Abstract
Background
Objective was to determine whether prophylactic low level laser therapy (LLLT) reduces the risk of severe mucositis as compared to placebo or no therapy.
Methods
MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials were searched until February 2014 for randomized controlled trials (RCTs) comparing prophylactic LLLT with placebo or no therapy in patients with cancer or undergoing hematopoietic stem cell transplantation (HSCT). All analyses used random effects models.
Results
Eighteen RCTs (1144 patients) were included. Prophylactic LLLT reduced the overall risk of severe mucositis (risk ratio (RR) 0.37, 95% confidence interval (CI) 0.20 to 0.67; P = 0.001). LLLT also reduced the following outcomes when compared to placebo/no therapy: severe mucositis at the time of anticipated maximal mucositis (RR 0.34, 95% CI 0.20 to 0.59), overall mean grade of mucositis (standardized mean difference −1.49, 95% CI −2.02 to −0.95), duration of severe mucositis (weighted mean difference −5.32, 95% CI −9.45 to −1.19) and incidence of severe pain (RR 0.26, 95% CI 0.18 to 0.37).
Conclusion
Prophylactic LLLT reduced severe mucositis and pain in patients with cancer and HSCT recipients. Future research should identify the optimal characteristics of LLLT and determine feasibility in the clinical setting.
Introduction
Oral mucositis is one of the most frequent and distressing complications observed in patients receiving cancer treatment [1]. Mucositis develops in approximately 20–40% of patients receiving conventional chemotherapy, 60–85% of patients undergoing hematopoietic stem cell transplantation (HSCT) and in nearly all patients with head and neck cancer receiving radiation [1]–[3]. Chemotherapy associated mucositis typically peaks at 7 to 14 days after the initiation of chemotherapy and resolves within a few days as compared to radiotherapy associated mucositis in head and neck cancer patients, which peaks at weeks 4 to 6 of treatment and usually lasts for weeks after completion of radiation [3]–[6]. Oral mucositis is associated with pain, infections, need for enteral or parental nutrition, impaired nutritional status and quality of life, increased duration and cost of hospital stay, and interruptions or dose reductions in chemotherapy or radiotherapy [1], [7]–[10]. Because of these implications, research has been focused on different preventive and treatment strategies for oral mucositis.
The Multinational Association of Supportive Care in Cancer (MASCC) and the International Society of Oral Oncology (ISOO) have recently published guidelines for the prevention of oral mucositis [11]. One recommended intervention was low level laser therapy (LLLT), also referred to as photobiomodulation, in patients receiving HSCT with or without total body irradiation (level of evidence II) and in patients receiving head and neck radiotherapy without concomitant chemotherapy (level of evidence III) [11]. These recommendations were based on a systematic review which included studies published up to December 2010 that did not synthesize the data [12]. In contrast to the MASCC/ISOO recommendations and systematic review, a Cochrane Collaboration systematic review published in 2011 found that there was only weak evidence from two small studies at risk for bias favoring LLLT for the prevention of mucositis [13]. In their conclusions, LLLT was not one of the two interventions (cryotherapy and keratinocyte growth factor) found to have evidence of benefit [13]. The authors recommended that more randomized controlled trials (RCTs) are required for interventions such as laser therapy [13].
Since both of these systematic reviewers were conducted, there have been several RCTs performed evaluating the effect of prophylactic LLLT on oral mucositis [14]–[21]. Synthesis of all the evidence with careful evaluation of the risk of bias would permit a better evaluation of the effect of LLLT and may also provide insight into factors which may explain heterogeneity of the effect of the intervention.
Our primary objective was to determine whether prophylactic LLLT reduces the overall risk of severe mucositis in children and adults with cancer or undergoing HSCT as compared to placebo or no therapy. Our secondary objectives were to determine whether prophylactic LLLT reduces the incidence of severe mucositis when maximum mucositis is anticipated, overall mean mucositis grade, duration of severe mucositis, incidence of any pain and severe pain, overall mean pain scores, proportion of patients requiring opioid analgesia and unplanned radiotherapy interruption, as compared to placebo or no therapy.
Materials and Methods
Data Sources and Searches
We developed a protocol for this review and followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [22]. Comprehensive searches for relevant trials using the Ovid platform in MEDLINE (from 1946 to February 17, 2014), EMBASE (from 1947 to February 17, 2014), Cochrane Central Register of Controlled Trials (to January2014), CINAHL (1983 to February 17, 2014), Web of Science (to February 17, 2014), SCOPUS (to February 2014), and LILACS (to February 2014) were performed without any language or publication status restriction. The search strategy included the following Medical Subject Heading terms: “mucositis”, “laser therapy”, “low-level laser therapy”, “phototherapy”, “light emitting diode”, “transplantation”, “chemotherapy” and “chemoradiotherapy”. Multiple synonyms, abbreviations, and related keywords for each of these terms were used for searching the databases. The search strategy is available as Appendix S1.
We also reviewed the conference proceedings of the International Society of Paediatric Oncology, American Society of Clinical Oncology, American Society of Hematology, American Society of Pediatric Hematology and Oncology, and Multinational Association of Supportive Care in Cancer from 2011 to 2013 to identify more recently completed studies. The reference lists of identified studies were also searched to identify further eligible studies.
Study Selection
We defined inclusion and exclusion criteria a priori. We included RCTs and quasi-RCTs in this review. Case–control studies, cohort studies, case reports, case series, animal studies, letters to editors, editorials, review articles and commentaries were excluded. Studies were included if the population consisted of patients with cancer or undergoing HSCT and patients were randomly assigned to receive prophylactic LLLT versus placebo, no therapy or usual care. Studies were excluded if: (1) allocation not randomly assigned; (2) absence of a placebo or no treatment group; (3) randomized chemotherapy cycles or left and right buccal mucosa within a patient rather than randomizing patients (as episodes would not be independent); and (4) duplicate publication. Studies included in the meta-analysis were not restricted by language or publication status.
Two reviewers (SO and GZ) independently evaluated the titles and abstracts of publications identified by the search strategy. Any publication considered potentially relevant by either reviewer was retrieved in full and assessed for eligibility. Inclusion of studies in this meta-analysis was determined by agreement of both reviewers. Discrepancies were resolved by a third reviewer (LS). Agreement of study inclusion between reviewers was evaluated using the kappa statistic. Strength of agreement was defined as slight (0.00 to 0.20), fair (0.21 to 0.40), moderate (0.41 to 0.60), substantial (0.61 to 0.80), or almost perfect (0.81 to 1.00) [23].
Type of Intervention
Interventions were internally (intraoral) or externally (extraoral) delivered LLLT given as prophylaxis for oral mucositis in any intensity, power, wavelength, energy density or schedule.
Outcomes
The primary outcome was the overall incidence of severe mucositis over the entire observation period. Severe mucositis was defined as grades 3 or 4 mucositis on a 5 point grading scale ranging from 0 to 4. Three instruments were graded in this fashion, namely the World Health Organization (WHO) scale, the Radiation Therapy Oncology Group (RTOG) scale, and the National Cancer Institute Common Terminology Criteria (NCI CTC) (version 2) [24]–[26]. NCI CTCAE (version 3 and 4) uses a grading scale which ranges from 1 to 5; for our purpose, severe mucositis was considered grades 3 to 5 [27], [28]. We also included the Tardieu mucositis scale which ranges from grades 0 to 3 [29]. Grades 2 and 3 on the Tardieu scale are similar to grades 3 and 4 according to the other mucositis grading scales and consequently, we classified Tardieu scale scores of grade 2 and 3 as severe mucositis. However, we conducted a sensitivity analysis excluding studies using the Tardieu scale to evaluate the robustness of the results. In studies reporting severe mucositis by more than one of these 5 point grading scales, the WHO scale was used for primary outcome analysis if available.
The secondary outcomes were: (1) incidence of severe mucositis (defined using the same approach as the primary outcome) at the time point when maximum mucositis was expected, namely at week 6±1 of radiotherapy or chemo-radiotherapy in head and neck cancer patients and at day 10±4 of chemotherapy or HSCT (from the days of chemotherapy initiation or stem cell infusion respectively); (2) overall mean mucositis grade or score over the observation period as measured by any mucositis grading scale including continuous scales such as the Oral Mucositis Assessment Scale (OMAS) [30]; and (3) duration of severe mucositis (defined using the same approach as the primary outcome). We also evaluated oral pain for studies that used an 11 point pain visual analogue scale (VAS) ranging from 0 to 10 [31]. We examined the incidence of any pain defined as a pain score more than 0; incidence of severe pain defined as a pain score more than 7 [32]; and overall mean pain score. Other outcomes were the proportion of patients requiring opioid analgesia and proportion of patients with unplanned radiotherapy interruption due to mucositis in head and neck cancer patients.
Risk of Bias Assessment
We used the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials [33]. This tool includes the following domains relevant to internal validity: selection bias, performance bias, detection bias, attrition bias and reporting bias. We evaluated the following sources of bias related to these domains: random number generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective outcome reporting. We a priori prioritized allocation concealment and blinding for stratified analyses [34]. For blinding, we evaluated whether participant, personnel and outcome assessors were blinded versus studies in which at least one of these groups was not blinded.
Data Extraction
A data abstraction form was developed by the authors and all information was abstracted in duplicate by two authors (SO and GZ). Where information was missing from a publication, the corresponding author was contacted and the missing information was requested.
Data Synthesis
We combined data at the study level for this meta-analysis. For dichotomous outcomes such as the overall incidence of severe mucositis, data were synthesized using the risk ratio (RR) as the effect measure with its 95% confidence interval (CI). Risk ratios less than 1 suggest that LLLT is better than placebo or no therapy in preventing oral mucositis. Number needed to treat was calculated as the inverse of the absolute risk difference between groups. For continuous outcomes with missing summary measures, we made the following assumptions to facilitate data synthesis: the mean can be approximated by the median; the range contains six standard deviations (SDs), the 95% CI contains four standard errors (SEs), and the interquartile range contains 1.35 SDs. Where continuous outcomes were measured using different scales (such as the mean mucositis grade), outcomes were synthesized using the standardized mean difference (SMD). Where continuous outcomes were measured on the same scale (such as the pain VAS), outcomes were synthesized using the weighted mean difference (WMD). A SMD or WMD less than 0 indicate that the mean mucositis or pain VAS scores were lower in the LLLT arm as compared to the placebo or no therapy arm. Effect sizes of dichotomous and continuous outcomes were weighted by the Mantel-Haenzel and inverse variance methods respectively. As we anticipated heterogeneity between studies, a random effects model was used for all analyses. Statistical heterogeneity between trials was assessed using the I2 value, which describes the percentage of total variation across studies due to heterogeneity rather than chance [33].
Potential publication bias was explored by visual inspection of funnel plots when at least 10 studies were available [33]. Publication bias occurs when small studies are published only if the results are positive. A funnel plot is a graph with the effect (RR, SMD or WMD in our analysis) on the x-axis, and the inverse of variance of the effect on the y-axis. Asymmetry, without studies in the bottom right corner, suggests publication bias. In the event of potential publication bias, we used the “trim and fill” technique to determine the impact of such potential bias [33]. With this technique, outlying studies are deleted, and hypothetical negative studies with equal weight are created.
In order to explore sources of heterogeneity, stratified analyses were planned a priori for the primary outcome only (to limit the number of analyses performed). Factors evaluated were: (1) population age (adult versus pediatric (age ≤18 years)/combined adult and pediatric); (2) underlying condition (head and neck cancer patients receiving radiotherapy or chemo-radiotherapy versus chemotherapy or HSCT); (3) intraoral versus extraoral laser delivery; (4) energy density of laser (≤4 J/cm2 versus >4 J/cm2); (5) blinding of patients, providers and assessors (yes versus no or unclear); and (6) adequate allocation concealment (yes versus no or unclear).
Meta-analyses were conducted using Review Manager 5.2 (Cochrane Collaboration, Nordic Cochrane Centre).
Results
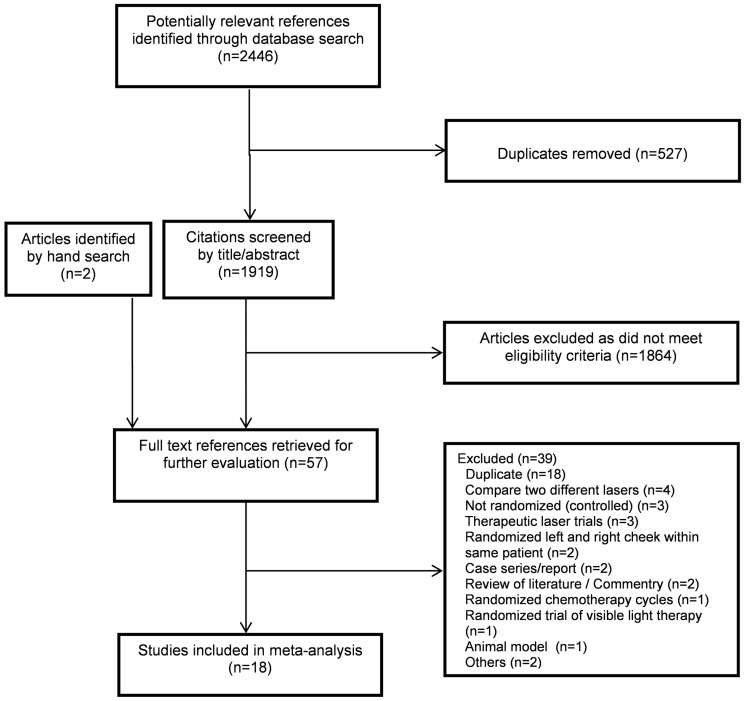
The flow diagram of trial identification, selection and reasons for exclusion is presented in Figure 1. A total of 2445 citations were identified by the search strategy; 18 studies met the eligibility criteria and were included in this systematic review [14]–[21], [35]–[48]. Agreement between reviewers regarding study inclusion was almost perfect (kappa 0.89, 95% CI 0.78 to 1.00). All studies except one were published as full text articles [38]. One study reported outcomes as a stratified analysis by underlying disease diagnosis and HSCT regimen [19] and thus, represented two separate analyses for a total of 19 prophylactic LLLT comparisons randomizing 1144 patients.
Figure 1. Flow diagram of trial identification and selection.
Table 1 lists the baseline characteristics of the studies, patient population, intervention and mucositis evaluation schedule and scale. The earliest trial was published in 1997 with 9 (47%) studies being published in 2012 and 2013. Half of the trials were from Brazil [14], [18], [20], [38]–[41], [43], [44], [47]. Eight trials were conducted in the HSCT population, 8 in head and neck cancer patients receiving radiotherapy or chemo-radiotherapy and the remainder in other patients receiving chemotherapy. One study was a solely pediatric trial [41]. Intraoral laser therapy was used in all except two trials [19]. With respect to laser source, InGaAlP (6 trials) and helium-neon (5 trials) were the most commonly used lasers. The mean wavelength and energy density of the lasers used across the trials was 660±34.7 nm and 3.3±1.3 J/cm2.
Table 1. Baseline characteristics of studies included in the meta-analysis*.
| First Author (reference) | Year Pub | Country | Age | Underlying Condition | Setting | No. Rando-mized | Type of Laser | Wave-length (nm) | Power Output (mW) | Irradiation Time per Spot (sec) | Energy per Spot (Joules) | Energy Density (J/cm2) | Laser Schedule | Oral Mucositis Evaluation Schedule | Mucositis Assessment Scale |
| Antunes [14], [36] | 2013 | Brazil | Adults | Head and neck cancer | Chemo-radio | 94 | InGaAIP | 660 | 100 | 10 | 1 | 4 | 5 sessions/week during radiation | Daily | WHO and OMAS |
| Arbabi-Kalati [15] | 2013 | Iran | Adults | Oncologic disorders | Chemo | 48 | Mustang | 630 | 30 | NA | NA | 5 | Prior to chemotherapy | Two times/week | WHO |
| Gautam [16], [37] | 2012 | India | Adults | Head and neck cancer | Chemo-radio | 239 | He-Ne | 632.8 | 24 | 125 | 3 | 3 | 5 sessions/week×45 days | Weekly | RTOG/EORTC |
| Gautam [17] | 2012 | India | Adults | Oral carcinoma | Chemo-radio | 121 | He-Ne | 632.8 | 24 | 145 | 3.5 | 3.5 | 5 sessions/week during radiation | Weekly | RTOG/EORTC |
| Gouvea de Lima [18] | 2012 | Brazil | Adults | Head and neck cancer | Chemo-radio | 75 | GaAlAs | 660 | 10 | 10 | 0.1 | 2.5 | 5 sessions/week during radiation | Every two weeks | NCI CTCv2 |
| Hodgson (a) [19] | 2012 | USA | Both | Hematologic, oncologic disorders | HSCT (allo, auto) | 40 | Infrared LED | 670±10 | 50 | 80 | 4 | 4 | Daily from day 0 to day +14 | Three times/week | WHO,NCI CTCAE and OMAS |
| Hodgson (b) [19] | 2012 | USA | Adults | Multiple myeloma | HSCT (auto) | 40 | Infrared LED | 670±10 | 50 | 80 | 4 | 4 | Daily from day 0 to day +14 | Three times/week | WHO,NCI CTCAE and OMAS |
| Oton-Leite [20], [48] | 2012 | Brazil | Adults | Head and neck cancer | Radio or Chemo-radio | 60 | InGaAlP | 685 | 35 | 25 | 0.8 | 2 | 5 sessions/week during radiation | Mid and at the end of treatment (week 3 and week 6) | WHO |
| Pires-Santos [38] | 2012 | Brazil | Adults | Breast cancer | Chemo | 12 | NA | NA | NA | NA | NA | NA | Day 0 to day +7 q 48 hours | NA | NA |
| Silva [21] | 2011 | Brazil | Both | Hematologic, oncologic disorders | HSCT (allo, auto) | 42 | InGaAIP | 660 | 40 | 4 | 0.16 | 4 | Daily from day −4 to day +4 | Daily | WHO |
| Chor [39] | 2010 | Brazil | Adults | NA | HSCT (auto) | 34 | AsGaAl | 660 | 50 | NA | NA | NA | Daily from day −7 to day 0 | Daily | Tardieu |
| Khouri [47] | 2009 | Brazil | Both | Hematologic disorders | HSCT (allo) | 22 | InGaAIP and GaAlAs | 660 and 780 | 25 | 10 | 0.25 | 6.3 | Daily until day +15 or day of engraftment | NA | WHO and OMAS |
| Antunes [40] | 2007 | Brazil | Adults | Hematologic Disorders | HSCT (allo, auto) | 38 | InGaAIP | 660 | 46.7 | 16.7 | 0.8 | 4 | Daily from day −7 until neutrophil recovery | Daily | WHO and OMAS |
| Cruz [41] | 2007 | Brazil | Children | Hematologic and solid malignancies | Chemo or HSCT (auto) | 62 | NA | 780 | 60 | NA | NA | 4 | Daily from start of chemo×5 days | Day +8 and day +15 | NCI CTC |
| Schubert [42] | 2007 | USA | Both | Hematologic, oncologic disorders | HSCT (allo, auto) | 47 | GaAlAs | 650 | 40 | 2 | 0.08 | 2 | Daily from day −1 of conditioning to day +2 | Two times/week | OMI [55] |
| Arun Maiya [35] | 2006 | India | Adults | Oral carcinoma | Radio | 50 | He-Ne | 632.8 | 10 | 180 | 1.8 | 1.8 | 5 sessions/week during radiation | Once at the end of treatment (week 6) | WHO |
| Lopes [43] | 2006 | Brazil | Adults | Head and neck cancer | Chemo-radio | 60 | InGaAlP | 685 | 35 | 58 | 2 | 2 | NA | Pretreatment, 4 weeks and at the end of therapy | NCI CTC |
| Bensadoun [44], [45] | 1999 | France | Adults | Head and neck cancer | Radio | 30 | He-Ne | 632.8 | 60 | 33 | 2 | 2 | 5 sessions/week during radiation | Weekly | WHO |
| Cowen [46] | 1997 | France | Adults | Hematologic malignancies | HSCT (auto) | 30 | He-Ne | 632.8 | 60 | 10 | 0.6 | 1.5 | Daily from day −5 to day −1 | Daily | Tardieu |
Abbreviations: Allo - allogeneic hematopoietic stem cell transplant; Auto-autologous hematopoietic stem cell transplant; Chemo – chemotherapy; EORTC-European Organization for Research and Treatment of Cancer; GaAIAs/AsGaAI – gallium-aluminium-arsenide/arsenate; He-Ne- helium-neon; HSCT – hematopoietic stem cell transplantation; InGaAIP – indium-gallium-aluminium phosphide; LED – light emitting diode; NA – not available; NCI CTC – National Cancer Institute Common Terminology Criteria; OMAS – Oral Mucositis Assessment Scale; OMI - Oral Mucositis Index; Pub – published; Radio- radiotherapy; RTOG – Radiation Therapy Oncology Group; VAS – visual analog scale; WHO – World Health Organization.
*There were 18 studies reporting 19 separate comparisons between low level light therapy and placebo/no therapy as one study stratified the population by underlying disease diagnosis and HSCT regimen.
Summary of the risk of bias of included studies is presented in Appendix S2. The number of studies at low risk of bias was as follows: for random sequence generation (n = 13, 68%), allocation concealment (n = 4, 21%), blinding of participants and personnel (n = 13, 68%), blinding of outcome assessor (n = 15, 79%), incomplete outcome data (n = 15, 79%) and selective outcome reporting (n = 13, 68%). There were four studies (21%) that were at low risk of bias across all domains.
Primary Outcome
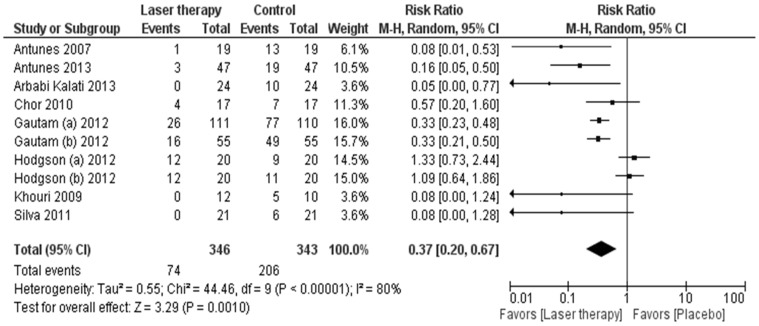
Ten studies encompassing 689 patients reported the overall incidence of severe mucositis using the WHO (n = 7), RTOG (n = 2) and Tardieu (n = 1) scales. Prophylactic LLLT reduced the risk of severe mucositis when compared to placebo or no therapy (RR 0.37, 95% CI 0.20 to 0.67; P = 0.001; Table 2 and Figure 2). The sensitivity analysis excluding the single study using the Tardieu scale did not affect the estimate of LLLT treatment effect (RR 0.34, 95% CI 0.18 to 0.65; P = 0.001).The absolute risk reduction in the incidence of severe mucositis with LLLT was −0.35 (95% CI −0.48 to −0.21; P<0.0001), resulting in a number needed to treat of three patients to prevent one episode of severe mucositis.
Table 2. Summary of outcomes of low level laser therapy as compared to placebo/no treatment.
| Outcome | Number Studies | Number Patients | Effect | 95% CI¥ | I2 | P |
| Overall incidence of severe (grade 3 or 4) mucositis | 10 | 689 | RR 0.37 | 0.20 to 0.67 | 80% | 0.001 |
| Incidence of severe (grade 3 or 4) mucositis at anticipated time of maximal mucositis* | 6 | 546 | RR 0.34 | 0.20 to 0.59 | 62% | 0.0001 |
| Overall mean grade of mucositis | 8 | 603 | SMD −1.49 | −2.02 to −0.95 | 86% | <0.0001 |
| Duration of severe (grade 3 or 4) mucositis | 3 | 361 | WMD −5.32 | −9.45 to −1.19 | 94% | 0.01 |
| Incidence of any pain | 7 | 591 | RR 0.89 | 0.76 to 1.04 | 96% | 0.15 |
| Incidence of severe pain** | 2 | 331 | RR 0.26 | 0.18 to 0.37 | 0% | <0.0001 |
| Overall mean pain scores | 5 | 222 | WMD −2.46 | −4.41 to −0.77 | 97% | 0.004 |
| Number of patients requiring opioid analgesia | 5 | 530 | RR 0.47 | 0.37 to 0.60 | 0% | <0.0001 |
| Unplanned radiotherapy interruption due to mucositis in head and neck cancer patients | 5 | 560 | RR 0.23 | 0.12 to 0.44 | 0% | <0.0001 |
Abbreviations: RR - risk ratio; SMD - standardized mean difference; WMD – weighted mean difference; CI – confidence interval;
*Maximum anticipated mucositis was week 6±1 in head and neck cancer radiotherapy/chemo-radiotherapy trials and day 10±4 in chemotherapy and hematopoietic stem cell transplantation trials (from date of chemotherapy initiation and stem cell infusion respectively).
** Severe pain defined as a visual analogue scale score >7.
All analyses used a random-effect model. A risk ratio <1 and a standardized mean difference or weighted mean difference <0 with 95% CIs that do not include 1 or 0 respectively, suggest that low level laser is better than placebo/no therapy.
Figure 2. Forest plot of overall incidence of severe (grade 3 or 4) mucositis.
Squares to the left of the vertical line indicate that low level laser therapy reduces mucositis. Horizontal lines through the squares represent 95% confidence intervals (CIs). The size of the squares reflects each study's relative weight, and the diamond represents the aggregate risk ratio and 95% CI.
Secondary Outcomes
Table 2 summarizes the secondary outcomes of the analysis. Synthesis of six studies encompassing 546 patients showed a reduced risk of severe mucositis with LLLT at the time of anticipated maximal mucositis (RR 0.34, 95% CI 0.20 to 0.59; P = 0.0001) (Table 2 and Appendix S3). Prophylactic LLLT also reduced the overall mean grade of mucositis and duration of severe mucositis (Table 2).
Table 2 also illustrates that LLLT was associated with a reduction in the incidence of severe pain, overall mean pain scores, and the proportion of patients requiring opioid analgesia. Finally, LLLT reduced unplanned radiation interruption in head and neck cancer patients.
Subgroup Analyses
Table 3 illustrates the stratified analyses for the primary outcome of severe mucositis. No interaction was seen between population age or underlying condition and the effect of LLLT. Studies using intraoral laser (RR 0.29, 95% CI 0.19 to 0.42) demonstrated a significantly larger reduction in severe mucositis compared to those using extraoral laser (RR 1.19, 95% CI 0.80 to 1.78; P for interaction <0.0001). There was a non-statistically significant larger effect of LLLT among studies utilizing >4 J/cm2 energy density as compared to ≤4 J/cm2 (P for interaction = 0.06). Studies with unclear or inadequate allocation concealment showed a larger treatment effect (P for interaction = 0.03).
Table 3. Effect of low level laser therapy as compared to placebo/no therapy on overall incidence of severe (grade 3 or 4) mucositis stratified by patient, laser and risk of bias characteristics.
| Subgroup | Number Studies | Number patients | RR | 95% CI¥ | P for interaction |
| Population Age | 0.90 | ||||
| Adult | 8 | 607 | 0.33 | 0.18 to 0.59 | |
| Pediatric or both adult/pediatric | 2 | 82 | 0.41 | 0.02 to 10.87 | |
| Underlying Condition | 0.85 | ||||
| Chemotherapy or HSCT | 7 | 264 | 0.35 | 0.13 to 0.98 | |
| Head and neck cancer radiotherapy/chemo-radiotherapy | 3 | 425 | 0.32 | 0.24 to 0.42 | |
| Type of Laser Delivery | <0.0001 | ||||
| Intraoral | 8 | 609 | 0.29 | 0.19 to 0.42 | |
| Extraoral | 2 | 80 | 1.19 | 0.80 to 1.78 | |
| Energy Density of Laser | 0.06 | ||||
| ≤4 J/cm2 | 8 | 619 | 0.43 | 0.23 to 0.78 | |
| >4 J/cm2 | 2 | 70 | 0.06 | 0.01 to 0.43 | |
| Participants, Personnel and Assessors Blinded | 0.11 | ||||
| Yes | 8 | 625 | 0.42 | 0.23 to 0.76 | |
| No or unclear | 2 | 64 | 0.08 | 0.01 to 0.56 | |
| Allocation Concealment Adequate | 0.03 | ||||
| Yes | 4 | 411 | 0.61 | 0.30 to 1.25 | |
| No or unclear | 6 | 278 | 0.16 | 0.07 to 0.41 |
Abbreviations: RR – risk ratio; CI – confidence interval; HSCT – hematopoietic stem cell transplantation.
All analyses used a random-effect model. A risk ratio <1 with 95% CIs that do not include 1, suggests that low level laser is better than placebo/no therapy.
Other Analyses
There were a sufficient number of studies for the primary outcome of severe mucositis to evaluate for publication bias. The funnel plot of risk of severe mucositis illustrated a potential for publication bias with an absence of studies in the right lower quadrant related to four studies (Figure 3) [15], [21], [40], [47]. When we used the “trim and fill” technique to account for this potential publication bias, the effect size of LLLT on severe mucositis was still statistically significant (RR 0.51, 95% CI 0.29 to 0.90; P = 0.0197). Funnel plots were not assessed for secondary outcomes because there were too few studies to permit these evaluations.
Figure 3. Funnel plot “trim and fill” technique assessing publication bias for overall incidence of severe mucositis.
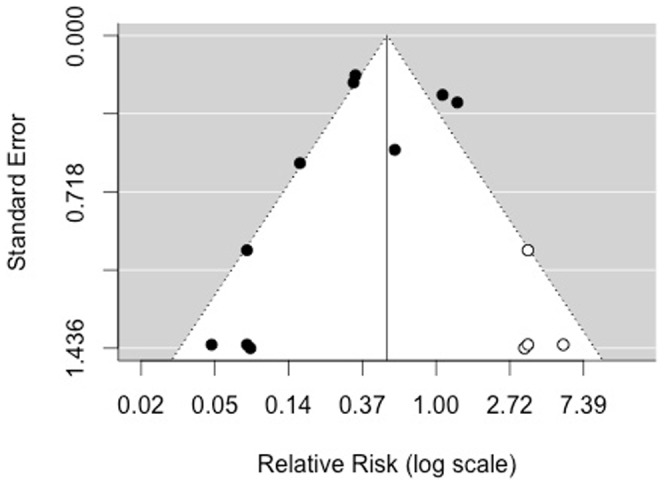
The x-axis represents the risk ratio for the effect of low level laser therapy and the y-axis represents the inverse of the variance of the effect. Estimated number of missing studies on right side = 4.
Discussion
Our meta-analysis demonstrated that prophylactic LLLT reduces the overall risk of severe mucositis and other measures of mucositis severity including the duration of severe mucositis in patients with cancer and in those undergoing HSCT. Low level laser therapy also reduced the risk of severe pain, overall mean pain scores, need for opioid analgesia and unplanned radiotherapy interruptions. The consistency of the effect among the primary and secondary outcome measures strengthens the confidence in these results.
In general, the risk of bias scores were favorable with 79% of studies blinding the outcome assessor and 68% of studies reporting adequate random sequence generation. However, only 21% of studies reported adequate allocation concealment. This finding is important as lack of allocation concealment has been associated with exaggerated treatment effects [34]. However, even in studies that reported adequate allocation concealment, the treatment effect of RR 0.61 likely represents a clinically meaningful benefit of LLLT. A second issue is the potential for publication bias. However, we demonstrated that even with the addition of hypothetical negative studies of equal weight, the effect was still statistically significantly with a RR 0.51. When put together, these issues highlight that LLLT is likely effective in the prevention of oral mucositis although reported treatment effects may be exaggerated.
The findings of our meta-analysis provide unique and clinically important information in comparison to three prior systematic reviews with conflicting conclusions about the effect of LLLT [12], [13], [49]. All three prior reviews included fewer randomized prophylactic LLLT trials for a variety of reasons including searching fewer databases, date of last update, and use of restrictive eligibility criteria [12], [13], [49]. Two reviews included 5 and 8 prophylactic LLLT studies. The third review included both prophylactic and therapeutic randomized and nonrandomized LLLT studies and did not attempt to synthesize data [12], [50]. The Cochrane Collaboration systematic review used robust methodology. However, it only included 5 studies and limited their outcome to the mucositis evaluation on day 28 of therapy, which may not be appropriate when combining head and neck radiotherapy or chemo-radiotherapy trials with other chemotherapy and HSCT trials [13].
In our stratified analysis, we found a statistically significant and qualitative interaction by extraoral versus intraoral LLLT administration. This effect is biologically plausible if there is inadequate delivery of dose to the target tissues due to absorption of power by more superficial non-target tissues [19], [51]–[54]. However, this result should be considered hypothesis generating as there are other differences in trial design which may explain these results. For example, the two extraoral laser trials used non-coherent light emitting diodes and initiated LLLT later in comparison to the other trials [21], [39], [42], [46]. Additionally, confidence in this analysis is limited since there were only two studies in the extraoral LLLT group.
The major strengths of our meta-analysis included rigorous methodology for identification of studies and synthesis of data. The primary outcome for our review was the overall incidence of severe mucositis throughout the observation period, a clinically relevant outcome. However, similar to many systematic reviews, our analysis was limited by the methodological quality and outcome reporting of the included studies. Only four studies were at low risk of bias across all six domains used to evaluate validity. The studies were relatively heterogeneous with respect to laser parameters, laser schedules, mucositis assessment scales, time point of assessments and outcome reporting. Finally, only one study was conducted exclusively in children, limiting generalizability to the pediatric population.
A major question that remains to be answered is the feasibility of intraoral LLLT for use in routine clinical practice. The administration of this intervention requires the utilization of specialized equipment, trained personnel, involvement of a multi-disciplinary team and co-operation of patients as manipulation of the oral cavity may be painful during mucositis. Little is known about whether this intervention can be implemented in most settings and further, whether the intervention demonstrates effectiveness in routine clinical practice.
In conclusion, prophylactic LLLT reduced severe mucositis and pain in patients with cancer and HSCT recipients. Future research should identify the optimal characteristics of LLLT and determine feasibility in the clinical setting.
Supporting Information
Search Strategies. Search strategies used in MEDLINE, EMBASE and EBM. Other database strategies are available on request.
(DOC)
Risk of bias assessment for included studies*.
(DOC)
Forest plot of incidence of severe (grade 3 or 4) mucositis at week 6±1 in head and neck cancer radiotherapy trials and at day 10±4 in chemotherapy or hematopoietic stem cell transplantation trials. Squares to the left of the vertical line indicate that low level laser therapy reduces mucositis. Horizontal lines through the squares represent confidence intervals (CIs). The size of the squares reflects each study's relative weight, and the diamond represents the aggregate risk ratio and 95% CI.
(TIF)
Acknowledgments
We wish to thank Elizabeth Uleryk for her tremendous support in conducting the literature search.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1. Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, et al. (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100: 1995–2025. [DOI] [PubMed] [Google Scholar]
- 2. Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, et al. (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66: 253–262. [DOI] [PubMed] [Google Scholar]
- 3. Blijlevens N, Schwenkglenks M, Bacon P, D'Addio A, Einsele H, et al. (2008) Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy–European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol 26: 1519–1525. [DOI] [PubMed] [Google Scholar]
- 4. Woo SB, Sonis ST, Monopoli MM, Sonis AL (1993) A longitudinal study of oral ulcerative mucositis in bone marrow transplant recipients. Cancer 72: 1612–1617. [DOI] [PubMed] [Google Scholar]
- 5. Vera-Llonch M, Oster G, Hagiwara M, Sonis S (2006) Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer 106: 329–336. [DOI] [PubMed] [Google Scholar]
- 6. Epstein JB, Beaumont JL, Gwede CK, Murphy B, Garden AS, et al. (2007) Longitudinal evaluation of the oral mucositis weekly questionnaire-head and neck cancer, a patient-reported outcomes questionnaire. Cancer 109: 1914–1922. [DOI] [PubMed] [Google Scholar]
- 7. McCann S, Schwenkglenks M, Bacon P, Einsele H, D'Addio A, et al. (2009) The Prospective Oral Mucositis Audit: relationship of severe oral mucositis with clinical and medical resource use outcomes in patients receiving high-dose melphalan or BEAM-conditioning chemotherapy and autologous SCT. Bone Marrow Transplant 43: 141–147. [DOI] [PubMed] [Google Scholar]
- 8. Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, et al. (2003) The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 98: 1531–1539. [DOI] [PubMed] [Google Scholar]
- 9. Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S (2007) Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer 15: 491–496. [DOI] [PubMed] [Google Scholar]
- 10. Peterman A, Cella D, Glandon G, Dobrez D, Yount S (2001) Mucositis in head and neck cancer: economic and quality-of-life outcomes. J Natl Cancer Inst Monogr 45–51. [DOI] [PubMed] [Google Scholar]
- 11. Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, et al. (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Migliorati C, Hewson I, Lalla RV, Antunes HS, Estilo CL, et al. (2013) Systematic review of laser and other light therapy for the management of oral mucositis in cancer patients. Support Care Cancer 21: 333–341. [DOI] [PubMed] [Google Scholar]
- 13. Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, et al. (2011) Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev CD000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antunes HS, Herchenhorn D, Small IA, Araujo CMM, Viegas CMP, et al. (2013) Phase III trial of low-level laser therapy to prevent oral mucositis in head and neck cancer patients treated with concurrent chemoradiation. Radiother Oncol 109: 297–302. [DOI] [PubMed] [Google Scholar]
- 15. Arbabi-Kalati F, Arbabi-Kalati F, Moridi T (2013) Evaluation of the effect of low level laser on prevention of chemotherapy-induced mucositis. Acta Medica Iranica 51: 157–162. [PubMed] [Google Scholar]
- 16. Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Vadhiraja BM (2012) Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients - a triple blinded randomized controlled trial. Radiother Oncol 104: 349–354. [DOI] [PubMed] [Google Scholar]
- 17. Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya GA (2012) Low level helium neon laser therapy for chemoradiotherapy induced oral mucositis in oral cancer patients - a randomized controlled trial. Oral Oncol 48: 893–897. [DOI] [PubMed] [Google Scholar]
- 18. Gouvea de Lima A, Villar RC, de Castro G Jr, Antequera R, Gil E, et al. (2012) Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: a phase III randomized study. Int J Radiat Oncol Biol Phys 82: 270–275. [DOI] [PubMed] [Google Scholar]
- 19. Hodgson BD, Margolis DM, Salzman DE, Eastwood D, Tarima S, et al. (2012) Amelioration of oral mucositis pain by NASA near-infrared light-emitting diodes in bone marrow transplant patients. Support Care Cancer 20: 1405–1415. [DOI] [PubMed] [Google Scholar]
- 20. Oton-Leite AF, Correa de Castro AC, Morais MO, Pinezi JCD, Leles CR, et al. (2012) Effect of intraoral low-level laser therapy on quality of life of patients with head and neck cancer undergoing radiotherapy. Head Neck 34: 398–404. [DOI] [PubMed] [Google Scholar]
- 21. Silva GBL, Mendonca EF, Bariani C, Antunes HS, Silva MAG (2011) The prevention of induced oral mucositis with low-level laser therapy in bone marrow transplantation patients: a randomized clinical trial. Photomed Laser Surg 29: 27–31. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 6 (7) e1000097 doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33: 159–174. [PubMed] [Google Scholar]
- 24.WHO (1979) WHO Handbook for Reporting Results of Cancer Treatment. Publication No. 48. Geneva: WHO.
- 25. Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31: 1341–1346. [DOI] [PubMed] [Google Scholar]
- 26. Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, et al. (2008) Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer. 16: 791–801. [DOI] [PubMed] [Google Scholar]
- 27.Higginson IJ, Armes J, Krishnasamy M (2004) Introduction, in fatigue in cancer. In: Armes J, Krishnasamy M, Higginson IJ, editors. Fatigue in Cancer. Oxford: Oxford University Press. pp. xvii–xxi.
- 28. Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, et al. (1997) Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol 34: 4–12. [PubMed] [Google Scholar]
- 29. Tardieu C, Cowen D, Thirion X, Franquin JC (1996) Quantitative scale of oral mucositis associated with autologous bone marrow transplantation. Eur J Cancer B Oral Oncol 32B: 381–387. [DOI] [PubMed] [Google Scholar]
- 30. Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH Jr, et al. (1999) Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer 85: 2103–2113. [DOI] [PubMed] [Google Scholar]
- 31. Collins JJ, Devine TD, Dick GS, Johnson EA, Kilham HA, et al. (2002) The measurement of symptoms in young children with cancer: the validation of the Memorial Symptom Assessment Scale in children aged 7–12. J Pain Symptom Manage 23: 10–16. [DOI] [PubMed] [Google Scholar]
- 32. Collins SL, Moore RA, McQuay HJ (1997) The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain 72: 95–97. [DOI] [PubMed] [Google Scholar]
- 33. Collins JJ, Byrnes ME, Dunkel IJ, Lapin J, Nadel T, et al. (2000) The measurement of symptoms in children with cancer. J Pain Symptom Manage 19: 363–377. [DOI] [PubMed] [Google Scholar]
- 34. Schulz KF, Chalmers I, Hayes RJ, Altman DG (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273: 408–412. [DOI] [PubMed] [Google Scholar]
- 35. Arun Maiya G, Sagar MS, Fernandes D (2006) Effect of low level helium-neon (He-Ne) laser therapy in the prevention & treatment of radiation induced mucositis in head & neck cancer patients. Indian J Med Res 124: 399–402. [PubMed] [Google Scholar]
- 36. Antunes HS, Herchenhorn D, Small I, Araujo C, Cabral E, et al. (2013) Cost-effectiveness of low-level laser therapy (LLLT) in head and neck cancer patients submitted to concurrent chemoradiation. Support Care Cancer 21: S199. [DOI] [PubMed] [Google Scholar]
- 37. Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Nigudgi S (2013) Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of life in head and neck cancer patients receiving chemoradiotherapy–a randomized controlled trial. Support Care Cancer 21: 1421–1428. [DOI] [PubMed] [Google Scholar]
- 38. Pires-Santos GM, Ferreira MFL, Oliveira SCPS, Monteiro JSC, Brugnera A, et al. (2012) Use of Laser photobiomodulation in the evolution of oral mucositis associated with CMF chemotherapy protocol in patients with breast cancer-Case Report. Medicina Oral, Patologia Oral y Cirugia Bucal 17: S252. [Google Scholar]
- 39. Chor A, Torres SR, Maiolino A, Nucci M (2010) Low-power laser to prevent oral mucositis in autologous hematopoietic stem cell transplantation. Eur J Haematol 84: 178–179. [DOI] [PubMed] [Google Scholar]
- 40. Antunes HS, de Azevedo AM, da Silva Bouzas LF, Adao CAE, Pinheiro CT, et al. (2007) Low-power laser in the prevention of induced oral mucositis in bone marrow transplantation patients: a randomized trial. Blood 109: 2250–2255. [DOI] [PubMed] [Google Scholar]
- 41. Cruz LB, Ribeiro AS, Rech A, Rosa LGN, Castro CG Jr, et al. (2007) Influence of low-energy laser in the prevention of oral mucositis in children with cancer receiving chemotherapy. Pediatr Blood Cancer 48: 435–440. [DOI] [PubMed] [Google Scholar]
- 42. Schubert MM, Eduardo FP, Guthrie KA, Franquin J-C, Bensadoun R-JJ, et al. (2007) A phase III randomized double-blind placebo-controlled clinical trial to determine the efficacy of low level laser therapy for the prevention of oral mucositis in patients undergoing hematopoietic cell transplantation. Support Care Cancer 15: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 43. Lopes CdO, Mas JRI, Zângaro RA (2006) Prevenção da xerostomia e da mucosite oral induzidas por radioterapia com uso do laser de baixa potência. Radiol bras 39: 131–136. [Google Scholar]
- 44. Bensadoun RJ, Franquin JC, Ciais G, Darcourt V, Schubert MM, et al. (1999) Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Support Care Cancer 7: 244–252. [DOI] [PubMed] [Google Scholar]
- 45. Bensadoun RJ, Ciais G (2002) Radiation-and chemotherapy-induced mucositis in oncology: results of multicenter phase III studies. J Oral Laser App 115–120. [Google Scholar]
- 46. Cowen D, Tardieu C, Schubert M, Peterson D, Resbeut M, et al. (1997) Low energy Helium-Neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: results of a double blind randomized trial. Int J Radiat Oncol Biol Phys 38: 697–703. [DOI] [PubMed] [Google Scholar]
- 47. Khouri VY, Stracieri ABPL, Rodrigues MC, Moraes DAd, Pieroni F, et al. (2009) Use of therapeutic laser for prevention and treatment of oral mucositis. Braz Dent J 20: 215–220. [DOI] [PubMed] [Google Scholar]
- 48. Oton-Leite AF, Elias LS, Morais MO, Pinezi JC, Leles CR, et al. (2013) Effect of low level laser therapy in the reduction of oral complications in patients with cancer of the head and neck submitted to radiotherapy. Spec Care Dentist 33: 294–300. [DOI] [PubMed] [Google Scholar]
- 49. Bjordal JM, Bensadoun RJ, Tuner J, Frigo L, Gjerde K, et al. (2011) A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support Care Cancer 19: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 50. Bowen JM, Elad S, Hutchins RD, Lalla RV (2013) Methodology for the MASCC/ISOO Mucositis Clinical Practice Guidelines Update. Support Care Cancer 21: 303–308. [DOI] [PubMed] [Google Scholar]
- 51. Stolik S, Delgado JA, Perez A, Anasagasti L (2000) Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J Photochem Photobiol B 57: 90–93. [DOI] [PubMed] [Google Scholar]
- 52. Enwemeka CS (2001) Attenuation and penetration of visible 632.8 nm and invisible infra-red 904 nm light in soft tissues. Laser Ther 95–101. [Google Scholar]
- 53. Hode L (2005) The importance of coherence. Photomed Laser Surg 23: 431–434. [DOI] [PubMed] [Google Scholar]
- 54. Enwemeka CS (2006) The place of coherence in light induced tissue repair and pain modulation. Photomed Laser Surg 24: 457. [DOI] [PubMed] [Google Scholar]
- 55. Schubert MM, Williams BE, Lloid ME, Donaldson G, Chapko MK (1992) Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer 69: 2469–2477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategies. Search strategies used in MEDLINE, EMBASE and EBM. Other database strategies are available on request.
(DOC)
Risk of bias assessment for included studies*.
(DOC)
Forest plot of incidence of severe (grade 3 or 4) mucositis at week 6±1 in head and neck cancer radiotherapy trials and at day 10±4 in chemotherapy or hematopoietic stem cell transplantation trials. Squares to the left of the vertical line indicate that low level laser therapy reduces mucositis. Horizontal lines through the squares represent confidence intervals (CIs). The size of the squares reflects each study's relative weight, and the diamond represents the aggregate risk ratio and 95% CI.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.