Abstract
Bypass of two arrest points is essential in the process of cellular immortalization, one of the components of the transformation process. Expression of human papillomavirus type 16 E6 and E7 together can escape both senescence and crisis, processes which normally limit the proliferative capacity of primary human keratinocytes. Crisis is thought to be mediated by telomere shortening. Because E6 stimulates telomerase activity and exogenous expression of the TERT gene with E7 can immortalize keratinocytes, this function is thought to be important for E6 to cooperate with E7 to bypass crisis. However, it has also been reported that E6 dissociates increased telomerase activity from maintenance of telomere length and that a dominant-negative p53 molecule can substitute for E6 in cooperative immortalization of keratinocytes with E7. Thus, to determine which functions of E6 are required to allow bypass of crisis and immortalization of keratinocytes with E7, immortalization assays were performed using specific mutants of E6, in tandem with E7. In these experiments, every clone expressing an E6 mutant capable of degrading p53 was able to bypass crisis and immortalize, regardless of telomerase induction. All clones containing E6 mutants incapable of degrading p53 died at crisis. These results suggest that the ability of E6 to induce degradation of p53 compensates for continued telomere shortening in E6/E7 cells and demonstrate that degradation of p53 is required for immortalization by E6/E7, while increased telomerase activity is dispensable.
Cellular immortalization is considered to be one of the major steps on the road to transformation (36). Many epithelial cell types, including keratinocytes, require bypass of two arrest signals in order to become immortal (17, 55, 80, 82). The first, senescence, is a p16INK4a-dependent arrest that is activated in epithelial cells in culture (8). As cells are passaged, they accumulate p16INK4a, a cyclin-dependent kinase inhibitor which blocks cell cycle progression by inhibiting the ability of cyclin D/cdk4/6 to phosphorylate and inactivate the repression by Rb on the E2F transcription factors, inducing a G1 arrest (20). The accumulation of p16INK4a may depend on telomere shortening for activation (61), or it may be a reflection of inadequate growth conditions in culture (80, 81) but, regardless, cells must bypass this arrest in order to become immortal in vitro (55, 82, 102).
When senescence is overcome, cells continue to grow until telomeres reach some critically short length, generally 5 to 7 kb for human cells, at which point chromosomal instability will occur if the cells continue to divide (5). Telomeres, repeat sequences at the ends of linear chromosomes, normally cap these ends and protect the cell from chromosomal fusion events and loss of upstream genetic material (13, 14). Telomere capping proteins can specifically suppress the DNA damage response, and loss of telomeric DNA as cells divide causes loss of the capped structure (50, 94). At this point, the cells come to crisis, an apoptotic process induced by the DNA damage response to these exposed telomere ends (50, 76). Crisis can be overcome by telomere length stabilization, either through telomerase activation (82), which restores telomere length and directly suppresses the DNA damage response (90), or activation of the alternative lengthening of telomeres (ALT) pathway for telomere maintenance, a recombination-based mechanism for telomere lengthening (19). Alternatively, crisis may be bypassed by loss of a normal apoptotic response to the short telomeres, such as through loss of p53 or ATM, an upstream regulator of the DNA damage response (24, 52). If both senescence and crisis are bypassed, the cells are immortalized. Depending on the specific mutations involved, these cells may stabilize their genomes and maintain a normal genetic complement, or they may display genomic instability, which may play a role in further carcinogenic changes (36).
The human papillomaviruses (HPVs) are the causative agent of cervical cancer, as well as other anogenital cancers and a subset of oral squamous cell carcinomas (64, 112). HPV infects basal keratinocytes, and the viral life cycle is tied to the differentiation program of the squamous epithelium (reviewed in reference 101). Carcinogenic progression is associated with an aberrant integration event of the viral genome into the host cell DNA, which causes loss of normal viral transcriptional regulation and overexpression of the E6 and E7 gene products of the virus (69). As such, it is worthwhile to consider the function of E6 and E7 independent of the whole viral genome, to understand what role these oncoproteins play in the absence of normal regulation by viral factors, such as the HPV E2 protein.
E6 and E7 have specific oncogenic potential, through interaction with major tumor suppressor pathways in the cell. E7 is known to bind and inactivate the Rb family of tumor suppressors (21, 72), which normally inhibit the E2F transcription factors (reviewed in reference 20), regulators of G1-to-S-phase cell cycle progression. This capability plays a major role in immortalization by the viral oncoproteins, as aging-dependent p16INK4a accumulation would normally lead to inactivation of E2F-mediated transcription (55). In the presence of E7, Rb repression is inhibited, which allows E2F factors to function, bypassing the p16INK4a arrest and thus senescence.
The E6 protein has many functions that may contribute to its oncogenic potential. Specifically relevant to immortalization, the classical function of E6 is binding to and inducing the degradation of the p53 tumor suppressor protein (87). This happens through the formation of a trimeric complex with the E6-AP, a cellular ubiquitin ligase which ubiquitinates p53 in the presence of E6 (44-46, 86, 109). The p53 protein is a convergence point for multiple signals that induce cell cycle arrest and apoptosis, including DNA damage (62, 91). As a transcription factor, p53 up-regulates target genes involved in coordinating these responses, such as p21CIP1, a CDK inhibitor acting on cyclin E/cdk2 complexes, and Bax, a proapoptotic member of the Bcl-2 family (9, 22, 67). Additionally, p53 may play a separate role in apoptosis at the mitochondria (65).
More recently, E6 has been shown to induce telomerase activity in keratinocytes (56, 100). This has been shown to be an E-box- and c-Myc-dependent phenotype (30, 63, 73, 107, 108). E6 increases transcription of the TERT gene, which encodes the catalytic subunit of the telomerase holoenzyme, a reverse transcriptase. The telomerase enzyme utilizes an RNA template, called TERC, to add telomere repeats to chromosomes (reviewed in reference 92). The TERT subunit of the enzyme is the limiting factor in telomerase activity, as the TERC molecule is ubiquitously expressed (11, 25), and overexpression of the TERT gene alone is sufficient to engender high levels of telomerase activity in all cell types examined (10, 55). In addition to its enzymatic activity, telomerase has been shown to directly interact with double-strand break-sensing proteins, the human homologues of the yeast Ku protein, Ku70 and Ku80, suppressing the potential DNA damage responses to telomere ends (4). Telomerase activity is present in ∼85% of human tumors, the rest utilizing the ALT pathway (34). Thus, telomere maintenance and telomerase would seem important for bypass of crisis and in many systems may play a major role in immortalization of cells.
Although it is clear that E6 does increase TERT expression and telomerase activity in cells, the activity levels engendered by E6 are substantially lower than those observed upon TERT overexpression in cells (55, 100). Corollary to that observation, telomeres continue to shorten over extended passage of E6, E7, or E6/E7 keratinocytes (100), rather than being extended or maintained as seen in TERT-expressing cells (41, 106). Thus, experiments were carried out to identify the required functions of E6 for bypass of crisis and immortalization of keratinocytes by E6/E7. Using a panel of previously characterized mutants of E6 in tandem constructs with E7, we performed clonal analysis of immortalization, which demonstrated that loss of p53 was required for bypass of crisis and immortalization, while activation of telomerase by E6 was insufficient to overcome crisis. These results suggest that there may be multiple mechanisms for cells to bypass crisis, through oncogenic lesions impinging on different points in the same pathway, not all of which require telomerase to be activated.
MATERIALS AND METHODS
Cell culture and infections.
Primary human foreskin keratinocytes (HFK) were isolated from neonatal foreskins and cultured in EpiLife medium supplemented with human keratinocyte growth supplement (Cascade Biologics). To generate stable cells expressing HPV type 16 (HPV-16) E6 mutants in conjunction with E7, the pBabe retroviral system was employed (57, 68). Using the ΦNX packaging cell line (American Type Culture Collection), vesicular stomatitis virus-pseudotyped amphotropic retrovirus was generated. Retrovirus encoding E6 and E7 in tandem or empty vector was used to infect second-passage HFK (counted from initial isolation from tissue samples) in 8 μg of Polybrene per ml of medium. Following selection for 4 days with 1.25 μg of puromycin per ml of medium, cells were expanded and clones were obtained by ring cloning one passage after selection (third passage). Cell populations, both mass and clones, were serially passaged, counting cells at each split and seeding a specified number of cells for continued passage as follows: 5 × 105 cells for lines A and C mass cultures on 10-cm dishes, 7.5 × 104 cells for line B mass cultures on 10-cm dishes, and 1 × 104 cells for the A, B, and C clonal lines on 12-well dishes. Cell counts were used to calculate population doublings (PDs) over time for both clones and mass cultures, using the formula {[log10(ending cell number) − log10(starting cell number)]/log102}. No correction was made for replating efficiency.
Generation of E6 mutant panel.
Mutagenesis of E6 was performed using the QuikChange kit (Stratagene) to alter E6 sequences to match previously described mutants (R8S/P9A/R10T and Δ123-127 [55, 56] and F125L and G134V [12, 60]). Wild-type HPV-16 E6/E7 in tandem in pGEM7Zf was mutagenized, and the resulting mutants were sequenced and then subcloned into the pBabe retroviral vector.
RT-PCR analysis.
RNA was prepared using the RNeasy kit (Qiagen). Reverse transcriptase (RT) reaction mixtures contained 5 μg of RNA, 8 mM dithiothreitol, 500 μM deoxynucleoside triphosphate (dNTP), 16.7 μM random hexamer primer, RNase inhibitor, 5 U of avian myeloblastosis virus RT, and buffer (Promega) and were incubated at 42°C for 60 min. PCRs were run with Promega buffer, 1.5 mM MgCl2, 200 μM dNTP, 10 μM primers, and 2.5 U of Taq polymerase (Promega). Primers for HPV-16 E6 were forward, 5′-GAG AGG ATC CAT GTT TCA GGA CCC ACA GG-3′, and reverse, 5′-CAT GAA TCC TTA CAG CTG GGT TTC TCT AC-3′. Primers for HPV-16 E7 were forward, 5′-CGG GAT CCA TCA TGC ATG GAG ATA C-3′, and reverse, 5′-GTG GAT CCG GTT TCT GAG AAC AGA TGG-3′. Primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were forward, 5′-CCC CTC TGC TGA TGC CCC CAT GTT-3′, and reverse, 5′-GAG CTT CCC GTC TAG CTC AGG GAT-3′. PCRs were run for 30 cycles of 94°C for 15 s, 54°C for 15 s, and 68°C for 45 s.
TRAP assay.
Telomeric repeat amplification protocol (TRAP) assays were done as previously described (63), after the protocol of Kim et al. (54). Cells were lysed in whole-cell lysis buffer (250 mM NaCl, 50 mM Tris [pH 7.5], 0.5% NP-40, 20% glycerol, protease inhibitor cocktail [Sigma]). TRAP PCR mixtures contained PCR buffer (Fermentas), 1.5 mM MgCl2, 1 mM EGTA, 0.1 μg of TS primer with a 5′-covalently linked biotin molecule (5′-biotin-AATCCGTCGAGCAGAGTT-3′) (Invitrogen), 5 μg of bovine serum albumin, 100 μM dNTP, 1 μg of T4 g32 single-strand binding protein (Roche Diagnostics Corporation), and 5 μg of cell extract. Reaction mixtures were incubated at room temperature for 30 min, and then 0.1 μg of CX primer (5′-(CCCTTA)3CCCTAA-3′) and 5 U of Taq DNA polymerase (Fermentas) were added. The reactions were subjected to 30 cycles of PCR (94°C for 40 s, 50°C for 40 s, and 72°C for 90 s), run on a 10% nondenaturing polyacrylamide gel, and transferred to Gene Screen Plus nylon membranes (NEN) in Tris-borate-EDTA. The membrane was washed with blocking buffer (5% sodium dodecyl sulfate [SDS], 17 mM Na2HPO4, 8 mM NaH2PO4), and streptavidin-horseradish peroxidase (Chemicon) was applied in blocking buffer at a 1:1,000 dilution. The membrane was washed with wash buffer I (1:10 dilution of blocking buffer) followed by wash buffer II (100 mM Tris [pH 9.5], 100 mM NaCl, 10 mM MgCl2), developed with the ECL Plus reagent (NEN), and detected using the ChemiImager 5500 (Alpha Innotech).
Western blot analysis.
Whole-cell lysates were prepared as described above. Fifty micrograms of whole-cell extract was run on SDS-10% polyacrylamide gel electrophoresis gels, transferred to nitrocellulose, and probed with the following antibodies: p16INK4a (clone G175-1239), p53 (clone DO-1), and p21 (clone 6B6) (BD PharMingen); and actin (C-2; Santa Cruz Biotechnology). Blots were imaged on the ChemiImager 5500 (Alpha Innotech), and images were quantitated with the FluorImager software package (version 2.1), using a local background method of calculation.
Southern blotting.
Genomic DNA was isolated from cells at specified passage using the Genomic-tip 20/G and the genomic DNA buffer set (Qiagen) following the manufacturer's protocol. DNA was quantitated, and 2.5 μg of DNA was digested with HinfI and RsaI restriction enzymes (NEB). Digestion reactions were run on 0.8% agarose gels, depurinated in 0.25 M HCl, denatured in 0.4 M NaOH, and transferred to Gene Screen Plus nylon membrane by passive transfer, using 0.4 M NaOH as transfer buffer (alkaline transfer). Membranes were dried, prehybridized in aqueous prehybridization solution (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 5× Denhardt's solution, 1% SDS, and 100 μg of sonicated salmon sperm DNA/ml), and probed with 105 cpm of aqueous prehybridization solution of a 51-nucleotide probe (5′-(CCCTAA)8CCC-3′) /ml end labeled to a specific activity of >108 dpm/μg. Blots were washed with high-stringency buffer (0.2× SSC, 0.1% SDS), and imaged on a PhosphorImager (Molecular Dynamics).
RESULTS
Ability of E6 to degrade p53 correlates with immortalization.
Because some studies suggested that loss of p53 alone (88), or in combination with gain of telomerase activity (82), was involved in keratinocyte immortalization, the requirement for p53 loss in E6/E7 immortalization was examined in parallel with the requirement for telomerase activation. A panel of E6 mutants was chosen, based on published determinations of the behavior of these mutants (12, 60), to clarify the roles of p53 degradation and telomerase up-regulation by E6 in E6/E7-mediated keratinocyte immortalization. As shown in Table 1, the E6 R8S/P9A/R10T mutant lacked the ability to induce p53 degradation in cells but could increase levels of telomerase activity comparable to wild-type E6. The E6 G134V protein induced the destruction of p53 but did not increase telomerase activity above baseline levels. E6 F125L could both degrade p53 and increase telomerase activity, while E6 Δ123-127 had neither function. Primary HFK stably expressing these E6 mutants with wild-type E7, in tandem, were generated using the pBabe retroviral vector system (68). The empty retroviral vector and wild-type E6 with E7 were included as controls. Fifteen clonal cell populations were isolated by ring cloning, five per experiment for each of three experiments, and both the mass cultures and clones were serially passaged, counted, and seeded at each split to calculate PD over time.
TABLE 1.
Function and behavior of E6 mutants in E6/E7 keratinocyte immortalization
| Mutant | Degrades p53 | Induces telomerase activity | Mass cultures (3 expt) | Clones (3 expt) | Average clonal PD |
|---|---|---|---|---|---|
| Vector | − | − | 0/3 | 0/15 | 28 (±8) |
| E6 WT/E7 | +a | + | 3/3 | 15/15 | >80 (imm)b |
| E6 R8S/P9A/R10T/E7 | − | + | 0/3 | 0/15 | 53 (±11) |
| E6 Δ123-127/E7 | − | − | 0/3 | 0/15 | 33 (±6) |
| E6 F125L/E7 | + | + | 3/3 | 15/15 | >80 (imm) |
| E6 G134V/E7 | + | − | 3/3 | 15/15 | >80 (imm) |
+, wild-type activity; −, no activity.
imm, immortalized.
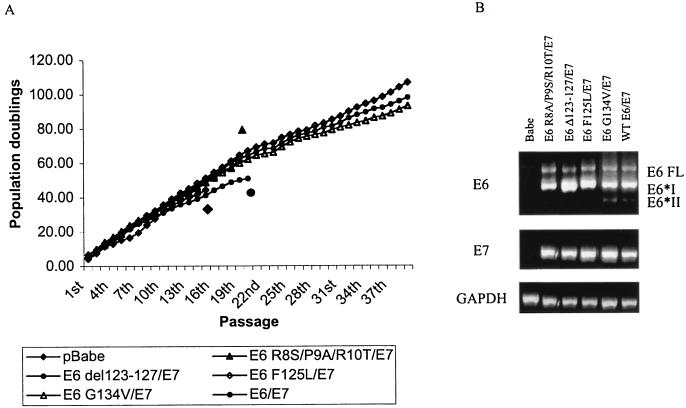
The control cells ceased to grow at an average PD level of 28 doublings (±8) for the clones (Table 1 and Fig. 1A). The wild-type E6/E7-expressing keratinocytes were able to grow far beyond the limits of the control cells, with clones achieving an average of more than 80 PD and mass cultures averaging greater than 100 PD before these experiments were stopped. Similarly, both the E6 F125L/E7- and E6 G134V/E7-expressing cells were able to grow for more than 80 PD for clones and greater than 100 PD for mass cultures, despite the fact that only the E6 F125L mutant increased telomerase activity in keratinocytes. In addition, although the E6 R8S/P9A/R10T/E7 cells showed an extended life span, with clonal PD averaging 53 (±11), this mutant was unable to sustain continued growth in culture, and all clones and mass cultures of these cells ultimately died. As expected, the E6 Δ123-127/E7-expressing cells were also not immortal, with an average clonal life span of 33 PD (±6). To confirm that all cultures were expressing both E6 and E7 as appropriate, RNA was purified from third-passage cells and RT-PCR was performed for E6, E7, and GAPDH as a control. As shown in Fig. 1B, all cell lines had similar levels of E6 and E7 expression, although the splicing pattern of the E6 mutants varied somewhat. To avoid the problem of being outside the linear range for quantitation of the PCRs, we carried out dilutions and observed the same relative levels of E6 mRNA in each sample (data not shown). Thus, the lack of immortalization by certain mutants cannot be attributed to differences in E6 or E7 expression levels. Taken together, these results demonstrate that the ability of E6 to degrade p53 is both necessary and sufficient for bypass of crisis and immortalization of keratinocytes with E7.
FIG. 1.
p53 degradation by E6 mutants is necessary and sufficient for immortalization with E7. Primary HFK were infected with retrovirus expressing the indicated E6 mutants, in tandem with E7, at the second absolute passage. Empty vector (pBabe) and wild-type E6/E7 cells were included as controls. Cells were counted after trypsinization and seeded at defined density for each passage. (A) Cumulative PDs are graphed as a function of time in culture, shown as passage number. For cell lines that did not immortalize, icons matching the legend were used to indicate the point at which these cells ceased to grow. The graph represents the averages of three independent experiments. (B) RT-PCR was performed to check for similar E6 and E7 expression in the mutant cell lines. As previously described (18, 93), multiple splice variants of E6 can be detected. Data shown are from line A. Similar results were obtained with lines B and C.
Acquisition of telomerase activity is not required for E6/E7 immortalization.
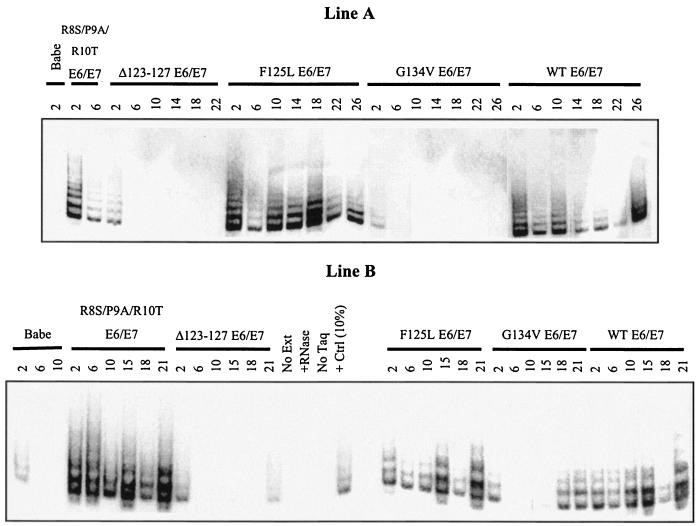
One possible explanation for the results described above is that the E6 mutants that degrade p53 acquire secondary mutations that induce telomerase activity over passage and, thus, these cells may require both loss of p53 and gain of telomerase activity for full immortality. To examine whether this indeed took place, telomerase activity was assayed over time in the various E6 mutant/E7 keratinocyte lines. As shown in Fig. 2, immediately following introduction of E6 and E7 into cells, telomerase levels in the E6 Δ123-127/E7 and E6 G134V/E7 cells were similar to those of vector controls. In contrast, cells expressing E6 R8S/P9A/R10T/E7, E6 F125L/E7, or wild-type E6/E7 had similar, increased levels of telomerase activity. Over their life span, there was some variation in the levels of telomerase activity in each of the cell lines, especially in the wild-type E6/E7 line A. However, it is clear that of the mutants, both the E6 R8S/P9A/R10T/E7 and E6 F125L/E7 cells had elevated telomerase activity throughout their life span, as expected. In contrast, the E6 Δ123-127/E7 cells showed no telomerase activation over their life span, similar to vector controls. Finally, the E6 G134V/E7 cells showed no telomerase activity at early passage in all lines examined. However, while lines A and C showed no reactivation of telomerase at later time points (passages 18, 22, and 26), line B keratinocytes did acquire telomerase activity upon extended passage (a low level at passage 15 and increasing at passages 18 and 21). This E6 mutant was sequenced using cDNA from early and late passages and was shown to maintain the G134V sequence. This suggests that while telomerase reactivation may accompany immortalization, it is not necessarily required for bypass of crisis. The E6 G134V/E7 cells can bypass crisis and go on to immortalization without reacquiring telomerase activity, as evidenced by the line A and C keratinocytes. This was also supported by the fact that all clones of the E6 G134V/E7 cells progressed to immortalization, rather than just a proportion of clones, suggesting that secondary genetic events are not required for unlimited growth, although they may take place in some of these populations.
FIG. 2.
Telomerase activity is induced as expected in E6 mutant/E7 keratinocytes over time. TRAP assays were performed to measure telomerase activity for each mutant, over time, indicated as passage number. Immediately following selection, the behavior of each mutant was as expected (Table 1), although levels varied somewhat over the lifetime of each culture. Results from lines A and B are shown. Line C was similar to line A.
Mutants that do not permit immortalization die at crisis.
To clarify the relative contributions of senescence and crisis to growth suppression of the various mutant E6/E7-expressing cells, markers of these processes were examined. Senescence is associated with, and may depend on, the characteristic accumulation of p16INK4a (8, 55, 80, 81). Crisis shows features of apoptosis, including the accumulation and activation of p53 protein by phosphorylation on specific residues, including serine 15, a target site of ATM/ATR kinases (50, 78). In addition, p21CIP1 levels generally increase in concert with p53 activation at crisis. Thus, the status of these proteins was examined.
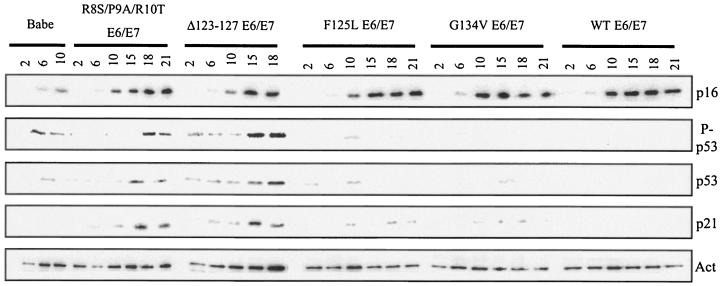
All cell lines containing an E6/E7 combination accumulated high levels of p16INK4a over time (Fig. 3). These cells continued to proliferate, however, because the expression of E7 bypasses the p16INK4a arrest (53, 55). The vector control cells, with no means to bypass this arrest signal, ceased to proliferate. The control cells had reduced levels of p16INK4a compared to the E6 wild-type and mutant plus E7 cells, but this was likely because the control cells are sensitive to these lower amounts of p16INK4a and cease to proliferate with less of this cell cycle inhibitor present. It is clear that all of the mutant E6/E7 cell lines accumulated p16INK4a with kinetics similar to wild-type E6/E7 cells. Thus, it is unlikely that this arrest signal is responsible for the growth cessation seen in the E6 R8S/P9A/R10T/E7 and E6 Δ123-127/E7 mutant cell lines.
FIG. 3.
p53 accumulation and activation accompany cell death at crisis in nonimmortalizing E6 mutant/E7 lines. Western blot assays were performed with 50 μg of total protein from the indicated cell lines, at the indicated passage numbers. Blots were probed for total p21CIP1 or phospho-p53, modified on serine-15, and then stripped and reprobed for total p53 and p16INK4a, followed by actin, as a loading control. Data shown are from line B. Similar results were obtained with lines A and C.
The alternative explanation is that the cell populations that do not immortalize are lost at crisis. As a measure of this, three markers of crisis were examined: activated p53, as measured by the amount of p53 phosphorylated at residue serine-15; total p53; and p21CIP1, a secondary marker of p53 activation and a potential mediator of growth arrest in these cells. The vector control cells again had relatively low levels of these proteins. E6 Δ123-127/E7 cells, which can survive past senescence, showed an induction of p53 protein, as well as increases in activated p53 and p21CIP1. Cells expressing E6 R8S/P9A/R10T/E7 showed induction of these crisis markers with different kinetics, with p53 and phospho-p53 levels increasing later in this mutant than in E6 Δ123-127/E7 cells. However, at passages 18 and 21, both mutant lines had similar levels of phospho-p53 and total p53. In contrast, because the E6 F125L/E7 and E6 G134V/E7 mutants retain the ability to degrade p53, cells that express these mutants with E7 showed little or no p53 protein, and hence little phosphorylated p53. There was a modest accumulation of p21CIP1 over time in the E6 F125L/E7 and E6 G134V/E7 mutant lines, compared to wild type, most likely by a p53-independent mechanism. However, p21CIP1 levels reached only ∼25% of those seen in the E6 R8S/P9A/R10T/E7 and E6 Δ123-127/E7 cells. Clearly, the distinction between those cell lines that survive and those that do not is the ability to bypass activation of crisis at later passages, dependent on the presence of p53.
Telomere lengths shorten in E6/E7 keratinocytes.
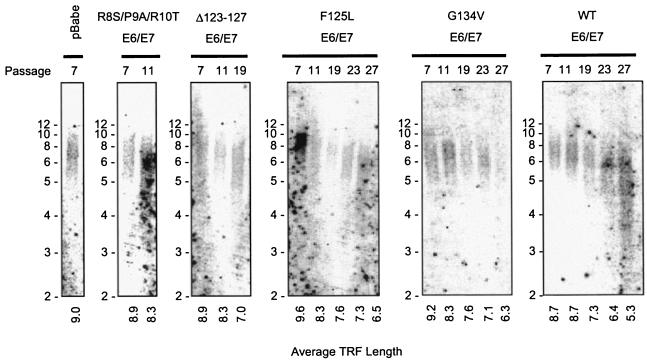
A possible alternative explanation for the ability of the E6 G134V/E7 cells to grow indefinitely without increased telomerase activity is that these cells may have, directly or indirectly, activation of the ALT pathway for telomere maintenance. Previous studies have shown that although E6 increased telomerase activity in keratinocytes, telomeres in E6 or E6/E7 cells were not increased in length (100), in contrast to the elongation of telomeres seen in TERT-expressing cells (41, 106). Over extended passage, it has been shown that telomeres shorten progressively in cells expressing E6 or E6/E7, in a manner similar to controls (100). However, it was possible that in the E6 G134V/E7 cells telomeres were elongated in some way, which might have allowed immortalization without telomerase activity. The hallmark of ALT activation is significantly elongated telomeres, from a normal range of 10 to 12 kb in early-passage cells to lengths greater than 23 kb in ALT cells (38). To determine if E6 G134V/E7 cells showed an extended telomere length, Southern blot analysis was performed to examine telomere restriction fragment lengths in the mutant cell lines. In agreement with published results, both the control cells and the wild-type E6/E7 cells showed progressive shortening of telomeres (Fig. 4). In addition, all of the mutant cell lines showed similar progressive shortening of telomeres, suggesting that the ALT pathway is not active in these cells. This argues that the E6/E7 wild-type and mutant cell lines are immortal not because of telomere elongation by telomerase activity but by a p53-dependent mechanism of bypassing the arrest associated with critical shortening of telomeres.
FIG. 4.
Telomere lengths shorten over the life span of wild-type and mutant E6/E7 cells. Southern blot analysis was performed for telomere restriction fragment (TRF) length on the pBabe vector control, mutant E6/E7, and wild-type E6/E7 keratinocyte lines. Passage number is indicated at the top of each lane. Average TRF length, indicated at the bottom of each lane, was determined using standard calculation methods (1), employing the formula [Σ(length) · (intensity)]/Σ(intensity) for each lane.
DISCUSSION
This study is the first to examine the role of E6 in keratinocyte (HFK) immortalization, the natural host cell of HPV infection. Mutational analysis of E6 has been performed in human mammary epithelial cells, which E6 can immortalize on its own, to understand the role of E6 in extending cellular life span (12, 55, 60). However, these studies are not directly comparable to the situation here, because human mammary epithelial and HFK cells are known to have differences in the mortality stages that block their proliferation (47, 55) and, importantly, they are not the host cell of HPV. It has been previously demonstrated that overexpression of the TERT gene engenders high levels of telomerase activity in cells, and telomeres are stabilized or lengthened in the presence of this high activity (41, 106). In contrast, E6/E7-expressing cells, while possessing elevated levels of telomerase activity relative to control cells, have substantially less activity than matched TERT-expressing cells (55, 100) and, consequently, do not maintain long telomeres as they age in culture (26, 100) (Fig. 4). Over time, E6/E7 cells come to have telomeres that are critically short and should induce crisis, but unlike control cells, wild-type E6/E7-expressing keratinocytes do not die at this point. With 100% efficiency, as measured by clonal survival (35, 43, 71) (Table 1), these cells bypass crisis and become immortal. Bypass of this arrest and apoptosis seems to depend on the presence of E6, because expression of E7 alone is incapable of immortalizing cells in the absence of feeder cells (35, 81).
The mutational analysis described herein suggests that the relevant contribution of E6 to E6/E7-mediated immortalization is the degradation of p53. Regardless of the telomerase activation by various mutants of E6, the ability of E6 to induce loss of the p53 protein was necessary and sufficient for keratinocyte immortalization with E7. These results are in concert with some previous studies examining which alterations were sufficient to supplement E7 expression and allow keratinocyte immortalization (88). The combination of a dominant-negative p53 (DN p53) mutant with E7 was sufficient for high-efficiency immortalization of keratinocytes, as measured by clonal immortalization. In these experiments, 100% of clones expressing the DN p53 with E7 grew indefinitely. In the context of the full-length HPV 16 genome, telomerase activation by the viral E6 protein is not required for immortalization (98). More recently, Rheinwald et al. examined the ability of DN p53 to supplement p16INK4a loss in keratinocyte immortalization (82). Although loss of both p53 and p16INK4a were required, in their system an additional alteration, the gain of high-level telomerase activity through the introduction of the TERT gene, was necessary to allow continuous growth of keratinocytes. These results, taken together with the work presented here, suggest that introduction of E7 affects cellular immortalization in ways other than just bypass of p16INK4a arrest. It is well established that E7 affects the cell cycle in multiple ways (28, 49, 70) and can allow bypass of a variety of arrest signals (7, 16, 27, 39, 48, 79, 83, 97), and so it is not surprising that it alters cell growth and immortalization differently than loss of p16INK4a alone. However, our results are seemingly contrary to those of others who showed that overexpression of telomerase plus E7 could immortalize keratinocytes (17, 55). The difference in the results may be solely due to the high levels of telomerase activity observed when the TERT gene is introduced into cells, with levels of activity 10-fold above that seen when E6 is introduced (55, 100) (data not shown).
Gain of telomerase and loss of p53 likely impinge on the same pathway at different points. It has been shown that loss of telomeric DNA induces a DNA damage-like response (14, 15, 29, 51, 75-77, 90, 94, 96, 99) and that downstream of this are cellular factors involved in response to many DNA damage response signals, the ATM kinase and its targets, including p53, which is directly phosphorylated by ATM (2, 59, 103, 104). In response to other DNA damage signals, such as UV irradiation or chemotherapeutic agents, cells arrest upon damage to attempt to repair it (31, 40, 66, 84, 103, 104, 110, 111). In the context of telomere shortening, because this is not repairable damage, cells with critically short telomeres are destroyed at crisis by apoptosis. This is postulated to control the genetic instability of cells with telomeres that are too short to function properly. It has been shown that cells with critically short telomeres have increased levels of end-to-end chromosomal fusions, as well as ring chromosomes, leading to aneuploidy and possibly potentiating cancer development (3, 37, 58, 74, 85, 95, 105).
TERT expression and consequent increases in telomerase activity in cells may bypass crisis in an indirect manner, by protecting telomeres so that crisis is never induced. In the case of HPV E6 and E7, telomerase activity levels may be too low to maintain telomere length, and thus these cells should undergo crisis. However, because crisis depends on activation of p53, E6/E7 keratinocytes avoid death at crisis because of the complete loss of p53 in these cells. The potential for alternative mechanisms to bypass cellular life span control pathways is not limited to HPV. Recent work by Seger et al. demonstrates that this is possible with cellular oncogenes as well (89). Seger et al. reported the generation of transformed human cells without addition of telomerase, through overexpression of MDM2, a normal regulator of p53 function that induces ubiquitylation and degradation of p53. This is in contrast to previous reports of transformation requiring simian virus 40 large and small T antigens, constitutive Ras activity, and gain of telomerase activity (23, 33) or loss of Rb function, gain of Ras, telomerase, and phosphatidylinositol 3-kinase activities (34). In these earlier models, telomerase activation was a necessary component, while Seger et al. demonstrated that there are alternative mechanisms to the same end.
Although telomerase activation may not be required for immortalization of keratinocytes, this function of E6 may be involved in the process of transformation in a manner unrelated to cellular immortalization. The fact that ∼90% of human tumors show telomerase activation suggests that this gene plays some role in cancer progression (34, 42). However, some data suggest that the role of telomerase activation is stabilization of what would otherwise be an overwhelmingly unstable genome (32, 62). Thus, telomerase activity may not be required for immortalization, but it may play a role in the transformation process. It has been demonstrated in models of cellular oncogenic transformation that even in cells with long telomeres, gained by activation of the ALT pathway for telomere maintenance, TERT expression confers greater oncogenic potential to these cells (6). Alternatively, telomerase activation by the HPV E6 protein may be a by-product of the necessary alteration of regulation of some other process, such as c-Myc transcriptional up-regulation (63, 108), which is known to increase telomerase levels but may also serve a function in the viral life cycle. Considering this, the role for telomerase activation by E6 in other phenotypes related to transformation remains to be examined.
Acknowledgments
We thank colleagues for their generous gifts of reagents, Laurel Baglia and Jennifer Changery for technical assistance, and Hartmut Land, Laurel Baglia, Craig Menges, Don Nguyen, and Angela Incassati for critical reading of the manuscript.
This work was supported by grant NIDR DE13526. H.R.M. was supported by the Rochester Training Program in Oral Infectious Diseases, PHS/NIDCR T32 DE07165.
REFERENCES
- 1.Allsopp, R. C., H. Vaziri, C. Patterson, S. Goldstein, E. V. Younglai, A. B. Futcher, C. W. Greider, and C. B. Harley. 1992. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 89:10114-10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, L., C. Henderson, and Y. Adachi. 2001. Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage. Mol. Cell. Biol. 21:1719-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artandi, S. E., S. Chang, S. L. Lee, S. Alson, G. J. Gottlieb, L. Chin, and R. A. DePinho. 2000. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406:641-645. [DOI] [PubMed] [Google Scholar]
- 4.Chai, W., L. P. Ford, L. Lenertz, W. E. Wright, and J. W. Shay. 2002. Human Ku70/80 associates physically with telomerase through interaction with hTERT. J. Biol. Chem. 277:47242-47247. [DOI] [PubMed] [Google Scholar]
- 5.Chan, S. W., and E. H. Blackburn. 2002. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene 21:553-563. [DOI] [PubMed] [Google Scholar]
- 6.Chang, S., C. M. Khoo, M. L. Naylor, R. S. Maser, and R. A. DePinho. 2003. Telomere-based crisis: functional differences between telomerase activation and ALT in tumor progression. Genes Dev. 17:88-100. (Erratum, 17:541.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, Y. E., L. Pena, G. C. Sen, J. K. Park, and L. A. Laimins. 2002. Long-term effect of interferon on keratinocytes that maintain human papillomavirus type 31. J. Virol. 76:8864-8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi, V., M. Cesnjaj, P. Bacon, J. Panella, D. Choubey, M. O. Diaz, and B. J. Nickoloff. 2003. Role of INK4a/Arf locus-encoded senescent checkpoints activated in normal and psoriatic keratinocytes. Am. J. Pathol. 162:161-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choisy-Rossi, C., P. Reisdorf, and E. Yonish-Rouach. 1998. Mechanisms of p53-induced apoptosis: in search of genes which are regulated during p53-mediated cell death. Toxicol. Lett. 102-103:491-496. [DOI] [PubMed] [Google Scholar]
- 10.Counter, C. M., W. C. Hahn, W. Wei, S. D. Caddle, R. L. Beijersbergen, P. M. Lansdorp, J. M. Sedivy, and R. A. Weinberg. 1998. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA 95:14723-14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Counter, C. M., M. Meyerson, E. N. Eaton, L. W. Ellisen, S. D. Caddle, D. A. Haber, and R. A. Weinberg. 1998. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene 16:1217-1222. [DOI] [PubMed] [Google Scholar]
- 12.Dalal, S., Q. Gao, E. J. Androphy, and V. Band. 1996. Mutational analysis of human papillomavirus type 16 E6 demonstrates that p53 degradation is necessary for immortalization of mammary epithelial cells. J. Virol. 70:683-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lange, T. 2001. Cell biology. Telomere capping—one strand fits all. Science 292:1075-1076. [DOI] [PubMed] [Google Scholar]
- 14.de Lange, T. 2002. Protection of mammalian telomeres. Oncogene 21:532-540. [DOI] [PubMed] [Google Scholar]
- 15.de Lange, T., and J. H. Petrini. 2000. A new connection at human telomeres: association of the Mre11 complex with TRF2. Cold Spring Harbor Symp. Quant. Biol. 65:265-273. [DOI] [PubMed] [Google Scholar]
- 16.Demers, G. W., E. Espling, J. B. Harry, B. G. Etscheid, and D. A. Galloway. 1996. Abrogation of growth arrest signals by human papillomavirus type 16 E7 is mediated by sequences required for transformation. J. Virol. 70:6862-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson, M. A., W. C. Hahn, Y. Ino, V. Ronfard, J. Y. Wu, R. A. Weinberg, D. N. Louis, F. P. Li, and J. G. Rheinwald. 2000. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doorbar, J., A. Parton, K. Hartley, L. Banks, T. Crook, M. Stanley, and L. Crawford. 1990. Detection of novel splicing patterns in a HPV16-containing keratinocyte cell line. Virology 178:254-262. [DOI] [PubMed] [Google Scholar]
- 19.Dunham, M. A., A. A. Neumann, C. L. Fasching, and R. R. Reddel. 2000. Telomere maintenance by recombination in human cells. Nat. Genet. 26:447-450. [DOI] [PubMed] [Google Scholar]
- 20.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 21.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papillomavirus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 22.el-Deiry, W. S. 1998. Regulation of p53 downstream genes. Semin. Cancer Biol. 8:345-357. [DOI] [PubMed] [Google Scholar]
- 23.Elenbaas, B., L. Spirio, F. Koerner, M. D. Fleming, D. B. Zimonjic, J. L. Donaher, N. C. Popescu, W. C. Hahn, and R. A. Weinberg. 2001. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15:50-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eller, M. S., G. Z. Li, R. Firoozabadi, N. Puri, and B. A. Gilchrest. 2003. Induction of a p95/Nbs1-mediated S phase checkpoint by telomere 3′ overhang specific DNA. FASEB J. 17:152-162. [DOI] [PubMed] [Google Scholar]
- 25.Feng, J., W. D. Funk, S. S. Wang, S. L. Weinrich, A. A. Avilion, C. P. Chiu, R. R. Adams, E. Chang, R. C. Allsopp, J. Yu, et al. 1995. The RNA component of human telomerase. Science 269:1236-1241. [DOI] [PubMed] [Google Scholar]
- 26.Fu, B., J. Quintero, and C. C. Baker. 2003. Keratinocyte growth conditions modulate telomerase expression, senescence, and immortalization by human papillomavirus type 16 E6 and E7 oncogenes. Cancer Res. 63:7815-7824. [PubMed]
- 27.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galloway, D. A., and J. K. McDougall. 1996. The disruption of cell cycle checkpoints by papillomavirus oncoproteins contributes to anogenital neoplasia. Semin. Cancer Biol. 7:309-315. [DOI] [PubMed] [Google Scholar]
- 29.Gatei, M., D. Shkedy, K. K. Khanna, T. Uziel, Y. Shiloh, T. K. Pandita, M. F. Lavin, and G. Rotman. 2001. Ataxia-telangiectasia: chronic activation of damage-responsive functions is reduced by alpha-lipoic acid. Oncogene 20:289-294. [DOI] [PubMed] [Google Scholar]
- 30.Gewin, L., and D. A. Galloway. 2001. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J. Virol. 75:7198-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard, P. M., E. Riballo, A. C. Begg, A. Waugh, and P. A. Jeggo. 2002. Nbs1 promotes ATM dependent phosphorylation events including those required for G1/S arrest. Oncogene 21:4191-4199. [DOI] [PubMed] [Google Scholar]
- 32.Hackett, J. A., and C. W. Greider. 2002. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene 21:619-626. [DOI] [PubMed] [Google Scholar]
- 33.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400:464-468. [DOI] [PubMed] [Google Scholar]
- 34.Hahn, W. C., and R. A. Weinberg. 2002. Modelling the molecular circuitry of cancer. Nat. Rev. Cancer 2:331-341. [DOI] [PubMed] [Google Scholar]
- 35.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1991. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J. Virol. 65:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 37.Hande, M. P., E. Samper, P. Lansdorp, and M. A. Blasco. 1999. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J. Cell Biol. 144:589-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henson, J. D., A. A. Neumann, T. R. Yeager, and R. R. Reddel. 2002. Alternative lengthening of telomeres in mammalian cells. Oncogene 21:598-610. [DOI] [PubMed] [Google Scholar]
- 39.Hickman, E. S., S. Bates, and K. H. Vousden. 1997. Perturbation of the p53 response by human papillomavirus type 16 E7. J. Virol. 71:3710-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirao, A., A. Cheung, G. Duncan, P. M. Girard, A. J. Elia, A. Wakeham, H. Okada, T. Sarkissian, J. A. Wong, T. Sakai, E. De Stanchina, R. G. Bristow, T. Suda, S. W. Lowe, P. A. Jeggo, S. J. Elledge, and T. W. Mak. 2002. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol. Cell. Biol. 22:6521-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holt, S. E., V. V. Glinsky, A. B. Ivanova, and G. V. Glinsky. 1999. Resistance to apoptosis in human cells conferred by telomerase function and telomere stability. Mol. Carcinog. 25:241-248. [PubMed] [Google Scholar]
- 42.Holt, S. E., and J. W. Shay. 1999. Role of telomerase in cellular proliferation and cancer. J. Cell. Physiol. 180:10-18. [DOI] [PubMed] [Google Scholar]
- 43.Hudson, J. B., M. A. Bedell, D. J. McCance, and L. A. Laiminis. 1990. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J. Virol. 64:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huibregtse, J. M., M. Scheffner, and P. M. Howley. 1991. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 10:4129-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huibregtse, J. M., M. Scheffner, and P. M. Howley. 1993. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol. Cell. Biol. 13:775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huibregtse, J. M., M. Scheffner, and P. M. Howley. 1993. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol. Cell. Biol. 13:4918-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang, X. R., G. Jimenez, E. Chang, M. Frolkis, B. Kusler, M. Sage, M. Beeche, A. G. Bodnar, G. M. Wahl, T. D. Tlsty, and C. P. Chiu. 1999. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 21:111-114. [DOI] [PubMed] [Google Scholar]
- 48.Jones, D. L., R. M. Alani, and K. Munger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones, D. L., and K. Munger. 1996. Interactions of the human papillomavirus E7 protein with cell cycle regulators. Semin. Cancer Biol. 7:327-337. [DOI] [PubMed] [Google Scholar]
- 50.Karlseder, J., D. Broccoli, Y. Dai, S. Hardy, and T. de Lange. 1999. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283:1321-1325. [DOI] [PubMed] [Google Scholar]
- 51.Karlseder, J., A. Smogorzewska, and T. de Lange. 2002. Senescence induced by altered telomere state, not telomere loss. Science 295:2446-2449. [DOI] [PubMed] [Google Scholar]
- 52.Khanna, K. K., M. F. Lavin, S. P. Jackson, and T. D. Mulhern. 2001. ATM, a central controller of cellular responses to DNA damage. Cell Death Differ. 8:1052-1065. [DOI] [PubMed] [Google Scholar]
- 53.Khleif, S. N., J. DeGregori, C. L. Yee, G. A. Otterson, F. J. Kaye, J. R. Nevins, and P. M. Howley. 1996. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc. Natl. Acad. Sci. USA 93:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 55.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 56.Klingelhutz, A. J., S. A. Foster, and J. K. McDougall. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79-82. [DOI] [PubMed] [Google Scholar]
- 57.Land, H., L. F. Parada, and R. A. Weinberg. 1983. Cellular oncogenes and multistep carcinogenesis. Science 222:771-778. [DOI] [PubMed] [Google Scholar]
- 58.Latre, L., L. Tusell, M. Martin, R. Miro, J. Egozcue, M. A. Blasco, and A. Genesca. 2003. Shortened telomeres join to DNA breaks interfering with their correct repair. Exp. Cell Res. 287:282-288. [DOI] [PubMed] [Google Scholar]
- 59.Lee, Y., M. J. Chong, and P. J. McKinnon. 2001. Ataxia telangiectasia mutated-dependent apoptosis after genotoxic stress in the developing nervous system is determined by cellular differentiation status. J. Neurosci. 21:6687-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu, Y., J. J. Chen, Q. Gao, S. Dalal, Y. Hong, C. P. Mansur, V. Band, and E. J. Androphy. 1999. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J. Virol. 73:7297-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lundberg, A. S., W. C. Hahn, P. Gupta, and R. A. Weinberg. 2000. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 12:705-709. [DOI] [PubMed] [Google Scholar]
- 62.Maser, R. S., and R. A. DePinho. 2003. Take care of your chromosomes lest cancer take care of you. Cancer Cell 3:4-6. [DOI] [PubMed] [Google Scholar]
- 63.McMurray, H. R., and D. J. McCance. 2003. Human papillomavirus type 16 E6 activates TERT gene transcription through induction of c-Myc and release of USF-mediated repression. J. Virol. 77:9852-9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMurray, H. R., D. Nguyen, T. F. Westbrook, and D. J. McCance. 2001. Biology of human papillomaviruses. Int. J. Exp. Pathol. 82:15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mihara, M., S. Erster, A. Zaika, O. Petrenko, T. Chittenden, P. Pancoska, and U. M. Moll. 2003. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11:577-590. [DOI] [PubMed] [Google Scholar]
- 66.Miyakoda, M., K. Suzuki, S. Kodama, and M. Watanabe. 2002. Activation of ATM and phosphorylation of p53 by heat shock. Oncogene 21:1090-1096. [DOI] [PubMed] [Google Scholar]
- 67.Moll, U. M., and A. Zaika. 2001. Nuclear and mitochondrial apoptotic pathways of p53. FEBS Lett. 493:65-69. [DOI] [PubMed] [Google Scholar]
- 68.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Munger, K. 2002. The role of human papillomaviruses in human cancers. Front. Biosci. 7:d641-d649. [DOI] [PubMed] [Google Scholar]
- 70.Munger, K., and P. M. Howley. 2002. Human papillomavirus immortalization and transformation functions. Virus Res. 89:213-228. [DOI] [PubMed] [Google Scholar]
- 71.Munger, K., W. C. Phelps, V. Bubb, P. M. Howley, and R. Schlegel. 1989. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 63:4417-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oh, S. T., S. Kyo, and L. A. Laimins. 2001. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J. Virol. 75:5559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Hagan, R. C., S. Chang, R. S. Maser, R. Mohan, S. E. Artandi, L. Chin, and R. A. DePinho. 2002. Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell 2:149-155. [DOI] [PubMed] [Google Scholar]
- 75.Pandita, T. K. 2002. ATM function and telomere stability. Oncogene 21:611-618. [DOI] [PubMed] [Google Scholar]
- 76.Pandita, T. K. 2001. The role of ATM in telomere structure and function. Radiat. Res. 156:642-647. [DOI] [PubMed] [Google Scholar]
- 77.Pandita, T. K., and S. Dhar. 2000. Influence of ATM function on interactions between telomeres and nuclear matrix. Radiat. Res. 154:133-139. [DOI] [PubMed] [Google Scholar]
- 78.Pandita, T. K., H. B. Lieberman, D. S. Lim, S. Dhar, W. Zheng, Y. Taya, and M. B. Kastan. 2000. Ionizing radiation activates the ATM kinase throughout the cell cycle. Oncogene 19:1386-1391. [DOI] [PubMed] [Google Scholar]
- 79.Pei, X. F., L. Sherman, Y. H. Sun, and R. Schlegel. 1998. HPV-16 E7 protein bypasses keratinocyte growth inhibition by serum and calcium. Carcinogenesis 19:1481-1486. [DOI] [PubMed] [Google Scholar]
- 80.Ramirez, R. D., B. S. Herbert, M. B. Vaughan, Y. Zou, K. Gandia, C. P. Morales, W. E. Wright, and J. W. Shay. 2003. Bypass of telomere-dependent replicative senescence (M1) upon overexpression of Cdk4 in normal human epithelial cells. Oncogene 22:433-444. [DOI] [PubMed] [Google Scholar]
- 81.Ramirez, R. D., C. P. Morales, B. S. Herbert, J. M. Rohde, C. Passons, J. W. Shay, and W. E. Wright. 2001. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rheinwald, J. G., W. C. Hahn, M. R. Ramsey, J. Y. Wu, Z. Guo, H. Tsao, M. De Luca, C. Catricala, and K. M. O'Toole. 2002. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell. Biol. 22:5157-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruesch, M. N., and L. A. Laimins. 1997. Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity. J. Virol. 71:5570-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saito, S., A. A. Goodarzi, Y. Higashimoto, Y. Noda, S. P. Lees-Miller, E. Appella, and C. W. Anderson. 2002. ATM mediates phosphorylation at multiple p53 sites, including Ser(46), in response to ionizing radiation. J. Biol. Chem. 277:12491-12494. [DOI] [PubMed] [Google Scholar]
- 85.Samper, E., F. A. Goytisolo, P. Slijepcevic, P. P. van Buul, and M. A. Blasco. 2000. Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang. EMBO Rep. 1:244-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 87.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 88.Sedman, S. A., N. L. Hubbert, W. C. Vass, D. R. Lowy, and J. T. Schiller. 1992. Mutant p53 can substitute for human papillomavirus type 16 E6 in immortalization of human keratinocytes but does not have E6-associated trans-activation or transforming activity. J. Virol. 66:4201-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seger, Y. R., M. Garcia-Cao, S. Piccinin, C. L. Cunsolo, C. Doglioni, M. A. Blasco, G. J. Hannon, and R. Maestro. 2002. Transformation of normal human cells in the absence of telomerase activation. Cancer Cell 2:401-413. [DOI] [PubMed] [Google Scholar]
- 90.Sharma, G. G., A. Gupta, H. Wang, H. Scherthan, S. Dhar, V. Gandhi, G. Iliakis, J. W. Shay, C. S. Young, and T. K. Pandita. 2003. hTERT associates with human telomeres and enhances genomic stability and DNA repair. Oncogene 22:131-146. [DOI] [PubMed] [Google Scholar]
- 91.Sharpless, N. E., and R. A. DePinho. 2002. p53: good cop/bad cop. Cell 110:9-12. [DOI] [PubMed] [Google Scholar]
- 92.Shay, J. W., Y. Zou, E. Hiyama, and W. E. Wright. 2001. Telomerase and cancer. Hum. Mol. Genet. 10:677-685. [DOI] [PubMed] [Google Scholar]
- 93.Shirasawa, H., M. H. Jin, K. Shimizu, N. Akutsu, Y. Shino, and B. Simizu. 1994. Transcription-modulatory activity of full-length E6 and E6*I proteins of human papillomavirus type 16. Virology 203:36-42. [DOI] [PubMed] [Google Scholar]
- 94.Smogorzewska, A., and T. de Lange. 2002. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 21:4338-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smogorzewska, A., J. Karlseder, H. Holtgreve-Grez, A. Jauch, and T. de Lange. 2002. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr. Biol. 12:1635-1644. [DOI] [PubMed] [Google Scholar]
- 96.Smogorzewska, A., B. van Steensel, A. Bianchi, S. Oelmann, M. R. Schaefer, G. Schnapp, and T. de Lange. 2000. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 20:1659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song, S., G. A. Gulliver, and P. F. Lambert. 1998. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc. Natl. Acad. Sci. USA 95:2290-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sprague, D. L., S. L. Phillips, C. J. Mitchell, K. L. Berger, M. Lace, L. P. Turek, and A. J. Klingelhutz. 2002. Telomerase activation in cervical keratinocytes containing stably replicating human papillomavirus type 16 episomes. Virology 301:247-254. [DOI] [PubMed] [Google Scholar]
- 99.Stansel, R. M., T. de Lange, and J. D. Griffith. 2001. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 20:5532-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stoppler, H., D. P. Hartmann, L. Sherman, and R. Schlegel. 1997. The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J. Biol. Chem. 272:13332-13337. [DOI] [PubMed] [Google Scholar]
- 101.Stubenrauch, F., and L. A. Laimins. 1999. Human papillomavirus life cycle: active and latent phases. Semin. Cancer Biol. 9:379-386. [DOI] [PubMed] [Google Scholar]
- 102.Tsutsui, T., S. Kumakura, A. Yamamoto, H. Kanai, Y. Tamura, T. Kato, M. Anpo, H. Tahara, and J. C. Barrett. 2002. Association of p16INK4a and pRb inactivation with immortalization of human cells. Carcinogenesis 23:2111-2117. [DOI] [PubMed] [Google Scholar]
- 103.Turenne, G. A., P. Paul, L. Laflair, and B. D. Price. 2001. Activation of p53 transcriptional activity requires ATM's kinase domain and multiple N-terminal serine residues of p53. Oncogene 20:5100-5110. [DOI] [PubMed] [Google Scholar]
- 104.Van, P. L., K. W. Yim, D. Y. Jin, G. Dapolito, A. Kurimasa, and K. T. Jeang. 2001. Genetic evidence of a role for ATM in functional interaction between human T-cell leukemia virus type 1 Tax and p53. J. Virol. 75:396-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Steensel, B., A. Smogorzewska, and T. de Lange. 1998. TRF2 protects human telomeres from end-to-end fusions. Cell 92:401-413. [DOI] [PubMed] [Google Scholar]
- 106.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 107.Veldman, T., I. Horikawa, J. C. Barrett, and R. Schlegel. 2001. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 75:4467-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veldman, T., X. Liu, H. Yuan, and R. Schlegel. 2003. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc. Natl. Acad. Sci. USA 100:8211-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 110.Xie, S., Q. Wang, H. Wu, J. Cogswell, L. Lu, M. Jhanwar-Uniyal, and W. Dai. 2001. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J. Biol. Chem. 276:36194-36199. [DOI] [PubMed] [Google Scholar]
- 111.Ye, R., A. Bodero, B. B. Zhou, K. K. Khanna, M. F. Lavin, and S. P. Lees-Miller. 2001. The plant isoflavenoid genistein activates p53 and Chk2 in an ATM-dependent manner. J. Biol. Chem. 276:4828-4833. [DOI] [PubMed] [Google Scholar]
- 112.zur Hausen, H. 1991. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology 184:9-13. [DOI] [PubMed] [Google Scholar]