Summary
Inflammatory bowel disease (IBD) is characterized by dysregulated intestinal immune homeostasis and cytokine secretion. Multiple loci are associated with IBD, but a functional explanation is missing for most. Here we found that pattern-recognition receptor (PRR)-induced cytokine secretion was diminished in human monocyte-derived dendritic cells (MDDC) from rs7282490 ICOSLG GG risk-carriers. Homotypic interactions between the co-stimulatory molecule ICOS and the ICOS ligand on MDDCs amplified nucleotide-binding oligomerization domain 2 (NOD2)-initiated cytokine secretion. This amplification required arginine residues in the ICOSL cytoplasmic tail that recruited the adaptor protein RACK1 and the kinases PKC and JNK leading to PKC, MAPK and NFκB activation. MDDC from rs7282490 GG risk-carriers had reduced ICOSL expression and PRR-initiated signaling and this loss-of-function ICOSLG risk allele associated with an ileal Crohn’s disease phenotype, similar to polymorphisms in NOD2. Taken together, ICOSL amplifies PRR-initiated outcomes, which may contribute to immune homeostasis.
Introduction
Inflammatory bowel disease (IBD) is characterized by dysregulated intestinal immune homeostasis and cytokine production. Host-microbial interactions are initially mediated by pattern-recognition-receptors (PRR); either loss-of-function or gain-of-function in PRR-mediated signaling and downstream outcomes can be associated with intestinal inflammation (Abraham and Medzhitov, 2011). For example, a loss in PRR pathways (e.g. the adaptor protein MyD88- or toll-like receptor 5 (TLR5)-deficient mice) (Rakoff-Nahoum et al., 2004; Vijay-Kumar et al., 2007) or a gain in PRR signaling (e.g. IRAK-M- or A20-deficient mice) (Biswas et al., 2011; Hammer et al., 2011) can result in an increased susceptibility to intestinal inflammation. A key role for host-microbial interactions is further highlighted by Crohn’s disease (CD)-associated NOD2 polymorphisms resulting in decreased nucleotide-binding oligomerization domain 2 (NOD2)-dependent signaling. These NOD2 polymorphisms likely increase risk for CD through multiple mechanisms (Abraham and Cho, 2009; Abraham and Medzhitov, 2011). In the intestinal environment, multiple other PRR are activated and the outcomes of their activation might be modulated by additional IBD risk loci. Although a number of loci have now been associated with IBD (Jostins et al., 2012), altered functions for the vast majority of these loci are unknown. One such region is at chromosome 21q22, encompassing the ICOSLG gene. ICOSLG region polymorphisms are associated with various immune-mediated diseases, including IBD (Franke et al., 2010; Jostins et al., 2012) and celiac disease (Dubois et al., 2010). Inducible T cell costimulator ligand (ICOSL), expressed on antigen-presenting cells (APC), interacts with ICOS on T cells (Ling et al., 2000) thereby contributing to T cell activation and differentiation (Dong et al., 2001; Hutloff et al., 1999; Yoshinaga et al., 1999). Consistently, ICOS- and ICOSL-deficient mice display a similar phenotype, characterized by dysregulated T cell polarization and B cell immunity (Sharpe, 2009; Simpson et al., 2010). Unlike the well-investigated role of ICOS signaling in adaptive immunity, how ICOSL signals in myeloid cells is poorly understood. ICOS-Ig treatment of mouse dendritic cells (DCs) results in p38 activation and interleukin-6 (IL-6) induction (Tang et al., 2009). However, additional ICOSL-initiated signaling pathways and the consequences of this signaling in DC are unclear. Moreover, a necessity for ICOSL signaling in PRR-initiated cytokine induction has not been described.
In this study, we identified a role for ICOSL-initiated signaling in PRR-mediated responses in primary human DC. ICOS was found to be expressed on monocyte-derived DC (MDDC), as well as on intestinal myeloid-derived cells, and ICOS:ICOSL interactions and downstream ICOSL-initiated signaling were required for amplification of PRR-induced cytokines. Upon stimulation with ICOS, ICOSL recruited protein kinase C (PKC), receptor for activated C kinase 1 (RACK1) and c-Jun N-terminal kinase (JNK) to form a multi-protein complex that amplifies downstream signaling and cytokine secretion. MDDC from ICOSLG region rs7282490 homozygous carriers of the IBD disease-risk allele (G) had reduced basal and PRR-induced ICOSL expression, decreased signaling in response to PRR and ICOSL stimulation, and decreased PRR-induced cytokine secretion relative to AA carriers. Finally, we found the loss-of-function ICOSLG rs7282490 risk allele leads to an ileal-associated CD phenotype that is similarly associated with loss-of-function NOD2 polymorphisms. Taken together, we establish loss-of-function consequences for the rs7282490 risk polymorphism in the ICOSLG region associated with IBD, and uncover a new role for ICOSL in amplification of PRR-induced cytokines.
Results
MDDC from CD-risk-associated ICOSLG carriers demonstrate decreased PRR-induced cytokine secretion
Given that lipopolysaccharide (LPS) upregulates ICOSL expression on macrophages (Aicher et al., 2000), ICOSLG region polymorphisms are associated with IBD (Franke et al., 2010; Jostins et al., 2012), and PRR play a critical role in proper cytokine regulation in the intestine, we asked whether ICOSL contributes to PRR-induced cytokine secretion in human MDDC. We first asked if the rs7282490 polymorphism downstream of the ICOSLG gene, and the most significantly IBD-associated polymorphism in the region (Jostins et al., 2012), might modulate these effects. We initially examined NOD2-induced cytokine secretion given the importance of NOD2 polymorphisms in CD (Abraham and Cho, 2009). We utilized muramyl dipeptide (MDP), the minimal bacterial peptidoglycan component specifically activating NOD2 (Girardin et al., 2003; Hedl and Abraham, 2011a, 2012; Hedl et al., 2007; Inohara et al., 2003). MDDC from 100 healthy individuals were stimulated with increasing concentrations of MDP, and IL-1β secretion was examined because of its role in amplifying PRR-mediated responses in human myeloid cells (Hedl and Abraham, 2011a) and high correlation with multiple other cytokines (Hedl and Abraham, 2012). We found less NOD2-induced IL-1β protein (log2 transformed) in MDDC from rs7282490 GG risk carriers compared to GA and AA carriers (Fig. 1A). Although the inhibitory effect of the rs7282490 GG polymorphism was seen at all three doses, it was most prominent at the lowest MDP concentration. The gram-positive bacterial cell wall contains both NOD2 and TLR2 ligands, and MDDC from rs7282490 GG risk carriers also demonstrated decreased TLR2-mediated IL-1β secretion relative to AA carriers (Fig. 1B). CD is associated with dysregulated responses to bacteria, and bacteria activate multiple PRR in addition to NOD2 and TLR2. Furthermore, NOD2 synergizes with TLRs to induce more robust cytokine secretion (Li et al., 2004; Netea et al., 2005). Therefore, we investigated whether the rs7282490 polymorphism affects cytokine secretion by multiple PRR in a separate cohort of 98 healthy individuals. MDDC from rs7282490 GG risk carriers secreted decreased amounts of IL-1β relative to GA and AA carriers after stimulation of TLR2, TLR3, TLR4, TLR5, TLR7 and TLR9 alone (Fig. 1C) or in combination (Fig. 1D) with NOD2. A similar decrease in tumor necrosis factor-α (TNF-α) and IL-10 was observed from rs7282490 carrier MDDC (Fig. S1). Taken together, our results show that the rs7282490 IBD-associated risk polymorphism in the ICOSLG region reduces PRR-initiated cytokine secretion.
Figure 1. MDDC from GG carriers of the CD-risk-associated rs7282490 ICOSLG region polymorphism have decreased cytokine secretion upon PRR stimulation relative to AA carriers.
Human MDDC (n=100) were stimulated for 24h with (A) 1, 10 or 100μg/ml of MDP or (B) 1, 10 or 100μg/ml of Pam3Cys. MDDC (n=98) were stimulated for 24h with 1μg/ml MDP (NOD2 ligand) and 1μg/ml Pam3Cys (TLR2 ligand), 0.1μg/ml polyI:C (TLR3 ligand), 0.01μg/ml lipid A (TLR4 ligand), 0.5ng/ml flagellin (TLR5 ligand), 0.1μg/ml CL097 (TLR7 ligand) or 0.1μg/ml CpG DNA (TLR9 ligand) for 24h (C) alone or (D) in combination. The data are represented as fold IL-1β induction (log2 transformed) upon PRR stimulation stratified on the rs7282490 genotype+SEM. *, p<0.05; **, p<0.01; ***, p<0.001.
Human MDDC and intestinal myeloid cells express ICOS and MDDC upregulate ICOSL upon NOD2 stimulation
Given the rs7282490 genotype-dependent effects on cytokine secretion in MDDC, we hypothesized that both ICOSL and its binding partner, ICOS, are expressed on primary human MDDC, allowing for ICOS:ICOSL engagement to promote cytokine secretion through homotypic DC interactions. Previous studies did not detect ICOS expression in mouse monocytes or dendritic cells (Hutloff et al., 1999). ICOS was not present on human monocytes (Fig. 2A), but was expressed by MDDC at steady state (Fig. 2A) and after MDP stimulation (data not shown). Specificity of the anti-ICOS antibody was demonstrated by decreased amounts of ICOS protein upon knock-down of ICOS in MDDC (Fig. 2B). Moreover ICOS expression on MDDC was within a range similar to that observed on activated primary human T cells (Fig. 2A). We further confirmed ICOS expression by flow cytometry using a second anti-ICOS antibody (clone ISA-3; data not shown), and by Western blot utilizing two distinct antibodies (Fig. 2C and Fig. S2A). In addition to studies on MDDC that were isolated at >95% purity using standard procedures, MDDC were isolated to >99.5% purity, as assessed by flow cytometry, and were found to express similar amounts of ICOS protein (Fig. S2B) and RNA (Fig. S2C). We confirmed RNA purity by ensuring expression of the MDDC marker CD11c, but not the T cell marker CD3, and further verified ICOSL mRNA expression using the highly purified MDDC (Fig. S2C). siRNA knockdown of ICOS also reduced ICOS mRNA expression in the highly purified MDDC (Fig. S2D). Finally, we examined ICOS expression on intestinal myeloid cells. ICOS was expressed on CD11c+CD3− intestinal myeloid-derived cells in amounts comparable to peripheral MDDC (Fig. 2D). In contrast, we did not observe ICOS expression on ex vivo CD3+ T cells in uninflamed intestine (data not shown), consistent with prior reports (Sato et al., 2004). As ICOS expression on peripheral monocyte-derived macrophages was similar to MDDC (data not shown), we also examined CD33+ cells in the intestine; CD33 is used as a marker of intestinal myeloid-derived cells, in particular macrophages (Kamada et al., 2008; Rogler et al., 1998). ICOS was expressed on CD33+ intestinal myeloid-derived cells to levels comparable to MDDC (data not shown).
Figure 2. Human MDDC and intestinal myeloid cells express ICOS and ICOSL is upregulated on MDDC upon NOD2 stimulation.
(A) Human monocytes (n=8), MDDC (n=11) and resting and activated primary human T cells (n=3) were stained for ICOS. A subset of MDDC (n=3) from the total cohort were run side-by-side in the same experiment as the T cells; MFI values were similar between these cell subsets. Representative flow cytometry plots with MFI values are shown. Summary graphs of ICOS expression + SEM. (B) MDDC were transfected with scrambled or ICOS siRNA for 48h. (Left) Representative flow cytometry plots with ICOS MFI values. (Right) Summary graph of ICOS expression + SEM (n=4). (C) ICOS expression by Western blot from two donors (clone ANC6C6-A3). Resting and activated primary human CD4+ T cells served as controls. The band migrating between ~22–27 kDa is consistent with the monomeric form of ICOS. GAPDH served as loading control. (D) Intestinal lamina propria cells were isolated from non-inflamed colonic resection specimens and gated on CD11c+CD3− cells. (Left) Representative flow cytometry plots with ICOS MFI values. (Right) Summary graph of ICOS expression + SEM (n=5). (E–F) MDDC were transfected with scrambled or ICOSL siRNA for 48h. (E) Representative flow cytometry plot of ICOSL expression from one of seven individuals with MFI as indicated. (F) ICOSL mRNA expression (n=7) as assessed by RT-PCR. (G–H) Human MDDC (n=8) were stimulated for (G) the indicated time points with 100 μg/ml MDP or (H) for 12h with indicated MDP doses. Left: Shown are representative flow cytometry plots with ICOSL MFI values as indicated. Right: Summarized data are represented as the fold ICOSL induction normalized to untreated cells (represented by the dotted line at 1) + SEM. (I) Shown is fold ICOSL mRNA expression (n=8) normalized to untreated MDDC (represented by the dotted line at 1) + SEM. scr, scrambled; Tx, treatment. *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1×10−4; ††, p<1×10−5.
ICOSL was also expressed by MDDC (Fig. 2E) and both ICOSL protein and mRNA were reduced in response to ICOSL siRNA-mediated knock-down (Fig. 2E&F). Furthermore, we found that ICOSL transcription and surface expression increased upon NOD2 stimulation (Fig. 2G, H&I). Thus, in addition to expressing ICOSL, MDDC and intestinal DC express ICOS.
PRR stimulation induces ICOS-ICOSL interactions on MDDC which in turn amplifies cytokine secretion
We next asked whether ICOSL and ICOS contribute to PRR-induced cytokine secretion. ICOSL expression was knocked down by siRNA (Fig. 2E&F) and NOD2-induced cytokine production upon stimulation with 100 μg/ml MDP was examined (Fig. 3A). This MDP dose results in peak induction of ICOSL expression (Fig. 2H) and approximates amounts of muramic acid in stool (Vavricka et al., 2004). Induction of the pro-inflammatory cytokines TNF-α, IL-8, IL-1β and IL-23, which are dysregulated in CD (Abraham and Cho, 2009), and the anti-inflammatory cytokine IL-10 in response to NOD2 stimulation was reduced upon ICOSL silencing (Fig. 3A). Silencing of ICOS (Fig. 2B) also decreased NOD2-induced cytokine secretion (Fig. 3B). These results were confirmed with three additional siRNAs against each ICOSL and ICOS eliminating the possibility of off-target effects (Fig. S3A). We further ensured cell viability was intact (Fig. S3B) and that functional responses were intact upon stimulation through an alternative pathway utilizing dectin ligands (Fig. S3C). Similar data were obtained using ICOSL and ICOS neutralizing antibodies (Fig. S3D). Therefore, these data demonstrate that both ICOS and ICOSL on MDDCs are critical for optimal NOD2-mediated cytokine secretion.
Figure 3. ICOSL and ICOS enhance NOD2-induced MAPK, NF-κB and PI3K activation and cytokine secretion in primary human MDDC.
MDDC (n=8) were transfected with scrambled, (A) ICOSL, or (B) ICOS siRNA and 48h later stimulated for 24h with 100μg/ml of MDP. (A,B) Cytokines were measured in supernatants + SEM. Significance was calculated compared to scrambled siRNA transfected, MDP-treated cells. Similar results were observed in an additional n=4. (C–F) MDDC (n=14–16) were left untreated or stimulated for 24h with (C) indicated doses of MDP, (D) 100μg/ml recombinant ICOSL or (E) 10μg/ml recombinant ICOS alone or combined with 1μg/ml MDP. Shown is cytokine concentration in supernatants + SEM or (F) synergy calculated as a fold increase of cytokine levels upon combined ICOS and MDP treatment divided by the sum of the cytokine levels upon treatment with each stimulus alone+SEM. Stimulation of cells with an IgG1 isotype control did not result in cytokine secretion (data not shown). (G–I) MDDC (n=6–12) were transfected with scrambled or ICOSL siRNA. 48h later, cells were stimulated for 15min with 100μg/ml MDP. Left: Representative flow cytometry plots with MFI values as indicated for (G) phospho-ERK, -p38 and -JNK, (H) phospho-IκBα or (I) phospho-Akt and phospho-p70S6K. Right: Summarized data are represented as the fold phospho-protein induction normalized to untreated, scrambled siRNA transfected cells+SEM. scr, scrambled, Tx, treatment; IC, ICOS; ICL, ICOSL. *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1×10−4; ††, p<1×10−5. Lines over adjacent bars indicate identical p values for these bars.
We next asked whether NOD2-mediated cytokine induction in MDDCs was regulated by ICOS- or ICOSL-initiated signaling. We first stimulated cells with 1 μg/ml MDP, a dose that resulted in low cytokine secretion (Fig. 3C), and asked if adding soluble recombinant ICOSL or ICOS, respectively, enhances this low cytokine secretion. Soluble ICOSL did not enhance the low amounts of cytokine secreted by MDDC in response to 1 μg/ml MDP (Fig. 3D). The soluble ICOSL was functional as it was able to costimulate primary T cells (data not shown). In contrast, soluble ICOS markedly enhanced NOD2-induced cytokine secretion (Fig. 3E&F). Notably, MDDC treatment with ICOS alone induced low cytokine secretion (Fig. 3E), indicating that ICOSL-initiated signaling is sufficient for cytokine induction. When ICOSL expression was silenced, ICOS treatment failed to increase NOD2-mediated cytokine secretion, clearly establishing ICOS-ICOSL interactions are required to enhance NOD2-induced cytokine secretion (Fig. S3E). The MDDC did not contain significant T cell contamination (ranging from 0.5–5%). Moreover, resting T cells express very low amounts of ICOS (Fig 2A&C). However, to ensure that amplification of PRR-induced cytokine secretion by ICOSL was mediated by ICOS on MDDC rather than ICOS on T cells, we added an increasing percentage (up to 10%) of purified, resting T cells to MDDC in which ICOS was silenced. This failed to increase NOD2-mediated cytokine secretion, whereas addition of MDDC (up to 10%) rescued the enhanced cytokine secretion; addition of intact MDDC to 50% almost completely restored cytokines (Fig. S3F). Therefore, in contrast to the low amount of ICOS on resting T cells, ICOS on MDDC is sufficient and functional in mediating downstream outcomes. To further verify that cell-derived ICOS can interact with ICOSL on MDDC to increase cytokine secretion, we confirmed that HeLa cells transfected with ICOS (Fig. S3G) enhanced MDP-induced cytokines relative to empty vector (EV)-transfected cells (Fig. S3F). Soluble ICOS was similarly able to rescue NOD2-induced cytokine secretion under conditions where ICOS expression in MDDC was reduced (Fig. S3F). Nuocytes are another ICOS-expressing population (Wong et al., 2012); however, these were not present in our MDDC population whereas we could detect nuocytes in intestinal lamina propria cells (data not shown). To further verify that the ICOS:ICOSL-mediated effects in MDDC were not contaminated with ICOS-expressing cell populations, we confirmed the ability of ICOS:ICOSL to augment MDP-induced cytokine secretion on highly purified MDDC (>99.5% purity as per cells examined in Fig. S2B–D) (Fig. S3H). Taken together, we establish that ICOS is expressed on MDDC and interacts with ICOSL to initiate ICOSL-mediated signaling which, in turn, augments NOD2-induced cytokine secretion from MDDC.
ICOSL signaling enhances NOD2-induced MAPK, NF-κB and PI3K activation in primary human MDDC
We next sought to define the signaling pathways activated upon ICOSL stimulation on MDDC. Previous studies have shown that ERK, p38 and JNK contribute to cytokine secretion upon PRR, including NOD2, stimulation (Hedl and Abraham, 2011a, 2012; Kawai and Akira, 2010; Kobayashi et al., 2005; Park et al., 2007; Pauleau and Murray, 2003; Vidal et al., 2001; Yang et al., 2007). We therefore measured their activation after MDDC were treated with low dose MDP, ICOS or combined MDP and ICOS. Similar to the cytokine data (Fig. 3E), ERK, p38 and JNK were all activated by low-dose MDP or ICOS treatment alone and activation was enhanced by combined ICOS and MDP treatment (Fig. S4A). We observed a similar pattern of activation in the NF-κB pathway (Fig. S4B), and the intermediates of the PI3K pathway Akt and mTOR (Fig. S4C), which all contribute to cytokine induction (Hedl and Abraham, 2011b, 2012; Kobayashi et al., 2005). Taken together, ICOSL synergizes with suboptimal NOD2 stimulation to increase activation of pathways critical for cytokine secretion, including the MAPK, NF-κB and PI3K signaling pathways.
Exogenous ICOS treatment was found to enhance cytokine-activating signaling pathways induced by low dose MDP (Fig S4A–C). We next asked whether endogenous ICOS:ICOSL interactions were necessary for MAPK, NF-κB and PI3K pathway activation in response to MDP. 100 μg/ml of MDP was used, a dose that promotes increased ICOSL expression (Fig. 2H) and which requires both ICOS and ICOSL for optimal NOD2-initiated cytokine secretion (Fig. 3A&B). Silencing of ICOSL in MDDC resulted in reduced activation of ERK, p38 and JNK after NOD2 stimulation (Fig. 3G), and the phospho-flow data were confirmed by Western blot (Fig. S4D). Similarly, activation of the NF-κB pathway (Fig. S4E,Fig. 3H), and the PI3K pathway (Fig. 3I) were attenuated in these cells. Taken together, ICOSL is required for optimal NOD2-initiated MAPK, NF-κB, and PI3K signaling in primary human MDDC. Having identified that ICOSL stimulation was required for optimal signaling (Fig. 3G–I) and cytokine induction (Fig. 3A) upon NOD2 stimulation of MDDC, we next asked if ICOSL contributed to cytokine induction upon stimulation of other PRR. We observed that ICOSL was required for optimal cytokine secretion upon TLR2, TLR3, TLR4, TLR5 and TLR9 stimulation of human MDDC (Fig. 4).
Figure 4. ICOSL is required for optimal cytokine secretion upon stimulation of multiple PRR.

Human MDDC (n=8) were transfected with scrambled or ICOSL siRNA. 48h later cells were stimulated for 24h with 10 μg/ml Pam3Cys (TLR2), 100 μg/ml polyI:C (TLR3), 0.1 μg/ml lipid A (TLR4), 5 ng/ml flagellin (TLR5) or 10 μg/ml CpG DNA (TLR9). Cytokine concentrations in the supernatants are shown + SEM. Significance was calculated compared to scrambled siRNA transfected, PRR-treated cells. We confirmed the results with TLR ligands in an additional n=4. Tx, treatment; scr, scrambled *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1×10−4; ††, p<1×10−5.
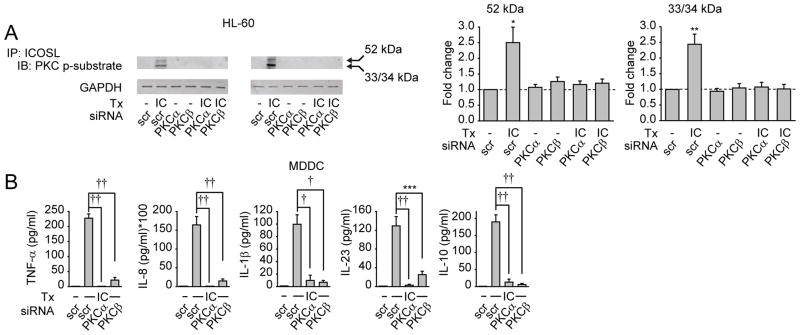
PKCα and PKCβ are required for phosphorylation of ICOSL and ICOSL-mediated cytokine induction
Unlike the well-investigated signaling through ICOS in T cells, ICOSL-initiated pathways are poorly understood. To better dissect the mechanisms through which ICOSL activation regulates PRR-induced signaling, we conducted an initial in silico analysis and identified serines and threonines in the ICOSL cytoplasmic region that might serve as putative PKC phosphorylation sites. To more clearly examine the role of PKCs in PRR signaling, we first established that PKCs were activated after ICOS and low dose (1 μg/ml) MDP treatment of MDDC, and that combined treatment synergized to increase PKC activation (Fig. S5A). Moreover, we verified that upon 100 μg/ml MDP treatment, knock-down of ICOSL impaired PKC activation (Fig. S5B). We then asked if PKC activation functions upstream of the signaling pathways activated upon ICOSL stimulation. PKC classes include conventional PKCs (such as PKCα, β and γ) and atypical PKCs (such as PKCζ). Upon inhibition of conventional PKCs and PKCζ in ICOS-stimulated MDDCs, MAPK, NF-κB and PI3K signaling was attenuated (Fig. S5C–E). The results of the pharmacological inhibition were subsequently confirmed with a siRNA approach presented below. Therefore, PKCs are required for signaling downstream of ICOSL. As ICOSL contains putative PKC phosphorylation sites, we next asked whether ICOS treatment led to PKC-dependent phosphorylation of ICOSL. Due to the cell number limitation of primary human monocytes and large numbers of cells required for immunoprecipitation experiments, we addressed this question in the human monocytic HL-60 cell line, which also upregulated ICOSL expression after treatment with PRR ligands such as LPS (Fig. S5F) and required ICOSL signaling for LPS-mediated cytokine induction (Fig. S5G). Low amounts of cytokine secretion were observed after ICOS treatment, which was mediated by ICOSL (Fig. S5H). Using an antibody recognizing phosphorylated PKC consensus sites, we found that ICOS treatment of HL-60 cells led to phosphorylation of PKC substrate motifs on ICOSL-corresponding bands (Fig. 5A). Ensembl analysis (ensembl.org) identifies four human ICOSL isoforms, the most common splice variants being the 52, 34 and 33 kDa variants (Ling et al., 2001). We observed bands migrating at the molecular weights corresponding to the sizes of these isoforms (Fig. 5A). We next asked which PKCs were required for phosphorylation of ICOSL. Conventional PKCs are critical for ICOSL-initiated signaling (Fig. S5C–E), and both PKCα and PKCβ, but not PKCγ, are expressed in myeloid cells (Webb et al., 2000) (data not shown). PKCα and PKCβ knock-down (Fig. S5I&J) reduced both ICOS-induced ICOSL phosphorylation in HL-60 cells and ICOSL-mediated cytokine secretion in MDDC (Fig. 5A&B). Thus after ICOS treatment, ICOSL is phosphorylated on PKC substrate sites in a PKCα- and PKCβ-dependent manner, and PKCα and PKCβ activation is in turn required for ICOSL-mediated downstream signaling and cytokine induction.
Figure 5. PKCα and PKCβ are required for phosphorylation of ICOSL and ICOSL-mediated cytokine induction.
(A) HL-60 cells were transfected with scrambled, PKCα or PKCβ siRNA as indicated and stimulated with 10μg/ml ICOS for 15min. Cell lysates were immunoprecipitated with anti-ICOSL antibody, and phosphorylation of PKC substrate motifs in ICOSL was assessed by Western blot. GAPDH expression from total lysates served as the loading control. Left: Two representative Western blots. Right: Summarized data (n=6) are represented as the fold phospho-protein induction normalized to untreated, scrambled siRNA-transfected cells for each of the 2 bands visualized (representing distinct ICOSL isoforms)+SEM. (B) MDDC (n=8) were transfected with scrambled, PKCα, or PKCβ siRNA and 48h later stimulated for 24h with 10μg/ml ICOS. Cytokine concentrations in supernatants are shown + SEM. scr, scrambled; IC, ICOS; p-, phospho-. *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1×10−4; ††, p<1×10−5.
Arginine residues in the ICOSL cytoplasmic tail are required for recruitment of signaling molecules
We next sought to define the mechanism through which ICOSL signaling affects downstream outcomes, and to define the structural requirements in ICOSL for these outcomes. The 34 kDa ICOSL isoform has a short cytoplasmic tail with two highly conserved arginine residues (R278 and R280) and two serine residues (S285 and S293) of which only S285 is conserved across several species (Fig. 6A). Arginine residues can serve as recruitment sites for PKC (Leventhal and Bertics, 1993). We therefore constructed ICOSL mutants in which the arginines and serines, alone or in combination, were replaced with alanines. We also generated an ICOSL mutant without an intracellular cytoplasmic tail in order to assess the importance of signaling initiated by the cytoplasmic tail. We expressed each of these ICOSL variants in HeLa cells, a cell line that did not express detectable surface ICOSL, and observed equivalent surface expression with each of the constructs in amounts comparable to MDDC (Fig. 6B).
Figure 6. Arginine residues in the proximal ICOSL cytoplasmic tail are required for the recruitment of signaling intermediates leading to cytokine secretion.
(A) Sequence alignments (CLUSTALW) of mammalian ICOSL cytoplasmic tail sequences and indicated mutated residues. (B) EV, WT ICOSL, tailless, S285A, S293A, S285A and S293A, R278A, R280A or R278A and R280A ICOSL mutants were transfected into HeLa cells. Surface ICOSL expression was measured 24h later by flow cytometry. Expression was comparable to primary MDDC (shown as control). (C–D) HeLa cells were transfected with WT ICOSL or ICOSL mutants and stimulated with 10μg/ml ICOS for 15min. Cell lysates were immunoprecipitated with anti-ICOSL antibody. (C) Phosphorylated PKC substrate, or PKCβ, RACK-1 and JNK recruitment was assessed by Western blot. (D) Phospho-PKCβ and phospho-Jun associated with ICOSL was assessed by Western blot. Normalized densitometry measurements are shown above the gels. (E) HeLa cells were transfected with WT ICOSL, and PKC was inhibited either through PKCβ siRNA transfection at the same time, or through 10μM bisindolylmaleimide at the time of ICOS treatment. Cells were then treated with 10μg/ml ICOS for 15min. Cell lysates were immunoprecipitated with anti-ICOSL antibody. Associated phospho-Jun was assessed by Western blot. (C–E) ICOSL expression from total lysates served as the loading control. (F) HeLa cells were transfected with EV, WT ICOSL or ICOSL mutants. 24h later cells were stimulated for 15min with 10μg/ml ICOSL. Top: Representative flow cytometry plots with MFI values as indicated. Bottom: Summarized data are represented as the fold phospho-protein induction normalized to untreated, WT ICOSL transfected cells+SEM. Significance is compared to ICOS-treated, WT ICOSL transfected cells. (G) WT (gray bars) or tailless (white bars) ICOSL and WT NOD2 were transfected into HeLa cells. 24h later, cells were stimulated with 10 μg/ml ICOS, 1 μg/ml MDP or a combination of both for 24h. The data are represented as IL-6 secretion in triplicate + SEM (representative of three independent experiments). Significance comparisons are between single vs combined treatment conditions as indicated, or between WT vs tailless ICOSL-transfected cells treated with the same stimuli indicated above white bars. (H) WT ICOSL or ICOSL mutants and WT NOD2 were transfected into HeLa cells. 24h later, cells were stimulated with (left) 10 μg/ml ICOS or (right) 10 μg/ml ICOS and 1 μg/ml MDP for 24h. The data are represented as IL-6 secretion in triplicate (representative of three independent experiments). Significance compared to (left) ICOS- or (right) ICOS- and MDP-treated, WT ICOSL-transfected cells is shown. scr, scrambled, Tx, treatment, WCL, whole cell lysate. **, p<0.01; ***, p<0.001; †, p<1×10−4; ††, p<1×10−5.
After ICOS treatment we observed phosphorylation of PKC substrate motifs (with a phosphorylated band corresponding to the 34 kDa ICOSL isoform) in cells expressing WT ICOSL (Fig. 6C). This phosphorylation was absent in the tailless, S285A, S285A and S293A, R278A, R280A, and R278A and R280A mutants, but remained intact in S293A mutants (Fig. 6C). Therefore, S285 is required for PKC substrate (serine) phosphorylation of ICOSL, and each of the upstream arginines is similarly required for this phosphorylation. We next asked what are the signaling events initiated by the ICOSL cytoplasmic tail that contribute to cytokine secretion. Conventional, activated PKCβ can recruit the scaffolding protein RACK1 and JNK resulting in c-Jun activation (Lopez-Bergami et al., 2005). RACK1 knock-down in primary MDDC decreased ICOSL-induced cytokine secretion (Fig. S6A), suggesting a role for RACK1 in ICOS:ICOSL outcomes. Similarly, JNK pathway activation is required for NOD2-induced (Hedl and Abraham, 2012) and ICOSL-induced cytokine secretion (data not shown) in primary myeloid cells. Upon ICOS treatment, PKCβ, RACK1 and JNK were each recruited to ICOSL; recruitment of these molecules was absent in tailless, R278A and R280A, but intact in S285A and S293A mutants (Fig. 6C). These findings are consistent with prior studies showing that arginine-containing peptides are sufficient for recruitment of conventional PKC (Leventhal and Bertics, 1993).
As the S285A ICOSL mutant resulted in a loss of PKC substrate phosphorylation in ICOSL, but did not affect PKCβ, RACK1 or JNK recruitment to ICOSL, we sought to define the role of S285 in ICOSL-mediated outcomes. Phosphorylation of PKC can modulate its ability to phosphorylate and activate downstream substrates, such as JNK (Lopez-Bergami et al., 2005). We therefore hypothesized that the phosphorylation of S285 in ICOSL might contribute to optimal activation of the PKCβ and JNK recruited to ICOSL. In the ICOS-induced protein complex associated with the S285A ICOSL mutant, there was a ~20–30% decrease in phosphorylated PKCβ and activation of JNK as assessed by phosphorylation of its c-Jun substrate (Fig. 6D). Consistent with the failure to recruit PKCβ and JNK to the ICOSL tail, phospho-PKCβ and phospho-c-Jun were not detected in association with tailless, R278A, R280A or R278A/R280A ICOSL mutants (Fig. 6D). We further verified the requirement for PKC activation in the phospho-c-Jun associated with ICOSL through both inhibition and knock-down of conventional PKC (Fig. 6E).
We next examined if the S285A, R278A or R280A ICOSL mutations leading to phosphorylation defects of signaling molecules within the ICOSL-associated complex also lead to cellular signaling defects. ICOS-dependent phosphorylation of JNK (Fig. S6B) and its substrate, c-Jun, (Fig. 6F) was diminished in S285A ICOSL mutants and more dramatically attenuated in R278A and R280A ICOSL mutants. Similar results were observed with activation of IκBα (Fig. 6F). Tailless ICOSL also resulted in a loss of ICOSL-initiated JNK and NF-κB pathway activation (Fig. 6F, Fig. S6B). Taken together, upon ICOS treatment, S285 in ICOSL is required for optimal phosphorylation of proteins recruited to ICOSL. In contrast, R278 and R280 are required for recruitment, and therefore phosphorylation, of proteins associated with ICOSL, which in turn, leads to activation of cytokine-inducing pathways after ICOS:ICOSL interactions.
We next examined how the ICOSL mutants affect ICOS:ICOSL-mediated cytokine induction and synergy with NOD2. In HeLa cells transfected with WT NOD2 and ICOSL, IL-6 was secreted after either MDP or ICOS treatment (Fig. 6G), and secretion was increased after combined MDP and ICOS treatment (Fig. 6G). This synergy was abolished if WT NOD2 was co-expressed with ICOSL lacking the cytoplasmic tail (Fig. 6G). Consistent with the recruitment of signaling complexes and phospho-kinase activation (Fig. 6C&F), IL-6 secretion was decreased in S285A ICOSL mutants after ICOS (Fig. 6H, left) or ICOS and MDP treatment (Fig. 6H, right), and IL-6 secretion was abolished in R278A and R280A ICOSL mutants upon ICOS treatment, and dramatically attenuated upon combined ICOS and MDP treatment (Fig. 6H). The S285A and S293A mutations combined did not further impair cytokine secretion relative to S285A (Fig. 6H), consistent with the failure of this combined mutant to further attenuate ICOSL-initiated signaling pathways (Fig. 6F, Fig. S6B). Similar results were observed with IL-8 secretion (data not shown). Taken together, the synergy in cytokine induction with NOD2 and ICOS:ICOSL stimulation is modulated by conserved arginine residues in the cytoplasmic tail of ICOSL which recruit the PKC-RACK1-JNK signaling complex.
The rs7282490 risk G allele in the ICOSLG region is associated with decreased ICOSL expression
We next sought to define the mechanism through which the rs7282490 polymorphism regulates PRR-induced cytokine secretion. As rs7282490 is downstream of the ICOSLG gene, we hypothesized that it might influence basal and/or induced ICOSL expression. We therefore assessed ICOSL expression in MDDC from AA, GA and GG rs7282490 individuals. MDDC from GG carriers expressed lower amounts of ICOSL mRNA (Fig. 7A) and protein (Fig. 7B) than MDDC from AA individuals, while GA carriers showed an intermediate ICOSL expression. MDP stimulation increases ICOSL expression (Fig. 2G–I) and the difference in ICOSL expression between GC and AA carriers were more pronounced upon MDP stimulation (Fig. 7A&B). To better assess if the rs7282490 risk allele regulated ICOSL induction by MDP, we reanalyzed the data by normalizing the amount of MDP-induced ICOSL mRNA and protein to that present in unstimulated cells. We found that NOD2-mediated ICOSL induction was clearly lower in GG carriers relative to AA homozygotes (Fig. 7C). The expression of the 52 kDa, 34 kDa and 33 kDa ICOSL isoforms underwent upregulation upon MDP treatment similar to that observed when using primers detecting a site common to all three ICOSL isoforms, with the maximum induction peak at 4 h (Fig. 2G & Fig. S7A). Furthermore, GG rs7282490 carriers showed decreased expression of all three ICOSL isoforms in untreated or MDP-treated MDDC relative to AA carriers (data not shown). Other genes in the region did not demonstrate altered expression based on rs7282490 genotype (Fig. S7B). Moreover, knock-down of these genes (Fig. S7C) did not regulate NOD2-induced cytokine secretion (Fig. S7D). To determine if the rs7282490 genotype, in turn, leads to differences in signaling, we measured induction of ERK activation in cells from AA and GG homozygotes, as these genotypes showed the most dramatic differential in ICOSL expression (Fig. 7A–C) and cytokine secretion (Fig. 1). We found less ERK activation in GG carriers after MDP, ICOS, and particularly combined MDP and ICOS treatment of MDDC compared to AA carriers (Fig. 7D). Therefore, consistent with the decreased cytokine production after PRR stimulation (Fig. 1), MDDC from disease-risk rs7282490 GG carriers demonstrate decreased basal and NOD2-induced ICOSL expression, as well as decreased ICOSL-initiated signaling.
Figure 7. The rs7282490 risk G allele in the ICOSLG region is associated with decreased basal and NOD2-induced ICOSL expression, decreased ERK activation and an increased likelihood of ileal Crohn’s disease.
(A–C) MDDC from rs7282490 AA, GA or GG carriers (n=8 for each group) were left untreated or stimulated with 100μg/ml MDP for (A) 4h or (B) 12h. Summarized data are represented as ICOSL (A) mRNA expression (expressed as the change in CT values from duplicate samples normalized to GAPDH and represented as a linear scale + SEM) or (B, right) protein expression, with average MFI values indicated above bars. (B, left) Representative flow cytometry plots of ICOSL expression with MFI values as indicated. (C) Summarized data are represented as fold NOD2-induced ICOSL mRNA and protein expression relative to untreated cells + SEM. (D) MDDC from rs7282490 AA or GG carriers (n=7 for each group) were stimulated for 15min with 10μg/ml ICOS or 1μg/ml MDP, either alone or in combination. Left: Representative flow cytometry plots of phospho-ERK with MFI values as indicated. Right: Summarized data are represented as the fold phospho-ERK induction normalized to untreated cells+SEM. (E) Top: The ICOSLG rs7282490 polymorphism was stratified on CD location using the allelic inheritance model or recessive inheritance model. Bottom: As a control, risk allele frequencies of NOD2 CD-risk polymorphisms were stratified on CD location using allelic inheritance model. Δ, change; OR, odds ratio. *, p<0.05; **, p<0.01; †, p<1×10−4; ††, p<1×10−5.
The rs7282490 risk G allele in the ICOSLG region is associated with increased likelihood of ileal CD
CD carriers of NOD2 loss-of-function polymorphisms demonstrate an increased ileal disease frequency (Fig. 7E) (Abraham and Cho, 2009). As ICOSL was found to amplify NOD2-initiated signaling and cytokine secretion (Fig. 1, 3, 6&7), we asked whether the CD risk loss-of-function G allele at rs7282490 in the ICOSLG region also shows an increased ileal distribution. We observed a higher frequency of the rs7282490 G allele in ileal disease CD compared to colonic CD (Fig. 7E). Because GG carriers demonstrate decreased PRR-induced cytokine secretion compared to AA and GA carriers, we examined a recessive inheritance model for ileal disease. Consistent with the functional effects on cytokine secretion, we observed a greater effect of the G allele on ileal location in the recessive compared to the allelic inheritance model (Fig. 7E).
Discussion
Disease-associated polymorphisms represent important functional perturbations that can define novel and unexpected gene functions in pathways fundamental to disease pathogenesis. In this study, we found ICOS:ICOSL signaling amplifies PRR-initiated signaling and cytokine secretion by primary human MDDC. We identified that ICOS is expressed on MDDC and intestinal DC, and that ICOS on MDDC can interact with ICOSL on MDDC leading to ICOSL-initiated signaling. The ability of costimulatory molecules to amplify PRR responses is relatively unexplored. We found that two arginines in the proximal cytoplasmic tail of ICOSL are required for recruitment of PKCβ, RACK1 and JNK to ICOSL, which leads to activation of the proteins in this recruited complex, and subsequent activation of cellular signaling and cytokine secretion. Given the impaired PRR-induced signaling and cytokine secretion in the absence of ICOS, we hypothesize that direct ICOS:ICOSL interactions lead to the vast majority of the ICOSL cytoplasmic tail-associated events during PRR stimulation. We found that MDDC from IBD-associated rs7282490 GG risk carriers in the ICOSLG region show decreased basal and NOD2-induced ICOSL expression. Consistently, these MDDC had decreased ICOSL-initiated signaling, which reduced amplification of PRR signaling and cytokine secretion (Fig. S7E). While MDDC from the rs7282490 risk carriers are only partially reduced in PRR-induced cytokine secretion due to a relative decrease in ICOSL expression, complete knock-down of ICOSL expression attenuates PRR-induced cytokine secretion even at high doses of PRR ligands. Similar to loss-of-function NOD2 risk individuals, loss-of-function ICOSLG risk GG carriers exhibit increased association with an ileal phenotype of CD.
Although inflammatory diseases are characterized by increased cytokine production, multiple loss-of-function polymorphisms in genes participating in inflammatory-inducing pathways within innate pathways are associated with CD (Abraham and Cho, 2009). Similarly, mice deficient in genes participating in innate response pathways (e.g. TLR5 (Vijay-Kumar et al., 2007), MyD88 (Rakoff-Nahoum et al., 2004)) can demonstrate inflammatory outcomes with enhanced colitis susceptibility, highlighting that proper initial innate responses are required for intestinal immune homeostasis. Enhanced inflammation in the context of loss-of-function in innate response pathways can occur for various reasons, including a failure to properly respond to or efficiently eliminate microbes (Feuillet et al., 2006; Grainger et al., 2013; Naiki et al., 2005) which can lead to inappropriately increased compensatory inflammatory responses. NOD2 loss-of function polymorphisms and ICOSLG rs7282490 represent two major IBD-risk polymorphisms resulting in reduced PRR-initiated cytokine secretion. Moreover, GG rs7282490 carriers show reduced cytokine secretion upon stimulation of a broad range of PRR, indicating that this polymorphism is both common and has global innate regulatory consequences. A role for the costimulatory molecule ICOSL in amplifying PRR-induced cytokine secretion on myeloid cells is particularly relevant to IBD given that myeloid cells mediate early microbial recognition in the intestine. Our findings identify a critical role for the rs7282490 ICOSLG region polymorphism associated with autoimmune/inflammatory diseases in amplifying PRR-initiated inflammatory signaling and cytokine secretion.
Experimental Procedures
Patient and control recruitment and genotyping
Informed consent was obtained per protocol approved by the institutional review board at Yale University. For cell studies in healthy controls, we recruited participants with no personal or family history of autoimmune/inflammatory disease, including psoriasis, SLE, rheumatoid arthritis, multiple sclerosis, type I diabetes mellitus, CD, and ulcerative colitis, or a history of HIV. Due to the limitation in primary human cells and the range of stimulation conditions we wished to interrogate, two separate cohorts of 100 and 98 individuals were recruited for NOD2 and TLR2 dose-response studies, and NOD2 and TLR synergy studies in MDDC, respectively. Genotyping for polymorphisms was performed by TaqMan SNP genotyping (Applied Biosystems, Foster City, CA) or Sequenom platform (Sequenom Inc., San Diego, CA). For association studies, a total of 2724 subjects were enrolled, including 1512 CD cases (542 ileal, 705 ileocolonic and 265 colonic cases) and 1212 healthy controls. CD patients were diagnosed by clinical, endoscopic, radiologic and histologic criteria. Medical histories were collected with the validated NIDDK IBD Genetics Consortium phenotype forms (Nguyen et al., 2006).
Primary human cell culture
Monocytes were purified and differentiated to MDDC as in (Hedl and Abraham, 2012). T cells were isolated using CD4 selection (Miltenyi Biotec), and activated with platebound anti-CD3 (5 μg/mL) and anti-CD28 (1 μg/mL) (R&D Systems) for 5 days. Activation was confirmed by proliferation and upregulation of the activation markers CD25 and CD69. Intestinal lamina propria cells were isolated from colonic resection specimens from uninvolved intestine in 5 non-inflammatory bowel disease patients undergoing surgery for diverticular disease or colon cancer. Briefly, intestinal resections were washed and then digested (RPMI containing 0.2 mM EDTA, beta-mercaptoethanol) in a 37°C shaker to remove epithelial cells. Epithelial cells and intraepithelial lymphocytes were discarded and intestines were washed, cut into 1mm2 pieces and incubated in a buffer consisting of RPMI, 75 μg/ml collagenase Type VIII, DNAse (Sigma-Aldrich) and HEPES for 45 min in a 37°C shaker. The cells were filtered through a 40 micron filter, selected on a ficoll gradient, washed, and analyzed by FACS.
MDDC and HL-60 cell stimulation
MDDC or HL-60 human myeloid cells were treated with MDP (Bachem, King of Prussia, PA), Pam3Cys, lipid A (Peptides International, Louisville, KY), flagellin, CL097, CpG, poly I:C (Invivogen, San Diego, CA), E. coli LPS (Sigma-Aldrich), ICOS and ICOSL (R&D Systems Inc.). Supernatants were assayed for TNF-α, IL-8, IL-10 (BD Biosciences), IL-23, or IL-1β (eBioscience, San Diego, CA) by ELISA.
Transfection of small interfering RNAs (siRNAs)
300 nM scrambled or ON-TARGETplus SMARTpool siRNA against ICOSL, ICOS, PKCα or PKCβ (Dharmacon, Lafayette, CO) (4 pooled siRNAs for each gene) were transfected into MDDC as in (Hedl and Abraham, 2012).
Constructions and transfection of ICOSL mutant vectors
ICOSL tailless (generated by inserting a stop codon), S285A, S293A, S285A and S293A, R278A, R280A, and R278A and R280A mutants were generated through site-directed mutagenesis (QuikChange Lightning kit; Agilent Technologies, Santa Clara, CA) of WT ICOSL cDNA clone in pReceiver vector (GeneCopoeia, Rockville, MD), and the mutations were confirmed by sequencing. HeLa cells were transiently transfected by Lipofectamine (Invitrogen) with 4 μg WT or mutant ICOSL vectors or empty vector +/− 50 ng WT NOD2 in pCDNA.3. 24h after transfection, cells were assayed for ICOSL surface expression or stimulated as indicated.
Protein detection
Phospho-proteins were detected with Alexa Fluor 647-labeled phospho-ERK, phospho-p38, phospho-JNK, phospho-Jun, phospho-Akt, phospho-p70-S6K, phospho-IκBα (Cell Signaling, Danvers, MA) and phospho-PKC (Santa Cruz Biotechnology, Santa Cruz, CA) along with isotype controls. ICOSL was measured using PE-labeled anti-ICOSL (BD Biosciences). ICOS was measured using APC-labeled mouse anti-ICOS antibody (R&D Systems, clone 669222) by flow cytometry or using mouse anti-ICOS antibody (Santa Cruz, clone ANC6C6-A3) by Western blot.
ICOSL was immunoprecipitated from HL-60 or transfected HeLa lysates with anti-ICOSL antibody (Abcam, Cambridge, MA)-bound to protein A Sepharose (Upstate Biotechnology). PKC-mediated phosphorylation was visualized by using an anti-PKC-substrate antibody (Cell Signaling) and ICOSL-associated proteins were examined with antibodies recognizing RACK1, PKCβ (R&D Systems Inc.), JNK, phospho-Jun or phospho-PKCβ (Cell Signaling). GAPDH antibody (EMD Biosciences, San Diego, CA) and ICOSL antibody (Abcam) were used to assess equal loading.
mRNA expression analysis
Quantitative PCR was performed as in (Hedl and Abraham, 2011a). Samples were normalized to GAPDH. Primers are available upon request.
Statistical analysis
Association of ICOSL and NOD2 variants with disease location in CD patients was tested under the allelic and recessive genetic inheritance models using the chi-squared test as implemented in PLINK (Purcell et al., 2007). Significance for experimental studies was assessed using two-tailed t-test. p < 0.05 was considered significant.
Supplementary Material
Highlights.
ICOS:ICOSL interactions on human DC amplify PRR-induced signaling and cytokines
Conserved arginines in the ICOSL tail recruit a PKC/RACK1/JNK signaling complex
ICOSLG risk SNPs decrease ICOSL expression, and PRR-induced signaling and cytokines
The ICOSLG risk polymorphism is associated with an ileal Crohn’s disease phenotype
Acknowledgments
This work was supported by the Broad Foundation and the NIH: R01DK077905, R56AI089789, DK099097, DK-P30-34989, U19-AI082713, U01 DK62429, U01 DK062422, R01 DK092235.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter JA, Clark EA. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- Biswas A, Wilmanski J, Forsman H, Hrncir T, Hao L, Tlaskalova-Hogenova H, Kobayashi KS. Negative regulation of Toll-like receptor signaling plays an essential role in homeostasis of the intestine. Eur J Immunol. 2011;41:182–194. doi: 10.1002/eji.201040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- Dubois PC, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, Zhernakova A, Heap GA, Adany R, Aromaa A, et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010;42:295–302. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19:713–721. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, Barrera J, Huang EJ, Hou B, Malynn BA, et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol. 2011;12:1184–1193. doi: 10.1038/ni.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedl M, Abraham C. Distinct Roles for Nod2 Protein and Autocrine Interleukin-1{beta} in Muramyl Dipeptide-induced Mitogen-activated Protein Kinase Activation and Cytokine Secretion in Human Macrophages. J Biol Chem. 2011a;286:26440–26449. doi: 10.1074/jbc.M111.237495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedl M, Abraham C. Secretory mediators regulate Nod2-induced tolerance in human macrophages. Gastroenterology. 2011b;140:231–241. doi: 10.1053/j.gastro.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedl M, Abraham C. IRF5 risk polymorphisms contribute to interindividual variance in pattern recognition receptor-mediated cytokine secretion in human monocyte-derived cells. J Immunol. 2012;188 doi: 10.4049/jimmunol.1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci U S A. 2007;104:19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- Leventhal PS, Bertics PJ. Activation of protein kinase C by selective binding of arginine-rich polypeptides. J Biol Chem. 1993;268:13906–13913. [PubMed] [Google Scholar]
- Li J, Moran T, Swanson E, Julian C, Harris J, Bonen DK, Hedl M, Nicolae DL, Abraham C, Cho JH. Regulation of IL-8 and IL-1beta expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–1725. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- Ling V, Wu PW, Finnerty HF, Bean KM, Spaulding V, Fouser LA, Leonard JP, Hunter SE, Zollner R, Thomas JL, et al. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J Immunol. 2000;164:1653–1657. doi: 10.4049/jimmunol.164.4.1653. [DOI] [PubMed] [Google Scholar]
- Ling V, Wu PW, Miyashiro JS, Marusic S, Finnerty HF, Collins M. Differential expression of inducible costimulator-ligand splice variants: lymphoid regulation of mouse GL50-B and human GL50 molecules. J Immunol. 2001;166:7300–7308. doi: 10.4049/jimmunol.166.12.7300. [DOI] [PubMed] [Google Scholar]
- Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C [corrected] Mol Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki Y, Michelsen KS, Schroder NW, Alsabeh R, Slepenkin A, Zhang W, Chen S, Wei B, Bulut Y, Wong MH, et al. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J Biol Chem. 2005;280:29242–29249. doi: 10.1074/jbc.M503225200. [DOI] [PubMed] [Google Scholar]
- Netea MG, Ferwerda G, de Jong DJ, Jansen T, Jacobs L, Kramer M, Naber TH, Drenth JP, Girardin SE, Kullberg BJ, et al. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174:6518–6523. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- Nguyen GC, Torres EA, Regueiro M, Bromfield G, Bitton A, Stempak J, Dassopoulos T, Schumm P, Gregory FJ, Griffiths AM, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. 2006;101:1012–1023. doi: 10.1111/j.1572-0241.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Nunez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23:7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rogler G, Hausmann M, Vogl D, Aschenbrenner E, Andus T, Falk W, Andreesen R, Scholmerich J, Gross V. Isolation and phenotypic characterization of colonic macrophages. Clin Exp Immunol. 1998;112:205–215. doi: 10.1046/j.1365-2249.1998.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Kanai T, Watanabe M, Sakuraba A, Okamoto S, Nakai T, Okazawa A, Inoue N, Totsuka T, Yamazaki M, et al. Hyperexpression of inducible costimulator and its contribution on lamina propria T cells in inflammatory bowel disease. Gastroenterology. 2004;126:829–839. doi: 10.1053/j.gastro.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS) Curr Opin Immunol. 2010;22:326–332. doi: 10.1016/j.coi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Tang G, Qin Q, Zhang P, Wang G, Liu M, Ding Q, Qin Y, Shen Q. Reverse signaling using an inducible costimulator to enhance immunogenic function of dendritic cells. Cell Mol Life Sci. 2009;66:3067–3080. doi: 10.1007/s00018-009-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 2004;127:1401–1409. doi: 10.1053/j.gastro.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Vidal VF, Casteran N, Riendeau CJ, Kornfeld H, Darcissac EC, Capron A, Bahr GM. Macrophage stimulation with Murabutide, an HIV-suppressive muramyl peptide derivative, selectively activates extracellular signal-regulated kinases 1 and 2, C/EBPbeta and STAT1: role of CD14 and Toll-like receptors 2 and 4. Eur J Immunol. 2001;31:1962–1971. doi: 10.1002/1521-4141(200107)31:7<1962::aid-immu1962>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BL, Hirst SJ, Giembycz MA. Protein kinase C isoenzymes: a review of their structure, regulation and role in regulating airways smooth muscle tone and mitogenesis. Br J Pharmacol. 2000;130:1433–1452. doi: 10.1038/sj.bjp.0703452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J Biol Chem. 2007;282:36223–36229. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.