Abstract
Background and Purpose
Several outcome prediction scores have been tested in patients receiving acute stroke treatment with previous generations of endovascular stroke treatment devices. The TREVO-2 trial was a randomized controlled trial comparing a novel endovascular stroke treatment device (the Trevo device) to a previous-generation endovascular stroke treatment device (the Merci device).
Methods
We used data from the TREVO-2 trial to validate the Totaled Health Risks in Vascular Events (THRIVE) score in patients receiving treatment with a third-generation endovascular stroke treatment device and to compare THRIVE to other predictive scores. We used logistic regression to model outcomes and compared score performance with receiver operating characteristic curve analysis.
Results
In the TREVO-2 trial, the THRIVE score strongly predicts clinical outcome and mortality. The relationship between THRIVE score and outcome is not influenced by either success of recanalization or the type of device used (Trevo versus Merci). The superiority of the Trevo device to the Merci device is evident particularly among patients with a low-to-moderate THRIVE score (0–5; 53.8% good outcome with Trevo versus 27.5% good outcome with Merci). In receiver operating characteristic curve analysis, the THRIVE score was comparable or superior to several other outcome prediction scores (HIAT, HIAT-2, SPAN-100, and iScore).
Conclusions
The THRIVE score strongly predicts clinical outcome and mortality in the TREVO-2 trial. Taken together with THRIVE validation data from patients receiving intravenous tissue-type plasminogen activator or no acute treatment, the THRIVE score has broad predictive power in patients with acute ischemic stroke, which is likely because THRIVE reflects a set of strong nonmodifiable predictors of stroke outcome. A free Web calculator for the THRIVE score is available at http://www.thrivescore.org.
Keywords: cerebral hemorrhage, forecasting, outcome assessment, stroke
Several ischemic stroke outcome scoring systems that predict ischemic stroke outcomes in patients undergoing endovascular stroke treatment (EST)1–4 have been developed and validated, and other scores predict stroke outcomes in patients receiving intravenous tissue-type plasminogen activator (tPA) or no acute treatment.5–10
The Totaled Health Risks in Vascular Events (THRIVE) score was one of the first ischemic stroke prediction tools developed.2 We originally developed the THRIVE score in the context of EST, using patients in the MERCI and Multi-MERCI trials.2 We subsequently validated the performance of the THRIVE score in EST using patients from the Merci Registry3 and then found that the THRIVE score performs equally well in patients receiving tPA or no acute stroke treatment, using data from the National Institute of Neurological Disorders and Stroke (NINDS) tPA trial9 and the Virtual International Stroke Trials Archive (VISTA).10
To date, no stroke outcome prediction scores have been tested in patients receiving the latest generation of EST devices, known as retrievable stents. These third-generation devices (the Trevo device and Solitaire device) have been shown to be superior to a previous-generation device (the Merci device) for both recanalization and clinical outcomes in 2 randomized controlled trials.11,12
Here, we examine the use of the THRIVE score in patients undergoing EST with either the Trevo device or the Merci device in the TREVO-2 trial. We examine the relationship between THRIVE and outcomes in TREVO-2, first among all patients, and then stratified by recanalization and treatment assignment. We then compare the performance of the THRIVE score to other clinical prediction scores in this setting.
Methods
Data Source and Subjects
We obtained demographic data, clinical data, 3-month functional outcomes on the modified Rankin Scale (mRS), and 3-month mortality from the TREVO-2 trial.11 TREVO-2 was a 178-patient randomized controlled trial of EST that compared outcomes among patients with large artery occlusion stroke treated with either the Trevo device or the Merci device, as previously presented in detail.11 Appropropriate institutional review board and regulatory approvals were obtained by participating centers in TREVO-2,11 and no data or protected health information was transmitted outside the trial databases to the investigators of this report. The TREVO-2 trial was registered with ClinicalTrials.gov, number NCT01270867.
Measurements
To calculate each patient’s THRIVE score, we noted their age, initial stroke severity as measured by the National Institutes of Health Stroke Scale (NIHSS) score, and the presence or absence of hypertension, diabetes mellitus, or atrial fibrillation. The THRIVE score assigns 1 point for age 60 to 79 years, 2 points for age ≥80 years, 2 points for NIHSS score 11 to 20, 4 points for NIHSS score ≥21, and 1 point each for hypertension, diabetes mellitus, and atrial fibrillation.2 Other clinical prediction scores (Houston Intra-Arterial Therapy [HIAT] score, HIAT-2, Stroke Prognostification using Age and NIH stroke scale [SPAN-100] and iScore) were calculated as follows: HIAT assigns 1 point each for age ≥75 years, NIHSS ≥18, and glucose ≥150 mg/dL.1 HIAT-2 assigns 2 points for age 60 to 79 years, 4 points for age ≥80 years, 1 point for NIHSS score 11 to 20, 2 points for NIHSS score ≥21, and 3 points for Alberta Stroke Program Early Computed Tomography Score ≤7.4 The SPAN-100 is a single-point score—the score is positive if the sum of the patient’s age and NIHSS is ≥100.7 For the iScore, the NIHSS was converted to Canadian Neurological Society (CNS) score according to the formula NIHSS=23–(2×CNS) with rounding to the nearest integer and a minimum score of zero, as previously described.13 The iScore (30-day version) was calculated as age in years+(10 points for men)+(105 points for CNS=0, 65 points for CNS=1–4, or 40 points for CNS=5–7)+(30 points for nonlacunar pathogenesis or 35 points for undetermined pathogenesis)+10 points for atrial fibrillation, +10 points for congestive heart failure, +10 points for cancer, +35 points for renal dialysis, +15 points for prestroke-dependent status, +15 points for admission glucose ≥135 mg/dL.8
Our outcomes measures were functional outcome on the mRS at 3 months (cut between a score of 2 and 3, such that good outcome is 0–2 and poor outcome is 3–6) and mortality by 3 months. Successful re-canalization was defined as a Thrombolysis in Cerebral Ischemia score of 2b or 3 at the completion of device use during the EST procedure.
Statistical Analysis
We analyzed categorical data in the contingency data with the Fisher exact test and continuous data with the nonparametric Wilcoxon rank-sum test. Logistic regression was performed using standard techniques to model good outcome (mRS, 0–2). Receiver operating characteristic (ROC) curves were constructed by plotting test sensitivity against (1–specificity). We compared pairwise score discrimination for 3-month outcomes by comparing the area under the curve for ROC curves using the χ2 statistic. In cases in which a particular score could only be calculated for a subset of patients in the total data set, only that subset of patients was used to perform the pairwise ROC curve comparison. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Role of the Funding Source
The original TREVO-2 trial was funded by Stryker Neurovascular, and the role of the funding source in the trial has been described in detail in the primary TREVO-2 article. The funding source had no role in the design of the current analysis or the writing of this article. Statistical work for the present study was performed by the second author, Dr Xiang of Prospect Analytical, on contract from Stryker Neurovascular for TREVO-2 statistics. Statistical analysis was performed in close consultation with the first author (A.C.F.).
Results
Patient Characteristics
Patient characteristics are summarized in Table 1, which displays patient age, NIHSS, comorbidities (hypertension, diabetes mellitus, and atrial fibrillation), and clinical prediction scores (THRIVE, HIAT, HIAT-2, Hemorrhage After Thrombolysis [HAT], SPAN-100, and iScore) according to assignment to the Trevo or Merci device.
Table 1.
Patient Characteristics
| Merci (n=88) | Trevo (n=90) | All (N=178) | P Value | |
|---|---|---|---|---|
| Age | 67.4±13.9 | 67.0±14.7 | 67.2±14.2 | 0.94 |
| NIHSS | 19 (14–22) | 18 (15–21) | 19 (15–21) | 0.65 |
| HTN | 76.1% (67/88) | 82.2% (74/90) | 79.2% (141/178) | 0.36 |
| DM | 37.5% (33/88) | 25.8% (23/89) | 31.6% (56/177) | 0.11 |
| AFib | 48.8% (42/86) | 42.7% (38/89) | 45.7% (80/175) | 0.45 |
| Women | 54.5% (48/88) | 60.0% (54/90) | 57.3% (102/178) | 0.54 |
| THRIVE score | 5 (4–6) | 5 (4–6) | 5 (4–6) | 0.60 |
| HIAT score | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.76 |
| HIAT-2 score | 5 (3–6.5) | 5 (3–6) | 5 (3–6) | 0.93 |
| SPAN-100 | 14.8% (13/88) | 21.1% (19/90) | 18.0% (32/178) | 0.33 |
| iScore | 178.5 (158.5–201) | 177 (158–194) | 177 (158–198) | 0.50 |
Age is presented as mean±SD. NIHSS and other ordinal scores are presented as median (interquartile range). Dichotomous values (comorbidities, sex, and SPAN-100) are presented as % (number out of total). Continuous data were compared by the nonparametric Wilcoxon rank-sum test, and categorical data were compared by Fisher exact test. AFib indicates atrial fibrillation; DM, diabetes mellitus; HIAT, Houston Intra-Arterial Therapy score; HTN, hypertension; NIHSS, National Institutes of Health Stroke Scale; SPAN-100, Stroke Prognostification using Age and NIH stroke scale; and THRIVE, Totaled Health Risks in Vascular Events.
THRIVE Score and Clinical Outcomes in TREVO-2
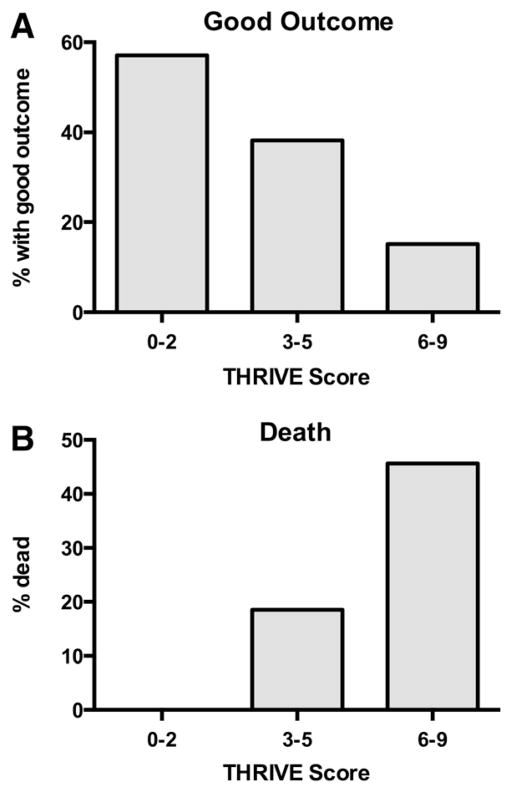
Increasing THRIVE score strongly predicts a decreasing chance of good outcome (mRS of 0–2 at 3 months; Figure 1A) and increased chance of death by 3 months (Figure 1B) among all patients in TREVO-2.
Figure 1.
Relationship between THRIVE score and clinical outcome and death in the TREVO-2 trial. A, Decreasing chances of good outcome (modified Rankin Scale [mRS], 0–2 at 3 months) with increasing levels of trichotomized THRIVE score (0–2, 3–5, 6–9). The good outcomes at each level are THRIVE 0 to 2, 57.1% (8/14); THRIVE 3 to 5, 38.2% (34/89); and THRIVE 6 to 9, 15.2% (10/66; P<0.001). B, Increasing chances of death by 3 months with increasing levels of trichotomized THRIVE score. The chance of death at each level is THRIVE 0 to 2, 0% (0/14); THRIVE 3 to 5, 18.5% (17/92); and THRIVE 6 to 9, 45.6% (31/68; P<0.001). THRIVE indicates Totaled Health Risks in Vascular Events.
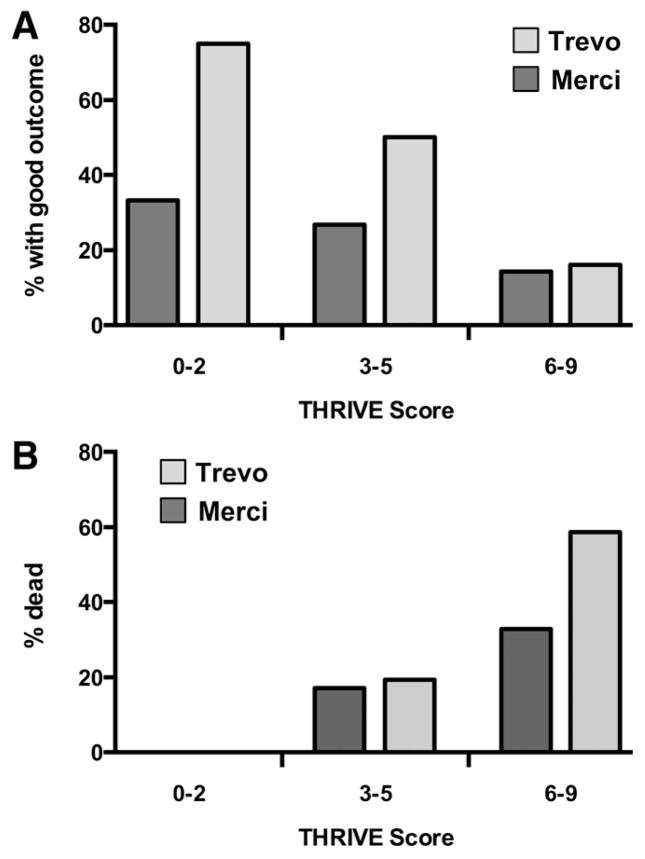
A similar relationship is seen when patients assigned to each device (Trevo versus Merci) are considered separately (Figure 2). Of note, there is a substantial difference in good outcomes between the Trevo and Merci device at low-to-moderate THRIVE scores (0–5; 53.8% versus 27.5% good outcome; P=0.009) but no detected difference in outcomes in the group with high THRIVE scores (6–9; 16.1% versus 14.3%; P=1.0; Figure 2A). Conversely, the overall increased mortality reported in the Trevo arm of TREVO-211 is found exclusively among patients with high THRIVE scores (6–9; 59.4% versus 33.3%; P=0.05) and not among patients with low-to-moderate THRIVE scores (0–5; 16.7% versus 15.4%; P=1.0; Figure 2B).
Figure 2.
THRIVE performance according to treatment assignment in TREVO-2 (Trevo device vs Merci device). A, Comparison of the rate of good outcome at 3 months at the 3 levels of trichotomized THRIVE score according to treatment assignment. In the Trevo device arm, good outcomes according to THRIVE level are THRIVE 0 to 2, 75% (6/8); THRIVE 3 to 5, 50% (22/44); and THRIVE 6 to 9, 16.1% (5/31). In the Merci device arm, good outcomes according to THRIVE level are THRIVE 0 to 2, 33.3% (2/6); THRIVE 3 to 5, 26.7% (12/45); and THRIVE 6 to 9, 14.3% (5/35). B, Comparison of the rate of death by 3 months at the 3 levels of trichotomized THRIVE score according to treatment assignment. In the Trevo device arm, rate of death according to THRIVE level is THRIVE 0 to 2, 0% (0/8); THRIVE 3 to 5, 19.6% (9/46); and THRIVE 6 to 9, 59.4% (19/32). In the Merci device arm, rate of death according to THRIVE level is THRIVE 0 to 2, 0% (0/6); THRIVE 3 to 5, 17.4% (8/46); and THRIVE 6 to 9, 33.3% (12/36). THRIVE indicates Totaled Health Risks in Vascular Events.
Independence of THRIVE Score and Recanalization or Treatment Assignment
We have previously found that THRIVE score predicts outcomes independent of recanalization therapy with intravenous tPA9,10 or recanalization success with EST.2,3 Specifically, the effect of THRIVE score is not modified by intravenous tPA administration or successful recanalization during EST, and the effect of intravenous tPA administration or successful recanalization on clinical outcomes is not modified by the patient’s THRIVE score.2,3,9,10
In TREVO-2, THRIVE score and recanalization are similarly independent and are without interaction (Table 2). The effect of THRIVE score on good outcome is similar in patients with or without successful recanalization (Table 2; models 1 and 2), and controlling for successful recanalization does not substantially alter the relationship between THRIVE score and good outcome (Table 2; models 3 and 4).
Table 2.
Independence of THRIVE Score and Recanalization
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Model 1: good outcome (among patients with successful recanalization) | |||
| THRIVE | 0.64 | 0.49–0.83 | 0.001 |
| Model 2: good outcome (among patients without successful recanalization) | |||
| THRIVE | 0.62 | 0.40–0.95 | 0.028 |
| Model 3: good outcome (all patients) | |||
| THRIVE | 0.62 | 0.50–0.77 | <0.001 |
| Model 4: good outcome (all patients) | |||
| THRIVE | 0.63 | 0.50–0.79 | <0.001 |
| Successful recanalization | 4.26 | 1.92–9.47 | <0.001 |
In models 1 and 2, THRIVE score predicts good outcome, stratified on successful recanalization (Thrombolysis In Cerebral Ischemia score 2b or 3). Model 1 includes only patients with successful recanalization, and model 2 includes only patients without successful recanalization. In models 3 and 4, all TREVO-2 patients are included. THRIVE and successful recanalization are each independent predictors of good outcome (model 4), and addition of successful recanalization to the model does not alter the relationship of THRIVE score and outcome (model 3 compared with model 4). CI indicates confidence interval; and THRIVE, Totaled Health Risks in Vascular Events.
The treatment assignment (Trevo device versus Merci device) also did not interact with the relationship between THRIVE score and outcome. The effect of THRIVE score on good outcome is similar in patients treated with the Trevo device and patients treated with the Merci device (Table 3; models 1 and 2). Controlling for the treatment arm (Trevo device versus Merci device) does not substantially alter the relationship between THRIVE score and good outcome (Table 3; models 3 and 4).
Table 3.
Independence of THRIVE Score and Treatment Arm (Trevo vs Merci)
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Model 1: good outcome (among patients treated with the Trevo device) | |||
| THRIVE | 0.50 | 0.35–0.71 | <0.001 |
| Model 2: good outcome (among patients treated with the Merci device) | |||
| THRIVE | 0.72 | 0.53–0.97 | 0.03 |
| Model 3: good outcome (all patients) | |||
| THRIVE | 0.62 | 0.50–0.77 | <0.001 |
| Model 4: good outcome (all patients) | |||
| THRIVE | 0.60 | 0.48–0.75 | <0.001 |
| Treatment Arm (Trevo vs Merci) | 2.91 | 1.39–6.08 | <0.001 |
In Models 1 and 2, THRIVE score predicts good outcome, stratified on treatment arm (Trevo vs Merci). Model 1 includes only patients assigned to the Trevo device, and model 2 includes only patients assigned to the Merci device. In Models 3 and 4, all TREVO-2 patients are included. THRIVE and treatment arm (Trevo device vs Merci device) are each independent predictors of good outcome (model 4), and controlling for treatment arm does not alter the relationship of THRIVE score and outcome (model 4 compared with model 3). CI indicates confidence interval; and THRIVE, Totaled Health Risks in Vascular Events.
THRIVE Score and Risk of Symptomatic Intracranial Hemorrhage
We have found that THRIVE score predicts the risk of intracranial hemorrhage (ICH) after tPA in the NINDS trial9 and in VISTA-Acute.10 We therefore explored whether THRIVE score predicts the risk of symptomatic ICH (sICH) among EST patients in TREVO-2. As shown in Table I in the online-only Data Supplement, no relationship between THRIVE score and sICH was found.
ROC Curve Analysis Comparing THRIVE With Other Outcome Prediction Scores
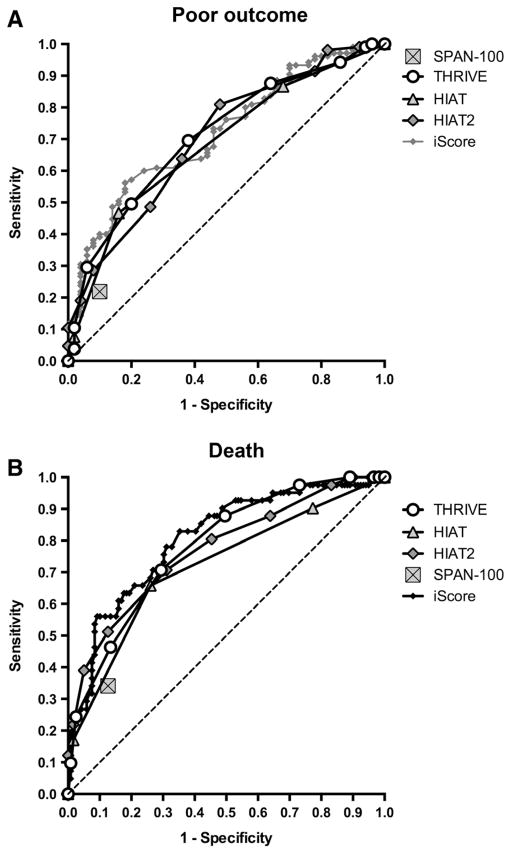
To better understand the relative use of available ischemic stroke outcome prediction scores, we used ROC curve analysis to compare the THRIVE Score to other scores for which sufficient data were available in TREVO-2 (HIAT, HIAT-2, SPAN-100, and iScore).
The ROC curves for the THRIVE score had a similar area under the curve in comparison to HIAT, HIAT-2, and iScore and were superior to the SPAN-100 (Figure 3A and 3B). For prediction of poor outcome at 3 months (mRS, 3–6), the THRIVE ROC area under the curve was 0.712, compared with 0.684 for HIAT (P=0.44), 0.703 for HIAT-2 (P=0.80), 0.719 for iScore (P=0.84), and 0.560 for SPAN-100 (P<0.001). For prediction of death by 3 months, the THRIVE ROC area under the curve was 0.780, compared with 0.718 for HIAT (P=0.06), 0.770 for HIAT-2 (P=0.79), 0.805 for iScore (P=0.35), and 0.608 for SPAN-100 (P<0.001).
Figure 3.
Comparison of THRIVE to other outcome prediction scores by receiver operating characteristic (ROC) curve analysis. A, ROC curves for score prediction of poor outcome at 3 months (modified Rankin Scale [mRS], 3–6), comparing THRIVE, HIAT, HIAT-2, iScore, and SPAN-100. Higher area-under-the-curve values in ROC analysis indicate better discrimination of the score for the measured outcome. SPAN-100 is represented by a single point because it is a dichotomous predictor. B, ROC curves for score prediction of death by 3 months (mRS, 0–2), comparing THRIVE, HAT, HIAT-2, iScore, and SPAN-100. HIAT indicates Houston Intra-Arterial Therapy score; ROC, receiver operating characteristic; SPAN-100, Stroke Prognostification using Age and NIH stroke scale; and THRIVE, Totaled Health Risks in Vascular Events.
Discussion
We find that the THRIVE score strongly predicts clinical outcomes among acute stroke patients treated with a third-generation EST device (the Trevo device) in the TREVO-2 trial. Among patients with a low-to-moderate THRIVE score (0–5), the superiority of the Trevo device versus the Merci device is particularly apparent. The performance of the THRIVE score in the TREVO-2 trial is similar to or superior to other predictive scores (HIAT, HIAT-2, SPAN-100, and iScore).
The THRIVE score has now been validated across all acute ischemic stroke treatment contexts: intravenous tPA treatment,9,10 EST,2,3 and no acute stroke treatment.9,10 In each of these contexts, the THRIVE score has been found to predict outcomes independent of recanalization therapy, such that there is no interaction between THRIVE and outcomes and between recanalization therapy and outcomes. It is likely that this consistent lack of interaction arises from the fact that the THRIVE score is composed of a set of nonmodifiable predictors of outcome.
The observation that the superiority of the Trevo device instead of the Merci device is particularly apparent among patients with a low-to-moderate THRIVE score (0–5) leads to the consideration that clinical trials of EST might benefit from using such a range of lower THRIVE scores as an inclusion criteria or prespecified subgroup analysis. To date, various approaches that might provide a greater benefit of EST to patients, such as patient selection based on advanced neuroimaging of the ischemic penumbra,14 have been used in EST trials. Although patient selection using advanced neuroimaging can identify a subset of patients with an extremely poor prognosis,14 initial attempts to use such techniques for randomized controlled trial patient selection have not yet led to improved outcomes.15 The possibility that the use of the THRIVE score may result in better discrimination of treatment options will require validation in future trials. Given the lack of statistical interaction between recanalization and THRIVE score, the greater magnitude of benefit of the more effective recanalization device at lower THRIVE scores is the result of the additive, but distinct, effects of THRIVE and recanalization on outcomes in these patients.
We also observed selective excess mortality with the Trevo device among patients with a high THRIVE score (6–9). This suggests that the imbalance in mortality seen in TREVO-211 may have resulted from enrollment of patients with high baseline risks because patients with a high THRIVE score have a high risk of mortality that is independent of recanalization therapy.2,3,9,10 Randomization of small numbers of subjects in a high baseline risk category should increase the odds of observing imbalances in outcomes that are unrelated to treatment assignment.
Although the THRIVE score predicts the risk of sICH after tPA,9,10 we do not find evidence in the TREVO-2 trial that THRIVE predicts sICH with either the Trevo or Merci device. Although this lack of an observed relationship may be because of limited sample size, it is consistent with a similar lack of relationship between THRIVE and sICH after use of the Merci device among patients in MERCI and Multi-MERCI trials and the Merci Registry (A.C.F., data on file). It seems likely that the THRIVE score predicts ICH after tPA because the components of THRIVE (age, stroke severity, and comorbities) affect the specific biological interactions of tPA with the ischemic neurovascular unit,16 whereas the risk of ICH in EST may be more related to mechanical risks and rapid reperfusion that may not be predicted a priori by THRIVE. Because mechanical complications of EST may drive sICH rates in this context, the probability of having such a complication may be related to chance and to the device itself to a greater extent than the factors that make up THRIVE (age, NIHSS, and comorbidities). Although a large proportion of patients in TREVO-2 did receive intravenous tPA before EST, patients with up-front intravenous tPA-related hemorrhage would not have qualified for enrollment because of this complication, so in this data set we cannot discuss the relationship between THRIVE, intravenous tPA, and EST hemorrhage risk.
The THRIVE score has advantages compared with other ischemic stroke prediction scores because of its relative simplicity and basis in clinical factors known to the clinician immediately on the patient’s presentation. To be of clinical use, an outcome prediction score should be easily determined by the bedside clinician, but at the same time, the score must also have sufficient granularity and predictive power across a range of values to predict outcomes adequately. The SPAN-1007 is an example of an easy-to-use score (obtained by adding age and NIHSS) that has high specificity but low sensitivity because of its extreme simplicity: it only allows a clinician to identify a small percentage of older patients with larger strokes that has a poor chance of outcomes. On the other end of the stroke prediction score landscape, there are higher complexity scores, such as iScore8 and Acute Stroke Registry and Analysis of Lausanne (ASTRAL),5 which yield a much broader range of possible scores but require more effort on the part of the clinician and include factors such as laboratory values and examination findings beyond the standard NIHSS. Other scores, such as HIAT-24 and hyperDense MCA, Rankin, Age, Glucose, OTT, NIHSS (DRAGON) score,6 also involve interpretation of neuroimaging findings, such as the Alberta Stroke Program Early Computed Tomography Score17 in HIAT-24 and the hyperdense artery sign and early infarct signs in DRAGON,6 and thus add complexity in determination for clinicians.
In summary, the THRIVE score is an easy-to-use predictive score to assess poststroke functional outcome and mortality across the full range of acute stroke treatment contexts, including several EST treatment options, treatment with intravenous tPA, and no acute treatment. The THRIVE score is in the public domain (Creative Commons license): free Web calculators are provided at http://www.thrivescore.org and http://www.mdcalc.com/thrive-score-for-stroke-outcome/.
Supplementary Material
Footnotes
Disclosures
Data were analyzed by the second author (Dr Xiang) based on specifications provided by the first author (Dr Flint) who designed the present study. Dr Flint is a member of the TREVO-2 Clinical Events Committee. Dr Xiang works for Prospect Analytical and performed contract statistical work for this analysis and the TREVO-2 trial (Stryker Neurovascular). Dr Lutsep served on the Executive Committee for the TREVO-2 trial (Stryker Neurovascular) and chairs the Data Safety Monitoring Board for the SENTIS trial (CoAxia). Dr Gupta has served on scientific advisory boards for Stryker Neurovascular, Covidien, and Rapid Medical. Dr Jovin has served on scientific advisory boards for Stryker Neurovascular, Covidien, CoAxia, and Neurointervention. Dr Albers has served on scientific advisory boards for Stryker Neurovascular, has consulted for Covidien, and has an equity interest in iSchemaView. Dr Liebeskind has served on scientific advisory boards for Stryker Neurovascular, Covidien, and CoAxia. Dr Sanossian was a member of the TREVO-2 Imaging Core Laboratory. Dr Smith has served on scientific advisory boards for Stryker Neurovascular. Dr Nogueira reports no conflicts.
References
- 1.Hallevi H, Barreto AD, Liebeskind DS, Morales MM, Martin SB, Abraham AT, et al. UCLA Intra-Arterial Therapy Investigators. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke. 2009;40:1780–1785. doi: 10.1161/STROKEAHA.108.535146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flint AC, Cullen SP, Faigeles BS, Rao VA. Predicting long-term outcome after endovascular stroke treatment: the totaled health risks in vascular events score. AJNR Am J Neuroradiol. 2010;31:1192–1196. doi: 10.3174/ajnr.A2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flint AC, Kamel H, Rao VA, Cullen SP, Faigeles BS, Smith WS. Validation of the Totaled Health Risks in Vascular Events (THRIVE) score for outcome prediction in endovascular stroke treatment. [Accessed July 1, 2013];Int J Stroke. 2012 Aug 29; doi: 10.1111/j.1747-4949.2012.00872.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1747-4949.2012.00872.x/abstract. [DOI] [PubMed]
- 4.Sarraj A. Optimizing prediction scores for poor outcome after intra-arterial therapy for anterior circulation acute ischemic stroke. Stroke; International Stroke Conference; New Orleans, LA. 2013. p. A205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ntaios G, Faouzi M, Ferrari J, Lang W, Vemmos K, Michel P. An integer-based score to predict functional outcome in acute ischemic stroke: the ASTRAL score. Neurology. 2012;78:1916–1922. doi: 10.1212/WNL.0b013e318259e221. [DOI] [PubMed] [Google Scholar]
- 6.Strbian D, Meretoja A, Ahlhelm FJ, Pitkäniemi J, Lyrer P, Kaste M, et al. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. Neurology. 2012;78:427–432. doi: 10.1212/WNL.0b013e318245d2a9. [DOI] [PubMed] [Google Scholar]
- 7.Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC. Stroke prognostication using age and NIH Stroke Scale: SPAN-100. Neurology. 2013;80:21–28. doi: 10.1212/WNL.0b013e31827b1ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saposnik G, Fang J, Kapral MK, Tu JV, Mamdani M, Austin P, et al. Investigators of the Registry of the Canadian Stroke Network (RCSN); Stroke Outcomes Research Canada (SORCan) Working Group. The iScore predicts effectiveness of thrombolytic therapy for acute ischemic stroke. Stroke. 2012;43:1315–1322. doi: 10.1161/STROKEAHA.111.646265. [DOI] [PubMed] [Google Scholar]
- 9.Kamel H, Patel N, Rao VA, Cullen SP, Faigeles BS, Smith WS, et al. The THRIVE score predicts ischemic stroke outcomes independent of thrombolytic therapy in the NINDS tPA trial. [Accessed July 1, 2013];J Stroke Cerebrovasc Dis. 2012 Nov 2; doi: 10.1016/j.jstrokecerebrovasdis.2012.08.017. http://www.strokejournal.org/article/S1052-3057(12)00311-4/abstract. [DOI] [PubMed]
- 10.Flint AC, Faigeles BS, Cullen SP, Kamel H, Rao VA, Gupta R, et al. THRIVE Score predicts ischemic stroke outcomes and thrombolytic hemorrhage risk in VISTA. Stroke. 2013;44:3365–3369. doi: 10.1161/STROKEAHA.113.002794. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 13.Nilanont Y, Komoltri C, Saposnik G, Côté R, Di Legge S, Jin Y, et al. The Canadian Neurological Scale and the NIHSS: development and validation of a simple conversion model. Cerebrovasc Dis. 2010;30:120–126. doi: 10.1159/000314715. [DOI] [PubMed] [Google Scholar]
- 14.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. DEFUSE 2 Study Investigators. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. MR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267:156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, et al. AJNR Am J Neuroradiol. 2001;22:1534–1542. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.