Abstract
Although polymorphisms in TLR receptors and downstream signaling molecules affect the innate immune response, these variants account for only a portion of the ability of the host to respond to microorganisms. To identify novel genes that regulate the host response to systemic lipopolysaccharide (LPS), we created an F2 intercross between susceptible (FVB/NJ) and resistant (129S1/SvImJ) strains, challenged F2 progeny with LPS via intraperitoneal injection, and phenotyped 605 animals for survival and another 500 mice for serum concentrations of IL-1β and IL-6. Genome-wide scans were performed on pools of susceptible and resistant mice for survival, IL-1β, and IL-6. This approach identified a locus on the telomeric end of the q arm of chromosome 9 (0–40 Mb) that was associated with the differences in morbidity and serum concentrations of IL-1β and IL-6 following systemic LPS in FVB/NJ and 129S1/SvImJ strains of mice. Fine mapping narrowed the locus to 3.7 Mb containing 11 known genes, among which are three inflammatory caspases. We studied expression of genes within the locus by quantitative RT-PCR and showed that Casp1 and Casp12 levels are unaffected by LPS in both strains, whereas Casp4 is highly induced by LPS in FVB/NJ but not in 129S1/SvImJ mice. In conclusion, our mapping results indicate that a 3.7-Mb region on chromosome 9 contains a gene that regulates differential response to LPS in 129S1/SvImJ and FVB/NJ strains of mice. Differences in the induction of Casp4 expression by LPS in the two strains suggest that Casp4 is the most likely candidate gene in this region.
Introduction
The innate immune system engages germline-encoded pattern-recognition receptors (PRRs) to detect a range of microbial motifs. PRRs are expressed by cells at the front line of host defense, including macrophages, dendritic cells, neutrophils, and epithelial cells, as well as cells of the adaptive immune system. Lipopolysaccharide (LPS), a major component of the cell wall of Gram-negative (GN) bacteria, is a pathogen-associated molecular pattern (PAMP) that is a potent stimulator of the innate immune response in mice and humans. Binding of LPS to the receptor complex, which consists of TLR4, CD14, and MD-2, initiates a signaling cascade that leads to translocation of NF-κB into the nucleus and the release of proinflammatory cytokines and chemokines (Akira and Takeda 2004; Takeuchi and Akira 2010). This inflammatory response affects development and progression of a number of diseases, including sepsis and septic shock.
Genetic factors play a significant role in the regulation of systemic inflammation. In addition to representing potential therapeutic targets for diseases such as sepsis, innate immune genes could also be used to identify individuals at highest risk for complications from sepsis. Polymorphisms in genes in the TLR4 pathway (Tlr4, Cd14, Irak4), downstream proinflammatory cytokines (TNF-α, IL-1β, IL-6), and genes in the coagulation/fibrinolysis cascade (Pai1, Factor V, Tafi) and other pathways (Mbl, heat shock proteins) have been shown to predispose individuals for increased susceptibility to sepsis and adverse outcomes (Cook et al. 2004a; Garantziotis et al. 2008; Papathanassoglou et al. 2006). Studies performed in mouse models support the theory that genetic background plays a significant role in development and progression of infection. For example, there is a range of responses among inbred strains of mice to inhaled (Lorenz et al. 2001) and systemic LPS (De Maio et al. 1998; Yang et al. 2009, 2010).
In fact, genetic studies in mice have been used extensively to identify novel innate immune genes that potentially contribute to host defense against microbial infections. Quantitative trait loci (QTLs) controlling various aspects of inflammation and survival in response to LPS (Cook et al. 2004b; Fulton et al. 2006; Matesic et al. 1999, 2000) and different species of Salmonella (Caron et al. 2002, 2005; Sebastiani et al. 1998) have been identified using F2 intercrosses between inbred strains of mice, recombinant inbred (RI) panels, and congenic strains. The emerging Collaborative Cross panel, which is derived from random mixing of eight founder strains, results in high phenotypic diversity and may enhance our ability to map causative loci underlying innate immune responsiveness in the future (Aylor et al. 2011).
Our recent work used whole-genome association mapping combined with gene expression profiling and RNA interference to identify novel candidate genes involved in the host response to systemic LPS (Yang et al. 2009). In this study and another previous study in our laboratory (Yang et al. 2010), the 129S1/SvImJ strain of mice was shown to be resistant while FVB/NJ mice were sensitive to systemic LPS. To identify genes that confer differential susceptibility of these two strains to systemic LPS, we created an F2 intercross between the susceptible (FVB/NJ) and resistant (129S1/SvImJ) strains. We used the F2 population to map a 3.7-Mb locus on Chr 9 that contains 11 known genes and controls differences in morbidity and production of IL-1β and IL-6. We showed that expression of Casp4 is highly induced by LPS in FVB/NJ but not in 129S1/SvImJ mice, suggesting that Casp4 is the most likely candidate gene in this locus.
Materials and methods
Ethics statement
Animal work was approved by the Institutional Animal Care and Use Committee (IACUC) at NIEHS. Every effort was made to ensure that discomfort, distress, and pained injury to animals was limited to that which is unavoidable in the conduct of scientifically sound research. Animals were monitored and cared for by veterinarians at NIEHS.
(129S1/SvImJ) × (FVB/NJ) F2 intercross
Female 129S1/SvImJ mice (Jackson Laboratories, Bar Harbor, ME) were bred with male FVB/NJ (Jackson Laboratories, Bar Harbor, ME) mice to create F1 animals (129S1/SvImJ × FVB/NJ). F2 progeny were generated by brother–sister mating of the F1 generation. Six- to eight-week-old male and female F2s were used for all experiments.
Mouse model
We used an established model of endotoxic shock in which mice are injected intraperitoneally with 125,000 EU/g [assessed by the chromogenic Limulus amebocyte lysate (LAL) kit; Cambrex, East Rutherford, NJ] of E. coli 0111:B4 LPS (Sigma, St. Louis, MO) or sterile saline control with no D-galactosamine sensitization (Buras et al. 2005). One group of mice (n = 605) was observed for clinical signs of morbidity for 5 days following LPS challenge and euthanized according to guidelines set by Morton and Griffiths (1985). The second group of animals was sacrificed 6 h post-LPS for serum and organ collection (n = 500). Serum cytokine concentrations were determined using standard ELISA kits and protocols (R&D Systems, Minneapolis, MN).
Genome-wide scan
Allele-specific genotyping by quantitative PCR (Germer and Higuchi 1999) was used to genotype pools of DNA from the most susceptible and resistant F2 mice at 300 SNP markers throughout the mouse genome. Pools (rather than individual samples) of DNA were used for genotyping to reduce the cost, and by using the most susceptible and resistant F2 mice, we were also able to increase the signal-to-noise ratio. Quantitative PCR was performed using 2 × SYBR® Green mix (Applied Biosystems, Foster City, CA) on a 7900 Sequence Detection System (Applied Biosystems). Genotyping data were plotted as %FVB DNA in each pool as a function of chromosomal location (Germer et al. 2000). The same genotyping methodology was applied for fine mapping of the locus on Chr 9 except that individual DNA samples were used and data were analyzed to look for the most informative recombinant events. All loci and primers designed using previously published strategy (Cook et al. 2004b; Germer and Higuchi 1999; Germer et al. 2000) are listed in the Supplementary Table 1.
Gene expression studies
RNA from lung, liver, and spleen of two to four animals intraperitoneally challenged with LPS or saline was extracted using the RNAEasy kit (Qiagen, Valencia, CA) and pooled prior to expression studies. Primers for Casp1, Casp4, and Casp12 were designed in Primer3 to create 100–150-bp fragments that span at least one exon–exon junction (Supplementary Table 2). RT-PCR was performed in a one-step reaction using Multiscribe reverse transcriptase and 2× SYBR Green mix (Applied Biosystems) on a 7900 Sequence Detection System (Applied Biosystems). Data were analyzed using the ΔΔCt method (Livak and Schmittgen 2001; Schmittgen and Livak 2008).
Sequencing
Primer pairs to generate overlapping amplicons for resequencing the proximal promoter (2 kb), exons of Casp4, and all putative NF-κB binding sites in the 5-kb upstream region were designed on sequences masked for repetitive elements, SNPs, and homology to other regions of the genome using Primer 3 (Supplementary Table 3). PCR followed by cycle sequencing reactions at 1/64 Big Dye reaction scale were performed. Magnetic bead cleaned (Agencourt Bioscience, Beverly, MA) cycle sequencing reactions were run on ABI 3730 sequencers (Applied Biosystems). Sequence data files were uploaded into PolyPhred for quality analysis and polymorphism detection (Montgomery et al. 2008). Publicly available sequence variation data were obtained from the mouse SNP collection in the Mouse Phenome Database (Jackson Laboratories, http://phenome.jax.org/SNP/). This database contains data from 22 data sets, including the Perlegen resequencing project.
Results
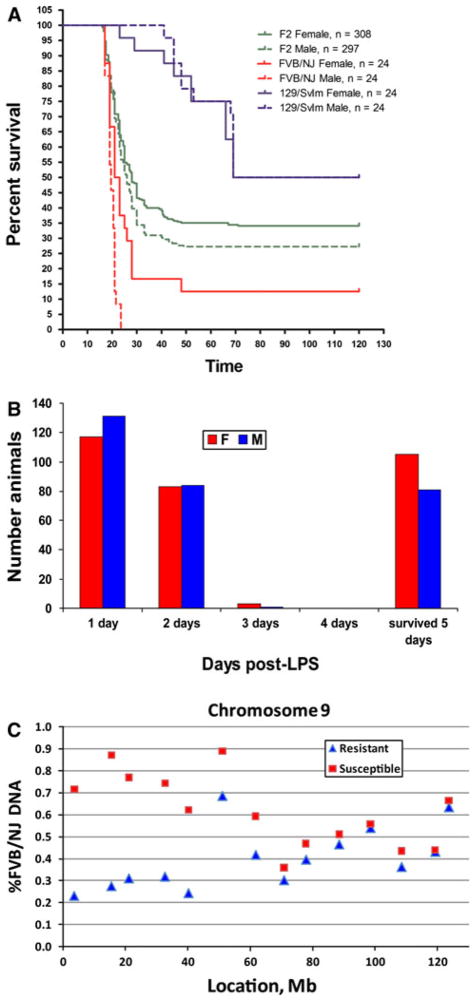
Our previous studies identified FVB/NJ as one of the strains of mice sensitive to systemic LPS challenge, while the 129S1/SvImJ strain of mice was shown to be resistant to systemic LPS (Yang et al. 2009, 2010). To identify genes that control strain difference in sensitivity to systemic LPS, we bred 605 F2 intercross mice [F1(129S1/SvImJ × FVB/NJ) × F1], challenged them with LPS systemically at 6–8 weeks of age, and followed them for morbidity over 5 days. Survival curves in Fig. 1a demonstrate that F2 animals showed an intermediate phenotype between susceptible and resistant parent strains and that there were minimal differences between males and females [P = 0.067 by Mantel–Cox test (Rosner 1995)]. The distribution of morbidity (70% of animals died within 48 h and the remaining 30% survived 5 days) (Fig. 1b) is very close to the expected 75%/25% distribution for a Mendelian trait and suggests that a single dominant gene accounts for the phenotypic difference between FVB/NJ and 129S1/SvImJ strains.
Fig. 1.
Mapping of morbidity phenotypes in [129S1/SvImJ × FVB/NJ F2] mice. a Kaplan–Meier survival curves for the sensitive FVB/NJ strains of mice (red), resistant 129S1/SvImJ mice (blue), and 129S1/SvImJ × FVB/NJ F2 progeny (green), with solid lines representing females and dashed lines representing males. F2 generation exhibits an intermediate phenotype between the two parental strains with a small difference (~5%) in morbidity of males and females. b Distribution of morbidity by day. Approximately 70% of male and female mice die within the first 2 days, while the remaining 30% survive for 5 days, suggesting that a single gene is responsible for the difference in morbidity between 129S1/SvImJ and FVB/NJ mice. c %FVB DNA in the sensitive DNA pool (<24 h survival, N = 240 mice; red squares) and resistant DNA pool (survived 5 days, N = 190; blue triangles) for SNP markers on chromosome 9. The locus is identified by the facts that %FVB DNA deviates from 50% and that sensitive and resistant pools diverge in their %FVB DNA between 0 and 40 Mb
We then undertook the pooled DNA genotyping approach to identify the locus responsible for differences in observed response in morbidity. We pooled DNA from 240 mice, both males and females, with <24 h of survival (susceptible pool) and 190 mice who survived 5 days (resistant pool). Genotyping of the two pools for 300 SNP markers ~10 Mb apart revealed a locus on Chr 9 (Fig. 1c) that is responsible for the difference in morbidity between the two parent strains, with the susceptible mice having a distinctly higher percent of FVB/NJ (susceptible strain) DNA at that locus compared to the resistant mice. We observe significant segregation of %FVB allele in sensitive and resistant pools at the five most proximal markers on Chr 9, suggesting the presence of a locus that controls differences in morbidity between the two parental strains between 0 and 40 Mb. No other loci were identified by the pooled genotyping approach (Supplementary Fig. 1).
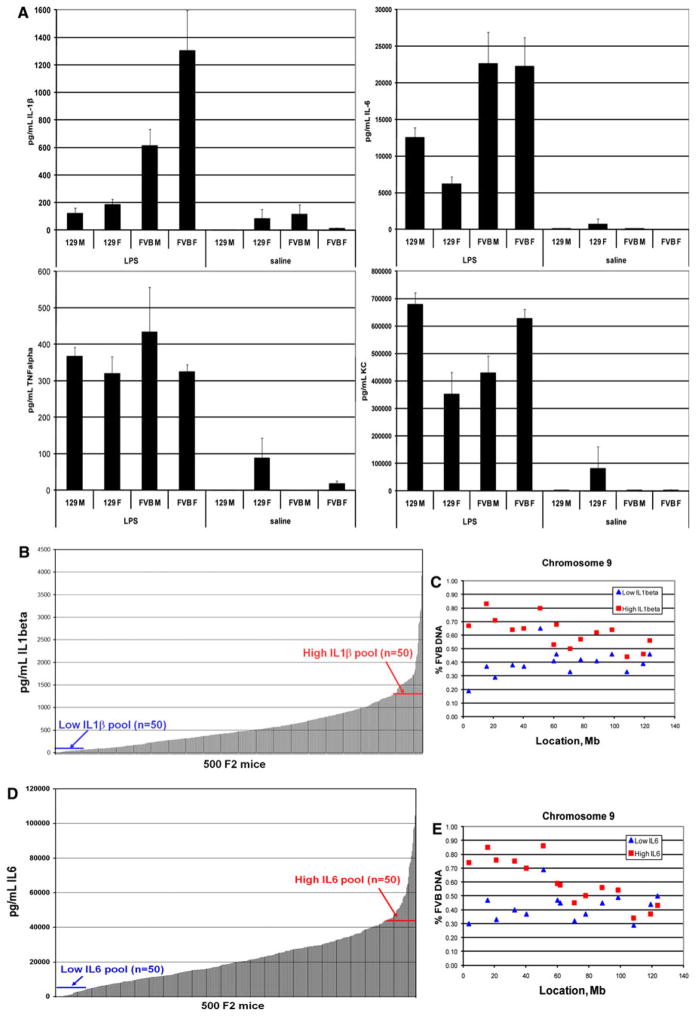
We next examined cytokine production in the parental strains and determined that both male and female mice differ significantly between the two strains in the production of IL-1β and IL-6 but not TNF-α and KC in response to intraperitoneal LPS (Fig. 2a). We then challenged another group of 500 F2 intercross mice, collected serum 6 h post-LPS, and measured serum concentrations of IL-1β and IL-6 to identify low and high responders (Fig. 2b for IL-1β and Fig. 2c for IL-6 data). The distributions of the IL-1β and IL-6 levels are continuous in the F2 population, suggesting that multiple genes may contribute to cytokine production phenotypes. We pooled DNA of 50 animals each with the lowest and highest IL-1β serum concentrations to make “low IL-1β” and “high IL-1β” pools. Genotyping of the two pools identified the same locus on Chr 9 that is responsible for differences in morbidity as the major locus controlling serum levels of IL-1β in FVB/NJ and 129S1/SvImJ mice (Fig. 2d). We applied the same mapping approach to the IL-6 trait. Using the above techniques, we mapped the differences in IL-6 production to the same locus 0–40 Mb on Chr 9 (Fig. 2e). In addition to the major locus on Chr 9, there appears to be another locus with a smaller effect on Chr 14 in both IL-1β and IL-6 genome-wide scans (Supplementary Figs. 2, 3). Interestingly, only 17 of 50 animals in the low IL-6 pool are in common with those in low IL-1β pool and 9 of 50 animals in the high IL-6 pool are in common with the high IL-1β pool, suggesting minimal overlap of these phenotypes. However, both phenotypes yielded the 0–40-Mb locus on Chr 9.
Fig. 2.
Mapping of cytokine phenotypes in [129S1/SvImJ × FVB/NJ] F2 mice. a Concentrations of IL-1β, IL-6, KC, and TNF-α in the serum of 129S1/SvImJ and FVB/NJ mice 6 h after systemic LPS challenge. There are significant differences in the production of IL-1β (two-tailed t-test P = 0.0004 for males and 0.002 for females) and IL-6 (two-tailed t-test P = 0.04 for males and 3 × 10−5 for females) between the two parental strains in response to LPS. No significant differences were observed in serum concentration of KC and TNF-α. b Serum concentrations of IL-1β in 500 129S1/SvImJ × FVB/NJ F2 mice in response to systemic LPS. Continuous nature of the phenotype suggests that there are multiple genes controlling differences in IL-1β production by 129S1/SvImJ and FVB/NJ strains of mice. c %FVB DNA in the high IL-1β DNA pool (N = 50; red squares) and low IL-1β pool (N = 50; blue triangles) for SNP markers on chromosome 9. d Serum concentrations of IL-6 in 500 129S1/SvImJ × FVB/NJ F2 mice in response to systemic LPS. Continuous nature of the phenotype suggests that there are multiple genes controlling differences in IL-1β production by 129S1/SvImJ and FVB/NJ strains of mice. e %FVB DNA in the high IL-6 DNA pool (N = 50; red squares) and low IL-6 pool (N = 50; blue triangles) for SNP markers on chromosome 9. The locus is identified by the facts that %FVB DNA deviates from 50% and that sensitive and resistant pools diverge in their %FVB DNA between 0 and 40 Mb
To fine map the locus on Chr 9, we sought to identify informative recombinant events in individual mice from sensitive and resistant DNA pools. This fine-mapping approach is predicated on the presence of a single locus with a dominant effect. We first individually genotyped all mice from sensitive (n = 240) and resistant (n = 190) pools for six markers that were used in the initial genome-wide screen to cover the first 40 Mb of Chr 9. This analysis identified a total of 72 mice, 51 resistant and 21 sensitive, with recombination events in this region; genotypes for the animals with recombination events that could be informative and that were selected for the next round of genotyping are shown in Table 1A. The most informative recombinants (animals 29185, 28923, 29029, 28807, 28878, and 28811) have recombination events between 3.6 and 9 Mb and therefore narrow the locus to the first 9 Mb of Chr 9. To further fine map this locus, we next genotyped 29 individual mice shown in Table 1A for additional markers in the first 9 Mb of Chr 9 at 0.5–1-Mb spacing. Recombination events in the first 9 Mb on Chr 9 are shown in Table 1B. The three most informative recombinants (29185, 28807, and 28878) from this analysis narrow the locus to 0–6.7 Mb as they have recombination events between markers at 6.01 and 6.72 Mb. Animals 28923, 28996, and 28111 (recombination between 7 and 7.78), and 29029 (7.78 and 8.01) provide additional support for this locus. Finally, we sequenced the SNP markers rs30061084 (6.00 Mb) and r229944622 (6.72 Mb) in the most informative three animals (29185, 28807, and 28878) to confirm genotyping data.
Table 1.
Fine mapping of the systemic LPS sensitivity locus on chromosome 9. (A) Genotyping of individual animals for the six markers on Chromosome 9 that were included in the initial genome-wide screen identified 51 resistant and 21 sensitive mice with recombination events in this region. Shown are genotypes for the top 19 resistant and 10 sensitive mice. (B) Final fine mapping using markers 0.5–1 Mb apart
| A | ||||||
|---|---|---|---|---|---|---|
| Mb | 3.6 | 9 | 15.5 | 21.2 | 32.7 | 40.2 |
| Marker/mouse ID | rs13477344 | rs30187167 | rs13480078 | Pde4a_AN8416_8 | Ets1_JC10087_1 | Scn3b_JC14774_22 |
| Resistant | ||||||
| 29185 | BB | AB | AB | AB | AB | |
| 28923 | BB | AB | AB | AB | AB | |
| 29029 | BB | AB | AB | AB | ||
| 28933 | BB | BB | AB | AB | AB | AB |
| 28835 | BB | BB | AB | AB | AB | AB |
| 28935 | BB | BB | AB | AB | AB | AB |
| 29051 | BB | BB | AB | AB | ||
| 28921 | BB | BB | AB | AB | AB | |
| 29028 | BB | BB | AB | AB | AB | AB |
| 28832 | BB | BB | AB | AB | AB | AB |
| 28763 | BB | BB | AB | AB | AA | AA |
| 28841 | BB | BB | AB | AB | AB | AB |
| 28938 | BB | BB | AB | AB | AB | |
| 29027 | BB | BB | AB | AA | AB | |
| 29034 | BB | BB | AB | AB | AB | AB |
| 29186 | BB | BB | AB | AB | AB | AB |
| 30099 | BB | BB | AB | AB | AA | AA |
| 30100 | BB | BB | AB | AB | AB | AB |
| 30109 | BB | BB | AB | AB | AB | |
| Sensitive | ||||||
| 28807 | AB | BB | BB | BB | BB | BB |
| 28878 | AB | BB | BB | BB | BB | BB |
| 28996 | AB | BB | BB | BB | BB | BB |
| 28811 | AB | BB | BB | BB | BB | BB |
| 28980 | AB | AB | BB | BB | BB | BB |
| 28785 | AB | AB | BB | BB | BB | BB |
| 28784 | AB | AB | BB | BB | BB | BB |
| 28874 | AB | AB | BB | BB | BB | BB |
| 28886 | AB | AB | BB | BB | BB | BB |
| 29122 | AB | AB | BB | BB | BB | BB |
| B | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mb | 3.10 | 3.71 | 4.04 | 4.32 | 4.75 | 5.01 | 6.01 | 6.72 | 7.00 | 7.78 | 8.01 | 8.31 | 8.63 | 9.00 | 9.60 | 10.03 |
| Marker/mouse ID | rs31198619 | rs13477344 | rs30153081 | rs31718188 | rs32210333 | rs30077564 | rs30061084 | rs29944622 | rs33767271 | rs29791639 | rs29697768 | rs29790948 | rs29840665 | rs30187167 | rs33751470 | rs36523876 |
| Resistant | ||||||||||||||||
| 29185 | BB | BB | BB | BB | BB | BB | BB | AB | AB | AB | AB | AB | AB | AB | AB | AB |
| 28923 | BB | BB | BB | BB | BB | BB | BB | BB | BB | AB | AB | AB | AB | AB | AB | AB |
| 29029 | BB | BB | BB | BB | BB | BB | BB | BB | BB | AB | AB | AB | AB | AB | AB | |
| 28933 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | AB | AB |
| 28835 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB |
| 28935 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB |
| 29051 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | |
| 28921 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | |||
| 29028 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB |
| 28832 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB |
| 28763 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | |
| 28841 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | |
| 28938 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB |
| 29027 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB |
| 29034 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB |
| 29186 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB |
| 30096 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | ||
| 30099 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | ||||
| 30109 | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | BB | ||
| Sensitive | ||||||||||||||||
| 28807 | AB | AB | AB | AB | AB | AB | BB | BB | BB | BB | BB | BB | BB | BB | BB | |
| 28878 | AB | AB | AB | AB | AB | BB | BB | BB | BB | BB | BB | BB | BB | BB | ||
| 28996 | AB | AB | AB | AB | AB | AB | AB | BB | BB | BB | BB | BB | BB | |||
| 28811 | AB | AB | AB | AB | AB | AB | BB | BB | BB | BB | BB | BB | BB | |||
| 28980 | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | BB | BB |
| 28785 | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | |
| 28784 | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | |||
| 28874 | AB | AB | AB | AB | AB | AA | AB | AB | AB | AB | AB | AB | AB | AB | ||
| 28886 | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | |||
| 29122 | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | |
Shown are only genotypes for individual mice with the most informative recombinant events (out of 30 animals that were genotyped for the markers shown)
The top three informative recombinations in animals 29185, 28807, and 28878 map the locus to 0–6.7 Mb. No SNP markers or genes are located on the telomere 0–3.1 Mb, narrowing down this locus to 3.1–6.7 Mb. “A” refers to the FVB/NJ and “B” refers to the 129/SvIm allele. Based on the genetic model, AA and AB genotypes (italic) are sensitive and BB genotype (bold) is resistant
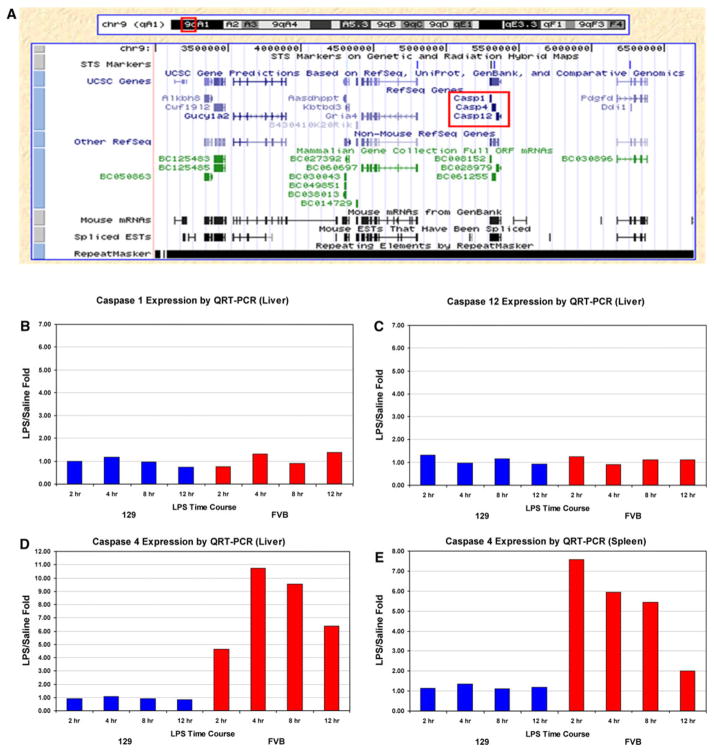
Our mapping narrows the locus to 0–6.7 Mb; however, the fact that there are no polymorphisms between the two parental strains (nor genes) in the first 0–3 Mb due to failure of genome assembly of this highly repetitive region further limits the locus to 3–6.7 Mb. This interval contains 11 known genes, including three inflammatory caspases (Fig. 3a). We studied the expression of Casp1, Casp4, and Casp12 in liver and spleen tissue of 129S1/SvImJ and FVB/NJ mice at four time points: 2, 4, 8, and 12 h post-LPS. Casp1 and Casp12 are not induced by LPS in either strain at any time point (Fig. 3b, c for liver data; spleen data not shown), while Casp4 is highly induced by LPS treatment in FVB/NJ but not in 129S1/SvImJ mice in liver and spleen at all four time points (Fig. 3d, e). We also measured Casp4 expression in livers of 40 F2 animals treated with LPS for 6 h (20 with low serum IL-1β/IL-6 and 20 with high serum IL-1β/IL-6), and showed that Casp4 expression levels in the liver segregate with serum cytokine phenotypes (Supplementary Table 5). On average, expression of Casp4 is 700-fold higher in livers of animals with high serum cytokine concentrations than in those with low levels of serum cytokines. These expression data suggest that Casp4 is the most viable candidate gene in this locus and that there is likely a polymorphism in either 129S1/SvImJ or FVB/NJ that may explain differential expression of Casp4 in response to LPS in the two strains of mice.
Fig. 3.
Candidate genes in the locus on Chr 9. a Genes contained in the novel LPS locus on Chr 9 between 3 and 6.7 Mb. The most likely candidates in the locus are the three inflammatory caspases. Expression of Casp1, Casp4, and Casp12 in liver and spleen tissue of 129S1/SvImJ and FVB/NJ mice in response to systemic LPS. b–e Casp1 and Casp12 are not induced by LPS in either strain at 2, 4, 8 and 12 h post-LPS (b, c for liver data; spleen data not shown), while Casp4 is highly induced by LPS treatment in FVB/NJ but not in 129S1/SvImJ mice in both liver and spleen at all time points (d, e). Shown are the mean with standard deviation values for LPS/saline fold changes for eight animals in each group (3 pools of 2–3 mice)
We next attempted to identify the sequence variant associated with expression differences in Casp4 in response to LPS in 129S1/SvImJ and FVB/NJ strains of mice. We queried the Mouse Phenome Database collection of publicly available SNPs for polymorphisms between the two strains of mice within the Casp4 gene and the upstream promoter region. This query identified 13 polymorphisms, with 11 intronic SNPs, 1 SNP in the 3′ UTR, and 1 SNP in the upstream promoter region (Table 2). The most likely candidate SNP that could account for changes in expression of Casp4 is the promoter SNP rs30020524. We used the Genomatix MatInspector software to identify transcription factor binding sites associated with the T allele in 129S1/SvImJ mice and the A allele in FVB/NJ mice at this locus. This analysis identified three putative transcription factor binding sites associated with the T allele (DM domain-containing transcription factors, Lim homeodomain factors, and vertebrate TATA binding protein factor), while the A allele is associated with four putative transcription factor binding sites (Brn-5 POU and Brn POU domain factors, yeast and plant TATA binding protein factors) (Supplementary Table 4). None of these transcription factors has been specifically associated with the response to LPS, suggesting that this polymorphism is unlikely associated with LPS-induced changes in expression of Casp4 in the two strains of mice.
Table 2.
Known polymorphisms in the caspase 4 gene between 129/SvIm and FVB/NJ strains of mice
| Mbp location (Build 37) |
NCBI gene annotation |
dbSNP rs |
dbSNP 128 SNP annotation |
129S1/ SvImJ |
129X1/ SvJ |
AKR/ J |
WSB/ EiJ |
FVB/ NJ |
A/J | BALB/ cByJ |
BTBR T + tf/ J |
C3H/ HeJ |
C57BL/ 6 J |
DBA/ 2 J |
KK/ HlJ |
NOD/ ShiLtJ |
NZW/ LacJ |
CAST/ EiJ |
MOLF/ EiJ |
PWD/ PhJ |
Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5.3069 | Casp1 intron9 | rs30020524 | Casp1 I Casp4 L |
T | T | T | T | A | A | A | A | A | A | A | A | A | A | T | T | (Celera2, Perlegen2) | |
| 5.3216 | Casp4 intron2 | rs32240574 | I | G | G | G | A | A | A | A | A | A | A | A | A | A | Perlegen2 NES11336258 | ||||
| 5.3220 | Casp4 intron2 | rs32240577 | I | C | C | C | T | T | T | T | T | T | T | T | T | T | T | T | T | Perlegen2 NES11336259 | |
| 5.3225 | Casp4 intron2 | rs3657871 | I | C | C | C | G | G | G | G | G | G | G | G | G | G | G | G | G | (Perlegen2, WICGR2) | |
| 5.3226 | Casp4 intron2 | rs3658578 | I | T | T | T | G | G | G | G | G | G | G | G | G | G | G | T | T | (Perlegen2, WICGR2) | |
| 5.3227 | Casp4 intron2 | rs3659109 | I | A | A | A | G | G | G | G | G | G | G | G | G | G | A | A | (Perlegen2, WICGR2) | ||
| 5.3227 | Casp4 intron2 | rs3659157 | I | T | T | T | A | A | A | A | A | A | A | A | A | A | (Perlegen2, WICGR2) | ||||
| 5.3230 | Casp4 intron3 | rs16783245 | I | T | T | T | C | C | C | C | C | C | C | C | C | C | Perlegen2 NES11336270 | ||||
| 5.3247 | Casp4 intron4 | rs3722091 | I | A | A | A | A | C | C | C | C | C | C | C | C | C | C | C | C | C | (Celera2, Perlegen2, WICGR2) |
| 5.3323 | Casp4 intron7 | rs30511331 | I | G | G | G | G | A | A | A | A | A | A | A | A | A | A | G | G | G | (Celera2, Perlegen2) |
| 5.3362 | Casp4 intron8 | rs31544867 | I | T | T | T | T | A | A | A | A | A | A | A | A | A | ? | A | A | A | (CGD2, Celera2, Perlegen2) |
| 5.3364 | Casp4 intron8 | rs31485595 | I | C | C | C | C | G | G | G | G | G | G | G | G | G | C | C | (Celera2, Perlegen2) | ||
| 5.3366 | Casp4 UTR | rs31661027 | U | G | G | G | A | A | A | A | A | A | A | A | A | A | A | A | (Celera2, Perlegen2) |
Thirteen polymorphisms between 129/SvIm and FVB/NJ strains and their allele distribution among the other 16 strains in the high-density SNP set in the Mouse Phenome Database (MPD).
This set consists of 7–9 million genomic locations for the 18 strains of mice
To check whether there are additional polymorphisms present that have not been captured in the database, we sequenced all of the exons and the 2-kb promoter region of Casp4 by Sanger sequencing. This additional sequence analysis did not identify any novel sequence variants. We also sequenced all predicted NF-κB binding sites in the additional 2–20 kb upstream of the transcriptional start site and NF-κB binding sites contained within introns of Casp4 but did not identify any additional SNPs. Finally, we queried the recently generated complete genome sequence data of inbred strains of mice available through the Wellcome Trust Sanger Center (http://www.sanger.ac.uk/resources/mouse/genomes/). At the present time, sequencing of the 129S1/SvImJ strain is completed but the sequence of the FVB/NJ strain is not available; however, given rapid advances in technologies and the decrease in the cost of genome sequencing, it is expected that this sequence will be available in the near future and that we will be able to identify additional polymorphisms in this interval that may play a role in differential sensitivity of the two strains of mice to systemic LPS.
Discussion
Using allele-specific genotyping of pooled DNA from an F2 intercross, we were able to identify a novel locus on Chr 9 that regulates murine response to systemic LPS. This locus is associated with both differences in morbidity and serum concentrations of IL-1β and IL-6 (but not TNF-α and KC) between FVB/NJ and 129S1/SvImJ strains of mice. The locus contains 11 known genes, including the three mouse proinflammatory caspases Casp1, Casp4, and Casp12. Expression patterns of the three inflammatory caspases within the locus suggest that Casp4 is the most plausible candidate gene.
Proinflammatory caspases Casp1, Casp4 (also known as Casp11 in mice), Casp5 (absent in mice), and Casp12 have a well-established role in innate immune response in mice and humans (Nadiri et al. 2006). Casp1 is the IL-1β converting enzyme (ICE) and cleaves pro-IL-1β, pro-IL-18 (IFNγ-inducing factor), and IL-33. As such, it is an integral part of inflammasomes, molecular platforms activated upon cellular infection or stress that trigger the maturation of proinflammatory cytokines such as IL-1β to engage in innate immune defenses (Schroder and Tschopp 2010). Inflammasomes are activated by a family of NOD-like receptors (NLRs) that recognize both PAMPs and danger-associated molecular patterns (DAMPs). Casp4 is involved in this process by cleaving Casp1, but its activation has not been studied extensively. The involvement of Casp12 in inflammasome activation is unclear as there is no known substrate for Casp12 at this time, but there is some evidence that Casp12 may be a negative regulator of the Casp1 pathway (Saleh et al. 2006). Dysregulation of inflammasome activation has been associated with a number of diseases, including but not limited to gout, sepsis, inflammatory bowel disease (IBD), and type II diabetes (Schroder and Tschopp 2010).
The role for all three proinflammatory caspases in the innate immune system is well established. Because of its crucial role in the activation of IL-1β and other proinflammatory cytokines, Casp1 is expressed at high levels in monocytes, macrophages, and neutrophils. Human Casp4 is present at high levels in most tissues, while expression of mouse Casp4 (Casp11) is below the detection limit in most tissues and cells. However, it has been shown that expression of mouse Casp4 is highly induced by LPS and IFNγ in macrophages (Schauvliege et al. 2002). Casp12 is expressed in all tissues, with highest levels in the lung, stomach, and small intestine. Both Casp1- and Casp4-deficient mice are resistant to LPS-induced endotoxic shock and do not produce cytokines such as IL-1β in response to LPS (Li et al. 1995; Wang et al. 1998). Casp12-deficient mice are resistant to peritonitis and septic shock and have enhanced bacterial clearance (Saleh et al. 2006).
In aggregate, our results and published results suggest that Casp4 is the most likely candidate gene in our locus. Casp4 was highly induced by LPS treatment in the liver and spleen of FVB/NJ but not 129S1/SvImJ mice in our study and was shown to be highly inducible by LPS in published studies (Schauvliege et al. 2002). Based on the fact that Casp4 is not induced by LPS in 129S1/SvImJ mice, it is likely that there is a sequence variant in this strain that alters transcription of LPS-inducible expression of Casp4, which in turn blocks production of IL-1β and IL-6 and renders the strain resistant to LPS-induced shock. These findings suggest that controlling the expression of Casp4 may represent a novel therapeutic approach to individuals with Gram-negative sepsis.
Taken together, our study strongly suggests that there is a yet to be described sequence variant in the 129S1/SvImJ strain of mice that reduces transcription of LPS-inducible expression of Casp4. One possible explanation for why we were unable to identify this sequence change is that the sequence change is outside of the proximal promoter that affects induction of Casp4 expression. Another possibility is that there are epigenetic mechanisms, either DNA methylation or histone modifications, involved in the control of LPS-inducible gene expression in these strains of mice. It is also possible that a microRNA on Chr 9 is interfering with the transcription of Casp4.
Our study identified one locus with a major effect but it is possible that there are other loci with smaller effects that account for differential susceptibility of FVB/NJ and 129S1/SvImJ strains of mice to systemic LPS. Although the morbidity phenotypes for the F2 generation suggest a Mendelian pattern of inheritance, cytokine secretion is a continuous phenotype in the F2 generation and therefore suggests a complex trait and involvement of multiple loci and genes. In addition to the locus on Chr 9, we identified a locus with a smaller effect on Chr 14 for both IL-1β and IL-6 traits. However, it is feasible that we missed other loci with smaller effects because the pooled genotyping approach we applied in this study would not reliably identify such loci. Moreover, the numbers of F2 progeny that we phenotyped (605 for morbidity and 500 for cytokine production) may have not been large enough to identify loci with small effects.
Supplementary Material
Acknowledgments
We thank Rachel Burton for technical assistance in the laboratory. This research was supported by the funding from the Intramural Program of the NIH/NHLBI and NIEHS extramural grants ES11375 and ES101946.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00335-011-9340-8) contains supplementary material, which is available to authorized users.
Contributor Information
Ivana V. Yang, Email: yangi@njhealth.org, Center for Genes, Environment and Health and Department of Medicine, National Jewish Health, 1400 Jackson Street, A650, Denver, CO 80206, USA. Department of Medicine, University of Colorado School of Medicine, Aurora, CO 80010, USA
Holly R. Rutledge, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27709, USA
Jun Yang, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27709, USA.
Laura A. Warg, Center for Genes, Environment and Health and Department of Medicine, National Jewish Health, 1400 Jackson Street, A650, Denver, CO 80206, USA
Sergio D. Sevilla, Project Management, Kendle International, Buenos Aires, Argentina
David A. Schwartz, Center for Genes, Environment and Health and Department of Medicine, National Jewish Health, 1400 Jackson Street, A650, Denver, CO 80206, USA. Department of Medicine, University of Colorado School of Medicine, Aurora, CO 80010, USA
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Aylor DL, Valdar W, Foulds-Mathes W, Buus RJ, Verdugo RA, Baric RS, Ferris MT, Frelinger JA, Heise M, Frieman MB, Gralinski LE, Bell TA, Didion JD, Hua K, Nehrenberg DL, Powell CL, Steigerwalt J, Xie Y, Kelada SNP, Collins F, Yang IV, Schwartz DA, Branstetter LA, Chesler EJ, Miller DR, Spence J, Liu EY, McMillan L, Sarkar A, Wang J, Wang W, Zhang Q, Broman KW, Korstanje R, Durrant C, Mott R, Iraqi FA, Pomp D, Threadgill D, Pardo-Manuel de Villena F, Churchill GA. Genetic analysis of complex traits in the emerging collaborative cross. Genome Res. 2011 doi: 10.1101/gr.111310.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- Caron J, Loredo-Osti JC, Laroche L, Skamene E, Morgan K, Malo D. Identification of genetic loci controlling bacterial clearance in experimental Salmonella enteritidis infection: an unexpected role of Nramp1 (Slc11a1) in the persistence of infection in mice. Genes Immun. 2002;3:196–204. doi: 10.1038/sj.gene.6363850. [DOI] [PubMed] [Google Scholar]
- Caron J, Loredo-Osti JC, Morgan K, Malo D. Mapping of interactions and mouse congenic strains identified novel epistatic QTLs controlling the persistence of Salmonella enteritidis in mice. Genes Immun. 2005;6:500–508. doi: 10.1038/sj.gene.6364234. [DOI] [PubMed] [Google Scholar]
- Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004a;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- Cook DN, Wang S, Wang Y, Howles GP, Whitehead GS, Berman KG, Church TD, Frank BC, Gaspard RM, Yu Y, Quackenbush J, Schwartz DA. Genetic regulation of endotoxin-induced airway disease. Genomics. 2004b;83:961–969. doi: 10.1016/j.ygeno.2003.12.008. [DOI] [PubMed] [Google Scholar]
- De Maio A, Mooney ML, Matesic LE, Paidas CN, Reeves RH. Genetic component in the inflammatory response induced by bacterial lipopolysaccharide. Shock. 1998;10:319–323. doi: 10.1097/00024382-199811000-00002. [DOI] [PubMed] [Google Scholar]
- Fulton WB, Reeves RH, Takeya M, De Maio A. A quantitative trait loci analysis to map genes involved in lipopolysaccharide-induced inflammatory response: identification of macrophage scavenger receptor 1 as a candidate gene. J Immunol. 2006;176:3767–3773. doi: 10.4049/jimmunol.176.6.3767. [DOI] [PubMed] [Google Scholar]
- Garantziotis S, Hollingsworth JW, Zaas AK, Schwartz DA. The effect of toll-like receptors and toll-like receptor genetics in human disease. Annu Rev Med. 2008;59:343–359. doi: 10.1146/annurev.med.59.061206.112455. [DOI] [PubMed] [Google Scholar]
- Germer S, Higuchi R. Single-tube genotyping without oligonucleotide probes. Genome Res. 1999;9:72–78. [PMC free article] [PubMed] [Google Scholar]
- Germer S, Holland MJ, Higuchi R. High-throughput SNP allele-frequency determination in pooled DNA samples by kinetic PCR. Genome Res. 2000;10:258–266. doi: 10.1101/gr.10.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lorenz E, Jones M, Wohlford-Lenane C, Meyer N, Frees KL, Arbour NC, Schwartz DA. Genes other than TLR4 are involved in the response to inhaled LPS. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1106–L1114. doi: 10.1152/ajplung.2001.281.5.L1106. [DOI] [PubMed] [Google Scholar]
- Matesic LE, De Maio A, Reeves RH. Mapping lipopolysaccharide response loci in mice using recombinant inbred and congenic strains. Genomics. 1999;62:34–41. doi: 10.1006/geno.1999.5986. [DOI] [PubMed] [Google Scholar]
- Matesic LE, Niemitz EL, De Maio A, Reeves RH. Quantitative trait loci modulate neutrophil infiltration in the liver during LPS-induced inflammation. FASEB J. 2000;14:2247–2254. doi: 10.1096/fj.99-1051com. [DOI] [PubMed] [Google Scholar]
- Montgomery KT, Iartchouck O, Li L, Loomis S, Obourn V, Kucherlapati R. PolyPhred analysis software for mutation detection from fluorescence-based sequence data. Curr Protoc Hum Genet. 2008;Chapter 7(Unit 7.16) doi: 10.1002/0471142905.hg0716s59. [DOI] [PubMed] [Google Scholar]
- Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- Nadiri A, Wolinski MK, Saleh M. The inflammatory caspases: key players in the host response to pathogenic invasion and sepsis. J Immunol. 2006;177:4239–4245. doi: 10.4049/jimmunol.177.7.4239. [DOI] [PubMed] [Google Scholar]
- Papathanassoglou ED, Giannakopoulou MD, Bozas E. Genomic variations and susceptibility to sepsis. AACN Adv Crit Care. 2006;17:394–422. doi: 10.4037/15597768-2006-4006. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of biostatistics. 4. Duxbury Press; Pacific Grove: 1995. [Google Scholar]
- Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR, Nicholson DW. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- Schauvliege R, Vanrobaeys J, Schotte P, Beyaert R. Caspase-11 gene expression in response to lipopolysaccharide and interferon-gamma requires nuclear factor-kappa B and signal transducer and activator of transcription (STAT) 1. J Biol Chem. 2002;277:41624–41630. doi: 10.1074/jbc.M207852200. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Sebastiani G, Olien L, Gauthier S, Skamene E, Morgan K, Gros P, Malo D. Mapping of genetic modulators of natural resistance to infection with Salmonella typhimurium in wild-derived mice. Genomics. 1998;47:180–186. doi: 10.1006/geno.1997.5116. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- Yang IV, Wade CM, Kang HM, Alper S, Rutledge H, Lackford B, Eskin E, Daly MJ, Schwartz DA. Identification of novel genes that mediate innate immunity using inbred mice. Genetics. 2009;183:1535–1544. doi: 10.1534/genetics.109.107540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IV, Alper S, Lackford B, Rutledge H, Warg LA, Burch LH, Schwartz DA. Novel regulators of the systemic response to lipopolysaccharide (LPS) Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.