Abstract
Background and Purpose
Collaterals at angiography before endovascular therapy were analyzed to ascertain the effect on a novel end point of successful revascularization without symptomatic hemorrhage in the Solitaire FR With the Intention for Thrombectomy (SWIFT) study.
Methods
Collateral grade (American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology) on baseline angiography was independently assessed, blind to other data, with statistical analyses delineating the relationship with clinical, laboratory, and imaging parameters.
Results
Angiographic data on collaterals were available in 119 of 144 subjects (mean age, 67±12 years; 52% woman; median National Institutes of Health Stroke Scale, 18 [range, 8–28]). Worse collaterals were noted in subjects with elevated baseline blood glucose (P=0.013) and those with elevated baseline systolic blood pressure (P=0.039). Multivariate predictors of partial or worse collaterals included absence of prior hypertension (odds ratio, 4.049, P=0.012), smoking history (odds ratio, 3.822; P=0.013), and higher blood glucose (odds ratio, 1.017; P=0.022). Collaterals were strongly related to Alberta Stroke Program Early CT Score (ASPECTS) at baseline (0–1: median 8 [3–10]; 2–9 [5–10]; 3–9 [7–10]; 4–9 [8–10]; P<0.001) and 24 hours (0–1: median 1 [0–5]; 2–6 [0–10]; 3–8 [0–10]; 4–8 [4–8]; P<0.001). Better collaterals were linked with Thrombolysis in Cerebral Infarction 2b/3 reperfusion (P=0.019), better median National Institutes of Health Stroke Scale at day 7/discharge (P<0.001), and better day 90 modified Rankin Scale (P<0.001). Better collateral grade was associated with successful revascularization without symptomatic hemorrhage, mean 2.3 (95% confidence interval, 2.1–2.5) versus 1.9 (95% confidence interval, 1.7–2.2), P=0.021.
Conclusions
Better collaterals were associated with lower glucose, lower blood pressure, smaller baseline infarcts in SWIFT, and greater likelihood of successful revascularization without hemorrhage and good clinical outcomes.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01054560.
Keywords: collaterals, endovascular therapy, stroke, revascularization
The extent of collateral perfusion is a potent determinant of recanalization and tissue fate after endovascular therapy for acute ischemic stroke.1 Collaterals at angiography have been associated with more extensive reperfusion, smaller infarcts, and less hemorrhagic transformation.2,3 Collateral circulation balances the hemodynamic impairment caused by arterial obstruction, yet the extent of collateral perfusion may vary considerably across individuals.4 Systematic evaluation of collaterals has revealed potential associations with age and medical comorbidities, yet prediction of collateral grade based on such clinical factors alone without definitive imaging may not be possible.5,6 Collateral grade may be routinely assessed at angiography before endovascular therapy, potentially enhancing prediction of subsequent revascularization and clinical outcomes.7 Prior analyses of collaterals, however, have demonstrated an association with recanalization after thrombolysis and mechanical thrombectomy with early generation devices, yet it remains unknown whether collaterals affect revascularization outcomes with stentriever technology.2,3,8,9 These devices have exhibited markedly increased recanalization rates, potentially reversing the effect of collaterals on recanalization.10,11 Collaterals may distinctly alter reperfusion without symptomatic hemorrhagic transformation, a physiological and clinically relevant end point recently used to define successful revascularization with stentriever devices.
We analyzed angiographic collateral grade before endovascular therapy in the Solitaire FR With the Intention for Thrombectomy (SWIFT) study to ascertain the potential effect of collaterals on the novel end point of successful revascularization without symptomatic hemorrhage, a metric of unqualified success with stentriever use.11
Methods
The SWIFT study was a randomized safety and efficacy study comparing use of the Merci device with the solitaire FR stentriever for arterial recanalization without hemorrhagic transformation in the setting of acute ischemic stroke.11 Detailed methods and results of this study have been previously published.11,12 In brief, patients were randomized to mechanical thrombectomy with Merci or solitaire FR within 8 hours of symptom onset after baseline imaging that excluded the presence of hemorrhage. No imaging or angiographic criteria were used to identify potential study candidates other than absence of extensive ischemia. Collateral grade was not prespecified or collected as part of the core laboratory adjudication process.
Post hoc evaluation of collateral grade on baseline angiography was conducted in our study, using the angiography archive established by the core laboratory. Two experienced readers, including a neuroradiologist and vascular neurologist with stroke imaging expertise, reviewed baseline angiography in all subjects enrolled in SWIFT, scored by consensus of the 2 readers. All diagnostic runs were evaluated for the presence of adequate information on collateral circulation with respect to the arterial occlusive lesion. Because cerebral angiograms documenting collaterals were not mandated as part of trial protocol, variability was noted from case to case in the completeness of such information. Collateral grades before endovascular treatment were assessed with the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology scale on angiography, blind to all other data.7 The American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology grading system is a 5-point scale: with 0=no collaterals visible to the ischemic site, 1=slow collaterals to the periphery of the ischemic site with persistence of some of the defect, 2=rapid collaterals to the periphery of the ischemic site with persistence of some of the defect and to only a portion of the ischemic territory, 3=collaterals with slow but complete angiographic blood flow of the ischemic bed by late venous phase, and 4=complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion. Use of this grading system and scale metrics has been previously reported.13 Cases with insufficient information on collateral status before treatment were excluded from subsequent analyses. These 2 readers also evaluated angiographic measures of recanalization and reperfusion. Recanalization of the arterial occlusive lesion was scored with the arterial occlusive lesion (AOL) score.13 Reperfusion of the corresponding arterial territory was scored with the Thrombolysis in Cerebral Infarction (TICI) scale, using 2/3 as the threshold for achieving reperfusion grade 2b or higher.7 Angiographic measures of collaterals, AOL recanalization, and TICI reperfusion were independently determined with disagreements resolved by consensus, blind to all other trial data. Alberta Stroke Program Early CT Score (ASPECTS) was scored on the computed tomography or MRI acquired immediately before treatment and on the required 24-hour imaging as previously reported.14
Statistical analyses were conducted by the SWIFT statisticians using clinical variables obtained from the main data set with angiography measures of collateral, AOL, and TICI scores obtained as part of this post hoc study. Clinical outcomes considered were symptomatic intracranial hemorrhage and functional independence at 90 days, defined as a modified Rankin Scale of 0, 1, or 2. Collateral score was treated as a categorical variable. Association of collateral grade with baseline characteristics and vascular and clinical outcomes was assessed via Fisher exact test for categorical variables and Kruskal–Wallis test for continuous variables. Baseline characteristics included comorbid conditions, demographics, and location and severity of stroke. Vascular outcomes were angiographic recanalization, defined as AOL score of 2 or 3, and angiographic reperfusion, defined as TICI of 2b or 3. Logistic regression was used to model outcome as a function of collateral grade and covariates selected using backward selection methodology. Baseline variables potentially associated with outcome (P<0.2) were considered for inclusion in the model. A significance level of P<0.05 was used to identify significant predictors of clinical outcomes, and odds ratios and 95% confidence intervals were estimated for each collateral grade.
Results
Angiographic data on collaterals were available in 119 of 144 subjects (mean age, 67±12 years; 52% woman; median National Institutes of Health Stroke Scale (NIHSS), 18 [range, 8–28]). Information on collaterals was not provided in 17 of 40 (42.5%) of internal carotid artery occlusions, 4 of 83 (4.8%) of proximal middle cerebral artery or M1 occlusions, and 4 of 4 (100%) of posterior circulation occlusions. Insufficient collateral data in these cases were because of omission of injections of potential collateral routes or limited temporal information without filming of late venous phase images. Collateral grade was 0 to 1 (none or marginal) in 32 (27%), 2 (partial) in 48 (40%), 3 (complete but delayed) in 35 (29%), and 4 (complete and early) in 4 (3%). Figure 1 reveals this diverse spectrum of collateral grades noted in SWIFT.
Figure 1.
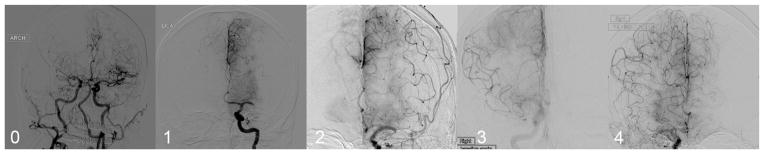
Examples of the broad distribution of American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology collateral grades noted in Solitaire FR With the Intention for Thrombectomy. Angiographic collaterals are demonstrated in 5 different cases: revealing grade 0 or no collaterals in right MCA occlusion, marginal or grade 1 collaterals in left MCA occlusion, grade 2 or partial collaterals in left MCA occlusion, grade 3 or slow but complete collaterals in right middle cerebral artery (MCA) occlusion, and grade 4 or rapid and complete collaterals in right MCA occlusion. ARCH indicates aortic arch injection; and LICA, left internal carotid artery.
Collateral grade or vigor was unrelated to age or sex of the subject. Elevated baseline blood glucose was noted with worse collaterals (P=0.013), with marginal or no collaterals (grade 0–1) revealing mean 154±65 mg/dL, partial collaterals (grade 2), mean 140±75 mg/dL; slow but complete collaterals (grade 3), mean 124±33 mg/dL; and complete, rapid collaterals (grade 4), mean 99±10 mg/dL. Similarly, elevated baseline systolic blood pressure was also associated with worse collaterals (P=0.039), marginal or no collaterals (grade 0–1) revealing mean 149±24 mm Hg, partial collaterals (grade 2) mean 137±23 mm Hg, slow but complete collaterals (grade 3) mean 137±24 mm Hg, and complete, rapid collaterals (grade 4) mean 138±10 mm Hg. Multivariate predictors of partial or worse collaterals included absence of prior hypertension (odds ratio, 4.049; P=0.012), smoking history (odds ratio, 3.822; P=0.013), and higher blood glucose (odds ratio, 1.017; P=0.022). Table I in the online-only Data Supplement illustrates the relationship of all variables with good clinical outcome in univariate and multivariate analyses. Subset analyses about the effect of collaterals were performed based on treatment modality and arterial occlusion site, with details in Table II in the online-only Data Supplement (Solitaire), Table III in the online-only Data Supplement (Merci), and Table IV in the online-only Data Supplement (middle cerebral artery occlusion).
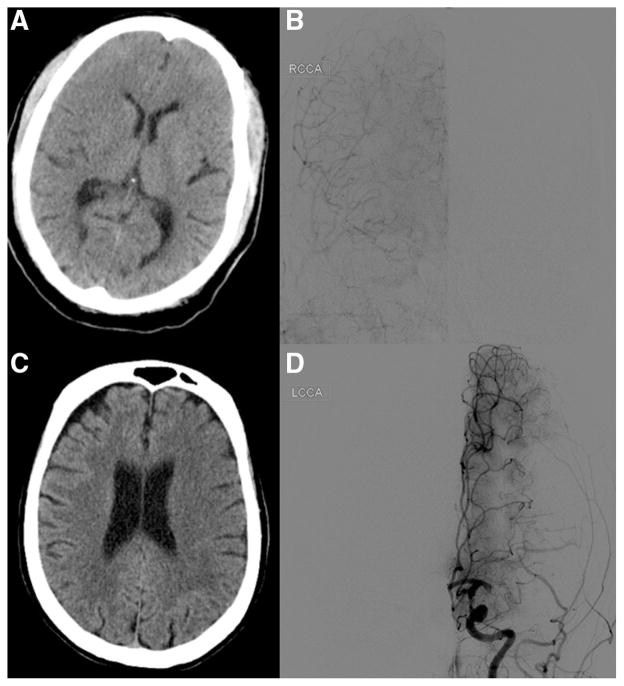
ASPECTS were evaluated at baseline in all 119 SWIFT subjects with collateral data and at 24 hours in 118 of 119 (99%) subjects. Because lower ASPECTS may indicate greater extent or severity of ischemia and worse collaterals, we analyzed ASPECTS at baseline with angiographic collateral grade. Collaterals were strongly related to ASPECTS at baseline (0–1: median 8 [3–10]; 2–9 [5–10]; 3–9 [7–10]; 4–9 [8–10]; P<0.001). Because the extent of collaterals at angiography may also indicate potential tissue fate, we analyzed collateral grade with 24-hour ASPECTS. Collaterals were strongly related to ASPECTS at 24 hours (0–1: median 1 [0–5]; 2–6 [0–10]; 3–8 [0–10]; 4–8 [4–8]; P<0.001). Figure 2 reveals an example where robust collaterals are associated with limited ischemic injury and another case where poor collaterals are linked with tissue fate of extensive infarction. Partial or worse collaterals were also associated with symptomatic hemorrhage (P=0.075).
Figure 2.
Alberta Stroke Program Early CT Score (ASPECTS) and collateral grade correlation in case examples. In right middle cerebral artery occlusion, preserved ASPECTS (A) is associated with robust or grade 4 collaterals (B). In a left middle cerebral artery occlusion, decreased ASPECTS (C) is associated with marginal or grade 1 collaterals (D). LCCA indicates left common carotid artery; and RCCA, right common carotid artery.
The relationship of collaterals with revascularization, including AOL recanalization and TICI reperfusion, revealed an interesting divergence. Failed recanalization (AOL, 0–1) with no downstream flow occurred in 7 subjects with grade 0 to 1 collaterals, 8 with grade 2, 1 with grade 3, and 0 with grade 4 collaterals. The extent of AOL recanalization, however, varied considerably, with the full spectrum of AOL recanalization demonstrated even in those with no or marginal collaterals (grade 0–1). Better collaterals were closely linked with TICI 2b/3 reperfusion (P=0.019).
Collaterals were strongly associated with clinical outcomes in SWIFT, including better median NIHSS at day 7/discharge (P<0.001) and better day 90 modified Rankin Scale (P<0.001). Using the combined definition of successful revascularization without symptomatic hemorrhage, better collaterals were an important indicator of subsequent outcomes, with mean collateral grade 2.3 (95% confidence interval, 2.1–2.5) versus 1.9 (95% confidence interval, 1.7–2.2), P=0.021. Figure 3 shows examples including poor collaterals associated with hemorrhagic transformation after recanalization and another case where robust collaterals were associated with full reperfusion without hemorrhage.
Figure 3.
Poor collaterals in a case of left middle cerebral artery occlusion (A) is followed by extensive ischemia and hemorrhagic transformation (B) after recanalization with marginal reperfusion. In another case of left middle cerebral artery occlusion, robust collaterals (C) is followed by complete or Thrombolysis in Cerebral Infarction 3 reperfusion (D) and good clinical outcome. LCCA indicates left common carotid artery.
Discussion
Collaterals were a pivotal factor in the revascularization and clinical outcomes of subjects enrolled in the SWIFT study, reinforcing the growing literature on collateral perfusion as a key variable in acute ischemic stroke.15,16 Collateral grade could be scored in the majority of SWIFT cases based solely on routine angiography acquired before endovascular therapy, despite lack of a formal protocol to acquire such information. In almost all middle cerebral artery occlusions, collaterals within the territory and from the ipsilateral anterior and posterior cerebral arteries could be demonstrated, except in cases where acquisition failed to include late venous phase images. In contrast, routine angiography runs of collaterals were available in only half of internal carotid artery occlusions and none of posterior circulation occlusions, where selective injections of contralateral or additional vessels are required. Our findings on the effect of collaterals suggest that acquiring such information on collaterals from selective injections or even noninvasive techniques before endovascular therapy may be important. The distribution of collateral grade in SWIFT, an endovascular therapy trial during the earliest stages of ischemia, reveals a relatively balanced mixture between those with none or poor collaterals, partial collaterals, and those with complete collateral filling of the territory. This finding underscores the marked heterogeneity of stroke pathophysiology across individuals, despite use of straightforward selection criteria.17
Our findings that link poor collateral flow with elevated blood glucose or hyperglycemia and elevated systolic blood pressure provide novel perspective on these routine parameters acquired in acute stroke that have been linked with worse outcomes.18,19 A graded association of lower blood glucose and blood pressure values was noted with better collateral flow, yet the relationship was largely driven by the acute hyperglycemia and hypertension noted in cases with poor collaterals. These findings warrant further investigation and suggest that acute hyperglycemia and hypertension in acute ischemic stroke may be indirect markers of poor collaterals, portending more extensive ischemia and worse outcomes after intervention. Interestingly, we did not find an association of collateral grade with demographic variables, yet the absence of prior hypertension and smoking history was strongly linked with poor collateral flow. Absence of prior hypertension and elevated blood pressure in acute stroke may, therefore, be a particularly informative surrogate of poor collateral flow. Smoking history has been previously associated with the degree of collateral sufficiency in coronary and peripheral ischemia, including the presence of more developed collaterals, with functionally limited perfusion of the territory.20
The link between the overall degree of collateral flow with ASPECTS suggests that this rapid algorithm on baseline imaging may grossly distinguish marked differences in collateral grade. The relatively marginal differences in median ASPECTS values for each category of collateral grade, however, suggest that this scale may not discern subtle differences in collaterals and that alternative noninvasive imaging approaches may be warranted for selection of optimal acute stroke therapy candidates.21–23 Collateral grade was linked with ASPECTS scores on 24-hour imaging obtained after endovascular therapy, indicating that the extent of ischemic injury may also be because of variation in collaterals at presentation. Hemorrhagic transformation was related to the presence of partial or worse collaterals, confirming prior studies.3
Revascularization after endovascular therapy, including both recanalization and reperfusion, was related to the degree of collaterals. AOL recanalization varied widely, even when poor collateral flow was noted at baseline. Successful reperfusion, exceeding two thirds of the ischemic territory, was highly associated with the collateral grade. These findings imply that the technical success of opening an artery with endovascular therapy may be possible even when poor collaterals are present, yet the extent of downstream reperfusion may be driven by the degree of collaterals.
Most importantly, the clinical outcomes after endovascular therapy in SWIFT were strongly tied to baseline collaterals, readily available from angiography before intervention. Early measures of neurological impairment, subsequent disability, and the novel metric of successful revascularization without symptomatic hemorrhage pioneered in SWIFT were all driven by collateral grade, demonstrated to vary considerably across individuals. These decisive results from a recent endovascular study using current stentriever device technology highlight the importance of ascertaining the degree of collaterals before treatment, although randomized studies are warranted to validate the role of collaterals in a prospective fashion. Although such information may be easily obtained from angiography as evidenced in our study, noninvasive multimodal imaging that includes, but extends beyond, ASPECTS may be used in the future to select patients for endovascular therapy optimally.22,23
Limitations of our retrospective study include the potential omission of critical variables from the data set that may be informative about collateral flow and the relationship with other clinical and imaging parameters. In addition, post hoc evaluation of collaterals was limited by the availability of angiography data, including dedicated injection of potential collateral routes, image quality, and temporal resolution. Specifically, the lack of contralateral injections in internal carotid artery occlusions and anterior circulation runs in posterior circulation cases substantially limited our ability to comment further on these common arterial lesions treated with endovascular therapy. Although consensus readings by 2 experienced angiography readers were used, inherent defects of the ASITN/SIR scale may preclude further discrimination in collateral grade and inter-rater reliability was not determined. Furthermore, bias may have been introduced by the same expert reviewers determining both collateral grade and revascularization, although a prespecified protocol dictated baseline collateral adjudication followed by revascularization determination. The retrospective nature of our study is also clearly influenced by any potential selection biases.
Conclusions
The degree of collateral circulation varied markedly across subjects in the SWIFT study, readily discernible from routine angiography acquired before endovascular therapy. Poor collaterals were linked with acute hyperglycemia and hypertension, known markers of poor outcomes in acute stroke, establishing an important pathophysiologic tie between these routine parameters and collateral flow. Collaterals demonstrated a profound effect on successful revascularization without hemorrhage and myriad clinical outcomes after endovascular therapy.
Supplementary Material
Acknowledgments
We extend our gratitude for the efforts of the Solitaire FR With the Intention for Thrombectomy (SWIFT) Investigators.
Sources of Funding
This work has been funded by National Institutes of Health (NIH)–National Institute of Neurological Disorders and Stroke (NINDS) Awards NIH/NINDS P50NS044378, K24NS072272, R01NS077706, and R13NS082049.
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.114.004781/-/DC1.
Disclosures
Some authors (Drs Liebeskind, Jahan, and Saver) were employed by the University of California, which holds a patent on retriever devices for stroke, at the time of this work. Dr Liebeskind is a consultant of Advisory Board, Modest, Stryker, and Covidien. Dr Jahan is a member of Speakers’ Bureau, Modest, and Stryker and is a consultant of Advisory Board, Modest, and Covidien. Dr Nogueira is a consultant of Advisory Board, Modest, Stryker/Concentric Medical Inc, Covidien/ev3 Neurovascular Inc, CoAxia Inc, Penumbra Inc, Rapid Medical Inc, Reverse Medical Inc, and Neurointervention Inc. Dr Zaidat is a consultant for Penumbra, Stryker, Covidien, and Microvention. Dr Saver is supported by research grant National Institutes of Health/National Institute of Neurological Disorders and Stroke P50NS044378 and is a consultant of Advisory Board and Covidien.
References
- 1.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, et al. UCLA Collateral Investigators. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. UCLA-Samsung Stroke Collaborators. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- 4.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 5.Singer OC, Haring HP, Trenkler J, Nolte CH, Bohner G, Reich A, et al. Age dependency of successful recanalization in anterior circulation stroke: the ENDOSTROKE study. Cerebrovasc Dis. 2013;36:437–445. doi: 10.1159/000356213. [DOI] [PubMed] [Google Scholar]
- 6.Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. [published online ahead of print March 28, 2013] [Accessed January 3, 2014];Ann Neurol. 2013 doi: 10.1002/ana.23906. http://onlinelibrary.wiley.com/doi/10.1002/ana.23906/abstract. [DOI] [PMC free article] [PubMed]
- 7.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology; Technology Assessment Committee of the Society of Interventional Radiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 8.Roberts HC, Dillon WP, Furlan AJ, Wechsler LR, Rowley HA, Fischbein NJ, et al. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke. 2002;33:1557–1565. doi: 10.1161/01.str.0000018011.66817.41. [DOI] [PubMed] [Google Scholar]
- 9.Liebeskind DS, Tomsick TA, Yeatts S, Foster LD, Carrozella J, Jovin TG, et al. Collaterals in the interventional management of stroke (IMS) III trial. Stroke. 2013;44:AWP5. doi: 10.1161/STROKEAHA.113.004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 12.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira R, et al. Solitaire with the intention for thrombectomy (SWIFT) trial: design of a randomized, controlled, multicenter study comparing the solitaire flow restoration device and the merci retriever in acute ischaemic stroke. [published online ahead of print November 6, 2012] [Accessed January 3, 2014];Int J Stroke. 2012 doi: 10.1111/j.1747-4949.2012.00856.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1747-4949.2012.00856.x/abstract. [DOI] [PubMed]
- 13.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liebeskind DS, Jahan R, Nogueira RG, Jovin TG, Lutsep HL, Saver JL, et al. Serial ASPECTS from baseline to 24 hours in swift: a novel surrogate endpoint for revascularization in acute stroke. Stroke. 2014;45:723–727. doi: 10.1161/STROKEAHA.113.003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am. 2005;15:553–73. x. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Liebeskind DS. Collateral perfusion: time for novel paradigms in cerebral ischemia. Int J Stroke. 2012;7:309–310. doi: 10.1111/j.1747-4949.2012.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011;31:1836–1851. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putaala J, Sairanen T, Meretoja A, Lindsberg PJ, Tiainen M, Liebkind R, et al. Post-thrombolytic hyperglycemia and 3-month outcome in acute ischemic stroke. Cerebrovasc Dis. 2011;31:83–92. doi: 10.1159/000321332. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Yáñez M, Castellanos M, Blanco M, García MM, Nombela F, Serena J, et al. New-onset hypertension and inflammatory response/poor outcome in acute ischemic stroke. Neurology. 2006;67:1973–1978. doi: 10.1212/01.wnl.0000247064.53130.91. [DOI] [PubMed] [Google Scholar]
- 20.Koerselman J, de Jaegere PP, Verhaar MC, Grobbee DE, van der Graaf Y SMART Study Group. Coronary collateral circulation: the effects of smoking and alcohol. Atherosclerosis. 2007;191:191–198. doi: 10.1016/j.atherosclerosis.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Tong E, Hou Q, Fiebach JB, Wintermark M. The role of imaging in acute ischemic stroke. Neurosurg Focus. 2014;36:E3. doi: 10.3171/2013.10.FOCUS13396. [DOI] [PubMed] [Google Scholar]
- 22.Wintermark M, Albers GW, Broderick JP, Demchuk AM, Fiebach JB, Fiehler J, et al. Stroke Imaging Research (STIR) and Virtual International Stroke Trials Archive (VISTA)-Imaging Investigators. Acute Stroke Imaging Research Roadmap II. Stroke. 2013;44:2628–2639. doi: 10.1161/STROKEAHA.113.002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wintermark M, Sanelli PC, Albers GW, Bello JA, Derdeyn CP, Hetts SW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: a joint statement by the American Society of Neuroradiology, the American College of Radiology and the Society of NeuroInterventional Surgery. J Am Coll Radiol. 2013;10:828–832. doi: 10.1016/j.jacr.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.