Abstract
Passively transferred neutralizing antibodies can block lentivirus infection, but their role in postexposure prophylaxis is poorly understood. In this nonhuman-primate study, the effects of short-term antibody therapy on 5-year disease progression, virus load, and host immunity were explored. We reported previously that postinfection passive treatment with polyclonal immune globulin with high neutralizing titers against SIVsmE660 (SIVIG) significantly improved the 67-week health of SIVsmE660-infected Macaca mulatta macaques. Four of six treated macaques maintained low or undetectable levels of virus in plasma, compared with one of ten controls, while two rapid progressors controlled viremia only as long as the SIVIG was present. SIVIG treatment delayed the de novo production of envelope (Env)-specific antibodies by 8 weeks (13). We show here that differences in disease progression were also significant at 5 years postinfection, excluding rapid progressors (P = 0.05). Macaques that maintained ≤103 virus particles per ml of plasma and ≤30 infectious virus particles per 106 mononuclear cells from peripheral blood and lymph nodes had delayed disease onset. All macaques that survived beyond 18 months had measurable Gag-specific CD8+ cytotoxic T cells, regardless of treatment. Humoral immunity in survivors beyond 20 weeks was strikingly different in the SIVIG and control groups. Despite a delay in Env-specific binding antibodies, de novo production of neutralizing antibodies was significantly accelerated in SIVIG-treated macaques. Titers of de novo neutralizing antibodies at week 12 were comparable to levels achieved in controls only by week 32 or later. Acceleration of de novo simian immunodeficiency virus immunity in the presence of passively transferred neutralizing antibodies is a novel finding with implications for postexposure prophylaxis and vaccines.
Human immunodeficiency virus (HIV) infection is usually accompanied by seroconversion and the temporal development of high, persistent levels of envelope (Env)-specific antibodies and neutralizing antibodies (NAbs). It was discovered nearly 2 decades ago that HIV-positive patients can make NAbs that recognize and neutralize sequentially divergent HIV (56). One mechanism for this broad response is the recognition of conserved conformational determinants on Env (42). The role of NAbs in viral control is still unclear, and the mechanism for their development has not been fully elucidated. Longitudinal studies of neutralization of autologous viruses after infection have shown that this response develops over a time frame of several months (28). There is then evidence for viral escape from NAbs, followed by subsequent neutralization of variants that arise in vivo (40). NAbs in many individuals increase in titer and the ability to recognize sequentially diverse Env proteins and neutralize multiple HIV isolates, although the virus continues to escape. The dynamic interplay of NAbs and escape variants is the subject of intense investigation to understand the underlying mechanisms of escape and control. This type of strong, high-titer NAb response that develops in many patients is considered desirable for vaccines to elicit, where preexisting immunity at the time of viral exposure may more effectively block or control infection.
Consistent with the character of the polyclonal NAbs in many individuals, some human monoclonal antibodies (MAbs) derived from HIV-infected patients neutralize multiple primary HIV isolates. An intensive search has yielded a handful of human MAbs with broad reactivity and neutralization capacity, and studies of these MAbs have advanced our understanding of determinants on Env that are targeted by NAbs with broad neutralizing capacity. The most potent of these are directed to linear or conformational determinants in gp41, such as 2F5 (29), linear or conformational determinants in hypervariable region 3 (V3), such as 447-D (11), the CD4 binding site, where IgG1-b12 is the most studied (3), or conserved carbohydrate residues, such as 2G12 (46). Many of these antibodies have shown additive or synergistic neutralization in vitro (22, 44, 45). However, this limited set of five or six MAbs may not represent the full complement present in a polyclonal antibody mixture derived from a patient or animal with broad neutralizing capacity, and the search for useful MAbs continues.
Experiments have been performed in model systems with neutralizing MAbs, high-avidity (“mature”) neutralizing polyclonal immunoglobulin G (IgG) such as HIVIG, and combinations thereof. These proof-of-principle experiments have been useful in analyzing the potential and limitations of these reagents. When given prophylactically, both polyclonal antibodies and MAbs with neutralizing activity can prevent or limit HIV, simian immunodeficiency virus (SIV), or simian-human immunodeficiency virus (SHIV) infection, as summarized in two recent reviews (14, 21). Using these reagents, studies have addressed potency or dose, timing, and specificity. The effectiveness of passive IgG or MAbs in the prophylactic mode has been demonstrated in primate (5, 7, 38, 39, 41) and SCID-hu mouse (8, 9) models. Importantly, combinations of IgG subclass human MAbs were shown to block oral (1) and vaginal (23) SHIV infection in macaques, demonstrating that IgG given parenterally can protect at mucosal surfaces. Three studies performed in collaboration with our group have directly addressed the active component, or specificity, and the mechanism of antibodies in preventing SHIV infection. These studies showed that HIVIG purified from the plasma of HIV-infected chimpanzees and capable of blocking SHIV-DH12 (41) can directly limit the infectivity of HIV type 1 (HIV-1) and SHIV in vivo (17). Nonneutralizing IgG was not effective, showing that NAbs are the active component in polyclonal HIVIG. The chimpanzee-derived HIVIG was specific for HIV-DH12 and protected macaques from SHIV-DH12 challenge only at doses that neutralized virus to 100% in vitro, in the range of 200 mg/kg of body weight (41).
Antibodies or sera obtained from HIV-infected patients have been tested for therapeutic effect in late-stage disease, and infusions were well tolerated. These studies have not conclusively shown beneficial effects (36), although there was some short-term benefit reported (51). Just as with drug intervention, the antiviral effects of IgG in later stages of infection are transient and dependent on the presence of agent, in the absence of specific improvements to the immune response. Subsequently more attention has been focused on early intervention, particularly in cases where timing of exposure is known, as with needle stick or perinatal exposures. Antibodies have been proposed as an alternative or adjunct to antiviral therapy to limit perinatal HIV infections, based on success with other viral infections such as hepatitis B. The inherent toxicities of the current drugs may limit their long-term use in adults and particularly in the perinatal setting. Even with the success of zidovudine in reducing transmission to newborns, it is not clear what toxicities or other difficulties the infant may experience (26). Protection of newborn macaques with passively transferred serum containing SIV-specific NAbs was fully effective in blocking oral infection (49). Vaccinated dam macaques transferred anti-SIV antibodies to their infants, which were subsequently protected from oral and conjunctival challenge. With breast-feeding, there is very significant additional risk of infection in the infant (30), and nontoxic therapies to limit infection via this route would be valuable. These data underscore the potential for either vaccinating (50) or passively treating pregnant females and/or newborns (24) to augment immunity. Recent efforts to test the effectiveness of HIVIG in limiting perinatal transmission were thwarted by a lower-than-predicted transmission rate (43).
Sustained suppression of plasma virus load is a key objective in therapies, as this is the strongest predictor of disease progression in lentivirus-infected humans (15, 25) and macaques (20, 53). Some evidence for viral suppression was obtained in prophylaxis studies in macaques that fell short of the desired end point. Even in cases where the antibodies failed to fully block SHIV infection, some clinical benefits accrued with high-titer NAbs present within hours to days of virus exposure. Passive intravenous transfer of the human neutralizing MAb IgG1-b12 provided dose-dependent protection to macaques vaginally challenged with the R5 virus SHIV-SF162P4. Complete protection against mucosal challenge with an R5 SHIV required essentially complete neutralization of the infecting virus (33), as seen in the SHIV-DH12 intravenous challenge studies (31, 41). Immediate postexposure prophylaxis blocked infection in infants using a cocktail of human MAbs (6) and in juveniles with HIVIG (32).
We have been interested in exploring the role of NAbs during the acute phase of infection, in an effort to model immediate postexposure prophylaxis. Our laboratory has performed a therapeutic study in the macaque model using polyclonal IgG obtained from a healthy SIV-infected nonprogressor with high-titer neutralizing activity (13). The interim analysis of this study at 18 months showed a beneficial effect of immediate postinfection therapy, which was associated with reductions in virus levels in plasma and dependent upon production of de novo antiviral immunity. Since that time, we have expanded the analysis of this study in an effort to determine the mechanism of passive IgG in contributing to the control of infection. Macaques were subsequently monitored for clinical, immunological, and virological parameters for 5 years posttreatment. In the work presented here, our expanded quantitative virological analyses show that virus in these macaques was controlled more effectively, as demonstrated by suppression of virus in blood and lymph nodes and a significantly longer time to disease progression (P = 0.05). We have shown that one SIVIG-treated macaque was cleared of the infection after intravenous challenge, as no virus was ever detected by any method. We report here that expanded immunological analyses led to a surprising finding. Cellular immunity was not different in the treated and control groups, but humoral immunity was strikingly different. Previously, we had observed a delay in the kinetics of binding antibody in SIVIG-treated versus control macaques. In a finding that has not been previously observed in SIV or HIV infection, the development of de novo NAbs in the passive IgG group was significantly accelerated (P = 0.001). We show that both the passive IgG and the de novo NAbs contributed to the rapid control of post-acute-phase viremia, resulting in delayed disease onset.
MATERIALS AND METHODS
IgG purification and administration.
Approximately 16 g of IgG was purified from 1.5 liters of plasma obtained by plasmapheresis from a single long-term nonprogressing Macaca mulatta macaque, E544, infected with the F236 isolate of SIVsm and remaining clinically healthy for more than 6 years (10). In addition, 18 g of IgG was purified from a pool of 1.8 liters of pooled normal plasma from simian retrovirus- and SIV-negative M. mulatta screened for the absence of any reactivity to SIV or simian retrovirus Env proteins. Plasma was pooled, heat inactivated (56°C, 1 h) prior to purification, and clarified by centrifugation to remove particulate matter at 54,000 × g at 4°C for 5 h. Clarified plasma was diluted 1:1 (vol/vol) with sterile 2× phosphate-buffered saline (PBS) and filtered through a Gelman Mini capsule filter (0.45-μm pore size). The resin for purification was a 500-ml-bed-volume XK 50/30 column of recombinant protein G Sepharose Fast-Flow agarose beads (Amersham Biosciences, Piscataway, N.J.). The column was equilibrated with 0.1 M NaPO4-0.15 M NaCl-0.01 M EDTA, pH 7.0, and washed with 5 bed volumes of PBS. Plasma was loaded at 5 ml/min. IgG was exhaustively bound at a flow rate of 5 ml/min, top to bottom, to the column by repeated cycling and monitoring of the unbound material, followed by 2 to 3 bed volumes of PBS (flowthrough). IgG was eluted using reverse flow of the pump (bottom to top) with 0.5 M acetic acid, pH 3.0, at a flow rate of 5 ml/min. Fractions (12 ml) were collected into tubes containing 2 ml of 3 M Tris, pH 9.0. Fractions were mixed within a few minutes of collection by inversion. Peak fractions were identified by optical density and electrophoresis on reducing sodium dodecyl sulfate-polyacrylamide gels; these were pooled and then concentrated by tangential flow filtration to 25 to 35 mg/ml using an Amicon model 800 and a 76-mm YM-30 membrane filter (13742). Material was dialyzed into PBS using Spectra-Por membranes with a 12,000- to 14,000-molecular-weight cut off. Final material was filter-sterilized using 0.2-μm filters and stored at −20°C until use. Levels of pyrogen in the IgG preparations were <5 endotoxin units/ml. Activity based upon binding to SIV Env gp160 and neutralization of SIVsmE660 in vitro was monitored at each step, and yields were typically 7.5 g per liter. Purity was determined by quantifying scans of silver-stained and Coomassie-stained gels and was in the range of 85 to 90%. No toxicities were associated with the intravenous administration of 170 mg/kg. Sixteen M. mulatta macaques were infected intravenously at a dose of 50 50% animal infectious doses with a macaque cell-grown stock of SIVsmE660 (10). Animals were left untreated (n = 4), treated with normal immune globulin (n = 6), or treated with SIV immune globulin (SIVIG, n = 6). Purified IgG was infused intravenously as a bolus at a dose of 170 mg/kg (25 to 30 ml) at 24 h and 2 weeks postinfection. Macaques were sedated for the slow infusion of approximately 20 min. Two additional uninfected animals served as controls: one was treated with SIVIG to measure the in vivo titer and decay of passively administered antibody; the second was untreated. Animals were monitored for clinical signs of disease, including lymph node palpation and measurement, weight, appetite, etc. Blood samples were taken at weekly, bimonthly, or monthly intervals to determine lymphocyte subsets, antibody responses, p27 Gag antigen levels in plasma, and viral load in plasma and in peripheral blood mononuclear cells (PBMC).
Assays for humoral immunity. (i) Recombinant Env and Gag antibody ELISAs.
Enzyme-linked immunosorbent assays (ELISAs) were performed as described previously (12, 16). Ninety-six-well plates were coated with purified recombinant SIV antigens. SIVsmH4 Env gp120 was produced in CHO cells as previously described (35). SIVmne Env gp160 was produced in vaccinia virus-infected BSC-40 cells; recombinant pseudovirion particles without Env were produced in vaccinia virus-infected BSC-40 cells; both were prepared as described previously (16, 37). Polystyrene 96-well flat-well plates (Maxisorp, Immulon; VWR, Brisbane, Calif.) were coated with antigen in carbonate-bicarbonate buffer (pH 9.5) at 2 μg/ml (100 μl/well) and incubated 4°C for 12 to 18 h. Wells were blocked with 1% normal goat serum in BLOTTO (5% skim dry milk in PBS) at 275 μl/well at 25°C for 1 h. Test plasma was diluted to a starting concentration of 1:50 or 1:100 in specimen diluent (0.1% [vol/vol] Triton X-100 in PBS, which also served as the wash solution), loaded at 100 μl/well, and incubated 30 min at 4°C. Plates were washed, detection was carried out with goat anti-monkey horseradish peroxidase conjugate (1:5,000; Cappel, ICN), and plates were developed with 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich, St. Louis, Mo.). Titers reported are interpolated midpoint titers normalized to a standard IgG (SIVIG, 50 μg/ml) run on each plate.
(ii) Antibody avidity assay.
Purified SIVmneE11S virions were harvested from CEMx174 cells and purified by centrifugation for use as native Env antigen (58). Sera were tested essentially as described by Cole et al. (4). Optical density (OD) values of sera bound in 1× PBS versus 8 M urea (three 5-min washes) were expressed as (average urea OD/average PBS OD) × 100. High avidity is defined as >50%, medium avidity is defined as 30 to 50%, and low avidity is defined as <30%.
(iii) Virus neutralization.
Virus neutralization was assessed using SIVsmE660 stock and CEMx174 cells. Titers shown are the plasma dilution that could inhibit cell killing by 50% in a dye-viability readout assay (27).
Assays for cellular immunity.
Cytotoxic-T-cell (CTL) responses were measured by using autologous B lymphoblastoid cell lines infected with recombinant vaccinia virus expressing SIV Gag as targets. Measurements were taken by using effector-to-target ratios of 10:1. Unfractionated and CD8+-cell-enriched or -depleted peripheral blood lymphocytes were tested for antigen-specific killing by using appropriate controls in standard chromium-release assays.
Virus load determinations.
Viral copy numbers in plasma were determined by quantitative-competitive PCR (QC-PCR), as described previously (53). For calibration of the assay to absolute numbers of viral particles, SIV tissue culture stocks are counted by transmission electron microscopy, and these results are compared with those obtained by QC-PCR. Briefly, plasma is cleared of platelets, and then virus is pelleted from the plasma, protein is extracted, and the viral RNA is reverse transcribed along with added internal RNA controls. Test and deletion plasmid templates at different ratios are amplified by the PCR. Amplified products are separated on 30-well 3% agarose high-throughput gels (ISS Corp.). The internal control PCR product contained an 83-bp deletion which enables discrimination between viral (336-bp) and internal control (253-bp) amplified products. Viral RNA levels are calculated from the dilution of sample that gives a visual signal equivalent to that of the internal control, and the limit of detection of this assay was 833 copies/ml. Viral RNA levels in a subset of plasma samples were quantified with a more sensitive version of this assay that has a limit of 300 copies per ml. Samples that were undetectable were assigned a value of 300, based on evaluation of selected subsets of samples.
Number of infected cells per million PBMC.
Virus load (per million PBMC) was determined by using standard cocultivation with normal human PBMC and a limiting dilution end point assay with four replicas per dilution. Virus production was measured by the Coulter SIV core antigen ELISA (Coulter Corporation, Hialeah, Fla.). The limit of detection is 1 infected cell per million PBMC.
Statistical analyses.
Kaplan Meier survival analysis and the log rank test were used to test for differences in the time to disease onset. To measure the average change from baseline in virus load levels during the period of interest, the area under the curve minus baseline was calculated for virus load after replacing values at the lower limit of detection of the assay by using the midpoint between zero and the lower limit (19). For differences in binary outcomes we used chi-square and Fisher's exact tests to determine statistical significance; for continuous outcomes we used the independent samples t test.
Veterinary care and clinical monitoring.
Macaques (M. mulatta, Indian origin) were cared for by the veterinary staff of The Children's Hospital Research Foundation at The Ohio State University. This facility meets NIH standards as stated in reference 29, ILAR recommendations, and AAALAC accreditation standards for animals of this species. For lentivirus-infected animals, housing is provided in BSL-3 containment facilities. Pair housing was employed and based upon macaque compatibility. Pair members were part of the same treatment group. At each blood drawing, macaques were weighed and lymph nodes were measured for lymphadenopathy. Macaques were observed for general health and alertness as well as clinical signs, including fever, dehydration, ataxia, etc. Blood samples were analyzed for complete blood count and lymphocyte subsets at each time point.
RESULTS
Study design and IgG characterization and administration.
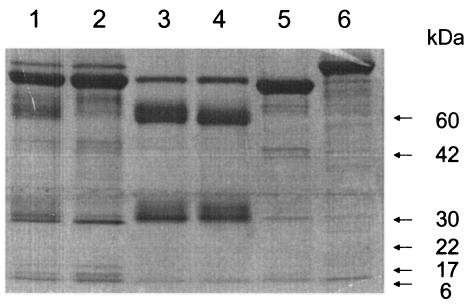
This study was designed to test the effectiveness of high-titer NAb provided for the first 4 weeks of infection, the duration of primary viremia in this model. Total IgG was purified from the plasma of a single M. mulatta (E544) animal infected with SIV and surviving more than 6 years without signs of AIDS. Macaque E544 was infected with isolate F236, which was also used to infect macaque 543. Blood from macaque 543 was used to infect macaque 660, from which the virus isolate SIVsmE660 was derived. Thus, the virus in E544 is a close relative of isolate E660. Sera from E544 possessed high-titer binding and neutralizing activity against SIVsmE660 and other closely related SIVsm viruses in vitro. SIVIG was purified from 1.8 liters of serially collected plasma samples from this animal, and normal IgG was prepared from the plasma of an uninfected M. mulatta animal. The purity of the IgG preparations was approximately 90% (Fig. 1). The neutralizing activity of this preparation was assessed against a panel of SIV isolates, and the purified IgG was very effective against SIVsmE660 and related isolates, neutralizing SIVsmE660 at 7 μg/ml. Neutralization of sequentially more distant isolates such as SIVmac239 was below detection, indicating the relatively narrow specificity of this SIVIG preparation. SIVIG was infused into one uninfected M. mulatta to verify the lack of infectivity of this preparation and to determine the titers of antibody achieved and persistence of anti-SIV antibody in vivo. As measured by both binding and virus neutralization, the SIVIG was undetectable by week 10. Sixteen M. mulatta juveniles of both sexes were then assigned to receive SIVIG, normal IgG, or no IgG, normalizing for gender and size. These macaques were infected intravenously with 50 50% animal infectious doses of SIVsmE660 on day 0 and then received infusions of the IgG preparations at 170 mg/kg, 1 day (24 h) and 14 days after infection. Six infected macaques received SIVIG, six received normal IgG, and four received no infusion after infection. The last group was included to control for potential immune activation due to the very large bolus of macaque IgG given to the macaques.
FIG. 1.
Biochemical characterization and purification of macaque IgG preparations An analysis of unpurified and purified preparations of M. mulatta total IgG by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4 to 12% gradient Tris-glycine gel, reducing conditions) is shown. Lanes: 1, plasma pool; 2, flowthrough from protein G column; 3, normal IgG peak fraction pool; 4, SIVIG IgG preparation; 5, human serum albumin; 6, human transferrin. Mobilities of standard molecular mass markers are noted on the right.
SIVIG treatment significantly delayed disease.
Clinical and laboratory findings at 250 weeks postinfection are summarized in Table 1. Macaques experienced no detectable effects from the bolus IgG infusion, based upon veterinary observation, blood chemistries, and lymphocyte subset analysis (data not shown). Onset of AIDS was defined based upon a combination of clinical and laboratory signs, summarized here. Typical signs were CD4+-cell decline to below 250 cells/μl, wasting and diarrhea, nervous effects such as ataxia, secondary infections that responded poorly to intervention, and pulmonary thromboembolism and thrombosis. Time to disease ranged from 16 to 20 weeks for rapid progressors and 28 to 65 weeks for progressors, with the average time to disease being 62 weeks (∼15 months) in the 10 controls. Based upon time to AIDS, macaques were categorized as rapid progressors (0 to 6 months to onset), progressors (6 months to 2.5 years), or slow progressors (more than 2.5 years, or more than twice the average time to disease in controls). Of the 10 macaques in the normal IgG and untreated control groups, 9 were euthanized with AIDS-related conditions and high levels of virus, and one remained disease free for the 5 years of the study. As is typically seen in this model of SIVsmE660 in rhesus macaques, 2 of 10 animals were rapid progressors to disease (213 in the untreated group and 192 in the normal Ig group). One of the 10 controls (no. 200) was a slow progressor with a low but detectable primary viremia (see Fig. 3). As reported previously in macaque SIV studies, rapid progressor 192 did not seroconvert and 213 failed to mount a significant de novo antiviral antibody response; both macaques succumbed to AIDS within the first 6 months of infection. There was no positive or negative effect of the normal Ig upon time to disease or euthanasia (Table 1). Thus, for the statistical analyses, these controls were considered one group of 10 control macaques.
TABLE 1.
Summary of clinical and laboratory status at 5 years postinfectiona
| Group | Macaque | Clinical and laboratory signs | Classification | Week of:
|
|
|---|---|---|---|---|---|
| Disease onset | Euthanasia | ||||
| Untreated | 213 | Wasting and diarrhea; involution of lymphoid tissue | Rapid progressor | 20 | 30 |
| 195 | Pulmonary thromboembolism, CD4+-cell decline | Progressor | 65 | 65 | |
| 182 | Pulmonary thrombosis; lymphoma; leukemia, CD4+-cell decline | Progressor | 64 | 78 | |
| 363 | Diarrhea, CD4+-cell decline | Progressor | 52 | 102 | |
| Normal Ig | 192 | Wasting and diarrhea; CD4+-cell decline | Rapid progressor | 16 | 18 |
| 184 | Wasting and diarrhea; ataxia; CD4+-cell decline | Progressor | 28 | 42 | |
| 104 | Lymphadenopathy; pneumonia | Progressor | 41 | 43 | |
| 88 | Wasting and diarrhea; CD4+-cell decline | Progressor | 49 | 53 | |
| 186 | Rash; secondary infections; CD4+-cell decline | Progressor | 52 | 156 | |
| 200 | Healthy | Slow progressor | >260 | >260 | |
| SIVIG | 204 | Wasting and diarrhea | Rapid progressor | 12 | 18 |
| 196 | Wasting | Rapid progressor | 16 | 21 | |
| 199 | CD4+-cell decline and weight loss | Progressor | 69 | 93 | |
| 185 | CD4+-cell decline | Slow progressor | 156 | 160 | |
| 176 | Healthy | Slow progressor | >260 | >260 | |
| 191 | Healthy, no evidence of infection | Slow progressor | >260 | >260 | |
CD4+-cell decline is defined as absolute CD4+-cell counts between 0 and 250 cells/mm3 for at least two consecutive time points and declining. Wasting is defined as ≥10% loss of body mass in 2 to 4 weeks. Secondary infections and pneumonia were treated with antibiotics. Macaques surviving longer than 260 weeks were censored at 260.
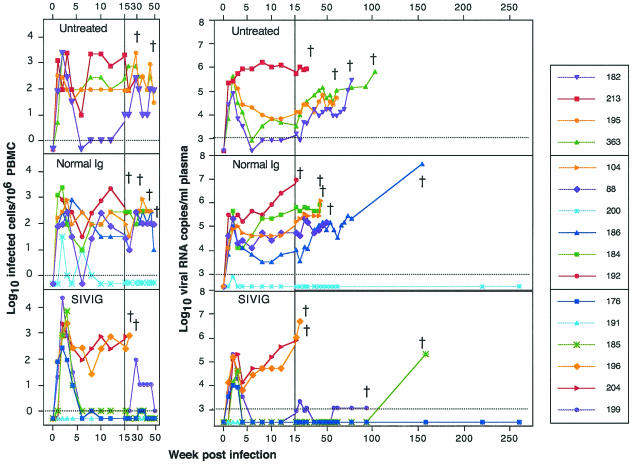
FIG. 3.
Longitudinal analysis of viral copy number in plasma and PBMC. The dagger indicates death of the macaque due to AIDS. (Left panels) Number of infected cells per million PBMC. Virus load in PBMC was determined by using standard cocultivation with normal human PBMC and limiting dilution endpoint assay with four replicas per dilution. The detection limit is 1 infected cell per million PBMC (100). Values less than 1 copy are graphed as 0.5 copy. Assays were completed through week 49 and are graphed with the first 15 weeks expanded, which is the same time scale as other virological and immunological assays. (Right panels) SIV RNA copies in plasma by QC-PCR. RNA equivalents per ml of plasma were determined by QC-PCR. The lower limit of detection is 300 copies per ml. Values below detection are graphed as 300 copies/ml. Data for the first 15 weeks are shown on an expanded scale to illuminate loads during primary viremia.
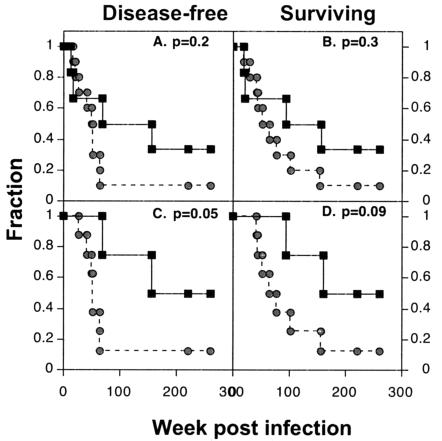
In the SIVIG treatment group, two macaques (no. 204 and 196) were euthanized at 18 to 20 weeks due to AIDS (33% rapid progressors with no de novo immunity). The four remaining SIVIG-treated macaques remained clinically healthy for 18 months or more. Macaque 191 was negative for virus by all assays, including nested-set long-terminal-repeat and Gag PCR of samples from lymph nodes and PBMC and thus appears to have prevented the intravenous challenge virus from infecting productively. Macaque 199 showed CD4+-cell decline at week 69 and was euthanized at week 93. Macaque 185 was healthy until week 156 (3 years postinfection). Macaques 176 and 191 remained healthy for the duration of observation (5 years). Four of six treated macaques versus one of ten controls showed no clinical signs through 18 months (P = 0.034, Fisher's exact test). Thus, the clinical health observed in the macaques in the SIVIG group can be attributed to the passive IgG treatment. When the log rank test is used on data through 5 years postinfection, there is a statistically significant difference in the rate of disease progression between the treated and control groups. The four Kaplan-Meier plots shown in Fig. 2 represent disease-free and surviving fractions for all macaques including rapid progressors (A and B) and for macaques that survived beyond 20 weeks (C and D; rapid progressors omitted). Based on these data, disease progression was significantly delayed by short-term SIVIG treatment if macaques survived beyond the first 20 weeks (P = 0.05, log-rank test). To better understand the effects of the passively transferred SIVIG, we examined (i) virus load in blood and tissues and (ii) de novo cellular and humoral immune responses as potential correlates of clinical outcome.
FIG. 2.
Kaplan-Meier plots of health and survival. Circles, controls; squares, SIVIG-treated animals. Data for the fraction that were disease free (all animals [A] and without rapid progressors [C]) and the fraction surviving (all animals [B] and without rapid progressors [D]) are shown. P values from the log rank test are noted in the upper right of each plot.
Virus levels in PBMC and plasma predict disease outcome.
Improved clinical outcome in treated and control macaques correlated with maintenance of low or undetectable virus load, a hallmark of slow progressors to disease in pathogenic animal models as well as in clinical HIV-1 infection. Several assays were used to determine the infection status of the macaques in this study. Virus production was monitored by p27 antigenemia by ELISA through week 81 (data not shown). Cells capable of virus replication were quantified using coculture of PBMC with normal macaque or human cells in a limiting-dilution assay (Fig. 3). For comparison, longitudinal analysis of virus load in plasma is also shown in Fig. 3. Virus load in plasma was predictive of clinical outcome in the SIVIG-treated macaques as well as untreated controls (integrated copies of viral DNA in PBMC were quantified using a QC-PCR assay). These data showed excellent agreement with the coculture data, with a similar stratification of levels of virus copy number following the acute phase (after 6 weeks) for corresponding animals; copy numbers ranged between 1 and 103 per μg of PBMC DNA (data not shown).
As expected, Gag antigen was detected in all of the control animals within the first 2 to 6 weeks of infection (data not shown), and all macaques were positive for RNA and cultivable virus during acute viremia. The rapid progressor macaques (no. 192 and 213) had very high levels of post-acute-phase virus in plasma and PBMC. Macaques euthanized with AIDS between 20 and 102 weeks had high and increasing levels of virus in the peripheral blood. The nonprogressor control macaque (no. 200) had a very low virus load during primary viremia, which was then undetectable in all assays but persistently positive by nested set PCR. The remaining seven macaques in the control groups had variable levels of acute and post-acute-phase viremia, which was predictive of disease onset and time of euthanasia. Nine of the ten controls had viremia above 50,000 particles/ml 1 year after infection, and all of these ultimately died of AIDS.
Gag antigen was not detected in any of the SIVIG-treated macaques during the first 12 weeks (data not shown), despite high levels of RNA in three of six macaques (no. 176, 185, and 199). Two rapid progressor macaques (no. 196 and 204) that died of AIDS in less than 30 weeks each had reductions of more than 1 log10 in peak viremia, followed by very high levels of virus rebound upon partial decay of the SIVIG, as determined by all viral assays. One SIVIG-treated macaque (no. 191) had virus that was never detectable in the periphery or by nested-set PCR of lymph node samples at 48 weeks postinfection, suggesting that the virus infection was not established in this macaque following intravenous exposure and SIVIG treatment. In three of six SIVIG-treated macaques (no. 176, 185, and 199), plasma and PBMC virus levels were moderate to high in the acute phase but dropped to levels at or below 103 copies/ml (the level of detection) from week 6 postinfection for at least 20 months following treatment. None of the macaques in the control group was able to control virus to this degree. All four of these macaques were disease free for 20 months, and three of these remained so for more than 3 years, two for more than 5 years. At week 69, macaque 199 showed CD4+-cell decline and was euthanized at week 93. The virus load remained at 103 copies/ml through week 81, and we never observed an increase.
Cultivable virus in lymph nodes was suppressed by treatment.
To address whether other tissue compartments might be sequestering virus reservoirs, we performed quantitative coculture with cells from peripheral blood and lymph nodes obtained from macaques that survived beyond the first 30 weeks. Frequencies of infected cells in the peripheral blood were reflected in the values found in lymph nodes at two time points during the first year (Table 2). There is close agreement among samples taken at similar time points, suggesting that the measure of virus in the periphery is a relatively good measure of the virus burden in the tissues sampled. These data, though limited, support the concept that the determination of viral burden in the periphery by coculture of PBMC or by PCR of plasma RNA is an accurate reflection of the infectious virus load in lymphoid tissue. However, two SIVIG-treated and one control macaque survived for long periods with low virus levels, only to eventually become viremic and die. Each of these animals had moderate to high levels of virus in PBMC or lymph node mononuclear cells (LNMC) during the first year, despite very low levels of virus in plasma. All macaques that survived beyond 3 years had 30 or fewer positive cells per million PBMC or LNMC.
TABLE 2.
Frequency of infected cells in lymph nodes and peripheral blood in survivors beyond 30 weeks
| Treatment | Macaque | Wk of disease onset | Frequency of infected cells ata:
|
|||
|---|---|---|---|---|---|---|
| Wk 31-32
|
Wk 48-49
|
|||||
| LNMC | PBMC | LNMC | PBMC | |||
| None | 195 | 65 | 300 | 100 | 90 | 30 |
| 182 | 64 | NA | 100 | 90 | 90 | |
| 363 | 52 | 900 | 300 | 90 | 90 | |
| Normal Ig | 184 | 28 | NA | 300 | — | — |
| 104 | 41 | NA | 300 | — | — | |
| 88 | 49 | NA | NA | 90 | 90 | |
| 186 | 52 | 100 | 100 | 90 | 10 | |
| 200 | >260 | NA | <1 | <1 | <1 | |
| SIVIG | 199 | 69 | 100 | 10 | 30 | 10 |
| 185 | 156 | NA | 1 | 30 | <1 | |
| 176 | >260 | 10 | 1 | NA | <1 | |
| 191 | >260 | NA | <1 | <1 | <1 | |
Number of cells capable of productive viral culture per million LNMC or PBMC; the lower limit of the assay is 1. NA, not available; —, macaque died.
Virological correlates of disease outcome.
We thus asked if other parameters were correlated with outcome: (i) peak virus during acute viremia, (ii) total acute virus burden, and (iii) total virus burden through euthanasia. We reported previously (53) that 16-week post-acute-phase plasma virus loads were predictive of outcome in this model. It was thus of interest to compare these data with correlative measures reported by others. In this study, as was reported by Lifson et al. (20), acute-phase cumulative plasma virus loads as measured by QC-PCR during the first 4 weeks were diminished 2.6-fold in SIVIG-treated animals compared with controls. Levels of virus in plasma ranged between 103 (the lower limit of the assay) and 107 particles/ml (Fig. 3). These levels are similar to those that have been described for HIV-1 in infected humans (15, 25, 34, 55). The QC-PCR data were analyzed using the independent samples t tests. Improvement in clinical outcome was correlated with two response variables: plasma virus load at euthanasia, which trended toward significance (P = 0.062), and total virus load calculated as area under the curve minus baseline censored at 260 weeks postinfection: for the SIVIG group, the value was 1 (95% confidence interval, −0.23 to 2.23), while for controls, it was 2.33 (1.59 to 3.08); P was 0.03.
Gag-specific CTLs were detected in macaques surviving beyond 1 year.
Effective cellular immunity has been shown to play a key role in reduction of primary virus by killing of virus-infected cells. In this experiment, we tested PBMC for CTL function at 5 months postinfection. These experiments are summarized in Tables 3 and 4. At this time point, 4 of the 12 remaining infected macaques had CD8+ effector cells specific for SIV Gag: no. 182 in the untreated control group, no. 186 and 200 in the normal Ig group, and no. 176 in the SIVIG group. PBMC from all other animals were negative at the time of the assay. CTL assays performed with CD8+-cell-enriched and -depleted cytotoxic lymphocytes showed that the lysis activity was associated with the CD8+-cell population (Table 4). Of the two macaques with measurable Gag-specific CTL in the control groups, no. 182 was euthanized at week 78 and no. 186 was euthanized at week 156.
TABLE 3.
Gag-specific CTLs at 16 to 20 weeks postinfection
| Animal | Group | Wk assayed | Effector cell stimulation | % 51Cr released by target cells infected with vaccinia virusa
|
|
|---|---|---|---|---|---|
| Wild type | Expressing SIV Gag | ||||
| 182 | Untreated | 19 | ConA | 0 | 32 |
| SIV Gag | 6 | 45 | |||
| SIV Gag 2 | 15 | 63 | |||
| 16 | ConA | 23 | 77 | ||
| SIV Gag | 14 | 73 | |||
| 200 | Normal Ig | 18 | ConA | 20 | 35 |
| SIV Gag | 25 | 46 | |||
| SIV Gag 2 | 18 | 39 | |||
| 20 | ConA | 14 | 24 | ||
| SIV Gag | 37 | 38 | |||
| SIV Gag 2 | 13 | 50 | |||
| 186 | Normal Ig | 18 | ConA | 4 | 12 |
| SIV Gag | 5 | 7 | |||
| SIV Gag 2 | 3 | 24 | |||
| 20 | ConA | 0 | 5 | ||
| SIV Gag | 6 | 12 | |||
| SIV Gag 2 | 12 | 37 | |||
| 176 | SIVIG | 19 | ConA | 2 | 24 |
| SIV Gag | 12 | 20 | |||
| SIV Gag 2 | 20 | 40 | |||
| 191 | SIVIG | 16 | ConA | 22 | 18 |
| SIV Gag | 16 | 36 | |||
| 19 | ConA | 26 | 21 | ||
| SIV Gag | 17 | 16 | |||
| SIV Gag 2 | 11 | 18 | |||
Effector cells were stimulated with concanavalin A (ConA) or once (SIV Gag) or twice (SIV Gag 2) with fixed peripheral blood lymphocytes infected with vaccinia virus expressing SIV Gag prior to the 51Cr release assay at an effector-to-target ratio of 10:1. Numbers in bold are significant.
TABLE 4.
Phenotype analysis of cytolytic cells
| Macaque | Effector cells | Flow cytometry
|
% 51Cr released by target cells infected with vaccinia virus
|
||
|---|---|---|---|---|---|
| % CD4+ | % CD8+ | Wild type | Expressing SIV Gaga | ||
| 182 | Whole | 19 | 54 | 14 | 64 |
| CD8+-cell depleted | 41 | 1 | 0 | 14 | |
| CD8+-cell enriched | 2 | 76 | 8 | 53 | |
| 186 | Whole | 1 | 51 | 7 | 34 |
| CD8+-cell depleted | 1 | 32 | 2 | 5 | |
| CD8+-cell enriched | 2 | 47 | 2 | 19 | |
| 200 | Whole | 1 | 50 | 12 | 47 |
| CD8+-cell depleted | 1 | 40 | 2 | 7 | |
| CD8+-cell enriched | 1 | 71 | 5 | 37 | |
All values in the column are significant.
Infused IgG delayed binding antibody and accelerated NAb production.
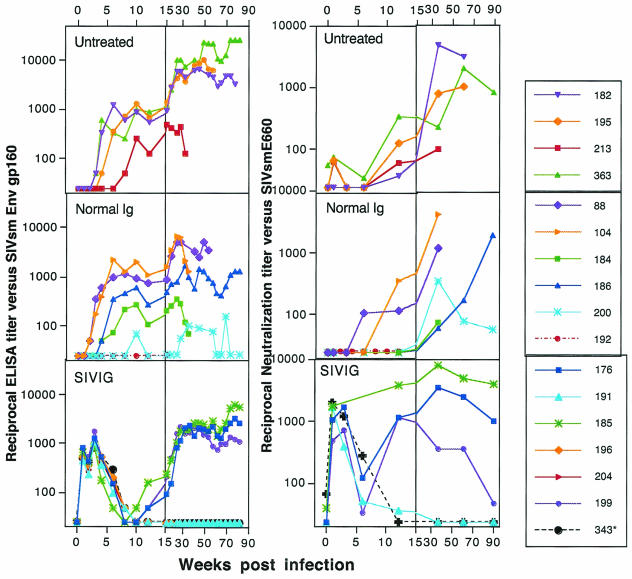
SIV Env- and Gag-specific antibodies and NAbs against the infecting virus, SIVsmE660, were monitored following infection and infusion. Binding antibody responses in the untreated and normal Ig groups followed the typical pattern of induction at 4 to 6 weeks postinfection (Fig. 4). The onset of de novo antibody production was at week 3 to 4 for the nine that produced antibodies; one macaque (no. 192, a rapid progressor) in the normal IgG group was antibody negative. Macaque 213, a rapid progressor euthanized at week 30, had a very weak de novo response. Generation of NAbs (Fig. 4) lagged behind the binding response, with detectable titers in a few animals by week 6 and several by week 12, and titers began to reach a plateau in some animals by week 30 to 50. Levels of de novo antibody produced in the infected animals are typical of the spectrum of immune responses usually seen in this infection (N. L. Haigwood and V. M. Hirsch, unpublished results).
FIG. 4.
Humoral immune responses to week 90. Data for the first 15 weeks are shown on an expanded scale to illustrate titers during primary viremia. (Left panels) SIV Env gp160-specific antibody. Env reciprocal midpoint titers were determined by using recombinant gp160 (SIVmne). The lower limit of detection is a titer of 50. Standard ELISA conditions were employed (12, 18), and all plates were normalized to a purified standard. Macaque 343 (asterisk) received an infusion of SIVIG without SIV infection. (Right panels) Neutralization of SIVsmE660. Virus neutralization was assessed with SIVsmE660 stock and CEMx174 cells. Titers are the plasma dilution that could inhibit cell killing by 50% in a dye viability readout assay (27).
The pattern and timing of both binding and NAbs were markedly different in the SIVIG group (Fig. 4). Blood samples were taken 1 week following each infusion; immediate postinfusion titers were not measured but can be inferred to be significantly higher than the titers shown here. The level of reconstituted passive anti-SIV Env-specific antibodies present during the peak of viremia (week 2) was equivalent to or greater than that seen in SIV-infected macaques at 8 to 10 weeks postinfection (Fig. 4). In the uninfected macaque (no. 343) receiving the SIVIG infusion, the titers of binding and NAbs were high after each infusion and decayed to baseline by weeks 8 to 10. Levels of NAb during acute viremia that were reconstituted by passive transfer were all greater than 1,000. All SIV-infected animals in the SIVIG group had the same profile of binding antibody as uninfected, SIVIG-treated macaque 343 for the first 6 weeks of infection. Thus, SIV infection itself had no measurable effect upon the activity or half-life of the infused antibodies. Decay of these antibody titers to baseline levels by weeks 8 to 10 following the second infusion can be seen in Fig. 4 (bottom panels). In the SIVIG group, de novo binding antibodies were detected in three animals starting at week 12, a delay of about 8 weeks relative to the controls; three macaques (no. 191, 196, and 204) were antibody negative. Surprisingly, NAbs were observed in three responders (176, 185, and 199) as early as week 12 at titers that were an order of magnitude higher than those in the control macaques at that same time postinfection. Passive transfer of NAb resulted in the more rapid and stable induction of high-titer NAb compared with that in control macaques. High-level, persistent NAbs were established in macaques 176 and 185; high but declining NAb levels were measured in macaque 199, which maintained a low virus load but declined clinically at week 69 and was euthanized at week 93. The induction of NAbs in the SIVIG group was accelerated by at least 12 weeks relative to controls, despite a lag in measurable Env-specific antibodies.
Inverse ratio of Env binding and NAb titers in SIVIG-treated macaques.
To more clearly understand the differences in levels of binding antibodies and NAbs, we analyzed the sera from macaques that developed NAbs during the study. These included no. 199, 185, and 176 in the SIVIG group, no. 182, 195, and 363 in the untreated group, and no. 88, 104, 184, 186, and 200 in the normal IgG group. Avidity was measured for all samples, using whole virus as a source of native Env antigen. When titers of antibody against whole-virus Env captured with concanavalin A were measured in sera from SIVIG-treated macaques, we again observed a drop in antibody titers with the decay of SIVIG, but not to baseline. Thus, whole-virus ELISA was somewhat more sensitive than the recombinant-gp160 ELISA. All three SIVIG-treated animals had medium to high avidity (40 to 50%) during weeks 0 to 8 that was a property of the SIVIG. With the onset of de novo responses, antibodies had very low avidity and developed medium to high avidity by week 50 postinfection. Percent avidity was not proportional to levels of NAbs. Among the control macaques, the three longest-surviving macaques, no. 88, 186, and 200, developed medium to high avidity within the same time frame (data not shown).
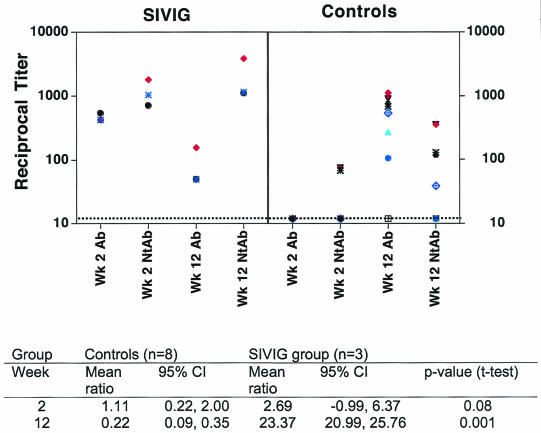
Env-gp160 binding and neutralizing titers at weeks 2 and 12 for each macaque are plotted in a scatter diagram in Fig. 5. Typically, at week 12, binding Ab levels are higher than NAb levels, as seen for the controls. In contrast, in the SIVIG-treated macaques, NAb titers are higher than binding-Ab titers in all cases. To analyze this statistically, we calculated the ratio of NAb to binding Ab in controls and SIVIG animals at weeks 2 and 12 and compared the means of these ratios. The mean ratio at week 2 in the controls was 1.11, close to 1, because levels of both types of antibodies were very low. In the SIVIG group at week 2, the ratio should be entirely due to the passively transferred SIVIG, and the mean ratio was 2.69. This reflects the mature nature of the SIVIG, which was obtained from a slow progressor 4 years after infection and has a high ratio of NAb to Env binding antibody. By week 12, in the controls, the mean ratio was 0.22, with negligible NAb and mainly binding activity. In contrast, in the SIVIG-treated animals at week 12, the ratio was 20.99, indicating to us a predominance of NAb. At week 12 these mean ratios were highly statistically significantly different between the controls and SIVIG animals (P = 0.001).
FIG. 5.
Scatter plot of Env-specific binding and SIVsmE660 NAbs at weeks 2 and 12 in macaques that seroconverted. Data are plotted from graphs shown in Fig. 4. SIVIG-treated animals were no. 176, 185, and 199; control macaques were no. 88, 104, 182, 184, 186, 195, 200, and 363. Note that some symbols overlap. Mean ratios are noted for each group at weeks 2 and 12, with 95% confidence intervals.
DISCUSSION
This study is the first demonstration that intensive, short-term postinfection therapy with neutralizing IgG can have long-term beneficial effects upon disease in a pathogenic primate lentivirus model. We found statistically significant delays in disease that were correlated with cumulative virus loads. Importantly, the treatment provided no benefit to two rapid progressors once the passive SIVIG material decayed. In M. mulatta animals infected with SIVsmE660, rapid progressors are typically 20% of the total, as seen in the 2 of 10 in the control group. The failure to mount virus-specific responses in rapid progressors is unexplained, and we have not developed a method for identifying these animals so that they can be eliminated from studies or distributed evenly between groups. In this study, the presence of two in the SIVIG group was useful in demonstrating the transient effects of passively transferred NAbs in this type of infection, as has been documented by others (2). The remaining four SIVIG-treated animals had virological and immunological profiles that were distinct from those of the controls and highly unusual. One macaque (no. 191) appears to have suppressed the virus to undetectable levels, suggesting that the SIVIG suppressed the virus to levels that may never cause pathogenesis, as seen in recent postexposure prophylaxis studies (6, 32). There was a CTL response to Gag at 16 weeks postinfection in this macaque, but no antibody response. This pattern has been observed in HIV infection, where extraordinarily low levels of virus were detected in seronegative patients with evidence of CTL activity (57). For macaques that experienced high levels of acute viremia, virological control after the first 6 to 8 weeks was dependent upon the establishment of effective de novo immunity, as the passively transferred SIVIG had decayed by week 8. These three macaques (no. 176, 185, and 199) suppressed viremia for various lengths of time, but their suppression was to a greater degree and for longer periods than that of any of the nine control group animals that experienced acute viremia at levels of >103 particles/ml. Responding macaques maintained low or undetectable plasma viral loads for 3, 4, or more than 5 years postinfection with IgG treatment given only during primary viremia. Further evidence of this high degree of suppression could be found in the low levels of cultivable virus in PBMC and LNMC.
The delay in disease in four of six SIVIG-treated macaques resulted in long-term survival for two of these (no. 176 and 191). In the three slow progressors that seroconverted, post-acute-phase viremia was suppressed by 2 to 3 log10 from the peak at 2 weeks of infection. Viral suppression by antiviral drugs during the acute phase of HIV-2 or SIV infection has also been shown to shift the balance toward viral control, in some cases preventing infection. High-dose, short-term antiviral therapy was tested in macaques infected with the highly pathogenic, macaque-adapted HIV-2-287 virus. Animals were treated at the time of viral exposure and for 16 weeks, followed by cessation of treatment. Five of six d4T-treated macaques and none of six controls controlled the virus and maintained normal CD4+ cell counts for 1 year (52). Similar benefits accrued with PMPA [(R)-9-(2-phosphonylmethoxypropyl)adenine] treatment of SIV-exposed macaques, where timing of administration affected the efficacy (47, 48). In these cases, reduction in viral burden would allow the more effective development of antiviral immunity, which in turn controls the infection, as seen with HIV-2-287 and d4T treatment, where CD8+-cell-specific factors were correlated with control (52).
The absence of detectable immune responses in rapid progressors to disease and their presence in slower progressors indicates an obligatory role for de novo response in controlling virus burden and disease progression. This study provides evidence that the types of immunity needed for control may differ depending upon the level of virus in the acute phase. Cellular immunity and low-level or no NAbs appear to be sufficient to control virus in the slow progressors, no. 200 in the control group and no. 191 in the SIVIG group. Macaques with Gag-specific CTL early in infection survived for 18 months (four of four) to >2 years (three of four) to >3 years (two of four). These observations support the hypothesis that CTL may be sufficient for virus control if the set point is low, as seen in exposed uninfected humans (57). However, we did not perform exhaustive analyses of cellular immunity in this study. Cellular immune responses were assessed early in infection by a chromium-51 release assay using in vitro restimulation of effector cells, an assay that was standard at the time of the initiation of this study. This assay is not quantitative and is therefore less useful than the more quantitative peptide enzyme-linked immunospot and intracellular cytokine assays that are available today. It is possible that evidence for a more pronounced role for T-cell immunity in virus control would be discerned by these assays. Determinants of HIV/SIV/SHIV-specific CTL in the setting of chronic infection include systemic viral antigen load, preservation of CD4+-T-lymphocyte counts, and genetic make-up (including major histocompatibility complex haplotype) as it pertains to the ability of the T cells of infected individuals to recognize particular viral proteins. This complexity of determinants has made it difficult for investigators to predict disease outcome on the basis of CTL response in the setting of chronic infection. Nonetheless, these data clearly demonstrate an association of SIV-specific cytolytic activity with better prognosis, and we have no indication that cellular responses were deleteriously affected by the treatment. The role that cellular responses play in limiting the spread of infection during passive IgG treatment should be more thoroughly analyzed in future studies.
The most remarkable differences in the SIVIG-treated seroconverters (macaques 176, 185, and 199) and the corresponding controls were the accelerated NAb response and the inverse relationship of de novo binding and NAbs at week 12 postinfection. This time was chosen for analysis because at this point controls have seroconverted but have not developed significant NAb titers (Fig. 4). It appears that the slope of NAb induction is similar in the SIVIG-treated and control macaques but that the timing of onset is significantly accelerated in the SIVIG animals. Titers of NAbs of >1,000 were measured by weeks 32 to 90 in a subset of control macaques concurrent with increasing levels of virus in plasma, as opposed to low-level to undetectable virus in plasma in the SIVIG macaques with similar NAb titers. We hypothesize that the success of this protocol was due, at least in part, to the use of two doses of SIVIG spaced 2 weeks apart to provide high titers of NAbs during the entire acute phase. The regimen may also have influenced the host immune responses. Most studies to date have utilized a single dose of IgG or MAb given at or near the time of virus exposure. We hoped that the maintenance of high titers of NAb for the first several weeks, beginning within hours or days of exposure, would increase the likelihood of controlling infection. If high-level, persisting NAbs are necessary to accelerate the de novo responses, then other groups might fail to observe the altered kinetics of responses. In attempting to understand these data, we have considered three main hypotheses for this novel finding.
One mechanism of action of HIVIG is to reduce the infectivity of HIV (17), and we think it likely that SIVIG similarly has a major antiviral effect in vivo in treated macaques. We showed that SIVIG reduced the peripheral virus load during the first few weeks of infection and resulted in post-acute-phase virus loads in cells and in plasma that were diminished by 3 to 4 log10 in responding animals. We hypothesize that the infectious virus particles produced in primary viremia are neutralized in vivo so that they cannot effectively seed additional sites to set up what has been termed steady-state replication. Evidence for this hypothesis can be found in the corroborating data obtained with lymph nodes and PBMC, showing that there were low levels of inducible virus by coculture.
A second hypothesis that we propose is that there is a more effective antigen presentation of virion antigens, particularly Env, in the presence of the NAbs found in the SIVIG. Passively transferred NAb binds to virions and, in addition to neutralizing them, may be more effectively presenting the viral Env determinants to antigen-presenting cells. The reason for proposing this hypothesis is that we must be able to explain the accelerated NAbs in the face of historical evidence from other systems, as well as our own data, showing delayed kinetics of binding antibodies. Complexes of antigen and NAb may be ideal vehicles for productive interactions with antigen-presenting cells. During the normal course of infection, peak virion production in the first few weeks proceeds without many antibodies present to bind up free virions. If the mature NAbs from the SIVIG could expose regions of Env to the immune system prior to mutational events that cover or shield key determinants (54), then a more effective set of NAbs might be produced early in infection.
Our third hypothesis is that these NAbs, by controlling viremia, may preserve the function of B cells and helper T cells, thereby facilitating the de novo response. It is clear that loss of T helper cells is key to loss of immune function and, ultimately, the ability to control infection. Exposure to many copies of virions without the devastation of infection may be an ideal method for eliciting cellular and humoral immune responses in these macaques that can effectively control the remaining virus. We do not have definitive evidence for any of the three ideas currently under consideration, and it is certainly possible that all three could contribute to this surprising response.
A question that was not addressed in this experiment was the breadth of NAbs that are effective in accelerating immunity. The SIVIG used was narrow in its neutralization specificity, neutralizing only SIVsm-related viruses. In addition, the SIVIG was matched to the challenge virus, because we wanted to optimize the chances for successful therapy. The use of “mismatched” HIVIG failed to block infection in previous studies (41), but these studies did not look at therapeutic applications. It will be important to examine this question in future studies. Our preliminary studies show that macaques 185 and 176 produced NAbs later in infection that were effective only against SIVsm viruses (N. L. Haigwood and D. C. Montefiori, unpublished data), suggesting that viral control was not dependent upon broadening of the immune response in these macaques. Our preliminary data indicate that the SIVIG-treated macaques did not diversify during the first year of infection (N. L. Haigwood, unpublished data). We plan to explore the development of autologous and heterologous NAbs in macaques with evidence of sequence divergence.
These results indicate that intervention early in infection with an agent that can neutralize virus can have long-lasting benefits for disease outcome in vivo. In this experiment, animals were treated with two large bolus infusions of IgG 1 and 14 days following infection, yet the effects of reduction of virus load to low or undetectable levels lasted for 3 to 5 years. A number of questions remain to be explored. It is tempting to speculate that NAbs elicited by vaccination at levels that are insufficient to provide sterilizing protection might play a similar role in accelerating de novo NAb maturation after virus exposure. These hypotheses require further studies for validation and discrimination. Ultimately, studies such as these support the testing of short-term therapies that are initiated during primary viremia. Therapies that control infection while enhancing effective antiviral immunity remain a key objective.
Acknowledgments
This study was supported by the Bristol-Myers Squibb Pharmaceutical Research Institute and by Public Health Service grants RR00166 (N.L.H.), AI-27757 (N.L.H.), and AI-20729 (N.L.L.).
We thank L. Stamatatos for helpful suggestions and critical reading of the manuscript, J. Klaniecki for recombinant SIV gp160, B. Travis for DNA QC-PCR assays, J. Ranchalis for RNA QC-PCR assays, P. Polacino for real-time PCR assays, K. McClelland for blood separations and database expertise, and P. Tarr for veterinary care at The Children's Hospital Research Foundation.
REFERENCES
- 1.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 2.Binley, J. M., B. Clas, A. Gettie, M. Vesanen, D. C. Montefiori, L. Sawyer, J. Booth, M. Lewis, P. A. Marx, S. Bonhoeffer, and J. P. Moore. 2000. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology 270:237-249. [DOI] [PubMed] [Google Scholar]
- 3.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. H. I. Parren, L. S. W. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, M. Lamacchia, E. Garratty, E. R. Stiehm, Y. J. Bryson, Y. Cao, J. P. Moore, D. D. Ho, and C. F. Barbas III. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 4.Cole, K. S., J. L. Rowles, B. A. Jagesrski, M. Murphey-Corb, T. Unangst, J. E. Clements, J. Robinson, M. S. Wyand, R. C. Desrosiers, and R. C. Montelaro. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 71:5069-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conley, A. J., J. A. I. Kessler, L. J. Boots, P. M. McKenna, W. A. Schleif, E. A. Emini, G. E. I. Mark, H. Katinger, E. K. Cobb, S. M. Lunceford, S. R. Rouse, and K. K. Murthy. 1996. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary isolate. J. Virol. 70:6751-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrantelli, F., R. Hofmann-Lehmann, R. A. Rasmussen, T. Wang, W. Xu, P. L. Li, D. C. Montefiori, L. A. Cavacini, H. Katinger, G. Stiegler, D. C. Anderson, H. M. McClure, and R. M. Ruprecht. 2003. Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS 17:301-309. [DOI] [PubMed] [Google Scholar]
- 7.Gardner, M., A. Rosenthal, M. Jennings, J. Yee, L. Antipa, and E. Robinson. 1995. Passive immunization of rhesus macaques against SIV infection and disease. AIDS Res. Hum. Retrovir. 11:843-854. [DOI] [PubMed] [Google Scholar]
- 8.Gauduin, M.-C., P. W. H. I. Parren, R. Weir, C. F. I. Barbas, D. R. Burton, and R. A. Koup. 1997. Passive immunization with a potent neutralizing human monoclonal antibody protects HU-PBL-SCID mice against challenge by primary isolates of human immunodeficiency virus type 1. Nat. Med. 3:1389-1393. [DOI] [PubMed] [Google Scholar]
- 9.Gauduin, M.-C., J. T. Safrit, R. Weir, M. S. C. Fung, and R. A. Koup. 1995. Pre- and postexposure protection against human immunodeficiency virus type 1 infection mediated by a monoclonal antibody. J. Infect. Dis. 171:1203-1209. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein, S., W. R. Elkins, W. T. London, A. Hahn, R. Goeken, J. E. Martin, and V. M. Hirsch. 1994. Immunization with whole inactivated vaccine protects from infection by SIV grown in human but not macaque cells. J. Med. Primatol. 23:75-82. [DOI] [PubMed] [Google Scholar]
- 11.Gorny, M. K., C. Williams, B. Volsky, K. Revesz, S. Cohen, V. R. Polonis, W. J. Honnen, S. C. Kayman, C. Krachmarov, A. Pinter, and S. Zolla-Pazner. 2002. Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize human immunodeficiency virus type 1 primary isolates from various clades. J. Virol. 76:9035-9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haigwood, N. L., P. L. Nara, E. Brooks, G. A. Van Nest, G. Ott, K. W. Higgins, N. Dunlop, C. J. Scandella, J. W. Eichberg, and K. S. Steimer. 1992. Native but not denatured recombinant HIV-1 gp120 generates broad spectrum neutralizing antibodies in baboons. J. Virol. 66:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haigwood, N. L., A. Watson, W. F. Sutton, J. McClure, A. Lewis, J. Ranchalis, B. Travis, G. Voss, N. L. Letvin, S.-L. Hu, V. M. Hirsch, and P. R. Johnson. 1996. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol. Lett. 51:107-114. [DOI] [PubMed] [Google Scholar]
- 14.Haigwood, N. L., and S. Zolla-Pazner. 1998. Humoral immunity to HIV, SIV, and SHIV. AIDS 12:S121-S132. [PubMed] [Google Scholar]
- 15.Hogevorst, E., S. Jurriaans, F. de Wolf, A. van Wijk, A. Wiersma, M. Valk, M. Roos, B. van Gemen, R. Coutinho, F. Miedema, and J. Goudsmit. 1995. Predictors for non- and slow progression in human immunodeficiency virus (HIV) type 1 infection: low viral RNA copy numbers in serum and maintenance of high HIV-1 p24-specific but not V3-specific antibody levels. J. Infect. Dis. 171:811-821. [DOI] [PubMed] [Google Scholar]
- 16.Hu, S.-L., K. Abrams, G. N. Barber, P. Moran, J. M. Zarling, A. J. Langlois, L. Kuller, W. R. Morton, and R. E. Benveniste. 1992. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science 255:456-459. [DOI] [PubMed] [Google Scholar]
- 17.Igarashi, T., C. Brown, A. Azadegan, N. Haigwood, D. Dimitrov, M. A. Martin, and R. Shibata. 1999. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nat. Med. 5:211-216. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, P. R., D. C. Montefiori, S. Goldstein, T. E. Hamm, J. Zhou, S. Kitov, N. L. Haigwood, L. Misher, W. T. London, J. L. Gerin, A. Allison, R. H. Purcell, R. M. Chanock, and V. M. Hirsch. 1992. Inactivated whole-virus vaccine derived from a proviral DNA clone of simian immunodeficiency virus induces high levels of neutralizing antibodies and confers protection against heterologous challenge. Proc. Natl. Acad. Sci. USA 89:2175-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Journot, V., G. Chene, P. Joly, M. Saves, H. Jacqmin-Gadda, J. M. Molina, and R. Salamon. 2001. Viral load as a primary outcome in human immunodeficiency virus trials: a review of statistical analysis methods. Control Clin. Trials 22:639-658. [DOI] [PubMed] [Google Scholar]
- 20.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascola, J. R. 2003. Defining the protective antibody response for HIV-1. Curr. Mol. Med. 3:209-216. [DOI] [PubMed] [Google Scholar]
- 22.Mascola, J. R., M. K. Louder, T. C. Van Cott, C. V. Sapan, J. S. Lambert, L. R. Muenze, B. Bunow, D. L. Birx, and M. L. Robb. 1997. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 71:7198-7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 24.McFarland, E. J., W. Borkowsky, T. Fenton, D. Wara, J. McNamara, P. Samson, M. Kang, L. Mofenson, C. Cunningham, A. M. Duliege, F. Sinangil, S. A. Spector, E. Jimenez, Y. Bryson, S. Burchett, L. M. Frenkel, R. Yogev, F. Gigliotti, K. Luzuriaga, and R. A. Livingston. 2001. Human immunodeficiency virus type 1 (HIV-1) gp120-specific antibodies in neonates receiving an HIV-1 recombinant gp120 vaccine. J Infect. Dis. 184:1331-1335. [DOI] [PubMed] [Google Scholar]
- 25.Mellors, J. W., L. A. Kingsley, C. R. Rinaldo, J. A. Todd, B. S. Hoo, R. P. Kokka, and P. Gupta. 1995. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann. Intern. Med. 122:573-579. [DOI] [PubMed] [Google Scholar]
- 26.Mofenson, L. M., J. S. Lambert, E. R. Stiehm, J. Bethel, M. W. III, J. Whitehouse, J. J. Moye, P. Reichenfelder, D. R. Harris, M. G. Fowler, B. J. Mathieson, G. J. Nemo, et al. 1999. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. N. Engl. J. Med. 34:385-393. [DOI] [PubMed] [Google Scholar]
- 27.Montefiori, D. C., T. W. Baba, A. Li, M. Bilska, and R. M. Ruprecht. 1996. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239delta3 in adult and infant rhesus monkeys. J. Immunol. 157:5528-5535. [PubMed] [Google Scholar]
- 28.Moog, C., H. J. A. Fleury, I. Pellegrin, A. Kirn, and A. M. Aubertin. 1997. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J. Virol. 71:3734-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muster, T., R. Guinea, A. Trkola, M. Purtscher, A. Klima, F. Steindl, P. Palese, and H. Katinger. 1994. Cross-neutralizing antibodies against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 68:4031-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.National Institutes of Health. 1985. Guide for the care and use of laboratory animals. DHEW publication NIH85-23. National Institutes of Health, Bethesda, Md.
- 30.Nduati, R., G. John, D. Mbori-Ngacha, B. Richardson, J. Overbaugh, A. Mwatha, J. Ndinya-Achola, J. Bwayo, F. E. Onyango, J. Hughes, and J. Kreiss. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283:1167-1174. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura, Y., T. Igarashi, N. Haigwood, R. Sadjadpour, R. J. Plishka, A. Buckler-White, R. Shibata, and M. A. Martin. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 76:2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura, Y., T. Igarashi, N. L. Haigwood, R. Sadjadpour, O. K. Donau, C. Buckler, R. J. Plishka, A. Buckler-White, and M. A. Martin. 2003. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc. Natl. Acad. Sci. USA 100:15131-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piatak, M., Jr., M. S. Saag, L. C. Yand, S. J. Clark, J. C. Kappes, K.-C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 35.Planelles, V., N. L. Haigwood, M. L. Marthas, K. A. Mann, C. Scandella, W. D. Lidster, J. R. Shuster, R. Van Kuyk, P. A. Marx, and P. A. Luciw. 1991. Functional and immunological characterization of SIV envelope glycoprotein produced in genetically engineered mammalian cells. AIDS Res. Hum. Retrovir. 7:889-898. [DOI] [PubMed] [Google Scholar]
- 36.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. H. I. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10:1-20. [DOI] [PubMed] [Google Scholar]
- 37.Polacino, P., V. Stallard, J. E. Klaneicki, D. C. Montefiori, A. J. Langlois, B. A. Richardson, J. Overbaugh, W. R. Morton, R. E. Benveniste, and S.-L. Hu. 1999. Limited breadth of the protective immunity elicited by simian immunodeficiency virus SIVmne gp160 vaccines in a combination immunization regimen. J. Virol. 73:618-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince, A. M., H. Reesink, D. Pascual, B. Horowitz, I. Hewlett, K. K. Murthy, K. E. Cobb, and J. Eichberg. 1991. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res. Hum. Retrovir. 7:971-973. [DOI] [PubMed] [Google Scholar]
- 39.Putkonen, P., R. Thorstensson, L. Ghavamzadeh, J. Albert, K. Hild, G. Biberfeld, and E. Norrby. 1991. Prevention of HIV-2 and SIVsm infection by passive immunization in cynomolgus monkeys. Nature 352:436-438. [DOI] [PubMed] [Google Scholar]
- 40.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 42.Steimer, K. S., C. J. Scandella, P. V. Skiles, and N. L. Haigwood. 1991. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to gp120. Science 254:105-108. [DOI] [PubMed] [Google Scholar]
- 43.Stiehm, E. R., J. S. Lambert, L. M. Mofenson, J. Bethel, J. Whitehouse, R. Nugent, J. J. Moye, M. Glenn Fowler, B. J. Mathieson, P. Reichelderfer, G. J. Nemo, J. Korelitz, W. A. Meyer III, C. V. Sapan, E. Jimenex, J. Gandia, G. Scott, M. J. O'Sullivan, A. Kovacs, A. Stek, W. T. Shearer, and H. Hammill. 1999. Efficacy of zidovudine and human immunodeficiency virus (HIV) hyperimmune immunoglobulin for reducing perinatal HIV transmission from HIV-infected women with advanced disease: results of Pediatric AIDS Clinical Trials Group protocol 185. J. Infect. Dis. 179:567-575. [DOI] [PubMed] [Google Scholar]
- 44.Thali, M., C. Furman, B. Wahren, M. Posner, D. D. Ho, J. Robinson, and J. Sodroski. 1992. Cooperativity of neutralizing antibodies directed against the V3 and CD4 binding regions of the human immunodeficiency virus gp120 envelope glycoprotein. J. AIDS 5:591-599. [PubMed] [Google Scholar]
- 45.Tilley, S. A., W. J. Honnen, M. E. Racho, T.-C. Chou, and A. Pinter. 1992. Synergistic neutralization of HIV-1 by human monoclonal antibodies against V3 loop and the CD4 binding site of gp120. AIDS Res. Hum. Retrovir. 8:461-467. [DOI] [PubMed] [Google Scholar]
- 46.Trkola, A., M. Purtsher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai, C.-C., K. E. Follis, T. W. Beck, A. Sabo, N. Bischofberger, and P. J. Dailey. 1997. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res. Hum. Retrovir. 13:707-712. [DOI] [PubMed] [Google Scholar]
- 48.Tsai, C. C., P. Emau, K. E. Follis, T. W. Beck, R. E. Benveniste, N. Bischofberger, J. D. Lifson, and W. R. Morton. 1998. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl)adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J. Virol. 72:4265-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Rompay, K. K., C. J. Beradi, S. DIllard-Telm, R. P. Tarara, D. R. Canfield, C. R. Valverde, D. C. Montefiori, K. S. Cole, R. C. Montelaro, C. J. Miller, and M. L. Marthas. 1998. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J. Infect. Dis. 177:1247-1259. [DOI] [PubMed] [Google Scholar]
- 50.Van Rompay, K. K., J. L. Greenier, K. S. Cole, P. Earl, B. Moss, J. D. Steckbeck, B. Pahar, T. Rourke, R. C. Montelaro, D. R. Canfield, R. P. Tarara, C. Miller, M. B. McChesney, and M. L. Marthas. 2003. Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251. J. Virol. 77:179-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vittecoq, D., S. Chevret, L. Morand-Joubert, F. Heshmati, F. Audat, M. Bary, T. Dusautoir, A. Bismuth, J. P. Viard, F. Barre-Sinoussi, J. F. Bach, and J. J. Lefrere. 1995. Passive immunotherapy in AIDS: a double-blind randomized study based on transfusions of plasma rich in anti-human immunodeficiency virus 1 antibodies vs. transfusions of seronegative plasma. Proc. Natl. Acad. Sci. USA 92:1195-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watson, A., J. McClure, J. Ranchalis, M. Scheibel, A. Schmidt, B. Kennedy, W. R. Morton, N. L. Haigwood, and S.-L. Hu. 1997. Early postinfection antiviral treatment reduces viral load and prevents CD4+ cell decline in HIV type 2-infected macaques. AIDS Res. Hum. Retrovir. 13:1375-1381. [DOI] [PubMed] [Google Scholar]
- 53.Watson, A., J. Ranchalis, B. Travis, J. McClure, W. Sutton, P. R. Johnson, S.-L. Hu, and N. L. Haigwood. 1997. Plasma viremia in macaques infected with SIV: plasma virus load early in infection predicts survival. J. Virology 71:284-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 55.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Jonson, E. E. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, M. S. Saag, and G. M. Shaw. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 56.Weiss, R. A., P. R. Clapham, J. N. Weber, A. G. Dalgleish, L. A. Lasky, and P. W. Berman. 1986. Variable and conserved neutralization antigens of human immunodeficiency virus. Nature 324:572-575. [DOI] [PubMed] [Google Scholar]
- 57.Zhu, T., L. Corey, Y. Hwangbo, J. M. Lee, G. H. Learn, J. I. Mullins, and M. J. McElrath. 2003. Persistence of extraordinarily low levels of genetically homogeneous human immunodeficiency virus type 1 in exposed seronegative individuals. J. Virol. 77:6108-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu, Y., K. Koo, J. D. Bradshaw, W. F. Sutton, L. R. Kuller, R. Bucala, D. Anderson, S. P. Mossman, F. Villinger, and N. L. Haigwood. 2000. Macaque blood-derived antigen-presenting cells elicit SIV-specific immune responses. J. Med. Primatol. 29:182-192. [DOI] [PubMed] [Google Scholar]