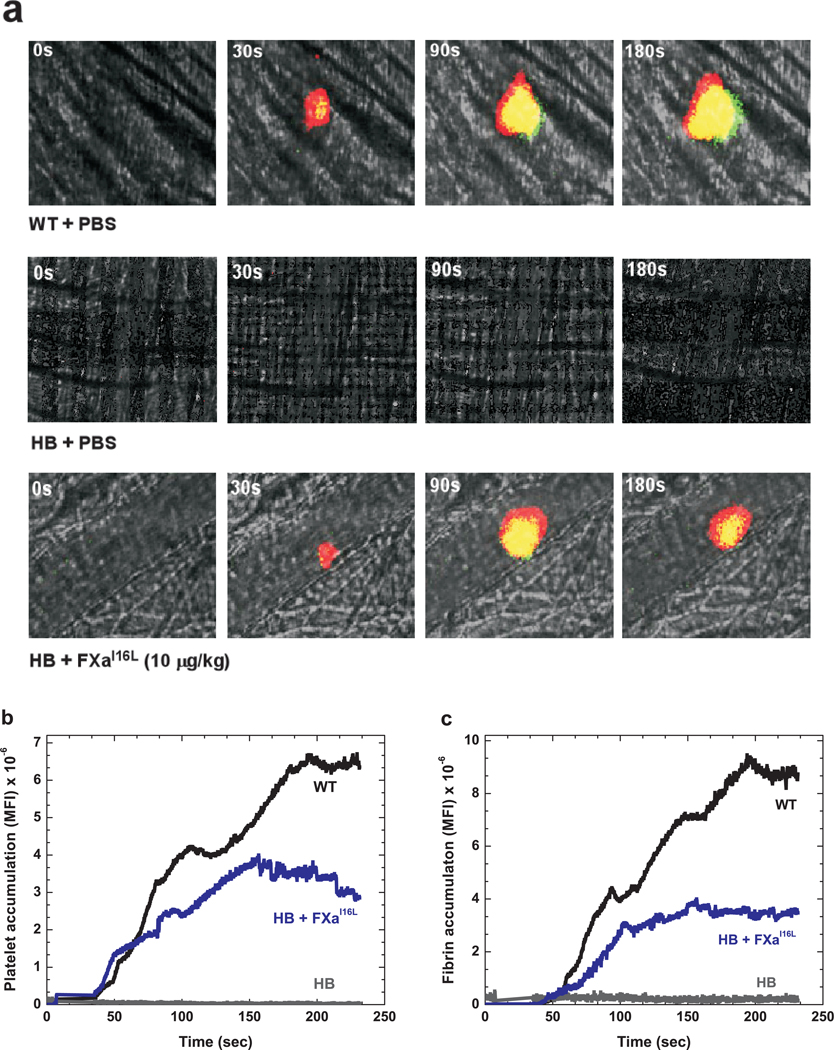
The efficacy of hFXaI16L was further assessed in the microcirculation through the kinetics of thrombus formation following laser injury to arterioles in the cremaster muscle. As shown in Fig. 3a, hFXaI16L (10 µg/kg or 0.2 nmol/kg, ~2.5 nM) infused 5 min prior to vessel injury, restored platelet (red) and fibrin (green) accumulation over time comparable to wt-mice (see also Supplemental Movie 1 and 2). Quantitative analysis revealed that hFXaI16L induced platelet accumulation was comparable to wt-mice whereas total fibrin deposition was reduced and slightly delayed (Fig. 3b,c). In contrast, little, if any, platelets or fibrin were detected in PBS-infused HB controls (Fig. 3a–c). At both 10 µg/kg (n = 3 mice/20 injuries) and 90 µg/kg (n = 8 mice/55 injuries) thrombus formation was observed 92% and 100% of the time, respectively. In addition, we explored the consequences of hFXaI16L infusion after laser injury. As shown in Supplemental Movie 3, there is no detectable clot formation in HB mice following laser injury. However, subsequent infusion of hFXaI16L (30 µg/kg) via a jugular vein cannulus rapidly resulted in platelet/fibrin accumulation at the previous injury site. Collectively, these data show that infusion of hFXaI16L either before or after laser injury restores hemostasis at the microcirculation level. Interestingly, in this model hFXaI16L was effective at a much lower dose (25–45-fold) than needed in the tail clip/FeCl3 models. While this could reflect sensitivity issues, it is also possible that differences in vessel size (~10-fold difference in diameter between carotid artery and arterioles), mechanism of clot formation, availability of FVa and/or the nature of the initiating injury could also contribute. Differences in efficacy between tail clip/FeCl3 and laser injury models has also been noted with FVIIa and when investigating how FV-Leiden influences the hemophilic phenotype20,22,23.
Figure 3.
Platelet and fibrin accumulation following laser-induced arteriole injury in wt-mice and hFXaI16L treated HB mice. In these experiments, PBS or protein was infused 5 min prior to vessel injury. In a, digital composite fluorescence and brightfield images of representative thrombi in wt-mice (+ PBS), HB (+PBS), and HB (+hFXaI16L, 10 µg/kg) mice prior to (0 sec) and 30, 90, and 180 sec after laser-induced injury of the blood vessel wall. Platelets (red) were detected by an Alexa555-labeled rat anti- CD41 F(ab)2 and fibrin (green) with Alexa488-labeled anti-fibrin antibody; areas of overlap are depicted by yellow. (b and c) Quantitative analysis of platelet and fibrin accumulation over time following laser injury in wt-mice (black; 20 thrombi; n = 3 mice), HB (grey; 20 thrombi; n = 3 mice), and HB + hFXaI16L (blue; 20 thrombi; n = 3 mice; 10 µg/kg) treated mice. Median fluorescence intensity (MFI) for platelet (b) and fibrin (c) fluorescence are plotted versus time. For both platelet (b) and fibrin (c) accumulation, there is no statistically significant difference (p > 0.1) in MFI when comparing values between wt-mice and HB + hFXaI16L at every 30 sec intervals by the Wilcoxon rank sum test. However, the MFI values (evaluated at 30 sec intervals) between wt-mice versus HB and HB + hFXaI16L versus HB are significantly different (p < 0.001).
Currently, FVIIa is the only recombinant bypassing biotherapeutic approved for treatment of bleeding episodes in hemophilia inhibitor patients3. It is generally thought that FVIIa at pharmacological doses binds to activated platelets and converts FX to FXa in a TF-independent manner, albeit at a very slow rate 24. This likely explains the need for frequent high dose infusions for an adequate hemostatic response3. The relative inefficiency of FVIIa in generating FXa at the platelet surface and the variability of procoagulant platelet populations from patient to patient may explain the difficulty in establishing a relationship between FVIIa plasma concentration and its hemostatic effect complicating optimal treatment25. Furthermore, high treatment cost and lack of clinically useful laboratory assays to monitor therapeutic responses suggest a need to compare effectiveness of other hemostatic agents to FVIIa.
To begin to evaluate this, the efficacy of hFXaI16L was compared to human FVIIa (hFVIIa) in the tail clip/FeCl3 injury models. Consistent with prior reports26, high levels of hFVIIa were needed to reduce blood loss following tail injury in both HB (Fig. 2c) and antibody-induced HA animals (Supplemental Fig. 5). For hFVIIa treated mice, reductions in blood loss occurred between 3–5 mg/kg (60–100 nmol/kg, ~0.6–1.0 µM) representing an approximately 10-fold increase compared to hFXaI16L. Similarly, high concentrations of hFVIIa were needed (3–5 mg/kg) to induce thrombus formation in HB mice in the FeCl3 injury model (Table 1). Like hFXaI16L, infusion of hFVIIa following injury to the carotid artery rapidly (2.5– 3.0 min) induced occlusive or transient thrombi.
While the effectiveness of hFXaI16L versus hFVIIa is compelling, the overall findings must be interpreted cautiously since human proteins were used with the potential for suboptimal species compatibility between clotting factors. This is particularly true with hFVIIa as it binds weakly, if at all, to murine TF23,27. To address this point, murine derivatives of FXaI16L (mFXaI16L) and FVIIa (mFVIIa) were expressed and purified. Recombinant mFVIIa has been recently shown functionally similar to hFVIIa except with respect to TF binding23,27. Biochemical characterization of purified mFXaI16L revealed that it has zymogen-like properties and can be rescued in assays where FVa is generated. For example, mFXaI16L has low activity (2–4%) in the absence of FVa compared to wt-mFXa as assessed by peptidyl substrate cleavage (Supplemental Fig. 6) yet the variant is as effective as wt-mFXa in a thrombin generation assay where FVa is generated in situ (Supplemental Fig. 7). Consistent with its zymogen-like properties, mFXaI16L has a much longer half-life in murine HB plasma compared to wt-mFXa indicating that, like hFXaI16L, it is partially protected from plasma-based inhibitors (Supplemental Fig. 8).
The efficacy of mFXaI16L and mFVIIa in HB mice were compared in the tail clip/FeCl3 injury models. As expected, mFXaI16L was able to substantially reduce blood loss in the tail clip assay; interestingly the dose required for efficacy was 4-fold less for mFXaI16L compared to the human protein. Only 100 µg/kg (2 nmol/kg, ~20 nM) reduced blood loss to that seen in wt-mice (Fig. 2d). Similarly, mFXaI16L was also clearly effective in the FeCl3 injury model with 5 out of 8 animals presenting with full occlusion at 50–100 µg/kg (Table 1). In contrast, high levels of mFVIIa were needed in both the tail clip (Fig. 2d) and FeCl3 injury models (Table 1). For mFVIIa treated mice, reductions in blood loss occurred only at 5 mg/kg, representing an approximately 50-fold increase in therapeutic dose compared to mFXaI16L. These within-species comparisons collectively indicate that FXaI16L is superior as a hemostatic bypass compared to FVIIa in hemophilic mice using established injury models. Although results with FXaI16L are certainly encouraging, additional parameters such as pharmacokinetic profile, dosing and safety will need to be assessed in a large animal model of hemophilia. Unlike the mouse model, the pharmacokinetics and dosing of FVIIa in hemophilic dogs is very similar to those parameters in humans. An important question in future experimentation is whether this 10–50-fold dose advantage of FXaI16L versus FVIIa seen in mice translates to the hemophilic dog model.
The high dose of FVIIa needed to ameliorate the hemophilic phenotype in mice supports the notion that the platelet-surface, TF-independent procoagulant mechanism predominates26. Unlike this mechanism, the efficacy of FXaI16L absolutely requires not only procoagulant cellular surfaces but the availability of its cofactor, FVa. We speculate that activated platelets play a key overall role in this process as they provide the necessary procoagulant membrane surface and release relatively large quantities of FV/FVa from α-granules25,28. Prior studies using isolated thrombin-activated platelets demonstrated that released platelet-derived FVa was sufficient to completely rescue the activity of hFXaI16L leading to rapid prothrombin activation2. The importance of activated platelets to the overall hemostatic potential of FXaI16L was seen in additional thromboelastography experiments (Supplemental Table 1). In the absence of an added extrinsic (TF) or intrinsic pathway activator, the addition of hFXaI16L with a direct platelet activator (ADP or collagen) to HB mouse whole blood completely normalized the coagulation parameters seen with hemophilic samples to values seen in normal blood. These data show that direct platelet activation with subsequent release of FV/FVa efficiently restores the activity of hFXaI16L to correct altered whole blood coagulation in the context of hemophilia.
Knowledge of the biochemistry of the FX zymogen to protease transition has provided a novel approach to circumvent limitations inherent to wt-FXa that preclude its use as a therapeutic bypass for hemophilia. Our innovative strategy of employing rational mutagenesis to make FXa more zymogen-like and thereby reducing function has yielded a remarkably effective agent for modulating hemostasis in hemophilic mice. The plasticity of zymogen-like FXa is central to this effectiveness, as enzyme function can be restored by FVa and activated cellular surfaces following vascular damage. This selective and localized enhancement of cell-surface prothrombinase rapidly produces thrombin at the injury site. The favorable safety profile of this variant in mice results from its extended half-life in plasma and poor reactivity towards other physiological substrates in comparison to wt-FXa. Any procoagulant therapeutic has the potential for thromboembolic complications and future studies in larger animals are needed. Furthermore, while the complexity of the immune response makes predictions difficult, it remains possible that an antibody response could be mounted against hFXaI16L despite a conservative amino acid change. Of note, evaluation of a FVIIa variant with three amino acid substitutions reported no development of antibodies in a phase I clinical trail30. Overall, the current data with FXaI16L provide proof-of-concept for a new class of bypassing agent with high hemostatic potential to treat patients with bleeding disorders. These studies also provide a general protein engineering strategy to modulate, either directly or indirectly, the activity of other serine proteases of biologic and therapeutic importance.
METHODS
Reagents
All tissue culture reagents were from Invitrogen (Carlsbad, CA) except insulin-transferrin-sodium selenite which was from Roche (Indianapolis, IN). Human factor deficient plasmas from individual donors were purchased from George King Biomedical, Inc. (Overland Park, KS). Automated activated partial thromboplastin time reagent (aPTT; TriniClot or Sta-C.K.Preset) was from Tcoag (Wicklow, Ireland) or Stago (Parsippany, NJ). Adenosine diphosphate (ADP) and collagen (collagen fibrils, type I from equine tendons) was obtained from Chrono-log Corp. (Havertown, PA). All reagents necessary to perform rotational thromboelastography (ROTEM) were obtained from Pentapharm (Durham, NC). Preparation and characterization of recombinant wt-hFXa, hFXaI16L, hFXaV17A, and mFVIIa has been previously described1,23. Recombinant wt-mFX and mFXI16L were expressed in human embryonic kidney 293 (HEK293) cells and prepared essentially as described1, except rather than employing immunopurification with an anti-FX antibody, the final purification step involved elution of mFX from a Poros HQ/20 column with a CaCl2 gradient (0 to 20 mM).
Antibodies
Rat anti-mouse CD41 antibody (clone MWReg 30) prepared as a F(ab)2 fragment was from BD Bioscience (San Jose, CA). Mouse anti-human fibrin monoclonal antibody (clone 59D8) which cross reacts with mouse fibrin has been previously described31,32. These antibodies were conjugated with Alexa555 or Alexa488 using the Alexa Fluor Protein Labeling Kit according to the manufacturer’s instructions (Molecular Probes/Invitrogen, Carlsbad, CA). The mouse anti-human FVIII antibody, GMA-8015, was from Green Mountain Antibodies (Burlington, VT). Plasma levels of mouse TAT (Enzygnost TAT, Dade Behring, Marburg Germany), D-dimer (Asserachrom D-Di; Diagnostica Stago), and fibrinogen (Innovative Research) were measured by ELISA according to the manufacturer’s guidelines.
Mice
Hemophilia B mice (Balb/c or C57BL/6 ) have been previously described and backcrossed for more than 5 generations onto the appropriate strain33,34. Wild-type mice used in control experiments were either purchased from Jackson Laboratory (Bar Harbor, ME) or generated as littermates to HB mice (C57BL/6 background, heterozygous female×wild-type male breeding format). To experimentally induce an HA phenotype, C57BL/6 mice purchased from Taconic Farms (Germantown, NY) were injected with an anti-FVIII antibody (GMA-8015; 4.0 mg/kg). This is an inhibitory antibody directed against the FVIII A2 domain and cross reacts with FVIII from multiple species. For all experiments, mice were between 6–10 weeks of age and weighed 20 to 30 g. Experimental approval was obtained from the Children’s Hospital of Philadelphia and Pfizer Institutional Animal Care and Use Committee.
Coagulation assays
Blood samples from mice obtained by tail-clipping were collected into one-tenth volume of 3.8% sodium citrate and immediately processed. Complete blood cell counts from individual mice were measured with an automated complete blood count analyzer with settings established for mouse blood (Hemavet, Drew Scientific Inc., Oxford, CT). Plasma was collected from whole blood following centrifugation and samples were evaluated fresh or snap frozen and stored at −80°C. The activated partial thromboplastin time (aPTT) on samples obtained from mice (wt or hemophilic) infused with FXa or PBS were assessed essentially as described35. For ex vivo half-life determinations, FXa (20 nM, final) was incubated in diluted hemophilic mouse plasma (1:4, 1:5, or 1:8) at room temperature and at various time points aliquots of the reaction were further diluted (0.1 nM FXa, final) in 20 mM Hepes, 0.15 M NaCl, 0.1% polyethylene glycol 8000, pH 7.5 (dilution buffer). Residual FXa bypassing activity was assessed over time using a modified aPTT clotting assay. In a typical assay, 50 µl of human HB plasma was mixed with an equal volume of aPTT reagent followed by a 180 second incubation period at 37°C. A 50 µl mixture of FXa (0.1 nM) in dilution buffer was then added to the solution and coagulation was initiated following the addition of 50 µl of 25 mM CaCl2. Time to clot formation was measured using a Start4 coagulation instrument (Diagnostica Stago, Inc., Parsippany, NJ).
Chromogenic substrate hydrolysis
All kinetic measurements were performed in 20 mM Hepes, 0.15 M NaCl, 0.1% (w/v) PEG-8000, 2 mM CaCl2, pH 7.5 (assay buffer). The kinetic parameters of peptidyl substrate hydrolysis (Spectrozyme Xa; American Diagnostica, Stamford, CT) were measured using increasing concentrations of substrate (10–500 µM) and initiated with either wt-mFXa (2.0 nM) or mFXaI16L (10 nM).
Rotational thromboelastography (ROTEM)
Whole blood (300 µl) from hemostatically normal wt or HB mice (Balb/c) was collected into citrate via tail clipping. Blood was transferred to a pre-warmed (37°C) ROTEM cup containing 0.2 M CaCl2 (20 µl) and 20 µl of coagulation activator (INTEM reagent; ADP, 25 µM; or collagen, 30 µg/mL). Bypassing protein or PBS (2 µl) to achieve the final indicated concentration (Supplemental Table 1) was immediately added. Data derived from the ROTEM machine was collected over 90 min and analyzed using the manufacturers software.
Thrombin generation assays
Thrombin generation in platelet poor murine plasma was determined essentially as described with some modifications due to the higher activity of coagulation inhibitors in mouse plasma2,19. Briefly, normal or HB murine (Balb/c) plasma (15 µl) was mixed with a tissue-factor reagent (10 µl; Technothrombin RB; 2 pM tissue factor/4.0 µM phospholipid) followed by the addition of wt-mFXa or mFXaI16L (25 µl). The reaction was immediately initiated following the addition of Z-Gly-Gly-Arg-AMC in 15 mM CaCl2 (50 µl; 0.5 mM, final) and the concentration of thrombin (at 33°C) was determined as described2.
Tail clip assay
Two different versions of the tail clip assay were employed. In the first, protein or PBS was initially infused via the tail vein approximately 5 min prior to injury. Mice were then anesthetized with isoflurane and then the tail was pre-warmed at 37°C and the distal portion transected at a diameter of 3 mm. The tail was then placed in a conical tube containing 10 mL or 14 mL of saline at 37°C and blood was collected for 10 min. In the second method, mice were anesthetized with isoflurane and then the tail was pre-warmed at 37°C and the distal portion transected at a diameter of 3 mm. The tail was then placed in a conical tube containing 14 mL of saline at 37°C and blood was collected for 2 min. Protein or PBS was then infused via a pre-inserted jugular vein cannulus. The injured tail was moved to a fresh tube of saline and blood was collected for an additional 10 min. Quantitative assessment of blood loss was determined by measuring total hemoglobin by absorbance at 575 nm following red cell lysis as described36. For some experiments, hemoglobin content was determined using the QuantiChromTM Hemoglobin Assay Kit (BioAssay Systems, Hayward, CA). For both determinations, quantitative assessment of hemoglobin content was converted to total blood loss (µl) using appropriately established standard curves using each method.
Ferric chloride carotid artery model
Ferric chloride-induced injury was performed according to published procedures20. Briefly, mice were anesthetized with pentobarbital and the carotid artery was exposed. A jugular vein cannula was established to allow for infusion of proteins. A miniature Doppler flow probe (Model 0.5VB; Transonic Systems, Ithaca, NY) was positioned around the exposed artery and baseline blood flow measurements were recorded. To induce an injury, a 2 mm2 piece of Whatman #1 filter paper soaked in 15% FeCl3 (0.92 M) was then applied to the adventitial surface of the artery for 2 minutes. The surface was then flushed with saline and blood flow was continuously monitored for up to 30 minutes. The time to carotid artery occlusion was defined as the time following application of FeCl3 until the blood flow has decreased by >90%. In some experiments involving HB animals, an injury was induced with FeCl3, blood flow was monitored for 10 minutes after which defined coagulation proteins were infused via a jugular vein cannula. Blood flow was monitored for an additional 20 minutes. In these experiments, the time to occlusion was defined as the time from infusion of clotting factors until the blood flow decreased by 90%.
Real-time in vivo imaging of thrombus formation
Evaluation of hemostasis following laser injury to the cremaster muscle has been previously described37 and our specific experimental system has been detailed20,32. Alexa555-labeled rat anti- CD41 F(ab)2 and Alexa488-labeled anti-fibrin antibody were administered via a jugular vein cannula to label platelets and fibrin, respectively. Based on empirical studies, antibodies were infused into mice at 0.2 mg/kg for the anti-CD41 Fab fragment and 0.1 mg/kg for the anti-fibrin antibody. Cremasteric arterioles of 30–50 µm were selected for study and vascular injury was induced using a pulse-nitrogen dye laser applied through the microscope objective. Brightfield and fluorescence images were collected over the course of 3–4 minutes at 4 frames/second and the data were analyzed with Slidebook 5 software (Intelligent Imaging Innovations, Denver, CO). The kinetics of clot formation was analyzed by determining median fluorescence intensity over time in approximately 18–20 thrombi from 3 animals/group.
Statistical analysis
All data are presented as mean ± SEM. An analysis of variance (ANOVA) followed by a Student-Newman-Keuls test was used to compare the treatment effects between groups. A P value of < 0.05 was considered statistically significant. For intravital experiments, data were considered nonparametric and presented as medians. In these experiments, the Wilcoxon rank sum test was used for statistical analysis.
Supplementary Material
Acknowledgements
This work was supported in part by NIH grant P01 HL-74124, Project 2, research funding from Pfizer (to R.M.C) and the Judith Graham Pool Postdoctoral Research Fellowship, National Hemophilia Foundation (to L.I). We would like to thank Leo Albert and Brenda Carito (Pfizer), and Allison Hannan (Children’s Hospital of Philadelphia) for their technical assistance. We are also grateful to Dr. Sriram Krishnaswamy (Children’s Hospital of Philadelphia/University of Pennsylvania) for useful suggestions and critical review of the manuscript.
Footnotes
Authorship Contributions
L.I. designed, coordinated, and performed experiments; R.T., P.M., G.P., H.K., A.S., J.L., and K.M.S. performed experiments; R.R.M. coordinated and performed experiments; V.C. designed and analyzed experiments; D.D.P. interpreted data; J.T., D.E., V.R.A., and R.M.C. designed experiments and analyzed data. L.I. and R.M.C wrote the paper. All authors discussed the results and commented on the manuscript.
Conflict of Interest Statement
R.M.C. receives licensing fees and research funding from Pfizer.
V.C., D.D.P., R.R.M., K.M.S., D.V.E., and J.F.T. are employees of Pfizer, Inc.
REFERENCES
- 1.Toso R, Zhu H, Camire RM. The conformational switch from the factor X zymogen to protease state mediates exosite expression and prothrombinase assembly. J. Biol. Chem. 2008;283:18627–18635. doi: 10.1074/jbc.M802205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunce MW, Toso R, Camire RM. Zymogen-like factor Xa variants restore thrombin generation and effectively bypass the intrinsic pathway in vitro. Blood. 2011;117:290–298. doi: 10.1182/blood-2010-08-300756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedner U. Mechanism of action, development and clinical experience of recombinant FVIIa. J. Biotechnol. 2006;124:747–757. doi: 10.1016/j.jbiotec.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Roberts HR, Ma AD. Overview of inherited hemorrhagic disorders. In: Colman RW, Marder VJ, Clowes AW, Gerorge JN, Goldhaber SZ, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 877–885. [Google Scholar]
- 5.Mann KG, Nesheim ME, Church WR, Haley PE, Krishnaswamy S. Surface dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76:1–16. [PubMed] [Google Scholar]
- 6.Mannucci PM. Back to the future: a recent history of haemophilia treatment. Haemophilia. 2008;14(Suppl 3):10–18. doi: 10.1111/j.1365-2516.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 7.DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br. J. Haematol. 2007;138:305–315. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 8.Darby SC, et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–99. J. Thromb. Haemost. 2004;2:1047–1054. doi: 10.1046/j.1538-7836.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 9.Turecek PL, Varadi K, Gritsch H, Schwarz HP. FEIBA: mode of action. Haemophilia. 2004;10:3–9. doi: 10.1111/j.1365-2516.2004.00934.x. [DOI] [PubMed] [Google Scholar]
- 10.Berntorp E. Differential response to bypassing agents complicates treatment in patients with haemophilia and inhibitors. Haemophilia. 2009;15:3–10. doi: 10.1111/j.1365-2516.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 11.Escobar MA. Health economics in haemophilia: a review from the clinician's perspective. Haemophilia. 2010;16(Suppl 3):29–34. doi: 10.1111/j.1365-2516.2010.02257.x. [DOI] [PubMed] [Google Scholar]
- 12.Monroe DM, Hoffman M, Oliver JA, Roberts HR. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br. J. Haematol. 1997;99:542–547. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 13.Butenas S, Brummel KE, Bouchard BA, Mann KG. How factor VIIa works in hemophilia. J. Thromb. Haemost. 2003;1:1158–1160. doi: 10.1046/j.1538-7836.2003.00181.x. [DOI] [PubMed] [Google Scholar]
- 14.Gitel SN, Medina VM, Wessler S. Inhibition of human activated Factor X by antithrombin III and alpha 1-proteinase inhibitor in human plasma. J. Biol. Chem. 1984;259:6890–6895. [PubMed] [Google Scholar]
- 15.Giles AR, Nesheim ME, Mann KG. Studies of Factors V and VIII:C in an animal model of disseminated intravascular coagulation. J. Clin. Invest. 1984;74:2219–2225. doi: 10.1172/JCI111648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giles AR, Mann KG, Nesheim ME. A combination of factor Xa and phosphatidylcholine-phosphatidylserine vesicles bypasses factor VIII in vivo. Br. J. Haematol. 1988;69:491–497. doi: 10.1111/j.1365-2141.1988.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 17.Khan AM, James MNG. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Prot. Sci. 1998;7:815–836. doi: 10.1002/pro.5560070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bode W, et al. The refined 1.9 Å crystal structure of human α-thrombin: Interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Trp insertion segment. EMBO J. 1989;8:3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tchaikovski SN, van Vlijmen BJ, Rosing J, Tans G. Development of a calibrated automated thrombography based thrombin generation test in mouse plasma. J. Thromb. Haemost. 2007;5:2079–2086. doi: 10.1111/j.1538-7836.2007.02719.x. [DOI] [PubMed] [Google Scholar]
- 20.Schlachterman A, et al. Factor V Leiden improves in vivo hemostasis in murine hemophilia models. J. Thromb. Haemost. 2005;3:2730–2737. doi: 10.1111/j.1538-7836.2005.01639.x. [DOI] [PubMed] [Google Scholar]
- 21.Turecek PL, et al. Factor VIII inhibitor-bypassing agents act by inducing thrombin generation and can be monitored by a thrombin generation assay. Pathophysiol. Haemost. Thromb. 2003;33:16–22. doi: 10.1159/000071637. [DOI] [PubMed] [Google Scholar]
- 22.Aljamali MN, et al. Long-term expression of murine activated factor VII is safe, but elevated levels cause premature mortality. J. Clin. Invest. 2008;118:1825–1834. doi: 10.1172/JCI32878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margaritis P, et al. Catalytic domain modification and viral gene delivery of activated factor VII confers hemostasis at reduced expression levels and vector doses in vivo. Blood. 2011;117:3974–3982. doi: 10.1182/blood-2010-09-309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weeterings C, Lisman T, de Groot PG. Tissue factor-independent effects of recombinant factor VIIa on hemostasis. Semin. Hematol. 2008;45:S12–S15. doi: 10.1053/j.seminhematol.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 2002;22:1381–1389. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 26.Tranholm M, et al. Improved hemostasis with superactive analogs of factor VIIa in a mouse model of hemophilia A. Blood. 2003;102:3615–3620. doi: 10.1182/blood-2003-05-1369. [DOI] [PubMed] [Google Scholar]
- 27.Petersen LC, et al. Characterization of recombinant murine factor VIIa and recombinant murine tissue factor: a human-murine species compatibility study. Thromb. Res. 2005;116:75–85. doi: 10.1016/j.thromres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Tracy PB, Eide LL, Bowie EJW, Mann KG. Radioimmunoassay of factor V in human plasma and platelets. Blood. 1982;60:59–63. [PubMed] [Google Scholar]
- 29.Giles AR, Nesheim ME, Hoogendoorn H, Tracy PB, Mann KG. The coagulant-active phospholipid content is a major determinant of in vivo thrombogenicity of prothrombin complex (Factor IX) concentrates in rabbits. Blood. 1982;59:401–407. [PubMed] [Google Scholar]
- 30.Moss J, Scharling B, Ezban M, Moller ST. Evaluation of the safety and pharmacokinetics of a fast-acting recombinant FVIIa analogue, NN1731, in healthy male subjects. J. Thromb. Haemost. 2009;7:299–305. doi: 10.1111/j.1538-7836.2008.03253.x. [DOI] [PubMed] [Google Scholar]
- 31.Weiler-Guettler H, et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J. Clin. Invest. 1998;101:1983–1991. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neyman M, Gewirtz J, Poncz M. Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood. 2008;112:1101–1108. doi: 10.1182/blood-2008-04-152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin HF, Maeda N, Smithies O, Straight DL, Stafford DW. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood. 1997;90:3962–3966. [PubMed] [Google Scholar]
- 34.Kung SH, et al. Human factor IX corrects the bleeding diathesis of mice with hemophilia B. Blood. 1998;91:784–790. [PubMed] [Google Scholar]
- 35.Margaritis P, et al. Novel therapeutic approach for hemophilia using gene delivery of an engineered secreted activated Factor VII. J. Clin. Invest. 2004;113:1025–1031. doi: 10.1172/JCI20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signaling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 37.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 2002;8:1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.