Abstract
Cell-based therapies for wound repair are limited by inefficient delivery systems that fail to protect cells from the acute inflammatory environment. Here, a biomimetic hydrogel system is described that is based on the polymer pullulan, a carbohydrate glucan known to exhibit potent antioxidant capabilities. It is shown that pullulan hydrogels are an effective cell delivery system and improve mesenchymal stem cell survival and engraftment in high-oxidative-stress environments. The results suggest that glucan hydrogel systems may prove beneficial for progenitor-cell-based approaches to skin regeneration.
Keywords: hydrogels, mesenchymal stem cells, redox polymers, skin engineering, wound healing
Introduction
Following cutaneous injury, wound healing proceeds through highly coordinated stages of repair to restore damaged tissues.[1] The initial phase is characterized by a massive influx of inflammatory cells that secrete abundant reactive oxygen species (ROS) to kill bacteria and defend the disrupted skin barrier.[2] However, excess ROS can be detrimental to wound repair, largely due to their high reactivity with key biologic pathways resulting in cell dysfunction and death.[3] Although free radicals are produced by all cells as part of normal metabolism, the inability to manage elevated levels of oxidative stress (whether due to increased ROS production or decreased antioxidant defenses) predictably leads to cell damage and an impaired response to injury.[4–6]
Stem cell-based applications for wound regeneration must enable delivered cells to function effectively in high ROS environments. Low levels of free radicals have been shown to function as important cell signaling mediators but high levels are thought to impede cell engraftment in ischemic wounds.[7,8] Although stem cells including mesenchymal stem cells (MSCs) may exhibit some intrinsic degree of antioxidant capacity,[9,10] this intracellular defense system may be rapidly overwhelmed in an inflammatory wound environment, resulting in poor cell survival and engraftment.[7,8] Thus, biomaterial-based cell delivery systems capable of mitigating these damaging ROS may improve the therapeutic utility of stem cells for skin regeneration.
Our laboratory recently developed a biomimetic hydrogel based on the carbohydrate pullulan, an alpha-(1–4)- and alpha-(1–6)-glucan (Figure 1A) produced by the fungus Aureobasidium pullulans.[11] We previously utilized this hydrogel system to improve early wound healing in a mouse model and examined its immunomodulatory properties on wound repair.[11] Glucans such as pullulan have been shown to be able to quench free radicals,[12–15] an important property that may prove useful for cell delivery into cutaneous wounds. In this study, we demonstrate that pullulan-based hydrogels exhibit important antioxidant properties that protect MSCsfrom oxidative damage both in vitro and in vivo. Moreover, our findings suggest that this novel cell delivery system may be a superior approach for MSC delivery as compared to local injection, the current “gold standard” for wound applications.
Figure 1.
Hydrogel delivery of MSCs. (A) Molecular structure of pullulan. (B) Antioxidant capacity of the pullulan polymer as measured using an ABTS assay. n = 3 for each data point. Statistical significance is relative to the spectrophotometric absorbance of hydrogen peroxide-free solution at the respective concentrations. (C) Antioxidant properties of the pullulan hydrogel system as measured using the Amplex Red assay kit. Incubation with the pullulan hydrogel decreased the amount of hydrogen peroxide at 30, 60, 120, and 240 min. Significance values are relative to control at the same concentration. (D) 1H NMR demonstrates increasing signal at δ = 8.46 in pullulan hydrogels following exposure to hydrogen peroxide, consistent with the oxidation of primary alcohol groups at C6. (E) Graph showing the increase in oxidation (aldehyde peak) of pullulan hydrogels over time, as measured by calculating the area under the signal at δ = 8.46. In contrast, dextran hydrogels (which lack a free C6 primary hydroxyl) exhibit less antioxidant capacity in this assay. Data are representative of three independent experiments. (F) Scanning electron micrographs demonstrating the differential morphology of plated MSCs (top row) compared to hydrogel-seeded MSCs (bottom row). Clusters of MSCs are shown within the three-dimensional environment of the porous hydrogel matrix. Scale bar 100 μm (left) and 15 μm (right). Values represent means ± SD. *p <0.05.
Experimental Section
Hydrogel Fabrication
Pullulan-based hydrogel scaffolds containing 5 wt% collagen were fabricated using a salt-induced phase inversion method as previously described.[11] Briefly, pullulan (200 000 kDa, Hayashibara Biochemical Laboratories, Okayama, Japan) was mechanically mixed with collagen type I in the presence of sodium trimetaphosphate under alkaline conditions and potassium chloride salt was used as a porogen. Dextran (2 000 000 kDa) hydrogels were fabricated in the same manner to use as controls in the nuclear magnetic resonance (NMR) studies. All fabrication reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted.
Cells and Animals
MSCs were harvested from 8–12 week old wildtype FVB/NJ (stock #001800,The Jackson Laboratory, Bar Harbor, ME, USA) female mice as previously described.[16] For bioluminescence experiments, MSCs were harvested from transgenic female mice (FVB background) that constitutively express firefly luciferase (luc) and enhanced green fluorescent protein (eGFP, gift of Dr. Christopher Contag, Stanford University). Luc/eGFP + MSCs were maintained under standard cell culture conditions in low-glucose phenol-red-free medium (Gibco-Invitrogen, Carlsbad, CA, USA) supplemented with 1% antibiotic/antimycotic (Gibco) and 10% fetal bovine serum (Gibco). Cells from passages three to six were used for all experiments. Media was changed to serum-free, phenol red-free media overnight prior to antioxidant experiments. Wildtype FVB/NJ female mice aged 8–12 weeks were used for subcutaneous implantation and ischemic excisional wound experiments. All animals were housed under standard conditions in accordance with Stanford University animal care guidelines.
Hydrogen Peroxide Studies
Hydrogen peroxide (30%, Sigma-Aldrich) was prepared in concentrations of 0, 10−4, and 10−3 M with sterile phosphate-buffered saline (PBS, Gibco). The antioxidant capacity of the pullulan polymer was tested using an antioxidant assay kit (Cayman Chemical Company, Ann Arbor, MI, USA). This assay is based on the ability of samples to inhibit the oxidation of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) by metmyoglobin. The antioxidant capacity of the pullulan hydrogel system was examined using the Amplex Red hydrogen peroxide assay kit (Invitrogen, Carlsbad, CA, USA). The pullulan hydrogel was added to standard concentrations of hydrogen peroxide for 30, 60, 120, 240 min. Spectrophotometric analysis was performed at 560 nm after removal of the hydrogel samples.
1H NMR
Pullulan or dextran hydrogels (1 g) in aqueous suspension were incubated with hydrogen peroxide (27 wt%) using CuSO4 (0.01 wt%) as a catalyst, similar to previously published methods.[17,18] Aliquots of hydrogel mixturewere taken at: 2, 30 min, 1, 2, and 20 h and immediately frozen to quench the reaction. 1H NMR was performed with water suppression at room temperature. Oxidation was measured by monitoring the intensity of the aldehyde peak at δ = 8.46 (reference peak at δ = 5.00 set to an integral value of 10). Similar methods were used to test dextran hydrogels as a control.
Microscopy
A live/dead assay (Calbiochem-EMD, Gibbstown, NJ, USA) was used as previously described.[11] Dihydroethidium (DHE, Molecular Probes-Invitrogen, Carlsbad, CA, USA) or CellTracker Green 5-chloromethylfluorescein diacetate (CMFDA, Molecular Probes-Invitrogen) was used at a loading dose of 10−5 M for 1 h prior to H2O2 administration. For scanning electron microscopy (SEM), MSCs were seeded onto coverslips or hydrogel scaffolds overnight and prepared for variable pressure SEM imaging as previously described.[11] For histologic processing, harvested tissue was embedded in Tissue-Tek optimum cutting temperature (OCT) (Sakura Finetek USA Inc., Torrance, CA, USA) for cryosectioning. 4′,6-Diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA, USA) was used for nuclear counterstaining. DHE was used to detect elevated ROS in OCT-embedded tissue flaps as previously described.[19] Images were obtained for fluorescence microscopy (Zeiss Axioplan 2 Imaging, Carl Zeiss, Inc. Thornwood, NY, USA) and processed in Adobe Photoshop CS3 (Adobe Systems Incorporated, San Jose, CA, USA) for publication.
Quantitative Polymerase Chain Reaction (qPCR) Analysis
Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) were used for in vitro transcriptional analyses. Assays used were for catalase (Mm00437992_m1) and β-actin (Mm01205647_g1).
In vitro Functional Studies
Intracellular catalase levels were measured using a catalase assay kit (Abcam Inc., Cambridge, MA, USA) per manufacturer’s instructions. One unit of catalase is the amount that decomposes 10−6 M of H2O2 per min at pH = 4.5 at 25 °C. The product was measured at 570 nm.
In vivo Wound Models in Mice
Anterior and posterior transverse full thickness incisions (1 cm) were made on the dorsum of a surgically prepped mouse. Mouse MSCs (2.5 × 105 cells) were delivered into the subcutaneous space below each wound by either 6 mm hydrogel scaffold disk (2 mm thickness, cells seeded overnight) or injection in 100 μL of sterile PBS. Incisions were closed with 6-0 absorbable suture (Vicryl, Ethicon, Cornelia, GA, USA). Full-thickness 3 mm punch biopsy wounds were created in the distal third of an ischemic peninsular flap in mice, similar to previously published models.[16,20] MSCs (2.5 × 105) were delivered via hydrogel scaffold or peri-wound subcutaneous injections in a total of 100 μL of sterile PBS immediately following injury (day 0). Animals were housed individually post-surgery. All animal procedures were approved by the Stanford University animal ethics committee.
Bioluminescent Imaging
DermaMatrix Acellular Dermis (Synthes, West Chester, PA, USA), a decellularized human skin-derived allograft, was used as a cell scaffold to compare with our hydrogel system. Pullulan-based hydrogels and DermaMatrix were cut into 1 cm squares prior to MSC seeding overnight into wells of a 24 well plate at 5 × 104 cells per well. Baseline measurements of luc activity (as a marker of cell viability) were taken with the IVIS 200 Series imaging system (Caliper Life Sciences, Hopkinton, MA, USA) after administration of 150 μg · mL−1 luciferin (Caliper Life Sciences) for 5 min. Wells were rinsed with PBS and changed to 0, 10−4, or 10−3 M H2O2 in serum-free phenol-red-free medium for 2 h. After each hour, 150 μg · mL−1 luciferin was added to fresh media for in vivo imaging system (IVIS) measurements. For in vivo imaging of MSCs, baseline bioluminescence was obtained with IVIS imaging immediately following subcutaneous delivery of MSCs. Luciferin (150 mg · kg−1) was injected subcutaneously at each wound site prior to obtaining images with IVIS.
Statistical Analysis
Statistical analysis was performed using Student’s unpaired t-test or one-way analysis of variance (ANOVA) with Tukey’s honestly significant difference (HSD) for multiple comparisons (Matlab, The MathWorks, Inc., Natick, MA). Values are represented as means ± standard deviation (SD). P-values <0.05 were considered statistically significant.
Results
Pullulan Polymer and Hydrogel System Exhibit Antioxidant Properties
To confirm the antioxidant potential of pullulan, we utilized an ABTS-based assay. Concentrations of pullulan from 1 to 5 × 10−4 M effectively prevented the oxidation of ABTS (Figure 1B), consistent with the known ability of glucans to function as antioxidants.[12,14,15] To test if this antioxidant capacity was retained in the hydrogel system, we incubated pullulan hydrogels in increasing concentrations of hydrogen peroxide using an Amplex Red assay. Pullulan hydrogels decreased the absorbance level of oxidized assay product at time points ranging from 30 to 240 min (Figure 1C), suggesting its ability to quench peroxide-induced oxidation reactions. Further, 1H NMR studies demonstrated increased signal at δ = 8.46 following exposure of pullulan hydrogels to hydrogen peroxide, consistent with oxidation of the C6 primary alcohol groups[18,21] (Figure 1D,E). In contrast, control hydrogels made with dextran, a branched glucan lacking free primary hydroxyl groups at C6, demonstrated less oxidation with hydrogen peroxide exposure (Figure 1E), suggesting a weaker ability to quench free radicals compared to pullulan hydrogels. Together, these studies indicate that pullulan-based hydrogels can act as antioxidant scaffolds, potentially by functioning as a “sacrificial” substrate for free radical reactions.
Seeded MSCs can be Viably Maintained in Porous Pullulan Hydrogels
To test the feasibility of using pullulan hydrogels as a cell delivery system, we seeded mouse MSCs onto these scaffolds in vitro. Seeded MSCs organized as viable clusters within the open porous scaffold and demonstrated a rounded cellular morphology (Figure 1F). In vitro live/dead assay confirmed that seeded MSCs remained greater than 95% viable for up to two weeks within the hydrogels (data not shown). These findings confirm that the hydrogel system is highly biocompatible with MSCs and can potentially be used as a bioengineered cell delivery system.
Pullulan/Collagen Hydrogels Protect MSCs from Oxidative Damage
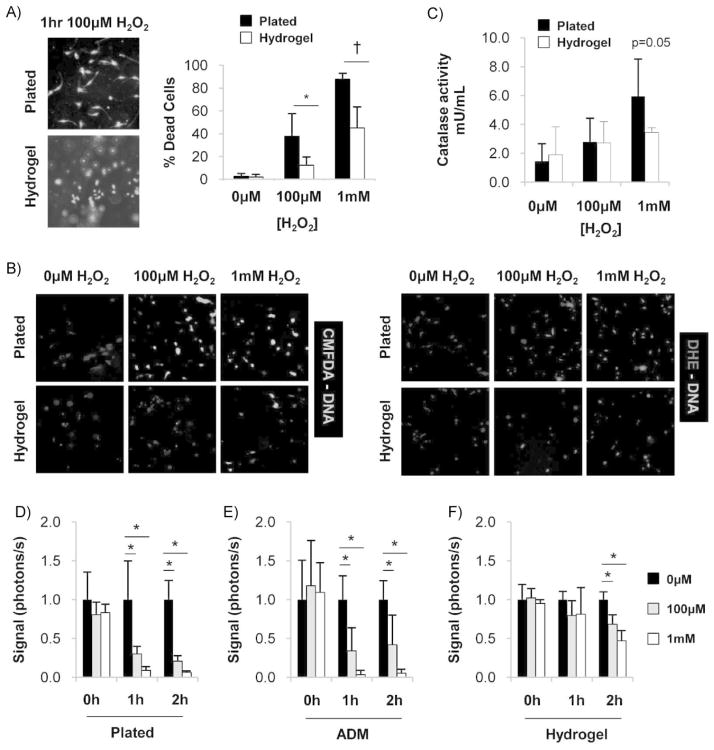
To test the ability of pullulan-based hydrogels to protect seeded MSCs from oxidative stress, we exposed MSCs to toxic levels of hydrogen peroxide.[3,10,22,23] MSCs seeded in hydrogels demonstrated improved survival compared to plated MSCs following exposure to 10−4 M (12.4 ± 7.2 vs. 37.8 ± 19.9% non-viable, p = 0.015) and 10−3 M hydrogen peroxide (45.11 ± 18.3 vs. 87.8 ± 5.3% non-viable, p <0.001) (Figure 2A).
Figure 2.
Pullulan-based hydrogels protect MSCs from ROS-induced damage. (A) Representative images (left) and quantification (right) of live/dead assay 1 h following exposure to hydrogen peroxide. n = 6. (B) Representative images of intracellular ROS activity as detected with CMFDA and DHE. Oxidized products are unable to be secreted from MSCs and are detected by wavelength-specific fluorescence. (C) Catalase activity of MSCs following 1 h exposure to hydrogen peroxide. n = 3. (D–F) Quantification of in vitro bioluminescence imaging of luc + MSCs seeded onto (D) standard tissue culture plates, (E) decellularized human dermis, and (F) pullulan-based hydrogels. n = 4. Values represent means ± SD. *p <0.05, †p <0.01.
To confirm intracellular oxidative damage, we performed CMFDA and DHE staining. CMFDA is oxidized by hydrogen peroxide, peroxynitrite, hydroxyl radical, and superoxide anion to fluoresce green, whereas DHE is selectively oxidized by superoxide anion to fluoresce red.[24] As expected, high concentrations of hydrogen peroxide were associated with increased intracellular ROS generation (Figure 2B, top). This pathologic effect was dramatically reduced in MSCs seeded within hydrogels (Figure 2B, bottom). Together, these studies suggest that pullulan-based hydrogels are capable of protecting seeded MSCs from high levels of free radicals, conditions that recapitulate the acute wound environment.
Pullulan Hydrogels Preserve MSC Catalase Function in the Setting of Elevated Hydrogen Peroxide Levels
We next assessed the activation of the antioxidant enzyme catalase, a critical enzyme that functions to convert hydrogen peroxide to water and oxygen. Catalase activity was increased when plated MSCs were exposed to high levels of hydrogen peroxide, with levels approaching statistical significance (5.94 ± 2.59 mU · mL−1 activity vs. 3.46 ± 0.31 mU · mL−1 no hydrogen peroxide, p = 0.053) (Figure 2C). In contrast, catalase activity in MSCs seeded in hydrogels only increased modestly under similar conditions, suggesting that the pullulan hydrogels were either protecting cells from hydrogen peroxide exposure or impairing MSC catalase activity.
To test the latter, we assayed gene expression levels of catalase in MSCs seeded onto pullulan hydrogels. There was a non-significant trend toward increased MSC catalase expression with pullulan hydrogels (1.69 ± 0.36-fold change, hydrogel vs. plated, p = 0.08), indicating that MSC catalase function is not significantly blocked by pullulan hydrogels. Further, this suggests that hydrogel-seeded MSCs do not activate catalase under high hydrogen peroxide conditions because they are not being exposed to high environmental levels of ROS. Together, these findings substantiate our biomaterial studies demonstrating the ability of pullulan to act as a free radical scavenger, thus minimizing the ability of exogenous ROS to damage cells seeded into the hydrogels.
Pullulan Hydrogels Exhibit Superior ROS Protection Compared to Acellular Dermal Matrix
Next, we examined whether a different type of scaffold (acellular dermal matrix, ADM), a biologic collagen-based scaffold used to treat burn injury,[25,26] exhibited anti-oxidant properties that could protect seeded MSCs. Luciferase-expressing MSCs seeded onto ADM demonstrated a significant decrease in bioluminescent signal following high levels of oxidative stress, indicating decreased cell viability and survival (Figure 2D,E). In contrast, pullulan hydrogels preserved MSC viability in this highly toxic environment (Figure 2F). There was a significant difference between group means as determined by one-way ANOVA at 1 h [10−6 M: F(2,9) = 6.53, p = 0.018; 10−3 M: F(2,9) = 18.58, p = 0.001] and 2 h [10−3 M: F(2,9) = 34.81, p <0.001]. Post hoc analysis using Tukey’s HSD (α = 0.05) confirmed that MSCs seeded in hydrogels exhibited greater viability compared to either ADM seeding or plating under these conditions. Taken together, these results indicate that the antioxidant capacity of pullulan hydrogels is largely due to its unique properties as a glucan-based biomaterial and suggests its superiority to purely collagen-based biologics from human or animal sources.
Pullulan Hydrogels Enhance MSC Survival in vivo
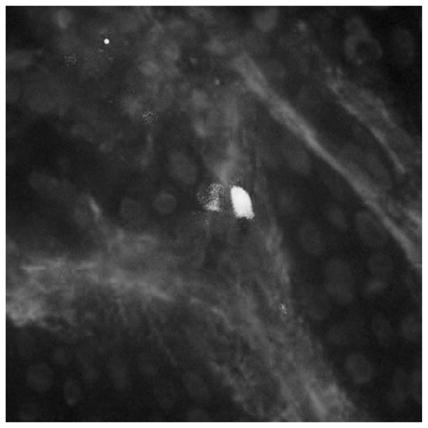
The acute wound environment is characterized by high free radical levels derived from infiltrating inflammatory cells and injured tissues.[1,3] To test the ability of pullulan hydrogels to deliver MSCs in vivo, we first utilized a subcutaneous implantation model. Compared to local injection, MSC delivery within hydrogels significantly prolonged cell survival and engraftment for up to 30 d (Figure 3A). Importantly, there was a dramatic decrease in cell signal within the initial days following injury (Figure 3B), suggesting that acute inflammatory responses (including ROS production) are a major cause of impaired cell survival following delivery. These results suggest that the pullulan hydrogel may protect seeded MSCs from these initial insults, thus enabling a greater proportion of cells to survive and subsequently engraft in the wound matrix (Figure 3C).
Figure 3.
Subcutaneous delivery of MSCs using pullulan hydrogels. (A) Representative images and (B) quantification of bioluminescent imaging of luc + MSCs delivered subcutaneously via injection (top row) or hydrogel scaffold (bottom row). Data are relative to bioluminescence at day 1 for the respective conditions. (C) Representative image of luc/eGFP + MSCs at day 10 post-injury delivered via hydrogel. Delivered MSCs were distributed homogeneously throughout the provisional dermal matrix as opposed to a subcutaneous location. Scale bar 20 μm. n = 4. Values represent means ± SD. *p <0.05.
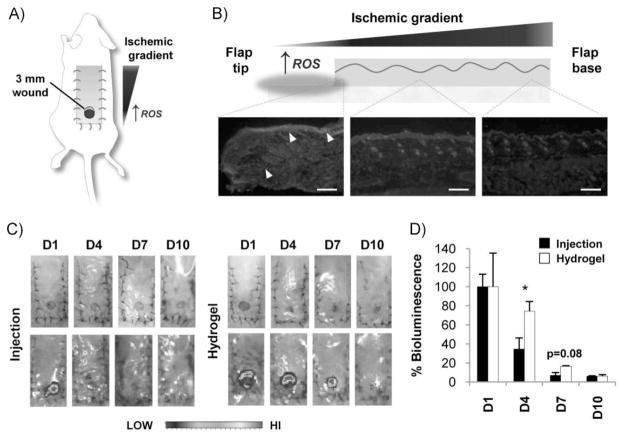
To test the effectiveness of pullulan hydrogel-mediated MSC delivery in a high ROS wound environment, we developed an ischemic excisional wound model in the mouse (Figure 4A). The creation of a peninsular skin flap produces an ischemic gradient[27] characterized by the highest levels of ROS at the distal tip wound site (Figure 4B). We compared the survival of luciferase-expressing MSCs delivered into this harsh wound environment using either local injection or pullulan hydrogels. Injection delivery produced a significant decrease in cell signal at day 4 post-injury (34.6% of day-1 bioluminescence) compared to hydrogel delivery of MSCs (Figure 4C–D) (74.3% of day-1 bioluminescence). By 7 d, wounds with injected MSCs demonstrated minimal signal (7.1% of day-1 bioluminescence) whereas hydrogel-MSC-treated wounds exhibited a non-significant trend toward greater signal (16.6% of day-1 bioluminescence, p = 0.08). Although the use of an ischemic excisional wound model was useful in evaluating the early survival and engraftment of delivered MSCs, the dramatic nature of this insult (open wound, ischemia, foreign body) precluded adequate evaluation of the beneficial effects of MSCs on wound healing. Despite this limitation, these findings indicate that hydrogel-mediated delivery of MSCs improves early engraftment and survival in an ischemic wound environment with high levels of ROS.
Figure 4.
Delivery of MSCs into ischemic excisional wounds. (A) Schematic of ischemic excisional wound model. An ischemic gradient is established with lowest levels of oxygen tension at the distal tip of the peninsular flap. (B) DHE immunofluoresence demonstrating highest levels of ROS at the ischemic flap tip. Arrowheads point to elevated ROS in the epithelial layer and dermal wound margin. Images were taken from the same flap at the same exposure from the distal, middle, and proximal third that corresponds to the left, middle, and right micrographs. Scale bar 200 μm. (C) Gross photographs (top) and IVIS bioluminescence imaging (bottom) of the ischemic excisional wound model following injection or hydrogel delivery of MSCs. (D) Quantification of bioluminescent imaging of luc + MSCs delivered via local injection or hydrogel scaffold. Data are relative to bioluminescence at day 1 for the respective conditions. n = 4. Values represent means ± SEM. *p <0.05.
Discussion
Poor stem cell engraftment has been implicated as a primary limitation of MSC-based therapies for wound healing.[28] Accordingly, biomaterial strategies have increasingly focused on developing scaffolds to improve cell delivery and facilitate regenerative healing.[1,29] For dermal reconstruction, an ideal engineered construct would recapitulate the biophysical environment of unwounded skin while effectively delivering stem cells capable of restoring skin cell populations.[29] However, the conditions that would benefit most from these cell-scaffold constructs (e.g., traumatic soft tissue injury or chronic wounds) are often highly toxic to both endogenous cells and exogenously delivered therapeutic cells due to ROS and other ischemia-related factors. Thus, multifaceted bioengineered systems designed specifically for the ischemic and inflammatory wound environment may prove essential for regenerative medicine.
We previously developed a pullulan-based biomimetic hydrogel that was highly biocompatible with multiple skin cell populations in vitro and exhibited potent immunomodulatory and angiogenic effects in vivo.[11] Because glucans such as pullulan are known to exhibit free radical quenching,[15] we proposed that our hydrogel system would protect seeded cells from oxidative stress. Consistent with this hypothesis, our findings indicated that pullulan-based hydrogels function primarily as a sacrificial substrate that reacts with ROS to protect seeded MSCs. This unique property may account for the improved survival of MSCs in pullulan hydrogels compared to collagen-based ADM following oxidative stress. Of note, the time points used for in vitro experiments (less than 2 h) may not have been sufficient to permit full exposure of seeded MSCs to hydrogen peroxide. However, the decrease in cell viability observed by 1 h in the ADM group suggests that inadequate diffusion of hydrogen peroxide is likely not a major confounding factor. The levels of hydrogen peroxide employed in this study (up to 10−3 M) are toxic to a wide range of cell types in vitro.[3,10] However, skin levels of hydrogen peroxide have been reported to be in this range,[22] thus substantiating the biological relevance of our stimuli.
Although glucans may have inductive effects on catalase expression in certain cell types,[30] our pullulan hydrogel scaffolds only weakly induced catalase expression in MSCs. MSCs are known to exhibit relatively high levels of catalase activity at baseline, potentially mitigating any biomaterial-inductive effects on gene expression.[10] Additionally, MSCs have intrinsic antioxidant properties that may underlie their therapeutic benefit in preclinical models.[10,31,32] However, the acute wound environment may still overwhelm these inherent antioxidant defense mechanisms and impair MSC survival post-delivery, highlighting the need for ROS-quenching systems in wound repair. Although we did not examine the role of ROS in regulating delivered cell differentiation, it is possible that quenching of exogenous ROS alters intracellular ROS signaling, a known regulator of MSC differentiation.[33] Future studies will examine how the pullulan hydrogel system affects intracellular oxidative stress pathways (including the family of superoxide dismutases) in delivered MSCs.
Other glucan biomaterials have demonstrated protective effects against oxidative injury in various animal models, including ROS-mediated liver damage,[34] traumatic spinal cord injury in rats,[12] and following burn injury.[35] Another engineered approach has been to incorporate antioxidant molecules directly into wound dressings. For example, collagen matrices impregnated with the antioxidants quercetin or curcumin have been successfully used to improve wound healing in rats.[36,37] Finally, investigators have even utilized synthetic antioxidants as a scaffolding substrate to demonstrate ROS-suppressing effects in vitro.[38] However, our pullulan-based hydrogels are unique in that seeded stem cells can be effectively delivered into highly toxic wound environments.
The use of glucans as a scaffold system for skin engineering has not been well-studied. Poly(lactide-co-glycolide) scaffolds containing β-glucans were highly biocompatible with human fibroblasts and adipose-derived stem cells,[39] but these cell-scaffold constructs were only evaluated in vitro. Gelatin/glucan scaffolds seeded with both human keratinocytes and fibroblasts improved full thickness wound healing in immunocompromised mice,[40] demonstrating the potential of glucan biomaterials for skin regeneration, albeit in a minimally inflammatory setting. In contrast, this is the first study to utilize glucan-based hydrogel scaffolds to deliver MSCs into high oxidative stress wounds in immunocompetent animals.
Preclinical studies have demonstrated the potential of ADM for stem cell delivery.[41,42] However, decellularized natural matrices are limited by availability, cost, ethical concerns, and the theoretical risk of disease transmission.[43] Further, our findings suggest that collagen-based scaffolds including ADM may lack effective antioxidant properties and may be less suitable for cell delivery in toxic environments. Although we did not test the use of ADM for MSC delivery in vivo, future studies will examine different ADM-like materials to determine if any particular dermal products exhibit significant antioxidant properties and how these native scaffolds compare to our hydrogel system. Further, other hydrogel-specific factors aside from ROS-quenching may contribute to improved MSC engraftment following delivery, including cell sequestration, provision of three dimensional matrix cues, and altered levels of extracellular oxygen. Collectively, these studies indicate that glucan-based biomaterial systems can potentially be used to deliver stem cells not only into cutaneous wounds but also in other high ROS settings such as myocardial infarction[44] or acute renal failure.[45] More broadly, we propose that cell-based applications for wound repair should be specifically designed to function in an inflammatory environment to fully enable the therapeutic potential of stem cells.
Conclusion
The glucan pullulan exhibits potent antioxidant properties which may prove beneficial for skin regeneration. In the setting of increased oxidative stress, MSCs seeded onto pullulan hydrogels exhibited improved viability and enhanced preservation of anti-oxidant defenses compared to controls. In vivo, pullulan-based hydrogel scaffolds significantly augmented the engraftment of MSCs into acute wounds compared to local injection. These experiments demonstrate the potential of engineered ROS-quenching constructs to deliver stem cells into inflammatory wound environments.
Acknowledgments
This work was supported by a grant to GCG from the United States Armed Forces Institute of Regenerative Medicine DOD #W81XWH-08-2-0032. The authors would like to thank Ms. Yujin Park for histologic processing and Dr. Lydia Joubert for performing SEM imaging.
Contributor Information
Victor W. Wong, Hagey Laboratory for Pediatric Regenerative Medicine, Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA
Kristine C. Rustad, Hagey Laboratory for Pediatric Regenerative Medicine, Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA
Jason P. Glotzbach, Hagey Laboratory for Pediatric Regenerative Medicine, Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA
Michael Sorkin, Hagey Laboratory for Pediatric Regenerative Medicine, Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA.
Mohammed Inayathullah, Biomaterials and Advanced Drug Delivery (BioADD) Laboratory, Stanford University School of Medicine, Stanford, CA, USA.
Melanie R. Major, Hagey Laboratory for Pediatric Regenerative Medicine, Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA
Michael T. Longaker, Hagey Laboratory for Pediatric Regenerative Medicine, Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA
Jayakumar Rajadas, Biomaterials and Advanced Drug Delivery (BioADD) Laboratory, Stanford University School of Medicine, Stanford, CA, USA.
Geoffrey C. Gurtner, Email: ggurtner@stanford.edu, Hagey Laboratory for Pediatric Regenerative Medicine, Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Nature. 2008;453:314. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. N Engl J Med. 1999;341:738. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Schafer M, Werner S. Pharmacol Res. 2008;58:165. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Khodr B, Khalil Z. Free Radic Biol Med. 2001;30:1. doi: 10.1016/s0891-5849(00)00378-6. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan MJ, Ceradini DJ, Gurtner GC. Antioxid Redox Signal. 2005;7:1476. doi: 10.1089/ars.2005.7.1476. [DOI] [PubMed] [Google Scholar]
- 6.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. J Biol Chem. 2008;283:10930. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelos MG, Kutala VK, Torres CA, He G, Stoner JD, Mohammad M, Kuppusamy P. Am J Physiol Heart Circ Physiol. 2006;290:H341. doi: 10.1152/ajpheart.00223.2005. [DOI] [PubMed] [Google Scholar]
- 8.Yao EH, Yu Y, Fukuda N. Curr Pharm Biotechnol. 2006;7:101. doi: 10.2174/138920106776597685. [DOI] [PubMed] [Google Scholar]
- 9.Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. J Dermatol Sci. 2008;49:133. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Valle-Prieto A, Conget PA. Stem Cells Dev. 2010;19:1885. doi: 10.1089/scd.2010.0093. [DOI] [PubMed] [Google Scholar]
- 11.Wong VW, Rustad KC, Galvez MG, Neofyotou E, Glotzbach JP, Januszyk M, Major MR, Sorkin M, Longaker MT, Rajadas J, Gurtner GC. Tissue Eng Part A. 2011;17:631. doi: 10.1089/ten.tea.2010.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kayali H, Ozdag MF, Kahraman S, Aydin A, Gonul E, Sayal A, Odabasi Z, Timurkaynak E. Neurosurg Rev. 2005;28:298. doi: 10.1007/s10143-005-0389-2. [DOI] [PubMed] [Google Scholar]
- 13.Novak M, Vetvicka V. J Immunotoxicol. 2008;5:47. doi: 10.1080/15476910802019045. [DOI] [PubMed] [Google Scholar]
- 14.Slamenova D, Labaj J, Krizkova L, Kogan G, Sandula J, Bresgen N, Eckl P. Cancer Lett. 2003;198:153. doi: 10.1016/s0304-3835(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 15.Tsiapali E, Whaley S, Kalbfleisch J, Ensley HE, Browder IW, Williams DL. Free Radic Biol Med. 2001;30:393. doi: 10.1016/s0891-5849(00)00485-8. [DOI] [PubMed] [Google Scholar]
- 16.Hamou C, Callaghan MJ, Thangarajah H, Chang E, Chang EI, Grogan RH, Paterno J, Vial IN, Jazayeri L, Gurtner GC. Plast Reconstr Surg. 2009;123:45S. doi: 10.1097/PRS.0b013e318191be4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aouf C, Harakat D, Muzart J, Estrine B, Marinkovic S, Ernenwein C, Le Bras J. ChemSusChem. 2010;3:1200. doi: 10.1002/cssc.201000143. [DOI] [PubMed] [Google Scholar]
- 18.McIntyre D, Vogel H. Starch. 1993;406 [Google Scholar]
- 19.Ozumi K, Tasaki H, Takatsu H, Nakata S, Morishita T, Koide S, Yamashita K, Tsutsui M, Okazaki M, Sasaguri Y, Adachi T, Nakashima Y. Atherosclerosis. 2005;181:55. doi: 10.1016/j.atherosclerosis.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 20.Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. J Biomed Biotechnol. 2011;2011:969618. doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Nooy A, Besemer A, van Bekkum H. Carbohydr Res. 1995;269:89. [Google Scholar]
- 22.Schallreuter KU, Gibbons NC, Zothner C, Abou Elloof MM, Wood JM. Biochem Biophys Res Commun. 2007;360:70. doi: 10.1016/j.bbrc.2007.05.218. [DOI] [PubMed] [Google Scholar]
- 23.Wilgus TA, Bergdall VK, Dipietro LA, Oberyszyn TM. Wound Repair Regen. 2005;13:513. doi: 10.1111/j.1067-1927.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 24.Eruslanov E, Kusmartsev S. Methods Mol Biol. 2010;594:57. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 25.Bello YM, Falabella AF, Eaglstein WH. Am J Clin Dermatol. 2001;2:305. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 26.Wainwright DJ. Burns. 1995;21:243. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 27.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Nat Med. 2004;10:858. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 28.Hocking AM, Gibran NS. Exp Cell Res. 2010;316:2213. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong VW, Gurtner GC. Plast Reconstr Surg. 2010;125:910. doi: 10.1097/PRS.0b013e3181cb6438. [DOI] [PubMed] [Google Scholar]
- 30.Kim YS, Ke F, Zhang QY. Fish Shellfish Immunol. 2009;27:336. doi: 10.1016/j.fsi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Fu X, Li H. Cell Tissue Res. 2009;335:317. doi: 10.1007/s00441-008-0724-3. [DOI] [PubMed] [Google Scholar]
- 32.Kemp K, Gray E, Mallam E, Scolding N, Wilkins A. Stem Cell Rev. 2010;6:548. doi: 10.1007/s12015-010-9178-6. [DOI] [PubMed] [Google Scholar]
- 33.Wang N, Xie K, Huo S, Zhao J, Zhang S, Miao J. J Cell Biochem. 2007;100:1548. doi: 10.1002/jcb.21139. [DOI] [PubMed] [Google Scholar]
- 34.Toklu HZ, Sehirli AO, Velioglu-Ogunc A, Cetinel S, Sener G. Eur J Pharmacol. 2006;543:133. doi: 10.1016/j.ejphar.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 35.Toklu HZ, Sener G, Jahovic N, Uslu B, Arbak S, Yegen BC. Int Immunopharmacol. 2006;6:156. doi: 10.1016/j.intimp.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Biomaterials. 2004;25:1911. doi: 10.1016/s0142-9612(03)00625-2. [DOI] [PubMed] [Google Scholar]
- 37.Gomathi K, Gopinath D, Rafiuddin Ahmed M, Jayakumar R. Biomaterials. 2003;24:2767. doi: 10.1016/s0142-9612(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 38.Wattamwar P, Mo Y, Wan R, Palli R, Zhang Q, Dziubla T. Adv Funct Mater. 2010;20:147. [Google Scholar]
- 39.Woo YI, Park BJ, Kim HL, Lee MH, Kim J, Yang YI, Kim JK, Tsubaki K, Han DW, Park JC. Biomed Mater. 2010;5:044109. doi: 10.1088/1748-6041/5/4/044109. [DOI] [PubMed] [Google Scholar]
- 40.Lee SB, Jeon HW, Lee YW, Lee YM, Song KW, Park MH, Nam YS, Ahn HC. Biomaterials. 2003;24:2503. doi: 10.1016/s0142-9612(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 41.Altman AM, Matthias N, Yan Y, Song YH, Bai X, Chiu ES, Slakey DP, Alt EU. Biomaterials. 2008;29:1431. doi: 10.1016/j.biomaterials.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Qi SH, Liu P, Xie JL, Shu B, Xu YB, Ke CN, Liu XS, Li TZ. Burns. 2008;34:385. doi: 10.1016/j.burns.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Glotzbach JP, Wong VW, Gurtner GC, Longaker MT. Curr Probl Surg. 2011;48:148. doi: 10.1067/j.cpsurg.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Becker LB. Cardiovasc Res. 2004;61:461. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 45.Lange C, Togel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Kidney Int. 2005;68:1613. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]