Abstract
Background
Controversy exists as to whether angiotensin (1–7) (Ang (1–7)) acts as a protective hormone against renal injury.
Methods
We compared the degree of improvement of hypertensive nephropathy following 8 weeks’ treatment with either the angiotensin II receptor type 1 antagonist olmesartan medoxomil or the cardioselective beta blocker atenolol in 8-week-old spontaneously hypertensive rats (SHRs).
Results
Both treatment regimens reduced mean blood pressure in a similar fashion, while bradycardia was present only in atenolol-treated SHRs. The heart weight:body weight ratio fell more in SHRs medicated with olmesartan versus those receiving atenolol. These changes were associated with increases in plasma Ang II in SHRs given the angiotensin II receptor blocker. At the end of treatment, plasma Ang (1–7) was higher in the olmesartan than atenolol or vehicle groups. The glomerular sclerosis (GS) index was lowered by olmesartan and atenolol compared with the vehicle group. While both olmesartan and atenolol attenuated renal perivascular collagen deposition (PVCD), the greatest effect was observed in SHRs receiving olmesartan. Elevations in plasma Ang (1–7) correlated negatively with reductions in GS or PVCD index, respectively.
Conclusions
While control of blood pressure remains a critical factor in the prevention of hypertensive nephropathy, Ang (1–7) may play a substantial role in preventing the structural changes in glomerulus through its effect on regulations of blood pressure and renal function.
Keywords: angiotensin (1–7), angiotensin II, angiotensin-converting enzyme 2, angiotensin receptor blockers, angiotensin receptors, atenolol, blood pressure, glomerulosclerosis
Introduction
High blood pressure has a substantial effect on the structure and function of the nephron leading to hypertensive nephrosclerosis, the second most common cause of end-stage renal disease [Hill, 2008]. Hypertensive nephrosclerosis is characterized by perivascular collagen deposition (PVCD) and vascular smooth muscle cells proliferation leading to increased intrarenal vascular resistance, glomerular ischemia, and glomerular sclerosis (GS) [Morel-Maroger et al. 1984]. Angiotensin II (Ang II) profibrotic actions via activation of type 1 receptors (AT1) enhance collagen deposition in the kidneys [Yiu et al. 2008]. Likewise Ang II receptor blockers have been shown in both experimental [Zhao et al. 2008] and clinical hypertension [Eijkelkamp et al. 2007] to retard the progression of nephropathy.
Beginning with an early report by Tikellis and colleagues, evidence continues to accumulate on the potential role of the angiotensin (1–7)/angiotensin-converting enzyme 2/mas-receptor axis (Ang (1–7)/ACE2/mas-R axis) as a counterbalancing mechanism to the profibrotic actions of Ang II [Tikellis et al. 2003]. Ang (1–7) is an endogenous vasodilator and antiproliferative hormone that is generated from either angiotensin I (Ang I) via several tissue peptidases or cleaved from Ang II by ACE2 [Ferrario et al. 2005b, 2005c]. In the kidney, Ang (1–7) vasodilates afferent arterioles [Ren et al. 2002], reduces renal plasma flow and increases absolute and fractional sodium excretion leading to increased diuresis and natriuresis [DelliPizzi et al. 1994], inhibits proteinuria in diabetic spontaneously hypertensive rat (SHRs), modulates the Na+-ATPase activity present in basolateral membranes of renal proximal tubules [Handa et al. 1996], induces stimulation of bicarbonate transport [Garcia and Garvin, 1994] and 6-keto-PGF1 alpha renal content [Hilchey and Bell-Quilley, 1995]. Renal ACE2 mRNA is reduced in several models of hypertension and diabetes [Batlle et al. 2008; Oudit et al. 2006] while ACE2 overexpression ameliorates progression of renal damage in diabetic subjects [Wong et al. 2007].
The opposing actions of Ang (1–7) on Ang II suggest that a part of the antihypertensive and renoprotective actions of blockade of angiotensin-converting enzyme (ACE) or Ang II receptor blockers may be mediated by the Ang (1–7)/ACE2/mas-R axis [Ferrario et al. 2005c]. This possibility remains controversial since Shao and colleagues recently reported that exogenous administration of Ang (1–7) to diabetic rats exacerbated the progression of renal injury [Shao et al. 2008]. With this in mind, the current study evaluated the comparative effect of the administration of an Ang II receptor antagonist versus a beta blocker in the progression of hypertensive nephropathy in the SHR.
Methods
Experimental protocol
Experiments were performed in 24 SHRs, all 8-week-old males (Charles River, Wilmington, MA, USA), in accordance with the guidelines set forth by Animal Care and Use Committee of the Wake Forest University School of Medicine. During the experiments, rats were housed individually under a 12-hour light/dark cycle in an AAALAC-approved facility and had free to access food and drinking water. Rats, randomly assigned to one of three treatment groups, were medicated with: (a) olmesartan (RNH-6270; Sankyo Pharmaceutical Company, Tokyo, Japan, 10 mg/kg body weight [BW]/day); (b) atenolol (Sigma, St. Louis, MO, USA, 30 mg/kg BW/day); or (c) vehicle (tap water) for 8 weeks. Olmesartan and atenolol were dissolved in 0.1% NaHCO3 + KHCO3 solution and distilled water, respectively, and given to the rats in the drinking water. The amount of drug drank by the rats was adjusted daily based on the water consumed during the preceding 24 h.
At the end of the treatment regimen, rats were weighed and then anesthetized with Inactin (Sigma, St. Louis, MO, 100 mg/kg BW given intraperitoneally [i.p.]). Mean arterial blood pressures and heart rate were measured with a computer-based data acquisition system (Biopac Instruments; BIOPAC Systems, Goleta, CA) by insertion of a plastic catheter (PE-50 Clay Adams; Becton Dickinson & Company, Sparks, MD) into the carotid artery and attachment of the catheter to a transducer. Following collection of arterial blood from a plastic catheter, the rats were sacrificed by decapitation for the collection of trunk blood. The heart was removed and weighed to determine the heart weight:body weight ratio. The kidney was removed and placed in 4% formalin solution.
Biochemistry
Plasma concentrations of Ang II and Ang (1–7) were determined by radioimmunoassay from blood collected into chilled tubes containing a mixture of 25 mmol/l ethylene-diamine tetraacetic acid (Sigma, St. Louis, MO, USA), 0.44 mmol/l 1,20-orthophenanthrolene monohydrate, 1 mmol/l Na+ para chloromercuribenzoate, and 3 mmol/l WFML (rat renin inhibitor: acetyl–His–Pro–Phe–Val–Statine–Leu–Phe) as described in detail elsewhere [Igase et al. 2005]. Measures of renal cortical ACE2 gene expression and activity were performed as described by us in detail elsewhere [Ferrario et al. 2005a].
Histological evaluation in kidney
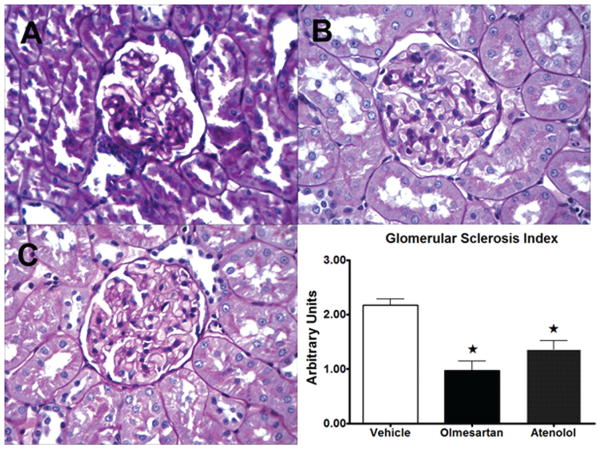
Sections of the kidneys were embedded in paraffin and 4 μm sections were transferred to subbed slides. After deparaffinization by sequential washes with xylene, ethanol (100%, 95%, and 75%), and double distilled water, tissue sections were stained with periodic acid-Schiff (PAS) and picrosirius red (PSR) and then examined by light microscopy. To assess the severity of GS in sections stained by PAS, the extent of the GS index was calculated by attributing a numerical injury score (scale: 0–4) to each glomeruli according to the apparent extent of the tuft area affected by mesangial expansion (mesangial cell proliferation and increase of extracellular matrix). An injury score of 0 means no damaged glomeruli, and 1, 2, 3, and 4 correspond to 1–30%, 31–60%, 61–90%, and 91–100% injured glomeruli, respectively. Fifty glomeruli were evaluated in each tissue slide.
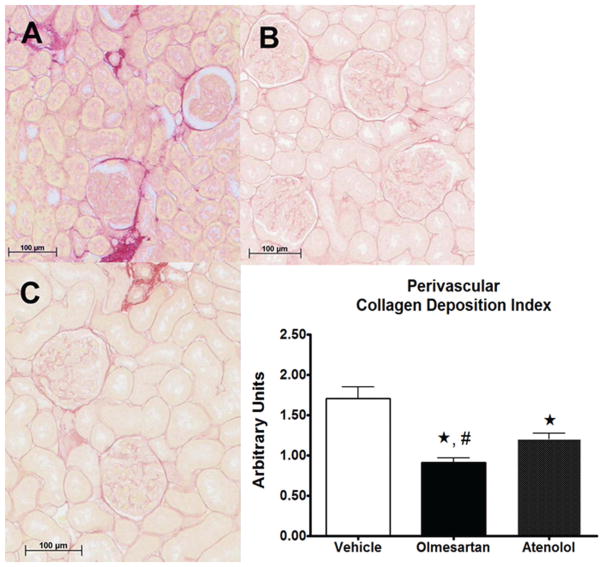
The severity of PVCD and the extent of PVCD index were evaluated in sections stained by PSR under light microscopy and photographed with a Zeiss AxioCam digital camera and AxioVision software (Zeiss), respectively. The digitized images at 20× magnifications were saved as JPEG files (1300 × 1030 pixels). Using Adobe Photoshop version 7.0 (Adobe System, San Jose, CA), the sum of pixels of PVCD stained by PSR and the sum of pixels of periglomerular artery were quantified in 10 arteries of the renal cortex from each kidney sample. The PVCD index was calculated as the sum of pixels of collagen deposition divided by the sum of pixels of the periglomerular artery. All indices were calculated for each rat as the weighted average of all individual scores.
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). The statistical analyses for mean blood pressure, heart rate, and body weight growth rate were performed using two-way analysis of variance (ANOVA) with repeated measure for time. Comparisons among three treatment groups were analyzed by ANOVA while post hoc multiple comparisons were determined by the unpaired Student’s t-test with appropriate correction of the significance level for multiple comparisons. Correlations between plasma concentrations of Ang (1–7) and renal histopathological parameters were analyzed by Pearson correlation analysis. Statistical significance was set to a p value < 0.05.
Results
Table 1 summarizes the effects of the administration of either olmesartan or atenolol on recorded variables. Both olmesartan and atenolol had equivalent effects in reducing the elevated blood pressure as compared with vehicle-treated SHRs while the antihypertensive effect of atenolol but not olmesartan was associated with bradycardia. The heart weight:body weight ratio, an index of cardiac hypertrophy, decreased more in SHRs given olmesartan than on those administered atenolol or vehicle. These changes occurred in the absence of corresponding alterations in body weight. Serum creatinine and urinary protein excretion at week 8 of the treatment period did not differ among SHRs given vehicle, olmesartan or atenolol (Table 1).
Table 1.
Main effect of treatment on hemodynamic, cardiac, and renal variables.
| Variables | Vehicle (n = 8) | Olmesartan (n = 8) | Atenolol (n = 8) |
|---|---|---|---|
| Mean blood pressure, mmHg | 173 ± 6 | 136 ± 6 | 146 ± 7 |
| p value | <0.05 | < 0.05 | |
| Heart rate, beats/min | 383 ± 8 | 391 ± 12 | 329 ± 10* |
| p value | n.s. | < 0.05 | |
| Body weight, g | 285 ± 4 | 301 ± 8 | 298 ± 5 |
| p value | n.s. | n.s. | |
| Heart weight, mg | 968 ± 14 | 873 ± 21# | 925 ± 14 |
| p value | < 0.05 | ||
| Heart weight:body weight ratio, mg/g | 3.40 ± 0.06 | 2.91 ± 0.05# | 3.11 ± 0.03 |
| p value | < 0.05 | < 0.05 | |
| Serum creatinine, mg/dl | 0.98 ± 0.18 | 0.92 ± 0.10 | 1.15 ± 0.16 |
| p value | |||
| Urinary protein, g/day | 1.78 ± 0.27 | 1.71 ± 0.44 | 1.72 ± 0.13 |
| p value | n.s. | n.s. |
Values are means ± standard error of the mean (SEM). p values denote statistical difference compared with vehicle-treated animals;
p < 0.05 versus atenolol;
p < 0.05 versus olmesartan;
n.s., not significant.
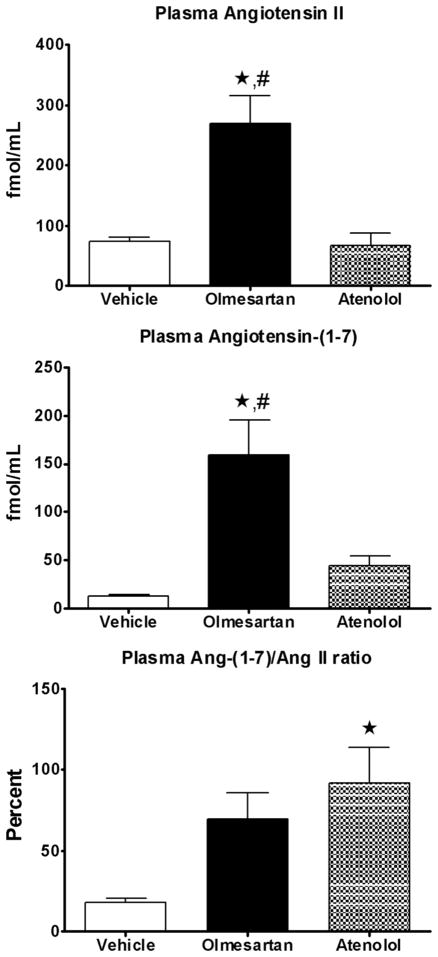
Figure 1 shows the effects of the treatment regimens on plasma concentrations of Ang II and Ang (1–7). Olmesartan but not atenolol-treatment was associated with increased plasma Ang II and Ang (1–7) compared with vehicle-treated rats (p < 0.05). The parallel increases in plasma Ang II and Ang (1–7) resulted in an increase in the Ang (1–7)/Ang II ratio that attained statistical significance in SHR medicated with atenolol (Figure 1). These changes occurred in the absence of alterations in plasma ACE activity. In addition, renal cortical ACE2 mRNA and ACE2 activity did not change with either treatment (data not shown).
Figure 1.
Angiotensin peptides concentrations in plasma after 8 weeks of treatment. Values are the mean ± standard error of the mean (SEM) of spontaneously hypertensive rats (SHRs) given vehicle (white bar), olmesartan (black bar), or atenolol (grey bar). *p < 0.05 versus vehicle; #p < 0.05 versus atenolol-treated SHRs.
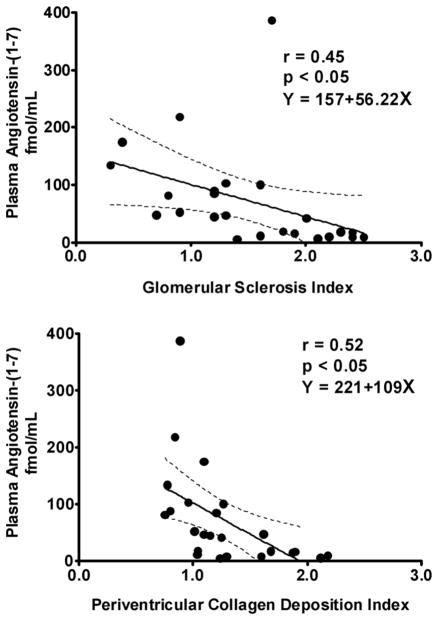
Representative cross sections of the renal tissues stained for PAS and PSR are shown in Figures 2 and 3, respectively. Both GS indices and PVCD indices were lower in both olmesartan and atenolol groups compared with vehicle (Figure 2 and 3). The reduction in PVCD indices in SHRs randomized to olmesartan was greater than that found in those receiving atenolol (Figure 3). Plasma levels of Ang (1–7) were inversely correlated with both GS and the PVCD indices (Figure 4), whereas there was no correlation of these indices with plasma Ang II levels.
Figure 2.
Representative cross sections of the renal cortical tissues stained for periodic acid-Schiff (PAS) in spontaneously hypertensive rats (SHRs) given vehicle (A), olmesartan (B), or atenolol (C) for 8 weeks. The renal cortical tissue from vehicle-treated SHRs shows greater mesangial cell proliferation and increase of extracellular matrix in comparison with those from olmesartan-treated or atenolol-treated SHRs, therefore glomerular sclerosis (GS) indices were lower in both olmesartan and atenolol groups compared with vehicle (graph). Values are the means ± standard error of the mean (SEM). *p < 0.05 versus vehicle group.
Figure 3.
Representative cross sections of the renal cortical tissues stained for picrosirius red (PSR) in spontaneously hypertensive rats (SHRs) given vehicle (A), olmesartan (B), or atenolol (C) for 8 weeks. The renal cortical tissue from vehicle-treated SHRs had substantially greater perivascular collagen deposition (PVCD) area in comparison with those from olmesartan-treated or atenolol-treated SHRs, therefore PVCD indices were lower in both olmesartan and atenolol groups compared with vehicle. The reduction in PVCD indices in SHRs randomized to olmesartan was greater than that found in those receiving atenolol (graph). Values are the mean ± standard error of the mean (SEM). *p < 0.01 versus vehicle group, #p < 0.05 versus atenolol group.
Figure 4.
Correlation between plasma angiotensin (1–7) (Ang (1–7)) levels and glomerular sclerosis (GS) index (top panel) and perivascular collagen deposition (PVCD) index (bottom panel). Plasma levels of Ang (1–7) were inversely correlated with both GS and the PVCD indices.
Discussion
When two different agents with different mechanisms of action but similar antihypertensive effect are employed to normalize the elevated blood pressure in SHRs, their effects in terms of changes in plasma angiotensin peptides and indices of renal structural remodeling are not equivalent. This interpretation is derived in the current study which showed that the equivalent antihypertensive actions of the Ang II receptor antagonist olmesartan medoxomil and the beta blocker atenolol was associated in olmesartan-treated SHRs with increased plasma Ang II and Ang (1–7) levels, and a more robust reversal of cardiac hypertrophy, GS, and renal collagen deposition. The current findings expand our previous observation showing a greater effect of olmesartan compared with atenolol in the reversal of cardiac and mesenteric artery vascular hypertrophy in SHRs [Yokoyama et al. 2005]. Since mesenteric arteries behave both structurally and functionally like intrarenal arteries [Li and Schiffrin, 1996], we sought to determine in the current study whether a similar result could be achieved in terms of reversal or glomerulosclerosis SHR medicated with the two agents. In agreement with this possibility, treatment with either olmesartan or atenolol attenuated the GS indices and PVCD indices within the renal cortex of SHRs, although the magnitude of the effect was significantly greater in SHRs medicated with the Ang II receptor blocker.
In this study, each treatment was started at age 8 weeks, a time at which rats are still transitioning into the established phase of sustained hypertension. After 8 weeks of treatment there were not significant differences of renal function estimated by serum creatinine or urinary protein excretion among SHRs medicated with either olmesartan or atenolol. Although GS indices in olmesartan-treated SHRs were lower than in rats medicated with atenolol, differences within the two treatments did not attain statistical significance. On the other hand, the reduction in PVCD in SHRs randomized to olmesartan was significantly greater than that found in rats receiving atenolol. These data suggest a greater impact of the treatment on the known profibrotic actions of Ang II.
As expected blockade of AT1 receptors is associated with increases in plasma Ang II levels which are the result of increased Ang II formation due to interruption of the negative feedback that Ang II exerts on renin synthesis and release and also blockade of AT1-mediated cellular uptake. The demonstration that the increases in circulating Ang II are also associated with an almost sevenfold increase in plasma Ang (1–7) levels are in keeping with our previous findings showing that the heptapeptide acts to oppose the actions of Ang II. Since in our study, the increases in Ang (1–7) occurs in the presence of AT1-receptor blockade, the data supports the view that Ang (1–7) may contribute directly to the antihypertensive and antifibrotic effects observed with the administration of olmesartan. This interpretation agrees with the findings of significant inverse correlations among plasma Ang (1–7) levels and either the GS index or PVCD index. Accumulating evidence suggest that Ang (1–7) functions as an endogenous inhibitor of the vasoconstrictor and proliferative actions of Ang II, as well as opposing the actions of the peptide on the regulation of renal function [Ferrario and Varagic, 2010]. Ang (1–7) is predominantly localized to the inner renal cortex and outer medulla and was found in proximal and distal tubules in SHRs [Ferrario et al. 2002]. Ang (1–7) stimulates synthesis and release of vasodilator prostaglandins, augments the metabolic actions of bradykinin, and increases the release of nitric oxide [Ferrario et al. 2010]. The present study provides new information of a renal vaso-protective role of Ang (1–7) by demonstrating that plasma concentrations of the peptide correlated with histological indices of renal damage.
The current finding is also in keeping with a previous report showing that Ang (1–7) contributes to the antihypertensive action of ACE inhibitors and Ang II receptor blockers. Although the Ang (1–7)/Ang II ratio, an index of the relative blood containing proportions of Ang (1–7) and Ang II, was markedly increased in both olmesartan and atenolol-treated SHRs, the higher Ang (1–7)/Ang II ratio achieved statistical significance only in rats medicated with atenolol. Nevertheless, the increased Ang (1–7)/Ang II ratio indicates that in response to the treatments, the proportion of circulating Ang (1–7) is now higher than the amounts of circulating Ang II. Given the results from prior studies, it would have been expected that blockade of AT1 receptors will be associated with increased ACE2 expression since this enzyme catalyzes Ang II into Ang (1–7). This did not occur in the present experiments as renal cortical ACE2 mRNA and activity were not different from vehicle in SHRs medicated with either olmesartan or atenolol. Because ACE2 measures were performed in these animals at a longer post-time treatment period (8 weeks) as compared with previous experiments (14 days), it is possible that increased ACE2 expression and activity in response to Ang II blockade may not be sustained. Ang (1–7) formation from Ang I is mediated by a several endopeptidases, particularly neprilysin and prolyl endopeptidase, the increased Ang (1–7) may be the result of enhanced activity in response to increase in Ang I. This interpretation agrees with the observation of increased plasma Ang (1–7) levels but not plasma Ang II in SHRs medicated with atenolol.
Boffa and colleagues suggested that blockade of Ang II receptors is associated with a greater effect on prevention of end-stage renal disease [Boffa et al. 2003]. Our study also agrees with this report that an olmesartan-induced effect may contribute to a blood pressure-independent reduction in renal pathology. Our findings demonstrated that blockade of AT1 receptors confers a more selective renal vasoprotective effect compared with atenolol despite roughly equal control of blood pressure. The more pronounced effects of olmesartan treatment on glomerular sclerosis, perivascular collagen deposition, and plasma Ang (1–7) suggest a contribution of Ang (1–7) in mediating the beneficial effects of AT1 receptor blockade on renal function.
In conclusion, our data demonstrate that the blood pressure lowering effect of both olmesartan and atenolol was associated with significant attenuation of GS and PVCD in the kidney of young SHRs. In addition, we now show that the beneficial effects of these treatments correlated with changes in plasma concentrations of Ang (1–7), a finding that provides additional support to an important role of this peptide in the modulation of renal function and disease. Our study encompasses the pharmacology of Ang (1–7) and expounds upon the peptide’s potential as a therapeutic agent against pathological processes both within and outside of the renovascular continuum.
Acknowledgments
The authors gratefully acknowledge the technical support and assistance of Dr Jewell Jessup and Ms Cindy Andrews in the experiments.
Funding
The research was supported by a grant from the National Heart, Lung and Blood Institute (grant number HL-51952) and an unrestricted educational grant from Sankyo Pharmaceutical Company (Tokyo, Japan). We also gratefully acknowledge grant support provided by Unifi, Inc., Greensboro, NC, and the Farley-Hudson Foundation, Jacksonville, NC.
Footnotes
Reprints and permissions: http://www.sagepub.co.uk/journalsPermissions.nav
Conflict of interest statement
The authors declare no conflicts of interest in preparing this article.
Contributor Information
Michiya Igase, Department of Geriatric Medicine, Ehime University Graduate School of Medicine, Shitsukawa, Toon City, Ehime 791-0295, Japan.
Hiroshi Yokoyama, Tagawa Municipal Hospital, Fukuoka, Japan.
Carlos M. Ferrario, Department of Surgery, Wake Forest University, School of Medicine, Winston-Salem, NC, USA.
References
- Batlle D, Soler MJ, Wysocki J. New aspects of the renin–angiotensin system: angiotensin-converting enzyme 2 - a potential target for treatment of hypertension and diabetic nephropathy. Curr Opin Nephrol Hypertens. 2008;17:250–257. doi: 10.1097/MNH.0b013e3282f945c2. [DOI] [PubMed] [Google Scholar]
- Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C. Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol. 2003;14:1132–1144. doi: 10.1097/01.asn.0000060574.38107.3b. [DOI] [PubMed] [Google Scholar]
- DelliPizzi AM, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin(1-7) Br J Pharmacol. 1994;111:1–3. doi: 10.1111/j.1476-5381.1994.tb14014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp WB, Zhang Z, Brenner BM, Cooper ME, Devereux RB, Dahlof B, et al. Renal function and risk for cardiovascular events in type 2 diabetic patients with hypertension: the RENAAL and LIFE studies. J Hypertens. 2007;25:871–876. doi: 10.1097/HJH.0b013e328014953c. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Ahmad S, Joyner J, Varagic J. Advances in the renin angiotensin system focus on angiotensin-converting enzyme 2 and angiotensin-(1-7) Adv Pharmacol. 2010;59:197–233. doi: 10.1016/S1054-3589(10)59007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Averill DB, Brosnihan KB, Chappell MC, Iskandar SS, Dean RH, et al. Vasopeptidase inhibition and Ang-(1-7) in the spontaneously hypertensive rat. Kidney Int. 2002;62:1349–1357. doi: 10.1111/j.1523-1755.2002.kid559.x. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005a;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, et al. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int. 2005b;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005c;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Varagic J. The ANG-(1-7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Renal Physiol. 2010;298:F1297–F1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia NH, Garvin JL. Angiotensin 1-7 has a biphasic effect on fluid absorption in the proximal straight tubule. J Am Soc Nephrol. 1994;5:1133–1138. doi: 10.1681/ASN.V541133. [DOI] [PubMed] [Google Scholar]
- Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1-7): in vivo and in vitro studies. Am J Physiol. 1996;270:F141–F147. doi: 10.1152/ajprenal.1996.270.1.F141. [DOI] [PubMed] [Google Scholar]
- Hilchey SD, Bell-Quilley CP. Association between the natriuretic action of angiotensin-(1-7) and selective stimulation of renal prostaglandin I2 release. Hypertension. 1995;25:1238–1244. doi: 10.1161/01.hyp.25.6.1238. [DOI] [PubMed] [Google Scholar]
- Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens. 2008;17:266–270. doi: 10.1097/MNH.0b013e3282f88a1f. [DOI] [PubMed] [Google Scholar]
- Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1-7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H1013–H1019. doi: 10.1152/ajpheart.00068.2005. [DOI] [PubMed] [Google Scholar]
- Li JS, Schiffrin EL. Effect of calcium channel blockade or angiotensin-converting enzyme inhibition on structure of coronary, renal, and other small arteries in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1996;28:68–74. doi: 10.1097/00005344-199607000-00011. [DOI] [PubMed] [Google Scholar]
- Morel-Maroger SL, Killen PD, Chi E, Striker GE. The composition of glomerulosclerosis. I. Studies in focal sclerosis, crescentic glomerulonephritis, and membranoproliferative glomerulonephritis. Lab Invest. 1984;51:181–192. [PubMed] [Google Scholar]
- Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, et al. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168:1808–1820. doi: 10.2353/ajpath.2006.051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- Shao Y, He M, Zhou L, Yao T, Huang Y, Lu LM, et al. Chronic angiotensin (1-7) injection accelerates STZ-induced diabetic renal injury. Acta Pharmacol Sin. 2008;29:829–837. doi: 10.1111/j.1745-7254.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, et al. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, et al. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu WH, Pan CJ, Ruef RA, Peng WT, Starost MF, Mansfield BC, et al. Angiotensin mediates renal fibrosis in the nephropathy of glycogen storage disease type Ia. Kidney Int. 2008;73:716–723. doi: 10.1038/sj.ki.5002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Averill DB, Brosnihan KB, Smith RD, Schiffrin EL, Ferrario CM. Role of blood pressure reduction in prevention of cardiac and vascular hypertrophy. Am J Hypertens. 2005;18:922–929. doi: 10.1016/j.amjhyper.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen SS, Chen Y, Ahokas RA, Sun Y. Kidney fibrosis in hypertensive rats: role of oxidative stress. Am J Nephrol. 2008;28:548–554. doi: 10.1159/000115289. [DOI] [PMC free article] [PubMed] [Google Scholar]