Abstract
Background
Currently, intensive lipid lowering is recommended in patients with atherosclerotic ischemic stroke or transient ischemic attack. However, the role of statin in cardioembolic stroke is unclear. We investigated the association of statin with pretreatment collateral status in cardioembolic stroke.
Methods
A collaborative study from two stroke centers in distinct geographic regions included consecutive patients with acute middle cerebral artery (MCA) infarction due to atrial fibrillation (AF) who underwent cerebral angiography. The relationship between pretreatment collateral grade and the use/dose of statin at stroke onset was assessed. The angiographic collateral grade was evaluated according to the ASITN/SIR Collateral Flow Grading System.
Results
Ninety-eight patients (76 statin-naïve, 22 statin users) were included. Compared with statin-naïve patients, statin users were older and more frequently had hypertension, hyperlipidemia and coronary heart disease. Excellent collaterals (grade 3–4) were more frequently observed in statin users (11 patients, 50%) than in statin-naïve patients (21 patients, 27.6%; p = 0.049). The use of atorvastatin 10 mg equivalent or higher doses of statin was associated with excellent collaterals (p for trend = 0.025). In multiple regression analysis, prestroke statin use was independently associated with excellent collaterals (odds ratio, 7.841; 95% confidence interval, CI, 1.96–31.363; p = 0.004).
Conclusions
Premorbid use of statin in AF patients is associated with excellent collateral flow. Although most statin trials excluded patients with cardioembolic stroke, our data suggests the possibility that statin may be beneficial in AF-related stroke.
Keywords: Atrial fibrillation, Collateral flow, Stroke, Statin, Arteriogenesis
Introduction
Statin is a hydroxymethyl glutaryl coenzyme A reductase inhibitor, which has pleiotropic effects on atherosclerotic plaque stabilization [1, 2]. In the current guideline, intensive lipid lowering with statin therapy is strongly recommended to reduce risk of stroke among patients with ischemic stroke or transient ischemic attack who have evidence of atherosclerosis [3]. In addition to reduced stroke recurrence, there is increasing evidence that poststroke statin use is also associated with favorable stroke phenotype and improved neurological outcome [4–6]. However, whether statin has a role in nonatherosclerotic stroke, such as atrial fibrillation (AF)-related stroke, is unknown because most clinical trials including the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial excluded patients with AF or other sources of cardiac embolism [7].
In patients with acute ischemic stroke, both antegrade flow and retrograde collateral flow maintain cerebral perfusion within the ischemic regions. We recently reported that the angiographic collateral grade determines the recanalization rate, hemorrhagic transformation and infarct growth after revascularization therapy [8–10]. The degree of recanalization depends on the pretreatment collateral circulation; patients with poor collateral flow displayed a low recanalization rate regardless of the mode of revascularization therapy or the site of the occlusion [9]. Poor collaterals are also associated with symptomatic hemorrhagic transformation and subsequent clinical deterioration after revascularization therapy [10]. Moreover, a serial diffusion-weighted imaging (DWI) study revealed infarct growth despite successful revascularization therapy if patients had poor collaterals [8].
Statin has been reported to enhance collateral flow in patients with acute ischemic heart disease and stroke [11, 12]. In this study, we investigated the association of statin with pretreatment collateral status in AF-related stroke. We compared the angiographic collateral flow between statin users and statin-naïve patients. In addition, we analyzed factors associated with pretreatment collateral status in AF-related stroke.
Methods
We retrospectively analyzed demographic, clinical, laboratory and radiographic data that were prospectively collected on consecutive patients who received endovascular therapy (intra-arterial thrombolytic therapy or mechanical therapy such as guidewire manipulation or a mechanical thrombectomy device) for acute cerebral ischemia. This study analyzed consecutive patients encountered at two university hospital stroke centers: UCLA Medical Center from May 2002 through July 2007, and Samsung Medical Center from July 2005 through July 2012. Inclusion criteria were: (1) symptoms of acute cerebral ischemia within 8 h of symptom onset, (2) acute ischemic lesions within the middle cerebral artery (MCA) territory on DWI, (3) conventional angiography performed for endovascular therapy, (4) M1 or proximal M2 occlusion documented by angiography, (5) availability of information about prestroke statin use, and (6) their stroke mechanism was classified as AF-related. Stroke classification was primarily based on the revised TOAST classification [13]. We defined AF-related stroke when the patients had nonvalvular AF but no other demonstrable etiologies. The type and dose of statin were recorded at the time of admission, and doses of statin were converted to an atorvastatin equivalent on the basis of estimates of their low-density lipoprotein-lowering potency [14]. Statin doses were categorized as atorvastatin 10 mg equivalent or higher, and lower doses. All patients gave written informed consent, and the relevant institutional review boards approved this study.
All patients underwent comprehensive diagnostic cerebral angiography including injection of both internal carotid arteries and the dominant vertebral artery, with image acquisition into the late venous phase to assess collateral circulation from all possible sources. The angiographic collateral grade was evaluated according to the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology Collateral Flow Grading System on baseline angiography [15]. This angiographic scale assigns patients to grade 0 (no collaterals visible to the ischemic site), 1 (slow collaterals to the periphery of the ischemic site with persistence of some of the defect), 2 (rapid collaterals to the periphery of ischemic site with persistence of some of the defect and to only a portion of the ischemic territory), 3 (collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase) and 4 (complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion). Two reviewers blinded to the patient information independently assessed the collateral grade. The κ-coefficient for interobserver agreement was 0.821. The opinion from a neuroradiologist was sought to resolve disagreements. Grade 3–4 was defined as excellent collaterals.
All patients underwent routine blood tests, electrocardiography, cardiac telemetry for at least 24 h, and echocardiography. Hemostatic markers of arterial prothrombotic tendency, including antiphospholipid antibodies, were measured in patients younger than 50 years of age or with stroke the cause of which was undefined after initial workup. All patients underwent magnetic resonance imaging, including DWI, if not contraindicated.
We analyzed the differences between the groups using the Pearson χ2 test or linear-by-linear association for categorical variables, and the Student t test or Mann-Whitney U test for continuous variables. The Spearman correlation coefficient was used to analyze the association of collateral grade with the time interval between symptom onset and conventional angiography. In addition, independent factors for excellent collateral flow were evaluated using logistic regression. Significant variables from univariate analyses (p < 0.2) were considered to represent explanatory variables and were entered in subsequent multivariate analyses. All statistical analyses were performed using SPSS for Windows, version 13.0 (SPSS, Chicago, Ill., USA). A p value <0.05 was considered statistically significant.
Results
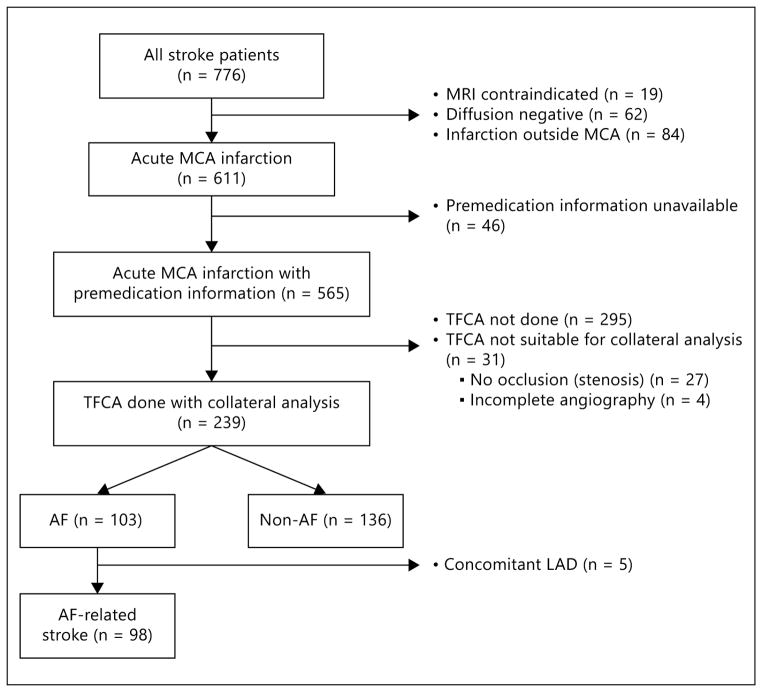
Of 776 patients with acute MCA infarctions on DWI, 98 (55 South Koreans and 43 southern Californians) were finally included in this study (fig. 1). Twenty-two patients were taking statin prior to the onset of stroke (atorvastatin 10 mg equivalent or higher, n = 14; lower dose statin, n = 8). The median time interval from symptom onset to angiography was 247.0 min (interquartile range, 193.0–334.5 min).
Fig. 1.
Flow diagram of patient selection. TFCA = Transfemoral cerebral angiography; LAD = large artery disease.
Patients’ baseline characteristics are listed in table 1. Compared with statin-naïve patients, statin users were older (p = 0.031) and more frequently had hypertension, hyperlipidemia and coronary heart disease (p = 0.032, p < 0.001 and p = 0.039, respectively). Statin users also showed lower levels of low-density lipoprotein-cholesterol and triglyceride than statin-naïve patients (p < 0.001 and p = 0.004, respectively). Excellent collaterals (grade 3–4) were more frequently observed in statin users (11 patients, 50%) than in statin-naïve patients (21 patients, 27.6%; p = 0.049). Moreover, the use of atorvastatin 10 mg equivalent or a higher dose was associated with excellent collaterals (p for trend = 0.025; fig. 2).
Table 1.
Patient characteristics according to statin use
| Statin-naïve patients (n = 76) | Statin users (n = 22) | p value | |
|---|---|---|---|
| Center | 0.102 | ||
| South Korean | 30 (39.5) | 13 (59.1) | |
| Southern Californian | 46 (60.5) | 9 (40.9) | |
| Age, years | 68.9±14.31 | 77±7.62 | 0.031 |
| Female gender | 48 (63.2) | 13 (59.1) | 0.729 |
| Hypertension | 43 (56.6) | 18 (81.8) | 0.032 |
| Diabetes | 11 (14.5) | 5 (22.7) | 0.345 |
| Current smoking | 7 (9.2) | 4 (18.2) | 0.260 |
| Dyslipidemia | 7 (9.3) | 14 (66.7) | <0.001 |
| Previous stroke | 20 (26.3) | 5 (22.7) | 0.734 |
| Previous coronary heart disease | 13 (17.1) | 9 (40.9) | 0.039 |
| NIH stroke scale | 17 [15–20] | 19 [14–23] | 0.343 |
| Systolic blood pressure, mm Hg | 155 [137–176] | 160 [145–170] | 0.327 |
| Time from onset to TFCA, min | 240 [191.5–328.5] | 263 [197–396] | 0.558 |
| Intravenous t-PA treatment | 43 (56.6) | 9 (40.9) | 0.223 |
| Blood pressure medication | |||
| β-Blocker | 19 (33.3) | 11 (57.9) | 0.058 |
| Ca2+ channel blocker | 8 (15.4) | 3 (15.8) | 1.000 |
| ARB/ACE inhibitor | 20 (28.6) | 9 (42.9) | 0.218 |
| Antithrombotic agent | 0.190 | ||
| Antiplatelets | 27 (36) | 12 (60) | 0.072 |
| Warfarin | 14 (18.7) | 4 (20) | 1.000 |
| Aspirin + warfarin | 3 (4) | 0 (0) | 1.000 |
| Laboratory findings, mg/dl | |||
| Glucose | 123.3±27.78 | 137.8±46.89 | 0.441 |
| Triglyceride | 90.3±47.82 | 121.2±47.82 | 0.004 |
| HDL cholesterol | 50.2±18.28 | 47.0±26.16 | 0.105 |
| LDL cholesterol | 104.3±31.67 | 77.1±18.16 | <0.001 |
| Excellent collateral | 0.049 | ||
| Absence | 55 (72.4) | 11 (50) | |
| Presence | 21 (27.6) | 11 (50) | |
Statistically significant p values are highlighted in italics. Values are represented as means ± SD, numbers with percentages in parentheses or medians with interquartile ranges in square brackets. ARB = Angiotensin II receptor blocker; ACE = angiotensin-converting enzyme; TFCA = transfemoral cerebral angiography; t-PA = tissue-type plasminogen activator; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Fig. 2.
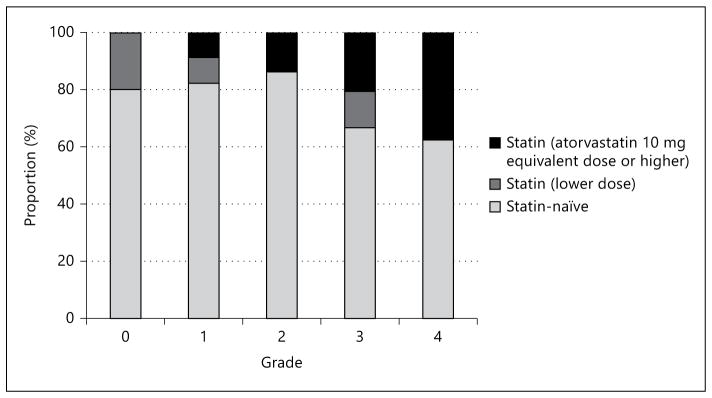
Relationship between pretreatment collateral grading and prior use of statin.
Logistic regression analysis was performed to further evaluate independent predictors for excellent collaterals (table 2). After adjusting for all the variables that showed a possible association (p < 0.2) in univariate analysis, only prestroke statin use (odds ratio, 7.841; 95% confidence interval, CI, 1.96–31.363; p = 0.004) was independently associated with excellent collateral flows.
Table 2.
Factors associated with excellent collaterals
| Pretreatment collateral flow
|
p | Estimated OR
|
p | ||||
|---|---|---|---|---|---|---|---|
| excellent (n = 32) | not excellent (n = 66) | crude | adjusted | 95% CI | |||
| Center | 0.986 | ||||||
| South Korean | 14 (43.8) | 29 (43.9) | |||||
| Southern Californian | 18 (56.3) | 37 (56.1) | |||||
| Age, years | 71.3±15.21 | 70.4±12.73 | 0.739 | ||||
| Female gender | 24 (75) | 37 (56.1) | 0.073 | 2.351 | 1.736 | 0.56–5.38 | 0.339 |
| Hypertension | 20 (62.5) | 41 (62.1) | 0.971 | ||||
| Diabetes | 6 (18.8) | 10 (15.2) | 0.652 | ||||
| Current smoking | 1 (3.1) | 10 (15.2) | 0.111 | 0.181 | 0.111 | 0.009–1.39 | 0.088 |
| Dyslipidemia | 8 (25) | 13 (20.3) | 0.601 | ||||
| Previous stroke | 8 (25) | 17 (25.8) | 0.936 | ||||
| Previous CHD | 4 (12.5) | 18 (27.3) | 0.109 | 0.381 | 0.45 | 0.104–1.946 | 0.285 |
| Systolic BP, mm Hg | 158 [140–192] | 155 [138–176] | 0.885 | ||||
| Intravenous t-PA use | 36 (56.3) | 16 (50.0) | 0.563 | ||||
| Antihypertensive medication | |||||||
| β-Blocker | 9 (33.3) | 21 (42.9) | 0.417 | ||||
| Ca2+ channel blocker | 4 (14.8) | 7 (15.9) | 0.902 | ||||
| ARB/ACE inhibitor | 7 (22.6) | 22 (36.7) | 0.176 | 0.504 | 0.342 | 0.107–1.099 | 0.072 |
| Antithrombotic agent | |||||||
| Antiplatelet agents | 15 (48.4) | 24 (37.5) | 0.370 | ||||
| Warfarin | 6 (19.4) | 12 (18.8) | 0.721 | ||||
| Both | 0 (0) | 3 (4.7) | 0.999 | ||||
| Any | 38 (59.4) | 21 (67.7) | 0.432 | ||||
| Statin use | 11 (34.4) | 11 (16.7) | 0.049 | 2.619 | 7.841 | 1.96–31.363 | 0.004 |
| Statin dose | |||||||
| Atorvastatin 10 mg equivalent or higher | 8 (25) | 6 (9.1) | 0.036 | ||||
| Lower | 3 (9.4) | 5 (7.6) | 0.559 | ||||
| Laboratory findings, mg/dl | |||||||
| Glucose | 117.9±31.86 | 130.7±33.43 | 0.081 | 0.986 | 0.985 | 0.969–1.002 | 0.085 |
| Triglyceride | 93±46.38 | 99.9±51.07 | 0.527 | ||||
| HDL cholesterol | 48.5±22.49 | 50±19.1 | 0.729 | ||||
| LDL cholesterol | 94.6±29.56 | 99.9±32.17 | 0.436 | ||||
Statistically significant p values are highlighted in italics. Values are represented as means ± SD, numbers with percentages in parentheses or median with interquartile range in square brackets. ‘Statin dose’ was excluded in the multivariate model due to large numbers of missing values. OR = Odds ratio; CI = confidence interval; CHD = coronary heart disease; BP = blood pressure; ARB = angiotensin II receptor blocker; ACE = angiotensin-converting enzyme; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
In statin users, excellent collateral flow was more frequently observed when conventional angiography was performed at a later time point after symptom onset (r = 0.491; 95% CI, 0.08–0.741; p = 0.028; fig. 3a). In contrast, no such correlation was observed in statin-naïve patients (r = 0.209; 95% CI, 0.003–0.398; p = 0.089; fig. 3b). Although the difference did not reach statistical significance, there was a trend for a positive correlation in the statin users.
Fig. 3.
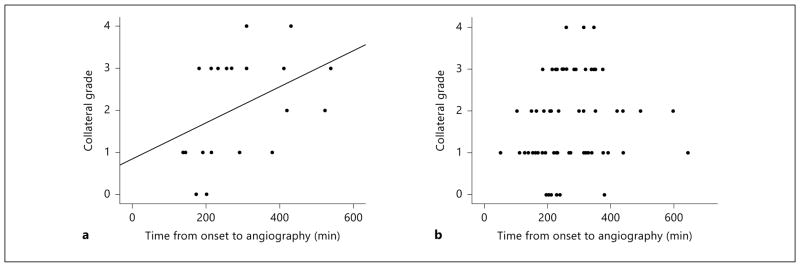
Scatterplots for correlation between interval from symptom onset to transfemoral cerebral angiography and angiographic collateral grade: statin users (a) and statin-naïve patients (b).
Discussion
The principal finding of this study is that prestroke use of statin is associated with excellent collateral flow in AF-related stroke. This result is in line with our previous findings that statin use is associated with better collateralization during acute stroke [11]. In the present study, however, we focused on AF-related stroke to assess poststroke collateral status after abrupt MCA occlusion.
Premorbid Statin Use Alone Determines Collateral Grade during Acute AF-Related Stroke
Collateral status in patients with AF-related stroke is usually poor because a large proximal artery is suddenly blocked by emboli and there is little time for collateral vessels to develop [16]. As a result, AF-related stroke is usually characterized by large cortical infarcts on DWI with severe perfusion defects on perfusion-weighted imaging [16]. Recanalization-oriented treatment is often limited and does not guarantee reperfusion [17]. The rate of bleeding after recanalization therapy is higher and the rate of recanalization is lower (large burden of clot) in AF-related stroke than in atherosclerotic stroke [18, 19]. Recent observational studies reported that the response to recanalizing therapy and its complication are largely affected by collateral status [10, 20]. The present study shows that the use of statin is associated with better collaterals in acute AF-related stroke.
The use of statin is associated with better outcome in atherosclerotic stroke [6, 21], which might be attributable to various effects of statin that include reversal of endothelial dysfunction, augmentation in the nitric oxide-mediated vasodilation, or plaque stabilization. However, clinical studies have shown inconsistent results concerning the correlation between premorbid statin use and collateral flow in patients with chronic coronary artery disease [12, 22]. In addition, an angiogenic effect of statin has not been proven in a chronic ischemia model [23]. Therefore, in atherosclerotic stroke, many factors other than statin medication may influence the vascular territory of the brain, which may include the degree and speed of atherosclerotic occlusion and the presence of stenotic lesions on collateral vessels.
Statin May Have Arteriogenesis-Promoting Potential Early after Stroke
Early preclinical studies have demonstrated that statin promotes angiogenesis by activating protein kinase Akt in a dose-dependent biphasic manner [24–26]. Recent preclinical and clinical observational studies showed that statin promotes new vessel formation via stem cell angiogenesis [25, 26] and increases circulating endothelial progenitor cells [27, 28]. In addition to angiogenesis/vasculogenesis, arteriogenesis (the growth of preexistent collateral arterioles into functional collateral arteries) is another form of vessel growth that requires a relatively short time to develop collateral formation and has more clinical relevance than angiogenesis/vasculogenesis [29–31]. Presently, good collaterals were evident in statin users within several hours after stroke onset, suggesting that statin may promote arteriogenesis. Prestroke statin use could also make existing collaterals more likely to be functional or prevent collateral failure in the setting of an abrupt vascular occlusion [32]. This finding is in line with a recent preclinical study that demonstrated that simvastatin promotes arteriogenesis after stroke [31]. A very recent perfusion-weighted imaging study that reported that prior use of statin is associated with good reperfusion might also support this finding [33].
There have been limited data regarding acute statin use and rapid collateral formation. However, several animal studies revealed an acute effect of statin on the restoration of endothelial dysfunction and vascular relaxation [25, 34]. Specifically, Kureishi et al. [25] showed a dose-dependent increment of Akt phosphorylation after 15 min of statin use and nitric oxide release from aortic endothelial cells after 1 h of statin use. Jorge et al. [34] reported rapid reversal of endothelial dysfunction in hypercholesterolemic rabbits with 4 days of pravastatin treatment. Together, these findings might explain our finding that statin is associated with rapid collateral development.
This finding is further supported by clinical studies that reported that, as well as prestroke statin use, early introduction of statin immediately after stroke is also associated with a good outcome. Randomized clinical trials [5, 35], large cohort studies [6, 21] and a metaanalysis [36] have linked statin use with reduced infarct size and more favorable outcome. However, insufficient data are available for patients with AF-related stroke. Two large ongoing clinical trials are evaluating the effects of statin therapy in acute ischemic stroke and will provide valuable information in the near future: the STARS07 (Stroke Treatment with Acute Reperfusion and Simvastatin) and the EUREKA (Effects of Very Early Use of Rosuvastatin in Preventing Recurrence of Ischemic Stroke) trials. However, patients with cardioembolic stroke were excluded in the EUREKA trial, and there was a lack of concern of stroke subtypes in the STARS07 study. Further studies are needed to test whether statin therapy is safe and effective in case of acute AF-related stroke by evaluating collateral status (conventional angiography) or tissue perfusion (perfusion- weighted imaging).
Limitations and Conclusions
Our study has limitations and should be interpreted with caution. The number of included patients was relatively small. It is a nonrandomized observational study and recruited acute stroke patients only who had proximal MCA occlusion on angiography. In addition, infarct growth on brain imaging and clinical outcome were not analyzed because of the small number of patients and many variables that might affect outcomes (including recanalization status). Therefore, although we have previously shown the impact of collaterals on various prognostic parameters [8–10], the present data do not clarify the impact of enhanced collaterals on clinical outcome after stroke in patients with AF-related stroke. Furthermore, information on the previous duration of statin therapy is not available in this study. Follow-up angiographies were not performed, which is another limitation in confirming long-term angiogenic effects of statin. Lastly, further studies are needed on the mechanisms of statin on arteriogenesis.
In summary, the premorbid use of statin in AF patients is associated with good collateral flow. Although most clinical trials have focused on the effects of statin against noncardioembolic stroke, our data raises the possibility that high-risk patients with AF for whom a statin is not indicated based on current guidelines might benefit from statin use. This hypothesis should be tested in a prospective trial. Further randomized trials to discern the effects of statins on cerebral arteriogenesis and collateral circulation in acute AF-related stroke are warranted.
Acknowledgments
Study Funding
This study was supported by the Korean Healthcare Technology R & D Project, Ministry of Health & Welfare (A110208).
Footnotes
Disclosure Statement
The authors report no disclosures.
References
- 1.Kwee RM, van Oostenbrugge RJ, Prins MH, Ter Berg JW, Franke CL, Korten AG, Meems BJ, van Engelshoven JM, Wildberger JE, Mess WH, Kooi ME. Symptomatic patients with mild and moderate carotid stenosis: plaque features at MRI and association with cardiovascular risk factors and statin use. Stroke. 2010;41:1389–1393. doi: 10.1161/STROKEAHA.109.575670. [DOI] [PubMed] [Google Scholar]
- 2.Otagiri K, Tsutsui H, Kumazaki S, Miyashita Y, Aizawa K, Koshikawa M, Kasai H, Izawa A, Tomita T, Koyama J. Early intervention with rosuvastatin decreases the lipid components of the plaque in acute coronary syndrome: analysis using integrated backscatter IVUS (ELAN study) Circ J. 2011;75:633. doi: 10.1253/circj.cj-10-0600. [DOI] [PubMed] [Google Scholar]
- 3.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 4.Bang OY, Ovbiagele B, Liebeskind DS, Restrepo L, Yoon SR, Saver JL. Clinical determinants of infarct pattern subtypes in large vessel atherosclerotic stroke. J Neurol. 2009;256:591–599. doi: 10.1007/s00415-009-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muscari A, Puddu GM, Santoro N, Serafini C, Cenni A, Rossi V, Zoli M. The atorvastatin during ischemic stroke study: a pilot randomized controlled trial. Clin Neuropharmacol. 2011;34:141–147. doi: 10.1097/WNF.0b013e3182206c2f. [DOI] [PubMed] [Google Scholar]
- 6.Ni Chroinin D, Callaly EL, Duggan J, Merwick A, Hannon N, Sheehan O, Marnane M, Horgan G, Williams EB, Harris D, Kyne L, McCormack PM, Moroney J, Grant T, Williams D, Daly L, Kelly PJ. Association between acute statin therapy, survival, and improved functional outcome after ischemic stroke: the North Dublin Population Stroke Study. Stroke. 2011;42:1021–1029. doi: 10.1161/STROKEAHA.110.596734. [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 8.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- 11.Ovbiagele B, Saver JL, Starkman S, Kim D, Ali LK, Jahan R, Duckwiler GR, Vinuela F, Pineda S, Liebeskind DS. Statin enhancement of collateralization in acute stroke. Neurology. 2007;68:2129–2131. doi: 10.1212/01.wnl.0000264931.34941.f0. [DOI] [PubMed] [Google Scholar]
- 12.Pourati I, Kimmelstiel C, Rand W, Karas RH. Statin use is associated with enhanced collateralization of severely diseased coronary arteries. Am Heart J. 2003;146:876–881. doi: 10.1016/S0002-8703(03)00413-7. [DOI] [PubMed] [Google Scholar]
- 13.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 14.Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study) Am J Cardiol. 1998;81:582–587. doi: 10.1016/s0002-9149(97)00965-x. [DOI] [PubMed] [Google Scholar]
- 15.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 16.Tu HT, Campbell BC, Christensen S, Collins M, De Silva DA, Butcher KS, Parsons MW, Desmond PM, Barber PA, Levi CR, Bladin CF, Donnan GA, Davis SM. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc Dis. 2010;30:389–395. doi: 10.1159/000316886. [DOI] [PubMed] [Google Scholar]
- 17.Soares BP, Chien JD, Wintermark M. MR and CT monitoring of recanalization, reperfusion, and penumbra salvage: everything that recanalizes does not necessarily reperfuse! Stroke. 2009;40:S24–S27. doi: 10.1161/STROKEAHA.108.526814. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K, Iguchi Y, Yamashita S, Shibazaki K, Kobayashi K, Inoue T. Atrial fibrillation as an independent predictor for no early recanalization after IV-t-PA in acute ischemic stroke. J Neurol Sci. 2008;267:57–61. doi: 10.1016/j.jns.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Seet RC, Zhang Y, Wijdicks EF, Rabinstein AA. Relationship between chronic atrial fibrillation and worse outcomes in stroke patients after intravenous thrombolysis. Arch Neurol. 2011;68:1454–1458. doi: 10.1001/archneurol.2011.248. [DOI] [PubMed] [Google Scholar]
- 20.Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, Harris GJ, Halpern E, Kemmling A, Koroshetz WJ. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–3005. doi: 10.1161/STROKEAHA.109.552513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flint AC, Kamel H, Navi BB, Rao VA, Faigeles BS, Conell C, Klingman JG, Sidney S, Hills NK, Sorel M, Cullen SP, Johnston SC. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke. 2012;43:147–154. doi: 10.1161/STROKEAHA.111.627729. [DOI] [PubMed] [Google Scholar]
- 22.Zbinden S, Brunner N, Wustmann K, Billinger M, Meier B, Seiler C. Effect of statin treatment on coronary collateral flow in patients with coronary artery disease. Heart. 2004;90:448–449. doi: 10.1136/hrt.2003.017871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boodhwani M, Mieno S, Feng J, Sodha NR, Clements RT, Xu SH, Sellke FW. Atorvastatin impairs the myocardial angiogenic response to chronic ischemia in normocholesterolemic swine. J Thorac Cardiovasc Surg. 2008;135:117–122. doi: 10.1016/j.jtcvs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 25.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMGCoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 27.Hibbert B, Ma X, Pourdjabbar A, Simard T, Rayner K, Sun J, Chen YX, Filion L, O’Brien ER. Pre-procedural atorvastatin mobilizes endothelial progenitor cells: clues to the salutary effects of statins on healing of stented human arteries. PLoS One. 2011;6:e16413. doi: 10.1371/journal.pone.0016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaumdally RJ, Goon PK, Varma C, Blann AD, Lip GY. Effects of atorvastatin on circulating CD34+/CD133+/CD45– progenitor cells and indices of angiogenesis (vascular endothelial growth factor and the angiopoietins 1 and 2) in atherosclerotic vascular disease and diabetes mellitus. J Intern Med. 2010;267:385–393. doi: 10.1111/j.1365-2796.2009.02151.x. [DOI] [PubMed] [Google Scholar]
- 29.Tongers J, Roncalli JG, Losordo DW. Role of endothelial progenitor cells during ischemia-induced vasculogenesis and collateral formation. Microvasc Res. 2010;79:200–206. doi: 10.1016/j.mvr.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis: a new concept for the treatment of arterial occlusive disease. Cardiovasc Res. 2001;49:543–553. doi: 10.1016/s0008-6363(00)00206-6. [DOI] [PubMed] [Google Scholar]
- 31.Zacharek A, Chen J, Cui X, Yang Y, Chopp M. Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke. 2009;40:254–260. doi: 10.1161/STROKEAHA.108.524116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grundmann S, Piek JJ, Pasterkamp G, Hoefer IE. Arteriogenesis: basic mechanisms and therapeutic stimulation. Eur J Clin Invest. 2007;37:755–766. doi: 10.1111/j.1365-2362.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- 33.Ford AL, An H, D’Angelo G, Ponisio R, Bushard P, Vo KD, Powers WJ, Lin W, Lee JM. Preexisting statin use is associated with greater reperfusion in hyperacute ischemic stroke. Stroke. 2011;42:1307–1313. doi: 10.1161/STROKEAHA.110.600957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorge PA, Osaki MR, de Almeida E. Rapid reversal of endothelial dysfunction in hypercholesterolaemic rabbits treated with simvastatin and pravastatin. Clin Exp Pharmacol Physiol. 1997;24:948–953. doi: 10.1111/j.1440-1681.1997.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 35.Montaner J, Chacon P, Krupinski J, Rubio F, Millan M, Molina CA, Hereu P, Quintana M, Alvarez-Sabin J. Simvastatin in the acute phase of ischemic stroke: A safety and efficacy pilot trial. Eur J Neurol. 2008;15:82–90. doi: 10.1111/j.1468-1331.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- 36.Biffi A, Devan WJ, Anderson CD, Cortellini L, Furie KL, Rosand J, Rost NS. Statin treatment and functional outcome after ischemic stroke: case-control and meta-analysis. Stroke. 2011;42:1314–1319. doi: 10.1161/STROKEAHA.110.605923. [DOI] [PMC free article] [PubMed] [Google Scholar]