Abstract
Background
There are multiple clinical and radiographic factors that influence outcomes after endovascular reperfusion therapy (ERT) in acute ischemic stroke (AIS). We sought to derive and validate an outcome prediction score for AIS patients undergoing ERT based on readily available pretreatment and posttreatment factors.
Methods
The derivation cohort included 511 patients with anterior circulation AIS treated with ERT at 10 centers between September 2009 and July 2011. The prospective validation cohort included 223 patients with anterior circulation AIS treated in the North American Solitaire Acute Stroke registry. Multivariable logistic regression identified predictors of good outcome (modified Rankin score ≤2 at 3 months) in the derivation cohort; model β coefficients were used to assign points and calculate a risk score. Discrimination was tested using C statistics with 95% confidence intervals (CIs) in the derivation and validation cohorts. Calibration was assessed using the Hosmer-Lemeshow test and plots of observed to expected outcomes. We assessed the net reclassification improvement for the derived score compared to the Totaled Health Risks in Vascular Events (THRIVE) score. Subgroup analysis in patients with pretreatment Alberta Stroke Program Early CT Score (ASPECTS) and posttreatment final infarct volume measurements was also performed to identify whether these radiographic predictors improved the model compared to simpler models.
Results
Good outcome was noted in 186 (36.4%) and 100 patients (44.8%) in the derivation and validation cohorts, respectively. Combining readily available pretreatment and posttreatment variables, we created a score (acronym: SNARL) based on the following parameters: symptomatic hemorrhage [2 points: none, hemorrhagic infarction (HI)1–2 or parenchymal hematoma (PH) type 1; 0 points: PH2], baseline National Institutes of Health Stroke Scale score (3 points: 0–10; 1 point: 11–20; 0 points: >20), age (2 points: <60 years; 1 point: 60–79 years; 0 points: >79 years), reperfusion (3 points: Thrombolysis In Cerebral Ischemia score 2b or 3) and location of clot (1 point: M2; 0 points: M1 or internal carotid artery). The SNARL score demonstrated good discrimination in the derivation (C statistic 0.79, 95% CI 0.75–0.83) and validation cohorts (C statistic 0.74, 95% CI 0.68–0.81) and was superior to the THRIVE score (derivation cohort: C statistic 0.65, 95% CI 0.60–0.70; validation cohort: C-statistic 0.59, 95% CI 0.52–0.67; p < 0.01 in both cohorts) but was inferior to a score that included age, ASPECTS, reperfusion status and final infarct volume (C statistic 0.86, 95% CI 0.82–0.91; p = 0.04). Compared with the THRIVE score, the SNARL score resulted in a net reclassification improvement of 34.8%.
Conclusions
Among AIS patients treated with ERT, pretreatment scores such as the THRIVE score provide only fair prognostic information. Inclusion of posttreatment variables such as reperfusion and symptomatic hemorrhage greatly influences outcome and results in improved outcome prediction.
Keywords: Prediction tools, Revascularization, Reperfusion
Introduction
Ischemic stroke is a leading cause of death and disability in the USA [1]. Early reperfusion remains a mainstay of acute therapy. Though not proven in randomized clinical trials, endovascular reperfusion therapy (ERT) may also benefit select patients with acute ischemic stroke (AIS) [2]. Several scores have been developed to improve patient selection for ERT [3–6] but none have included treatment factors to predict outcome after ERT. It is well established that reperfusion success is an important driver of good outcomes in endovascular treatment studies [7–10]. Despite disappointing recent trials of ERT, these continued to show the strong association between reperfusion success and good functional outcome [11, 12]. Other factors such as age, medical history, laboratory and radiographic findings, treatment success and complications may also influence outcomes. A valid prediction score may have clinical utility if it can both improve patient selection for ERT and predict long-term patient outcome after ERT.
We sought to develop and validate a simple outcome prediction score for anterior circulation AIS patients treated with ERT based on readily available pre- and posttreatment variables. We utilized a large multicenter cohort for score derivation and the North American Solitaire Acute Stroke (NASA) registry for score validation.
Methods
Data Sources
We analyzed a retrospective registry of consecutive patients treated with endovascular therapy at 10 tertiary stroke centers from September 2009 to July 2011. Participating hospitals submit information on consecutive ischemic stroke patients treated with ERT. Details of the registry have been described previously [13]. Only de-identified information was used for the purposes of this analysis after internal review board approval from each participating center.
Inclusion criteria for this study included patients with AIS who presented within 8 h of symptom onset with anterior circulation large vessel occlusions. Data were analyzed regarding demographics (age and sex), previous medical history (hypertension, atrial fibrillation, diabetes mellitus and dyslipidemia), radiographic interpretation of hemorrhages, location of arterial occlusion on angiography, reperfusion status and clinical outcomes. Successful re-perfusion was defined as a Thrombolysis In Cerebral Ischemia (TICI) score of 2b or higher on the final angiographic image and was rated by the treating interventionalist. Symptomatic hemorrhage was defined as a parenchymal hematoma (PH) type 2 using the European Cooperative Acute Stroke Study definition [14]. The Alberta Stroke Program Early CT Score (ASPECTS) [15] was based on available pretreatment CT scans, and final infarct volume was measured using the ABC/2 approach [16]. Outcomes were assessed by trained stroke neurologists at each site, and a modified Rankin score of less than or equal to 2 at 90 days was considered a good clinical outcome. Of 556 patients with anterior circulation large artery occlusions treated with ERT in the registry, 511 had complete data for inclusion in the derivation cohort. In a subset of patients, pretreatment ASPECTS (n = 263) and final infarct volume (n = 312) were available. From the NASA prospective cohort [17], we acquired similarly defined data to serve as the validation dataset for the prediction score. Of the 354 patients in the NASA registry, 223 patients with anterior circulation large artery occlusions treated with ERT within 8 h of onset had complete data and were included in the analysis.
Statistical Analysis
Using univariable tests, we assessed associations between pre- and post treatment variables using χ2 tests (or Fisher’s exact test if appropriate) for categorical variables and t tests (or Mann-Whitney test if appropriate) for continuous variables. A multivariable logistic regression analysis was performed in the derivation cohort to identify independent predictors of good outcome. Candidate variables were selected based on statistically significant univariable relationships (p<0.20) with good outcome (modified Rankin score 0–2 at 90 days) after ERT. Continuous variables were then categorized into appropriate clinically relevant strata (cut points). The multivariable final model included important predictors based on statistical significance (p<0.05). The calibration of the final model was tested using the Hosmer-Lemeshow test.
A risk score was developed by multiplying each independent predictor’s β coefficient in the final model by 1.5 and rounding to the nearest integer. For each patient, the weighted integers were summed to obtain a total risk score with a range from 0 to 11 points. The discriminatory ability of the model was assessed using the C statistic, or the probability that a randomly selected patient who experienced an outcome will have a higher predicted probability of having that outcome occur compared to another randomly selected patient who did not experience that outcome. A C statistic value of 0.5 indicates that the model is no better than chance, while a value of 1.0 indicates that the model has perfect discrimination. Calibration was visually assessed by comparing plots of predicted versus observed good outcome. The risk score was then validated by assessing discrimination and calibration in the validation cohort (NASA). We performed several subgroup analyses in the patients from the derivation cohort for whom imaging data were collected. We compared C statistics for pretreatment models such as the Totaled Health Risks in Vascular Events (THRIVE) score, posttreatment models including final infarct volume and combined pre- and posttreatment models. Lastly, we assessed the net reclassification improvement for our prediction score compared to the THRIVE score [18]. All p values are two-sided, with p<0.05 considered statistically significant. Analyses were performed using IBM SPSS software version 21 (Armonk, N.Y., USA).
Results
The derivation cohort consisted of 511 patients [mean age 65.3 ± 15.0 years; 48.1% male; median National Institutes of Health Stroke Scale (NIHSS) score 18]. The sites of arterial occlusion were the middle cerebral artery (M1 60.7%, M2 10.8%) and the internal carotid artery (ICA; 28.6%). In this cohort, 233 patients (45.6%) received intravenous tissue plasminogen activator (t-PA) prior to ERT. TICI 2b/3 reperfusion was achieved in 300 patients (58.7%), and symptomatic hemorrhage (PH2) occurred in 36 (7.0%). Good outcome at 3 months was noted in 186 patients (36.4%) of the derivation cohort. The validation cohort was comparable with regard to age (68.1 ± 15.0 years) and sites of occlusion (ICA: 26.0%; M1: 61.0%; M2 13.0%), but the symptomatic hemorrhage rate was higher (12.6%) and final TICI grade 3 was more common (35.0%). One hundred patients (44.8%) of the validation cohort achieved a good outcome (table 1).
Table 1.
Clinical and treatment characteristics of the derivation (n= 511) and validation (n= 223) cohorts
| Derivation cohort | Validation cohort | p value | |
|---|---|---|---|
| Mean age (SD), years | 65.3 (15.0) | 68.1 (15.0) | 0.02 |
| Male, n | 246 (48.1) | 100 (45.0) | 0.44 |
| Atrial fibrillation1, n | 161 (31.7) | 103 (46.2) | <0.01 |
| Hypertension, n | 359 (70.3) | 171 (76.7) | 0.07 |
| Diabetes mellitus, n | 116 (22.7) | 57 (25.6) | 0.40 |
| Median initial NIHSS score (IQR) | 18 (14–22) | 18 (14–22) | 0.90 |
| Vascular location, n | 0.48 | ||
| ICA | 146 (28.6) | 58 (26.0) | |
| Middle cerebral artery (M1) | 310 (60.7) | 136 (61.0) | |
| Middle cerebral artery (M2) | 55 (10.8) | 29 (13.0) | |
| Intravenous t-PA prior to ERT, n | 231 (45.3) | 114 (51.4) | 0.13 |
| Interhospital transfer, n | 284 (55.6) | 96 (52.5) | 0.47 |
| PH2 sICH, n | 36 (7.0) | 28 (12.6) | 0.02 |
| Final TICI grade, n | <0.01 | ||
| 0 | 84 (16.4) | 18 (8.1) | |
| 1 | 36 (7.0) | 6 (2.7) | |
| 2a | 91 (17.8) | 36 (16.1) | |
| 2b | 227 (44.4) | 84 (37.7) | |
| 3 | 73 (14.3) | 78 (35.0) | |
| Good outcome, n | 186 (36.4) | 100 (44.8) | 0.03 |
Values in parentheses represent percentages, except where indicated otherwise. IQR= Interquartile range; sICH= symptomatic hemorrhage.
Data missing in 3 patients in the derivation cohort.
In the derivation cohort, univariable analysis identified age, baseline NIHSS score, location of occlusion, intravenous t-PA use, reperfusion grade and hemorrhage as associated with outcome (table 2). Patients with good outcome at 90 days were younger (62.6 vs. 66.9 years, p<0.01), had lower baseline NIHSS scores (16 vs. 19, p<0.01), received intravenous t-PA more commonly (51.4 vs. 41.8%, p= 0.04), differed by location of occlusion (M2 vs. M1 vs. ICA: 56.4 vs. 36.5 vs. 26.5%; p<0.01), had higher reperfusion grades (TICI 2b/3 vs. TICI 0/1/2a: 82.8 vs. 44.9%; p<0.01) and had fewer postprocedure PH2 hemorrhages (2.7 vs. 9.5%, p<0.01). There were trends for association between poor outcome and hypertension (p= 0.08) and diabetes mellitus (p= 0.11). In the subset of patients in whom final infarct volume was measured (n= 312), final infarct volume was a strong predictor of outcome (23.5 vs. 91.0 ml, p<0.01). Lastly, in a subset of patients with pretreatment ASPECTS recorded (n= 263), ASPECTS >7 was strongly associated with good outcome (84.5 vs. 61.3%, p<0.01).
Table 2.
Comparison of baseline clinical and treatment characteristics in association with good outcome in the full derivation cohort (n= 511)
| Good outcome (n= 186) | Poor outcome (n= 325) | p value | |
|---|---|---|---|
| Mean age (SD), years | 62.3 (15.0) | 67.0 (14.8) | <0.01 |
| Male, n | 89 (47.8) | 157 (48.3) | 0.92 |
| Atrial fibrillation1, n | 56 (30.1) | 105 (32.6) | 0.56 |
| Hypertension, n | 122 (65.6) | 237 (72.9) | 0.08 |
| Diabetes mellitus, n | 35 (18.8) | 81 (24.9) | 0.11 |
| Median initial NIHSS score (IQR) | 16 (12–20) | 19 (16–22) | <0.01 |
| Vascular location, n | <0.01 | ||
| ICA | 42 (22.6) | 104 (32.0) | |
| Middle cerebral artery (M1) | 113 (60.8) | 197 (60.6) | |
| Middle cerebral artery (M2) | 31 (16.7) | 24 (7.4) | |
| Intravenous t-PA prior to ERT, n | 95 (51.4) | 136 (41.8) | 0.04 |
| Interhospital transfer, n | 99 (53.2) | 185 (56.9) | 0.42 |
| PH2 sICH, n | 5 (2.7) | 31 (9.5) | <0.01 |
| Final TICI grade, n | <0.01 | ||
| 0 | 9 (4.8) | 75 (23.1) | |
| 1 | 5 (2.7) | 31 (9.5) | |
| 2a | 18 (9.7) | 73 (22.5) | |
| 2b | 116 (62.4) | 111 (34.2) | |
| 3 | 38 (20.4) | 35 (10.8) |
Values in parentheses represent percentages, except where indicated otherwise. IQR= Interquartile range; sICH= symptomatic hemorrhage.
Data missing in 3 patients.
In the full data derivation cohort, age, baseline NIHSS score, location of occlusion, reperfusion grade and symptomatic hemorrhage remained independent predictors of good outcome while diabetes was marginally significant (p= 0.06) in multivariable analyses; intravenous t-PA and hypertension were no longer significant (table 3). A score including age, NIHSS score, reperfusion status, symptomatic hemorrhage and location of occlusion [score= −0.262 + 0.675(TICI 2b or 3 reperfusion status) – 0.012(age) – 0.128(initial NIHSS score) + 0.778(M2 clot) – 0.722(symptomatic hemorrhage)] showed good discrimination (C statistic 0.80, 95% CI 0.76–0.84).
Table 3.
Final multivariable logistic regression model of good outcome at 3 months (modified Rankin score 0–2) according to the SNARL score in the full derivation cohort (n= 511)
| Category | β coefficient | Points (0–11) | Adjusted OR (95% CI) | p value |
|---|---|---|---|---|
| Symptomatic hemorrhage (PH2) | ||||
| Yes | reference | 0 | – | <0.01 |
| No | 1.44 | 2 | 4.21 (0.08–0.71) | |
| Baseline NIHSS score | ||||
| >20 | reference | 0 | – | <0.01 |
| 10–20 | 0.83 | 1 | 2.29 (1.42–3.68) | |
| <10 | 2.14 | 3 | 8.46 (3.54–20.21) | |
| Age | ||||
| >80 years | reference | 0 | – | <0.01 |
| 60–79 years | 0.78 | 1 | 2.18 (1.18–4.03) | |
| <60 years | 1.05 | 2 | 2.85 (1.51–5.39) | |
| Reperfusion (TICI 2b or 3) | ||||
| No | reference | 0 | – | <0.01 |
| Yes | 2.03 | 3 | 7.57 (4.66–12.31) | |
| Location of occlusion (M2 or distal) | ||||
| No | reference | 0 | – | <0.01 |
| Yes | 0.92 | 1 | 2.52 (1.29–4.93) | |
The Hosmer-Lemeshow test showed good fitness (8 groups and 6 degrees of freedom; p= 0.58).
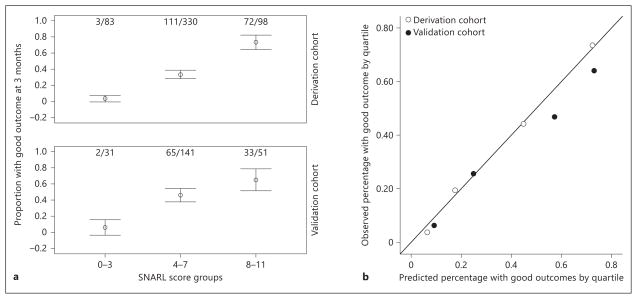
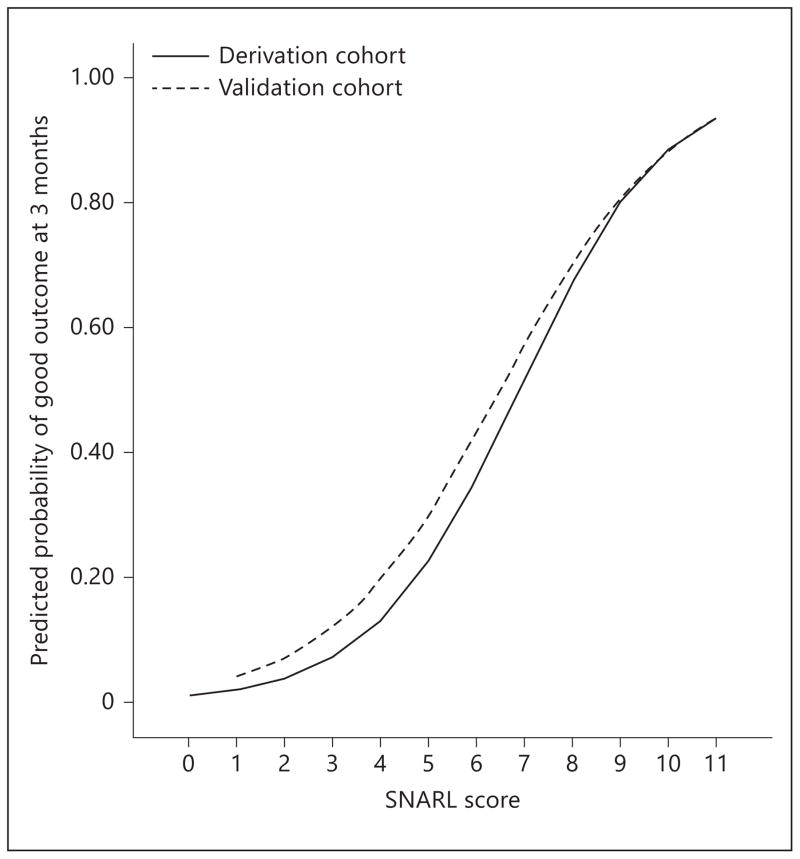
After simplification of continuous variables into relevant strata and weighting by strength in the model, a prediction score (acronym: SNARL) was developed (table 3). The Hosmer-Lemeshow test showed that the model was well calibrated (derivation cohort: χ2 = 5.66, p= 0.58; validation cohort: χ2 = 0.22, p= 0.98). The simplified SNARL score demonstrated good discrimination in the derivation cohort (C statistic 0.79, 95% CI 0.75–0.83) and validation cohort (C statistic 0.74, 95% CI 0.68–0.81); a comparison of these yielded no difference (p= 0.22). Furthermore, this simplified categorical variable model was not significantly different from the continuous variable model (p= 0.37). The observed probability of good outcome in groups of patients divided according to SNARL scores (0–3, 4–7, 8–11) is shown in figure 1a for the derivation and validation cohorts separately, while a plot of observed to predicted outcomes is shown in figure 1b. The predicted probability of good outcome by SNARL score in each cohort is shown in figure 2. We compared the SNARL score to the THRIVE score in the derivation cohort and validation cohorts and found the SNARL score was superior (C statistic 0.79 vs. 0.65 and 0.59, p<0.01 for both comparisons). The reclassification of outcomes based on THRIVE score versus the SNARL score is shown in table 4; the net reclassification improvement was 34.8%.
Fig. 1.
a Proportions are shown with 95% CIs for good outcome (modified Rankin score 0–2) in 3 groups divided according to the SNARL score (0–3, 4–7, 8–11) in both derivation and validation cohorts. Numbers shown above each graph are absolute numbers in each bin. b Calibration of the prediction tool in the derivation and validation samples according to observed versus predicted rate of good outcome at 3 months in quartiles of predicted probability.
Fig. 2.
Predicted probability of good outcome (modified Rankin score 0–2) across the range of the SNARL score in the derivation and validation datasets.
Table 4.
Reclassification of outcome comparing THRIVE and SNARL scores among 731 patients in both the derivation and validation cohorts with all elements available (shaded cells show reclassification)
| THRIVE score classification | SNARL score classification good outcome
|
|||
|---|---|---|---|---|
| <30% probability | 30–50% probability | >50% probability | total | |
| <30% probability | 23 | 10 | 11 | 44 |
| 30–50% probability | 43 | 30 | 82 | 155 |
| >50% probability | 4 | 22 | 61 | 87 |
|
| ||||
| Total | 70 | 62 | 154 | 286 |
| poor outcome
|
||||
|---|---|---|---|---|
| <30% probability | 30–50% probability | >50% probability | total | |
| <30% probability | 97 | 20 | 13 | 130 |
| 30–50% probability | 152 | 33 | 55 | 240 |
| >50% probability | 31 | 7 | 37 | 75 |
|
| ||||
| Total | 280 | 60 | 105 | 445 |
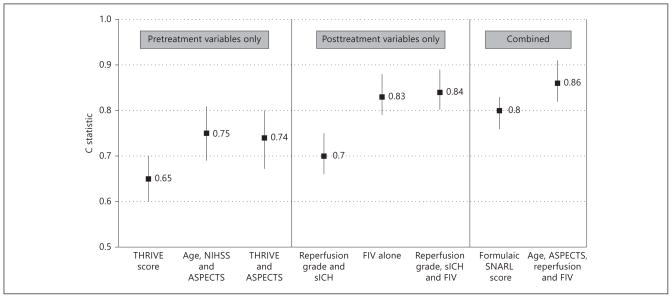
We performed several explorations in an attempt to further improve discrimination (fig. 3). These showed that generally, posttreatment models were superior to pretreatment prediction models. A scoring system with age, ASPECTS, reperfusion status and final infarct volume (C statistic 0.86, 95% CI 0.82–0.91) was significantly better than the simpler SNARL score (p= 0.04). However, since ASPECTS and infarct volumes were not uniformly captured in the derivation cohort, we did not include them for consideration in the validation cohort.
Fig. 3.
Comparison of C statistics for several pretreatment, posttreatment and combined prediction models in the derivation cohort. sICH= Symptomatic hemorrhage; FIV= final infarct volume.
Discussion
In this study, we developed and validated a prediction tool for outcome following ERT. The 12-point SNARL score combines 5 readily available pre- and posttreatment factors (symptomatic hemorrhage, baseline NIHSS score, age, reperfusion grade and location of occlusion) and demonstrated good discrimination and calibration in 2 independent cohorts. It was superior to the pretreatment THRIVE score but inferior to scores that incorporate final infarct volume. The simplified score performed similarly to a more complex formulaic score using continuous variables. The SNARL score may provide clinicians a tool to discuss expectations of outcomes with patients and their families and make early decisions regarding post-stroke care.
Previous scores have focused on pretreatment clinical, laboratory and radiographic predictors of outcomes with good results (C statistics ranging from 0.69–0.76) [3–6, 19]. These scoring systems serve primarily to select patients who are most likely to benefit from ERT, omit the strong influence of treatment factors on that prediction and may not serve to guide clinicians on functional outcome following ERT. Scores including posttreatment variables such as final infarct volume show excellent discrimination for outcome after MCA infarction [20–22]. Our study confirms that posttreatment variables, especially reperfusion status, symptomatic hemorrhage and final infarct volume, have significant influence on outcomes and should be included in prognostication [7, 10]. Furthermore, our study suggests that the THRIVE score may erroneously predict the outcome in one third of patients.
While the SNARL score is simple, it also excludes several variables that could further improve its discrimination. Clinical improvement at 24–36 h could provide a surrogate for long-term outcome but was not available in our datasets. In addition, factors such as sedation and mechanical ventilation may limit examination and also bias the NIHSS score towards those in whom measurement was feasible (i.e. milder strokes). Patients with smaller baseline infarct volumes [23], greater ratios of penumbra to core infarct tissue [24, 25] and collateral flow distal to the occlusion may be more likely to benefit from reperfusion strategies [26, 27]; these radiographic variables were not uniformly available in the derivation cohort. Likewise, medical factors including hyperglycemia, blood pressure and complications such as pneumonia contribute to long-term outcome after ischemic stroke [28, 29]. Lastly, the intensity and timing of rehabilitation would be expected to influence poststroke disability [30, 31]. Outcomes following ERT are also likely dependent on time to and mode of reperfusion [32]. Ascertaining reperfusion time is often challenging in clinical practice due to the nature of the procedure, whereby slow and steady reperfusion or partial followed by complete reperfusion may be impossible to time exactly. As the technology evolves, the recent advances in retrievable stents may further improve the technique, allowing for earlier and higher rates of reperfusion with less hemorrhage risk [8, 9]. Despite these missing elements, improvement in discrimination, if included, is likely to be small.
Besides the lack of the aforementioned parameters, our study has other limitations. As mentioned above, post-treatment neurologic impairment was not measured and might also be a strong predictor of 3-month outcome. Clinical and radiographic data such as reperfusion status and 3-month outcomes were entered by local sites without central adjudication or blinding, raising the potential for bias. Grading of TICI scores can demonstrate significant interobserver variability, depending on whether the primary arterial occlusion was completely or partially recanalized and the presence of distal emboli. Grading of final reperfusion can also demonstrate site bias in favor of better scores, compared to central adjudication, as demonstrated in the SWIFT trial [9]. However, most sites have participated in large endovascular clinical trials, and consensus definitions were used to define key variables such as TICI grade and symptomatic hemorrhage. Site characteristics such as volume of cases per year and experience may also drive outcomes but were not included in this study [33]. We also do not know how many patients were excluded from treatment, thus preventing us from defining the denominator population considered for ERT. Nevertheless, the proportion with good outcomes in our derivation cohort (36%) is similar to those in embolectomy studies [7, 10] but lower than those in the more recent retrievable stent studies [8, 9]. Finally, missing data and data-dependent predictor selection may have introduced biases, and model assumptions may have been erroneous if they did not behave in an additive or linear manner.
The SNARL score is a simple 12-point scoring system to predict 3-month disability after ERT for anterior circulation AIS. It was derived from a multicenter registry and was validated in a large prospective contemporary registry of the Solitaire stent retriever. Further external validation is warranted.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Appendix
NASA Site Investigators
Medical College of Wisconsin/Froedtert Hospital, Milwaukee, Wisc.: Osama O. Zaidat, Brian-Fred Fitzsimmons, John R. Lynch, Marc A. Lazzaro, Alicia C. Castonguay, Mohammad Issa; Emory University School of Medicine, Atlanta, Ga.: Rishi Gupta, Raul G. Nogueira, Chung-Huan Johnny Sun; St. Luke’s Kansas City, Kansas City, Mo.: Coleman O. Martin, William E. Holloway; Delray Medical Center, Delray Beach, Fla.: Nils Mueller-Kronast; California Pacific Medical Center, San Francisco, Calif.: Joey English, Nobl Barazangi, Christine Wong, Charlene Chen, Oana Spataru, Ann Bedenk, Megan Morrow, David Tong; Baptist Cardiac and Vascular Institute, Miami, Fla.: Italo Linfante, Guilherme Dabus, Eugene Lin, Edgar Samaniego; Alexian Brothers Medical Center, Elk Grove Village, Ill.: Tim W. Malisch, Franklin Marden; Oregon Health and Science University, Portland, Oreg.: Hormozd Bozorgchami; Wayne State University School of Medicine, Detroit, Mich.: Andrew Xavier; West Virginia University Hospital, Morgantown, W.Va.: Ansaar Rai, Jennifer Domico; Vanderbilt University Medical Center, Nashville, Tenn.: Michael T. Froehler; University of Iowa, Iowa City, Iowa: Jeri Sieren, Heena Olalde; Provena Saint Joseph Medical Center, Joliet, Ill.: Aamir Badruddin; Boston Medical Center, Boston, Mass.: Thanh N. Nguyen, Alexander M. Norbash, Hesham Masoud, Judith Clark; Desert Regional Medical Center, Palm Springs, Calif.: Muhammad A. Taqi, Tom Wolfe, Ajeet Sodhi; University of Kansas Medical Center, Kansas City, Kans.: Michael G. Abraham; Texas Stroke Institute, Plano, Tex.: Vallabh Janardhan; University of Texas Health Science Center, Houston, Tex.: Hashem Shaltoni; UT Southwestern Medical Center, Dallas, Tex.: Roberta Novakovic, G. Lee Pride Jr., Kim L. Rickert, Babu G. Welch, Jonathan A. White; Massachusetts General Hospital, Boston, Mass.: Albert J. Yoo, Thabele M. Leslie-Mazwi, Joshua A. Hirsch; University of Louisville Medical School, Louisville, Ky.: Alex Abou-Chebl; University of Texas, Houston, Tex.: Peng Roc Chen, Aditya Sanzgiri; Methodist Neurological Institute, Houston, Tex.: Gavin Britz; Duke University Medical Center, Durham, N.C.: Abhishek Agrawal; St. Louis University, St. Louis, Mo.: Ritesh Kaushal; University of Missouri, Columbia, Mo.: Ashish Nanda.
Footnotes
Disclosure Statement
T.G. Jovin: Consultant/Advisory Board, Modest, Concentric Medical, CoAxia, EV3, Neurointerventions; R.G. Nogueira: Consultant/Advisory Board, Modest, EV3, Concentric Medical, CoAxia, Rapid Medical; D.S. Liebeskind: Consultant/Advisory Board, Modest, CoAxia, Concentric Medical; O.O. Zaidat: Consultant/Advisory Board, Modest, EV3, Stryker Neurovascular, Penumbra; R. Gupta: Consultant/Advisory Board, Modest, Concentric Medical, CoAxia, Rapid Medical, Codman Corporation.
None of the other authors have any relevant interests to declare.
References
- 1.Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 2.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 3.Hallevi H, Barreto AD, Liebeskind DS, et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke. 2009;40:1780–1785. doi: 10.1161/STROKEAHA.108.535146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flint AC, Kamel H, Rao VA, et al. Validation of the Totaled Heath Risks In Vascular Events (THRIVE) score for outcome prediction in endovascular stroke treatment. Int J Stroke. 2014;9:32–39. doi: 10.1111/j.1747-4949.2012.00872.x. [DOI] [PubMed] [Google Scholar]
- 5.Flint AC, Cullen SP, Faigeles BS, Rao VA. Predicting long-term outcome after endovascular stroke treatment: the Totaled Health Risks in Vascular Events score. AJNR Am J Neuroradiol. 2010;31:1192–1196. doi: 10.3174/ajnr.A2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fargen KM, Chaudry I, Turner RD, et al. A novel clinical and imaging based score for predicting outcome prior to endovascular treatment of acute ischemic stroke. J Neuro-interv Surg. 2013;(suppl 1):i38–i43. doi: 10.1136/neurintsurg-2012-010513. [DOI] [PubMed] [Google Scholar]
- 7.Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 8.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): A randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 10.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 11.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth KN, Terry JB, Nogueira RG, et al. Advanced modality imaging evaluation in acute ischemic stroke may lead to delayed endovascular reperfusion therapy without improvement in clinical outcomes. Neurointerv Surg. 2013;5(suppl 1):i62–i65. doi: 10.1136/neurintsurg-2012-010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 15.Hill MD, Rowley HA, Adler F, et al. Selection of acute ischemic stroke patients for intra-arterial thrombolysis with prourokinase by using ASPECTS. Stroke. 2003;34:1925–1931. doi: 10.1161/01.STR.0000082483.37127.D0. [DOI] [PubMed] [Google Scholar]
- 16.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaidat OO, Castonguay AC, Gupta R, et al. North American Solitaire Stent Retriever Acute Stroke Registry: post-marketing revascularization and clinical outcome results. J Neurointerv Surg. 2013 doi: 10.1136/neurintsurg-2013-010895. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Sarraj A, Albright K, Barreto AD, et al. Optimizing prediction scores for poor outcome after intra-arterial therapy in anterior circulation acute ischemic stroke. Stroke. 2013;44:3324–3330. doi: 10.1161/STROKEAHA.113.001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liggins JT, Yoo AJ, Mishra NK, et al. A score based on age and DWI volume predicts poor outcome following endovascular treatment for acute ischemic stroke. Int J Stroke. 2013 doi: 10.1111/ijs.12207. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vora NA, Shook SJ, Schumacher HC, et al. A 5-item scale to predict stroke outcome after cortical middle cerebral artery territory infarction: validation from results of the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Stroke. 2011;42:645–649. doi: 10.1161/STROKEAHA.110.596312. [DOI] [PubMed] [Google Scholar]
- 22.Zaidi SF, Aghaebrahim A, Urra X, et al. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke. 2012;43:3238–3244. doi: 10.1161/STROKEAHA.112.671594. [DOI] [PubMed] [Google Scholar]
- 23.Yoo AJ, Verduzco LA, Schaefer PW, et al. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 25.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold M, Mattle S, Galimanis A, et al. Impact of admission glucose and diabetes on re-canalization and outcome after intra-arterial thrombolysis for ischaemic stroke. Int J Stroke. 2012 doi: 10.1111/j.1747-4949.2012.00879.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Johnston KC, Li JY, Lyden PD, Hanson SK, Feasby TE, Adams RJ, et al. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. RANTTAS Investigators. Stroke. 1998;29:447–453. doi: 10.1161/01.str.29.2.447. [DOI] [PubMed] [Google Scholar]
- 30.Langhorne P, Wagenaar R, Partridge C. Physiotherapy after stroke: more is better? Physiother Res Int. 1996;1:75–88. doi: 10.1002/pri.6120010204. [DOI] [PubMed] [Google Scholar]
- 31.Salter K, Jutai J, Hartley M, et al. Impact of early vs delayed admission to rehabilitation on functional outcomes in persons with stroke. J Rehabil Med. 2006;38:113–117. doi: 10.1080/16501970500314350. [DOI] [PubMed] [Google Scholar]
- 32.Khatri P, Abruzzo T, Yeatts SD, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta R, Horev A, Nguyen T, et al. Higher volume endovascular stroke centers have faster times to treatment, higher reperfusion rates and higher rates of good clinical outcomes. J Neurointerv Surg. 2013;5:294–297. doi: 10.1136/neurintsurg-2011-010245. [DOI] [PubMed] [Google Scholar]