Abstract
Objective
α-klotho, a protein with anti-aging properties, has been involved in important biological processes, such as calcium/phosphate metabolism, resistance to oxidative stress, and nitric oxide production in the endothelium. Recent studies have suggested a role of α-klotho in endocrine regulation of mineral metabolism and postnatal growth in infants. Yet, the role of α-klotho during pregnancy remains largely unknown. The aim of this study was to determine whether maternal plasma concentration of α-klotho changes during pregnancy and evaluate its expression in pregnancies complicated by small-for-gestational age (SGA) and/or preeclampsia (PE).
Study design
This cross-sectional study included patients in the following groups: (1) non pregnant women (n=37); (2) uncomplicated pregnancy (n=130); (3) PE without an SGA (PE; n=58); (4) preeclampsia with an SGA neonate (PE and SGA; n=52); and (5) SGA neonate without preeclampsia (SGA; n=52). Plasma concentrations of α-klotho were determined by ELISA.
Results
The median plasma α-klotho concentration was higher in pregnant than in non-pregnant women. Among women with an uncomplicated pregnancy, the median plasma concentration of α-klotho increased as a function of gestational age (Spearman Rho=0.2; p=0.006). The median (interquartile range) plasma concentration of α-klotho in women with PE and SGA [947.6 (762 – 2013) pg/mL] and SGA without PE [1000 (585 – 1567) pg/mL] were 21% and 17% lower than that observed in women with an uncomplicated pregnancy [1206.6 (894 – 2012) pg/mL], (p=0.005 and p=0.02), respectively. Additionally, there were no significant differences in the median plasma concentration of α-klotho between uncomplicated pregnancies and women with PE without an SGA neonate (P=0.5).
Conclusion
Maternal plasma concentration of α-klotho was higher during pregnancy than in a non-pregnant state. Moreover, the median maternal plasma concentration of α-klotho was lower in mothers who delivered an SGA neonate than in those with an uncomplicated pregnancy regardless of the presence or absence of preeclampsia.
Keywords: Hypertension in pregnancy, metabolism, fetal growth restriction, lifespan, pregnancy
Introduction
Kuro-o et al. first reported the discovery of a gene with anti-aging properties in mice [1]. The gene was named “klotho” after Clotho, a mythological Greek goddess who was responsible for spinning the thread of human life from birth to death. Inactivation of the gene klotho in mice (kl−/kl−) produces a phenotype resembling human aging, characterized by postnatal growth retardation, arteriosclerosis, gonadal atrophy, osteopenia, altered calcium/phosphate metabolism, pulmonary emphysema, infertility, and a shortened life span, when compared with wild type mice [1–3]. In contrast, overexpression of the klotho gene extends the lifespan of mice by 20 – 30%, and makes them more resistant to oxidative stress [2,4,5].
In humans, the klotho gene encodes a type I single transmembrane protein (α-klotho) that shares 86% of amino acid sequence with the klotho mice protein [5,6]. The α-klotho protein exists in two forms: a transmembrane protein predominantly expressed in renal distal tubular cells, parathyroid glands, the choroid plexus, and adipose tissue [1,7–9]; and a secreted form which is produced by proteolytic cleavage of the extracellular domain of the transmembrane form or by alternative mRNA splicing [6,8–10]. The isoforms have different biological properties; the transmembrane portion of α-klotho plays a key role regulating phosphate and calcium metabolism as an obligate co-receptor of fibroblast growth factor-23 (FGF-23) which increases the urinary excretion of phosphorus and inhibits the synthesis of active vitamin D (1,25-dihydroxyvitamin D3) in the kidney [6,10–15]. The secreted form of α-klotho (130 KD) is found in blood, urine, and cerebrospinal fluid, and is involved in important biological processes, such as angiogenesis [16,17], energy metabolism [18,19], nitric oxide production in endothelium [20,21], synthesis of antioxidant enzymes [22,23], and protection against endothelial dysfunction [24].
Preeclampsia (PE) and pregnancies with small for gestational age (SGA) fetuses are two of the “great obstetrical syndromes” [25–27]. Both are associated with adverse perinatal outcomes and can occur simultaneously in some cases [28–47]. We, and others, have previously observed that pregnancies with SGA and PE share several pathophysiologic derangements, including abnormal placentation [27,48–54], utero-placental ischemia [55–57], exaggerated oxidative stress [58–66], and maternal endothelial cell dysfunction [39,67–81]. However, the precise mechanisms and pathways that determine why some patients develop preeclampsia and/or SGA remain unclear.
Recent studies have suggested a role of α-klotho in the endocrine regulation of mineral metabolism and postnatal growth in infants [82]. However, the biology of α-klotho during pregnancy is largely unexplored. We hypothesized that α-klotho may play a role during pregnancy complications due to its function in nitric oxide production and protection against endothelial dysfunction/oxidative stress. Therefore, the objective of this study was to determine if maternal plasma concentrations of α-klotho are different in: 1) uncomplicated pregnancies compared to non-pregnant women; and 2) pregnancies complicated with PE and/or SGA compared to uncomplicated pregnancies.
Methods
Study Design
This cross-sectional study was conducted by searching the Detroit Medical Center/Wayne State University and Perinatology Research Branch clinical database and bank of biological samples. Women in the following groups were included: (1) non-pregnant women; (2) those with an uncomplicated pregnancy; (3) women with PE without an SGA neonate; (4) women with PE and an SGA neonate; and (5) women with an SGA neonate but without PE. Women with multiple gestations, chronic hypertension, renal disease, and fetuses affected with chromosomal and/or congenital anomalies were excluded. Study participants were enrolled in research protocols at Hutzel Women’s Hospital, Detroit, MI, USA and provided written informed consent prior to the collection of information and blood samples. The Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Human Investigation Committee of Wayne State University approved the collection of samples and their utilization for research purposes.
Clinical definitions
Patients were considered to have an uncomplicated pregnancy if they met the following criteria: (1) gestational age at venipuncture between 20 – 42 weeks; (2) no medical, obstetrical, or surgical complications; (3) absence of labor at the time of venipuncture; and (4) delivery of a normal term (≥ 37 weeks) neonate whose birth weight was between the 10th and 90th percentile for gestational age. Non-pregnant women were enrolled in the secretory phase of their menstrual cycle. They were not taking oral contraceptives, and had no history of acute or chronic inflammatory conditions. Preeclampsia was defined as the presence of hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on at least two occasions, 4 hours to 1 week apart) and proteinuria (≥ 300 mg in a 24-hour urine collection or one dipstick measurement ≥ 2+) [83,84]. Severe preeclampsia was defined as previously described [85]. Patients with PE were classified as preterm (<37 weeks) or term (≥37 weeks), according to the gestational age at which PE was diagnosed. The diagnosis of SGA was based on an ultrasonographic estimated fetal weight and confirmed by a birthweight below the 10th percentile for gestational age, according to the reference range proposed by Alexander et al. [86,87].
Sample collection and human klotho immunoassay
Blood was collected into tubes containing EDTA. Samples were centrifuged and stored at −70°C. Concentrations of α-klotho were measured using ELISA (Immuno-Biological Laboratories, Inc. Minneapolis, MN, USA). Inter- and intra-assay coefficients of variation were: 4.6% and 3.9%, respectively; the sensitivity of the assays was 48 pg/mL. Laboratory technicians were masked to pregnancy outcomes.
Doppler velocimetry of the uterine and umbilical arteries
Pulse-wave and color Doppler ultrasound examinations of the uterine (UT) and umbilical arteries (UA) were performed in a subset of patients with PE and/or SGA as previously described (Acuson, Sequoia, Mountain View, CA) [88]. Uterine artery Doppler velocimetry was considered as abnormal if the mean (average of right and left) resistance index (RI) was above the 95th percentile for gestational age (using the reference range proposed by Kurmanavicius) [89]. Umbilical artery Doppler velocimetry was defined as abnormal if either the pulsatility index (PI) was above the 95th percentile for gestational age (using the reference range proposed by Arduini and Rizzo) [90] or in the presence of abnormal waveforms (absent or reversed end-diastolic velocities) [91].
Statistical Analysis
The Kolmogorov-Smirnov test and visual plot inspection were used to assess the normality of continuous data distribution. The Kruskal-Wallis test was used to compare differences in the distribution of arithmetic variables among groups. The Chi-square test was used to examine differences in proportions. Logarithmic transformation was performed to meet the assumptions of linear regression. General linear models were constructed to evaluate the relationship between log transformed α-klotho concentration and study groups, adjusting for potentially confounding variables (gestational age at venipuncture, maternal age, race, nulliparity, and smoking status). Model reduction was performed based on the plausibility of regression coefficients, association between independent variables, and the magnitude of change in the association between study group and predicted geometric mean of α-klotho concentrations. Two-sided p-values <0.05 were considered statistically significant. Statistical analyses were performed using SAS version 9.3 (SAS, Cary, NC, USA).
Results
Demographic and clinical characteristics of the study population [non-pregnant women (n=37), uncomplicated pregnancies (n=130), PE only (n=58), PE and SGA (n=52), and SGA without PE (n=52)] are displayed in Table 1. Nulliparous women were more common among women with pregnancy complications (SGA, and PE) than those with an uncomplicated pregnancy. There was no significant difference in the median gestational age at venipuncture among the groups (p=0.43; Table 1). Otherwise, clinical and demographic characteristics were similar across study groups. Among women with PE, 62.7% (69/110) were considered as having preterm PE, 81% (89/110) had severe PE, and 47% (52/110) delivered an SGA neonate.
Table 1.
Demographic and Clinical Characteristics of the study population
| Characteristic | Non pregnant Women (n=37) | Uncomplicated pregnancy (n=130) | Preeclampsi without SGA newborns (n=58) | Preeclampsia with SGA newborns (n=52) | Pregnancies with SGA newborns (n=52) | P value |
|---|---|---|---|---|---|---|
| Age (years) | 26 (24 – 31) | 25 (21 – 29) | 24 (19 – 30) | 23 (19–28) | 24 (20–29) | 0.05 |
| Race | 0.52 | |||||
| Black | 21 (56.8%) | 102 (78.5%) | 46 (79.3%) | 45 (86.5%) | 45 (86.5) | |
| White | 11 (29.7%) | 15 (11.5%) | 6 (10.3%) | 6 (11.5%) | 4 (7.7%) | |
| Hispanic | 1 (2.7%) | 7 (5.4%) | 4 (6.9%) | 1 (1.9%) | 1 (1.9%) | |
| Other | 4 (11.1%) | 6 (4.6%) | 2 (3.4%) | 0 | 2 (3.8%) | |
| Nulliparity | 22 (59.5%) | 35 (26.9%) | 38 (65.5%) | 29 (55.8%) | 26 (50%) | <0.0001 |
| Smoking | 3 (10.7%) | 22 (17.1%) | 7(12.1%) | 8 (15.4%) | 16 (30.8%) | 0.73 |
| GA at venipuncture (weeks) | NA | 38 (31.4 – 39.1) | 35.1 (30.1 – 38.7) | 36 (32.4 – 38.5) | 36.9 (32.7 – 38.4) | 0.43 |
| GA at delivery (weeks) | NA | 39.3 (38.7 – 40.3) | 35.8 (31.1 – 38.7) | 36 (30.7 – 38.6) | 37.2 (33.6–38.5) | <0.0001 |
| Birth weight (grams) | NA | 3352 (3120 – 3630) | 2620 (1740–3180) | 1860 (1450 – 2502) | 2060 (1510 – 2380) | <0.0001 |
GA: Gestational age. SGA: Small for gestational age. Values are expressed as number (percentage) or median (interquartile range). Comparisons among groups were performed using the Kruskal-Wallis test for continuous variables and the Chi-square test for categorical variables. A p value <0.05 was considered statistically significant. Missing data were: Race, n=1; Smoking, n=1
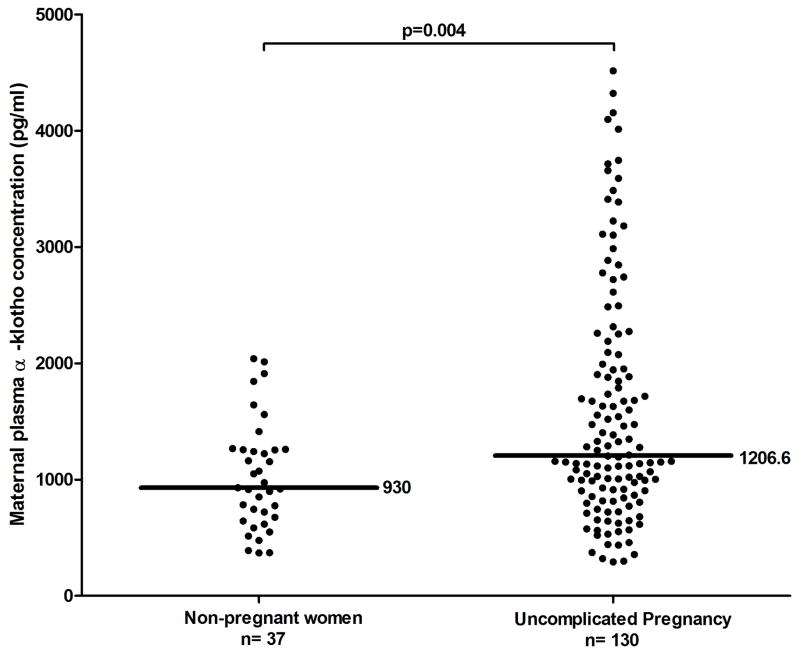
The median (interquartile range, IQR) α-klotho plasma concentration in women with uncomplicated pregnancies was higher than to that of non-pregnant women [1206 (894 – 2012) pg/mL vs. 930 (660 – 1258) pg/mL; (p=0.004)] (Figure 1). Among non-pregnant women, there was a correlation between plasma α-klotho concentrations and maternal age (Spearman’s Rho −0.5; p=0.001). In contrast, such relationship was not observed in uncomplicated pregnancies (Spearman’s Rho −0.14; p=0.1). The median plasma concentration of α-klotho among women with an uncomplicated pregnancy increased as a function of gestational age (Spearman Rho=0.2; p=0.006).
Figure 1.
Plasma concentration of α-klotho in non-pregnant women and in women with an uncomplicated pregnancy. Women with an uncomplicated pregnancy have a higher median plasma concentration of α-klotho than non-pregnant women [1206 pg/ml IQR: (894 – 2012) vs. 930 pg/ml IQR: (660–1258); p=0.004].
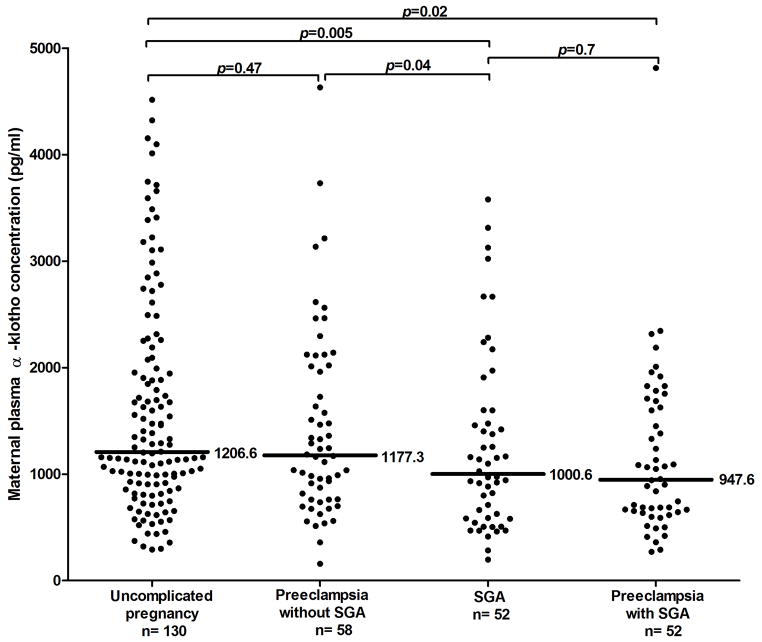
The unadjusted median plasma α-klotho concentration differed significantly among the groups of pregnant women (Kruskal-Wallis test p=0.01). Compared to women with an uncomplicated pregnancy, women with PE and SGA and those with isolated SGA had median plasma concentrations of α-klotho that were 21% and 17% lower, respectively (p=0.005 and p=0.02) (Figure 2). There was no significant difference in the median maternal plasma concentrations of α-klotho between women with PE who delivered an AGA neonate and those with uncomplicated pregnancies (p=0.47).
Figure 2.
Plasma concentration of α-klotho in patients with uncomplicated pregnancy, SGA, and preeclampsia, with and without SGA. Women with PE and SGA [947.6 pg/ml IQR: (762 – 2013)] and those with SGA without PE [1000.6 pg/ml IQR: (585 – 1567)] had a lower median plasma concentration of α-klotho than uncomplicated pregnancies [1206 pg/ml IQR: (894 – 2012)]; p=0.02 and p =0.005, respectively). However, there was no a significant difference in the median concentration of α-klotho between patients with PE without an SGA neonate [1177.3 pg/ml IQR: (762.4 – 2013.4)] and women with uncomplicated pregnancies (p=0.47).
After multivariable adjustments, differences in the mean maternal plasma α-klotho concentrations among groups remained significant. Holding the effect of potentially confounding variables constant (gestational age at venipuncture, maternal age, nulliparity, race, and tobacco use), the predicted geometric mean of α-klotho concentration was 25% and 30% lower in the isolated SGA group and in the PE and SGA groups, respectively, compared to uncomplicated pregnancies (both p<0.01). There was no significant difference in the predicted geometric mean of α-klotho concentration between women with PE who did not deliver an SGA neonate, and the uncomplicated pregnancy group (p=0.15).
Sub-classification (mild versus severe) of women with PE who did not deliver an SGA neonate by severity (mild vs. severe) did not alter the lack of difference in maternal plasma concentration of α-klotho compared to that of uncomplicated pregnancies. Study group differences in predicted geometric mean α-klotho concentrations also remained consistent across groups defined by gestational age at venipuncture (<34, 34 – 37, and >37 weeks). Effect modification terms used to examine whether the overall relationship between study group and geometric mean α-klotho concentrations differed according to gestational age at venipuncture: there were no significant differences, regardless of whether gestational age at venipuncture was examined as a continuous variable (p=0.32) or if it was categorized as ±37 weeks (p=0.87), or <34, 34–37, and >37 weeks (p=0.34). Overall, multivariable adjustment strengthened the association between SGA and maternal plasma α-klotho concentration compared to uncomplicated pregnancies (β, −0.25, increased by 16%).
Based on the association between lower maternal plasma α-klotho concentration and delivery of an SGA neonate, we constructed additional models to evaluate the relationship between α-klotho and birthweight in normal pregnancies. Holding the effect of gestational age at venipuncture constant, the relationship between maternal plasma α-klotho concentration and birthweight as a continuous variable was not statistically significant (p > 0.10).
Among SGA pregnancies [with PE (n=52) and without PE, (n=52)], Doppler velocimetry of the umbilical and uterine artery was performed in 78.8% (82/104) and 76% (79/104) of women, respectively. Among pregnancies complicated by SGA (with or without PE), 21.9% (18/82) had abnormal UA Doppler velocimetry and 64.5% (51/79) had abnormal UT Doppler velocimetry.
Table 2 shows that there were no significant differences in the median plasma α-klotho concentration among patients who delivered an SGA neonate whether UT Doppler velocimetry was normal or abnormal (p=0.31). Nor were there differences when comparing plasma α-klotho concentration of patients with isolated SGA (0.39) or, separately, those with PE and SGA (p=0.65) by UT Doppler velocimetry abnormality. In contrast, among patients who delivered an SGA neonate (with and without PE), the median plasma α-klotho concentration was lower in patients with abnormal UA Doppler velocimetry (p=0.006). Yet, this association was found only among isolated SGA (p=0.008), and not in the PE and SGA group (p=0.34).
Table 2.
Median (IQR) plasma α-klotho concentrations (pg/ml) in pregnancies with an SGA neonate, according to the results of uterine and umbilical artery Doppler velocimetry
| Category | n | Uterine Artery Doppler Velocimetry
|
p value | n | Umbilical Artery Doppler Velocimetry
|
p value | ||
|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | Normal | Abnormal | |||||
| SGA alone | 42 | 1267 (554 – 1830) | 932 (583 – 1307) | 0.39 | 43 | 1256 (815 – 1939) | 730.6 (564 – 906) | 0.008* |
| Preeclampsia+SGA | 37 | 980 (686 – 1782) | 953 (650 – 1525) | 0.65 | 39 | 1065 (680 – 1685) | 974 (621 – 1088) | 0.34 |
| Total | 79 | 1156 (612 – 1830) | 943 (635 – 1450) | 0.31 | 82 | 1162 (685 – 1703) | 810 (587 – 1056) | 0.006* |
Uterine artery (UT) Doppler velocimetry was defined as abnormal if the mean RI was >95th percentile for gestational age. Umbilical artery (UA) Doppler velocimetry was defined as abnormal if either the PI >95th percentile for gestational age or if absent or reversed end-diastolic velocity was present.
p<0.05
Multivariable regression analyses revealed that adjusting for potential confounders (gestational age at venipuncture, maternal age, nulliparity, race, and tobacco use), the association between study group (isolated SGA, PE and SGA, or uncomplicated pregnancy) and plasma α-klotho concentration did not vary significantly by UA or UT Doppler velocimetry abnormality (both p > 0.6).
Discussion
Principal findings of this study
The median plasma α-klotho concentration: 1) is higher in uncomplicated pregnant women than in non-pregnant women; 2) increases as a function of gestational age; 3) is lower in pregnancies with an SGA neonate (with or without PE) than in women with an uncomplicated pregnancy; and 4) is not significantly different between women with PE who did not deliver an SGA neonate and those with uncomplicated pregnancies, regardless of the severity of preeclampsia.
Klotho in health and disease
In humans, the circulating concentration of α-klotho increases with age [20,92,93], and after the age of 40 years, the concentration gradually declines [20]. The plasma concentration of α-klotho depends on the rate of synthesis (mainly in the kidney), and the proteolytic cleavage and subsequent release of the extra-membrane portion of the protein into the bloodstream. Secretion of α-klotho is determined by the presence of members of the A Disintegrin and Metalloproteinase (ADAM) family, principally ADAM10 and ADAM17, proteins responsible for the shedding of membrane proteins from the cellular surface [94]. Indeed, it has been reported that activation of ADAMs 10 and 17 produces an increase in the shedding of α-klotho, whereas the metalloproteinase inhibitor TAPI-1 decreases shedding of α-klotho to the extracellular fluid [8,94]. Furthermore, it has been proposed that insulin regulates α-klotho secretion, stimulating the activation of ADAM10 and/or ADAM17, resulting in an increase in the release of α-klotho [95].
α-klotho is excreted mainly in the urine, and the total amount of it’s excretion has been linked with the magnitude of the functioning nephrons in patients with chronic kidney disease [96]. Although the mechanisms which regulate the concentration of α-klotho are not fully understood, lower concentrations of α-klotho in plasma have been associated with age-related diseases, such as acute and chronic kidney disease [7,96–99], dyslipidemia [100], diabetes [99], and cancer [92,101]. Indeed, a growing body of evidence suggests that decreased plasma concentrations of α-klotho may be considered a candidate marker for endothelial dysfunction in cardiovascular and metabolic disorders [24,102–104].
Klotho expression during pregnancy
Our results indicate, for the first time, that pregnant women have a higher median plasma concentration of α-klotho than non-pregnant women, and that the plasma concentration of α-klotho increases as a function of gestational age. Knockout mice for α-klotho (kl−/kl−) are infertile, and there is no published information about the behavior of this protein in maternal blood during pregnancy. The precise origin of α-klotho during pregnancy is unknown. The changes in the expression of α-klotho during human pregnancy could be explained by two mechanisms: 1) modifications in the expression of α-klotho gene in the mother; and 2) production of α-klotho by the placenta with continuous transfer to the maternal circulation.
Knockout mice for α-klotho (kl−/kl−) have normal intrauterine development and are indistinguishable at birth from their wild type littermates [1], suggesting that the presence of the gene in fetuses is not essential for intrauterine development. This observation was supported by Ohyama et al. who reported that in rats, α-klotho expression in the fetal kidney is negligible, and increases considerably only after birth [105]. Moreover, prior studies suggest that gene expression and protein synthesis occur mainly in the placenta and not in the fetus [1,106].
In humans, Ohata et al. reported that serum samples from the umbilical vein of neonates at birth had higher concentrations of α-klotho compared with neonates of 4 days of age, mothers, and adult volunteers [106]. Additionally, Ohata et al. demonstrated the expression of α-klotho in syncytiotrophoblast and in the connective tissue of villi using immunohistochemistry [106].
Recently, Godang et al. reported a higher concentration of α-klotho in neonatal umbilical cord plasma compared to maternal plasma at 32–34 weeks of gestation and confirmed the expression of α-klotho in the syncytiotrophoblast [107]. The syncytiotrophoblast is the epithelial layer which covers the highly vascular embryonic placental villi, and is in direct contact with maternal blood [108]. The expression of α-klotho in the syncytiotrophoblast and its subsequent shedding by ADAM 17 – reported to be present in the syncytiotrophoblast [109]- could favor the release of the protein directly into the maternal circulation and explain the higher plasma concentration of α-klotho during pregnancy. The expression and synthesis of α-klotho protein in the placenta is of interest, since α-klotho is implicated in adipogenesis [19,110], angiogenesis [16,17], calcium metabolism [111,112], antioxidant effects [23], glucose metabolism [19], insulin signaling [4], and phosphate metabolism [113].
Additionally, the median (IQR) plasma concentration of α-klotho in non-pregnant women [930 (660–1258) pg/ml] reported herein is similar to that reported in women 24 hours postpartum (768 ± 261 pg/ml) [106] indicating that after delivery of the placenta, the maternal plasma concentration of α-klotho returns to pre-pregnancy levels.
Klotho expression in pregnancies complicated with preeclampsia and/or an SGA neonate
We report a novel finding that the median maternal plasma concentration of α-klotho is lower in women who delivered an SGA neonate, regardless of the presence or absence of PE. Although both PE and SGA are associated with failure of physiologic transformation of the spiral arteries [27,48–53,114], the factors which determine which women would develop PE and/or SGA remains unknown. The placenta is the principal regulator of nutrient transfer to the fetus and is a source of endocrine signals that affect the maternal and fetal metabolism. It has been proposed that the repetitive hypoxia/ischemia-reperfusion injury results in an exaggerated oxidative stress in the trophoblast, which could lead to: 1) epigenetic alterations in the placenta gene expression with subsequently impaired placental function [65,115,116]; and 2) endoplasmic reticulum (ER) stress which may affect placenta protein synthesis [115].
Recent evidence suggests that molecular adaptation and consequences of exaggerated oxidative stress in the trophoblast varies between pregnancies complicated with PE and/or SGA [39,65,117]. Pregnancies complicated by fetal growth restriction (with or without PE) have evidence of increased oxidative stress in placental tissue compared to that of pregnancies with PE alone [117,118]. The resulting ER stress is proposed to activate intracellular messengers, such as the eukaryotic translation initiation factor 2α (EIF2α), which is capable of attenuating mRNA translation, leading to a reduction in the production of placenta-derived proteins and reduced proliferation of trophoblast-like cell lines [117]. We hypothesize that the ER stress reported in placentas from pregnancies complicated with fetal growth restriction may alter the production of α-klotho in the syncytiotrophoblast, which has also been reported for other proteins related to placental function, such as placental growth factor [119], placental growth hormone [120], and placental lactogen [120,121].
A recent study reported that α-klotho mRNA expression was decreased in placentas from pregnancies with PE compared to that from normal pregnancies [122]. However, whether patients with PE also had SGA neonates was not reported in this study [122]. The concentrations of ADAM10 and ADAM17, which are responsible for the shedding of α-klotho into the bloodstream, has been reported to be higher in placenta from pregnancies complicated with preeclampsia compared to that in placentas from normal pregnancies [109,123]. However, our study did not observe a different plasma concentration of α-klotho in preeclampsia unless the fetus was also affected by SGA.
Consequences of the lower expression of α-klotho protein on postnatal growth
The deletion of α-klotho (kl−/kl−) in mice is associated with osteoporosis and postnatal growth retardation, expressed clinically as a lack of weight gain and a paucity of adipose tissue [1]. Recently, a role of α-klotho as a regulator of in vitro adipose adipocyte maturation has been described [19]. Plasma concentrations of α-klotho has been reported to be higher in full term neonates compared to preterm neonates in the early neonatal period (14 and 28 days), with a positive correlation with anthropometric parameters (body weight and length). This suggests that α-klotho increases as postnatal age advances, and may have a role in the regulation of postnatal growth [82].
Additionally, α-klotho has been identified as an endocrine factor capable of regulating calcium and phosphate metabolism in the kidney and bone [3,13,111,124,125]. During pregnancy, calcium and phosphate are actively transported across the placenta to the fetal circulation against a gradient, since fetal concentrations of calcium and phosphate are higher compared to those of the mother [126]. In the trophoblast, calcium is transported primarily via the transient receptor potential cation channel subfamily V (TRPV5 and TRPV6), which are key apical calcium channels demonstrated to be activated by α-klotho [10,111,112,126]. Lower production of α-klotho in pregnancies affected by SGA could be associated with impaired metabolism of calcium and phosphate in the fetus.
Strengths and Limitations
The cross-sectional nature of this study does not allow the establishment of a temporal relationship between changes in a-klotho concentrations and the development of SGA. This study provides the first evidence that maternal plasma concentrations of a-klotho are lower among women who deliver an SGA neonate, but not in cases with PE alone, when compared to women with an uncomplicated pregnancy.
Conclusion
The plasma concentration of α-klotho is increased in human pregnancy, unchanged in preeclampsia, and lower in the plasma of mothers who deliver SGA neonates, regardless of the presence or absence of preeclampsia.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104:229–237. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 4.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuro-o M. Klotho. Pflugers Arch. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 6.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 7.Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, et al. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–871. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- 8.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuro-o M. Klotho in health and disease. Curr Opin Nephrol Hypertens. 2012;21:362–368. doi: 10.1097/MNH.0b013e32835422ad. [DOI] [PubMed] [Google Scholar]
- 11.Nabeshima Y, Imura H. alpha-Klotho: a regulator that integrates calcium homeostasis. Am J Nephrol. 2008;28:455–464. doi: 10.1159/000112824. [DOI] [PubMed] [Google Scholar]
- 12.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 13.Huang CL, Moe OW. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch. 2011;462:185–193. doi: 10.1007/s00424-011-0950-5. [DOI] [PubMed] [Google Scholar]
- 14.Kuro-o M. Overview of the FGF23-Klotho axis. Pediatr Nephrol. 2010;25:583–590. doi: 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 15.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 17.Fukino K, Suzuki T, Saito Y, Shindo T, Amaki T, Kurabayashi M, Nagai R. Regulation of angiogenesis by the aging suppressor gene klotho. Biochem Biophys Res Commun. 2002;293:332–337. doi: 10.1016/S0006-291X(02)00216-4. [DOI] [PubMed] [Google Scholar]
- 18.Mori K, Yahata K, Mukoyama M, Suganami T, Makino H, Nagae T, Masuzaki H, et al. Disruption of klotho gene causes an abnormal energy homeostasis in mice. Biochem Biophys Res Commun. 2000;278:665–670. doi: 10.1006/bbrc.2000.3864. [DOI] [PubMed] [Google Scholar]
- 19.Razzaque MS. The role of Klotho in energy metabolism. Nat Rev Endocrinol. 2012;8:579–587. doi: 10.1038/nrendo.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl) 2004;117:742–747. [PubMed] [Google Scholar]
- 21.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 22.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–241. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–1601. doi: 10.1111/j.1532-5415.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero R. Prenatal medicine: the child is the father of the man 1996. J Matern Fetal Neona. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 26.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neona. 2009;22:633–635. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 27.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Seminars in perinatology. 1988;12:302–323. [PubMed] [Google Scholar]
- 29.Xiao R, Sorensen TK, Williams MA, Luthy DA. Influence of pre-eclampsia on fetal growth. J Matern Fetal Neona. 2003;13:157–162. doi: 10.1080/jmf.13.3.157.162. [DOI] [PubMed] [Google Scholar]
- 30.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba’aqeel H, Farnot U, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921–931. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 31.van Eerd EA, Roex AJ, Nikpoor P, Dekker GA. Adverse perinatal outcome and maternal risk factors in population versus customized defined SGA babies. J Matern Fetal Neona. 2012;25:369–373. doi: 10.3109/14767058.2011.579210. [DOI] [PubMed] [Google Scholar]
- 32.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 33.Gotsch F, Romero R, Kusanovic JP, Chaiworapongsa T, Dombrowski M, Erez O, Than NG, et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble Tie-2. J Matern Fetal Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203–212. doi: 10.1081/PRG-120021066. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giapros V, Drougia A, Krallis N, Theocharis P, Andronikou S. Morbidity and mortality patterns in small-for-gestational age infants born preterm. J Matern Fetal Neona. 2012;25:153–157. doi: 10.3109/14767058.2011.565837. [DOI] [PubMed] [Google Scholar]
- 38.Jelin AC, Cheng YW, Shaffer BL, Kaimal AJ, Little SE, Caughey AB. Early-onset preeclampsia and neonatal outcomes. J Matern Fetal Neona. 2010;23:389–392. doi: 10.1080/14767050903168416. [DOI] [PubMed] [Google Scholar]
- 39.Mirza FG, Strohsnitter WC, Rivera J, Gyamfi-Bannerman C. Intrauterine growth restriction with abnormal umbilical artery Dopplers: a harbinger for preeclampsia? J Matern Fetal Neona. 2012;25:2658–2661. doi: 10.3109/14767058.2012.704443. [DOI] [PubMed] [Google Scholar]
- 40.Nanthakomon T, Uerpairojkit B. Outcome of small-for-gestational-age fetuses according to umbilical artery Doppler: is there any yield from additional middle cerebral artery Doppler? J Matern Fetal Neona. 2010;23:900–905. doi: 10.3109/14767050903353208. [DOI] [PubMed] [Google Scholar]
- 41.Jelin AC, Cheng YW, Shaffer BL, Kaimal AJ, Little SE, Caughey AB. Early-onset preeclampsia and neonatal outcomes. J Matern Fetal Neonatal Med. 2010;23:389–392. doi: 10.1080/14767050903168416. [DOI] [PubMed] [Google Scholar]
- 42.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstetrics and gynecology. 2010;116:1302–1309. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 43.Schneider S, Freerksen N, Maul H, Roehrig S, Fischer B, Hoeft B. Risk groups and maternal-neonatal complications of preeclampsia--current results from the national German Perinatal Quality Registry. Journal of perinatal medicine. 2011;39:257–265. doi: 10.1515/jpm.2011.010. [DOI] [PubMed] [Google Scholar]
- 44.Adinma ED. Maternal and perinatal outcome of eclampsia in tertiary health institution in Southeast Nigeria. J Matern Fetal Neonatal Med. 2013;26:211–214. doi: 10.3109/14767058.2012.722708. [DOI] [PubMed] [Google Scholar]
- 45.Cosar H, Ozer E, Topel H, Kahramaner Z, Turkoglu E, Erdemir A, Sutcuoglu S, et al. Neuronal apoptosis in the neonates born to preeclamptic mothers. J Matern Fetal Neonatal Med. 2013 doi: 10.3109/14767058.2013.770463. [DOI] [PubMed] [Google Scholar]
- 46.Samuel A, Lin C, Parviainen K, Jeyabalan A. Expectant management of preeclampsia superimposed on chronic hypertension. J Matern Fetal Neonatal Med. 2011;24:907–911. doi: 10.3109/14767058.2010.535874. [DOI] [PubMed] [Google Scholar]
- 47.van Eerd EA, Roex AJ, Nikpoor P, Dekker GA. Adverse perinatal outcome and maternal risk factors in population versus customized defined SGA babies. J Matern Fetal Neonatal Med. 2012;25:369–373. doi: 10.3109/14767058.2011.579210. [DOI] [PubMed] [Google Scholar]
- 48.Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspectives in nephrology and hypertension. 1976;5:115–127. [PubMed] [Google Scholar]
- 49.Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clinics in obstetrics and gynaecology. 1977;4:573–593. [PubMed] [Google Scholar]
- 50.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:876–881. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 51.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 52.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. British journal of obstetrics and gynaecology. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 53.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. American journal of obstetrics and gynecology. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 54.Madazli R, Benian A, Ilvan S, Calay Z. Placental apoptosis and adhesion molecules expression in the placenta and the maternal placental bed of pregnancies complicated by fetal growth restriction with and without pre-eclampsia. J Obstet Gynaecol. 2006;26:5–10. doi: 10.1080/01443610500363840. [DOI] [PubMed] [Google Scholar]
- 55.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. American journal of physiology Heart and circulatory physiology. 2008;294:H541–550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 56.Ogge G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, Kim CJ, Hassan SS. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39:641–652. doi: 10.1515/JPM.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.George EM, Granger JP. Linking placental ischemia and hypertension in preeclampsia: role of endothelin 1. Hypertension. 2012;60:507–511. doi: 10.1161/HYPERTENSIONAHA.112.194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 59.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 60.Myatt L, Kossenjans W, Sahay R, Eis A, Brockman D. Oxidative stress causes vascular dysfunction in the placenta. The Journal of maternal-fetal medicine. 2000;9:79–82. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<79::AID-MFM16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 61.Potdar N, Singh R, Mistry V, Evans MD, Farmer PB, Konje JC, Cooke MS. First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. Bjog. 2009;116:637–642. doi: 10.1111/j.1471-0528.2008.02096.x. [DOI] [PubMed] [Google Scholar]
- 62.Madazli R, Benian A, Aydin S, Uzun H, Tolun N. The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia. J Obstet Gynaecol. 2002;22:477–480. doi: 10.1080/0144361021000003573. [DOI] [PubMed] [Google Scholar]
- 63.Cindrova-Davies T. Gabor Than Award Lecture 2008: pre-eclampsia - from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009;30 (Suppl A):S55–65. doi: 10.1016/j.placenta.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 64.Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertension in pregnancy: official journal of the International Society for the Study of Hypertension in Pregnancy. 2002;21:205–223. doi: 10.1081/PRG-120015848. [DOI] [PubMed] [Google Scholar]
- 65.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30 (Suppl A):S43–48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou X, Zhang GY, Wang J, Lu SL, Cao J, Sun LZ. A novel bridge between oxidative stress and immunity: the interaction between hydrogen peroxide and human leukocyte antigen G in placental trophoblasts during preeclampsia. American journal of obstetrics and gynecology. 2012;206:447 e447–416. doi: 10.1016/j.ajog.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 68.Chaiworapongsa T, Kim JC, Kim YM, Conoscenti G, Park KH, Edwin S, Yoon BH, Romero R. 9 Preeclampsia is characterized by a soluble selectin profile consistent with leukocyte and endothelial cell activation. Am J Obstet Gynecol. 2001;185:S74. [Google Scholar]
- 69.Friedman SA, Schiff E, Emeis JJ, Dekker GA, Sibai BM. Biochemical corroboration of endothelial involvement in severe preeclampsia. Am J Obstet Gynecol. 1995;172:202–203. doi: 10.1016/0002-9378(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 70.Chaiworapongsa T, Romero R, Yoshimatsu J, Espinoza J, Kim YM, Park K, Kalache K, et al. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;12:19–27. doi: 10.1080/jmf.12.1.19.27. [DOI] [PubMed] [Google Scholar]
- 71.Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dekker GA, van Geijn HP. Endothelial dysfunction in preeclampsia. Part I: Primary prevention. Therapeutic perspectives. J Perinat Med. 1996;24:99–117. doi: 10.1515/jpme.1996.24.2.99. [DOI] [PubMed] [Google Scholar]
- 73.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. American journal of hypertension. 1991;4:700–708. doi: 10.1093/ajh/4.8.700. [DOI] [PubMed] [Google Scholar]
- 74.Dekker GA, van Geijn HP. Endothelial dysfunction in preeclampsia. Part II: Reducing the adverse consequences of endothelial cell dysfunction in preeclampsia; therapeutic perspectives. J Perinat Med. 1996;24:119–139. doi: 10.1515/jpme.1996.24.2.119. [DOI] [PubMed] [Google Scholar]
- 75.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30 (Suppl A):S32–37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Seminars in reproductive endocrinology. 1998;16:17–31. doi: 10.1055/s-2007-1016249. [DOI] [PubMed] [Google Scholar]
- 77.Bretelle F, Sabatier F, Blann A, D’Ercole C, Boutiere B, Mutin M, Boubli L, et al. Maternal endothelial soluble cell adhesion molecules with isolated small for gestational age fetuses: comparison with pre-eclampsia. Bjog. 2001;108:1277–1282. doi: 10.1111/j.1471-0528.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 78.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neona. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soto E, Romero R, Richani K, Espinoza J, Chaiworapongsa T, Nien JK, Edwin SS, et al. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neona. 2010;23:646–657. doi: 10.3109/14767050903301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vasey PA, McMahon L, Paul J, Reed N, Kaye SB. A phase II trial of capecitabine (Xeloda) in recurrent ovarian cancer. Br J Cancer. 2003;89:1843–1848. doi: 10.1038/sj.bjc.6601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrozella L, Mahendroo M, Timmons B, Roberts S, McIntire D, Alexander JM. Endothelial microparticles and the antiangiogenic state in preeclampsia and the postpartum period. Am J Obstet Gynecol. 2012;207:140, e120–146. doi: 10.1016/j.ajog.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siahanidou T, Garatzioti M, Lazaropoulou C, Kourlaba G, Papassotiriou I, Kino T, Imura A, et al. Plasma soluble alpha-klotho protein levels in premature and term neonates: correlations with growth and metabolic parameters. Eur J Endocrinol. 2012;167:433–440. doi: 10.1530/EJE-12-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002 American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77:67–75. [PubMed] [Google Scholar]
- 84.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 85.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 86.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 87.Seeds JW. Impaired fetal growth: definition and clinical diagnosis. Obstet Gynecol. 1984;64:303–310. [PubMed] [Google Scholar]
- 88.Chaiworapongsa T, Romero R, Kusanovic JP, Mittal P, Kim SK, Gotsch F, Than NG, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol. 2010;35:155–162. doi: 10.1002/uog.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurmanavicius J, Florio I, Wisser J, Hebisch G, Zimmermann R, Muller R, Huch R, Huch A. Reference resistance indices of the umbilical, fetal middle cerebral and uterine arteries at 24–42 weeks of gestation. Ultrasound Obstet Gynecol. 1997;10:112–120. doi: 10.1046/j.1469-0705.1997.10020112.x. [DOI] [PubMed] [Google Scholar]
- 90.Arduini D, Rizzo G. Normal values of Pulsatility Index from fetal vessels: a cross-sectional study on 1556 healthy fetuses. J Perinat Med. 1990;18:165–172. doi: 10.1515/jpme.1990.18.3.165. [DOI] [PubMed] [Google Scholar]
- 91.Trudinger BJ, Cook CM, Giles WB, Ng S, Fong E, Connelly A, Wilcox W. Fetal umbilical artery velocity waveforms and subsequent neonatal outcome. Br J Obstet Gynaecol. 1991;98:378–384. doi: 10.1111/j.1471-0528.1991.tb13428.x. [DOI] [PubMed] [Google Scholar]
- 92.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2011;66:794–800. doi: 10.1093/gerona/glr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, Iwazu K, et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012;13:155. doi: 10.1186/1471-2369-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, Ohashi N, et al. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant. 2011;26:800–813. doi: 10.1093/ndt/gfq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wan M, Smith C, Shah V, Gullet A, Wells D, Rees L, Shroff R. Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol Dial Transplant. 2012 doi: 10.1093/ndt/gfs411. [DOI] [PubMed] [Google Scholar]
- 99.Devaraj S, Syed B, Chien A, Jialal I. Validation of an immunoassay for soluble Klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol. 2012;137:479–485. doi: 10.1309/AJCPGPMAF7SFRBO4. [DOI] [PubMed] [Google Scholar]
- 100.Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, Kurabayashi M, Kuroo M. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57:738–746. doi: 10.1007/s000180050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee J, Jeong DJ, Kim J, Lee S, Park JH, Chang B, Jung SI, et al. The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol Cancer. 2010;9:109. doi: 10.1186/1476-4598-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC, Dietz HC. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154–1161. doi: 10.1086/375035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim Y, Kim JH, Nam YJ, Kong M, Kim YJ, Yu KH, Lee BC, Lee C. Klotho is a genetic risk factor for ischemic stroke caused by cardioembolism in Korean females. Neurosci Lett. 2006;407:189–194. doi: 10.1016/j.neulet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 104.Moe SM. Klotho: a master regulator of cardiovascular disease? Circulation. 2012;125:2181–2183. doi: 10.1161/CIRCULATIONAHA.112.104828. [DOI] [PubMed] [Google Scholar]
- 105.Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, et al. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251:920–925. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 106.Ohata Y, Arahori H, Namba N, Kitaoka T, Hirai H, Wada K, Nakayama M, et al. Circulating levels of soluble alpha-Klotho are markedly elevated in human umbilical cord blood. J Clin Endocrinol Metab. 2011;96:E943–947. doi: 10.1210/jc.2010-2357. [DOI] [PubMed] [Google Scholar]
- 107.Godang K, Froeslie KF, Henriksen T, Isaksen GA, Voldner N, Lekva T, Ueland T, Bollerslev J. Umbilical cord levels of sclerostin, placental weight and birth weight are predictors of total Bone Mineral Content in neonates. Eur J Endocrinol. 2012 doi: 10.1530/EJE-12-0531. [DOI] [PubMed] [Google Scholar]
- 108.Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25:114–126. doi: 10.1016/j.placenta.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 109.Ma R, Gu Y, Groome LJ, Wang Y. ADAM17 regulates TNFalpha production by placental trophoblasts. Placenta. 2011;32:975–980. doi: 10.1016/j.placenta.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chihara Y, Rakugi H, Ishikawa K, Ikushima M, Maekawa Y, Ohta J, Kida I, Ogihara T. Klotho protein promotes adipocyte differentiation. Endocrinology. 2006;147:3835–3842. doi: 10.1210/en.2005-1529. [DOI] [PubMed] [Google Scholar]
- 111.Woudenberg-Vrenken TE, van der Eerden BC, van der Kemp AW, van Leeuwen JP, Bindels RJ, Hoenderop JG. Characterization of vitamin D-deficient klotho−/− mice: do increased levels of serum 1,25(OH)2D3 cause disturbed calcium and phosphate homeostasis in klotho−/− mice? Nephrol Dial Transplant. 2012;27:4061–4068. doi: 10.1093/ndt/gfs177. [DOI] [PubMed] [Google Scholar]
- 112.Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transplant. 2008;23:3397–3402. doi: 10.1093/ndt/gfn291. [DOI] [PubMed] [Google Scholar]
- 113.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. Faseb J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sheppard BL, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:695–705. doi: 10.1111/j.1471-0528.1981.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 115.Koukoura O, Sifakis S, Spandidos DA. DNA methylation in the human placenta and fetal growth (review) Mol Med Report. 2012;5:883–889. doi: 10.3892/mmr.2012.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol. 2008;173:451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lian IA, Loset M, Mundal SB, Fenstad MH, Johnson MP, Eide IP, Bjorge L, et al. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta. 2011;32:823–829. doi: 10.1016/j.placenta.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benton SJ, Hu Y, Xie F, Kupfer K, Lee SW, Magee LA, von Dadelszen P. Can placental growth factor in maternal circulation identify fetuses with placental intrauterine growth restriction? Am J Obstet Gynecol. 2012;206:163, e161–167. doi: 10.1016/j.ajog.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 120.Canete P, Monllor A, Pineda A, Hernandez R, Tarin JJ, Cano A. Levels of heat shock protein 27 in placentae from small for gestational age newborns. Gynecol Obstet Invest. 2012;73:248–251. doi: 10.1159/000334408. [DOI] [PubMed] [Google Scholar]
- 121.Mannik J, Vaas P, Rull K, Teesalu P, Rebane T, Laan M. Differential expression profile of growth hormone/chorionic somatomammotropin genes in placenta of small- and large-for-gestational-age newborns. J Clin Endocrinol Metab. 2010;95:2433–2442. doi: 10.1210/jc.2010-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giannubilo SR, Cecati M, Saccucci F, Corradetti A, Emanuelli M, Tranquilli AL. PP035 Placental klotho protein in preeclampsia: A possible link to long term outcomes. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health. 2012;2:260–261. doi: 10.1016/j.preghy.2012.04.146. [DOI] [PubMed] [Google Scholar]
- 123.Yang Y, Wang Y, Zeng X, Ma XJ, Zhao Y, Qiao J, Cao B, et al. Self-control of HGF regulation on human trophoblast cell invasion via enhancing c-Met receptor shedding by ADAM10 and ADAM17. J Clin Endocrinol Metab. 2012;97:E1390–1401. doi: 10.1210/jc.2012-1150. [DOI] [PubMed] [Google Scholar]
- 124.Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, Cass TA, et al. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest. 2012;122:4710–4715. doi: 10.1172/JCI64986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, Kaneko I, Haussler MR, Kerr Whitfield G. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414:557–562. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mitchell DM, Juppner H. Regulation of calcium homeostasis and bone metabolism in the fetus and neonate. Curr Opin Endocrinol Diabetes Obes. 2010;17:25–30. doi: 10.1097/MED.0b013e328334f041. [DOI] [PubMed] [Google Scholar]