Abstract
The ability of interleukin-7 (IL-7) and IL-15 to expand and/or augment effector cell functions may be of therapeutic benefit to human immunodeficiency virus (HIV)-infected patients. The functional effects of these cytokines on innate HIV-specific immunity and their impact on cells harboring HIV are unknown. We demonstrate that both IL-7 and IL-15 augment natural killer (NK) function by using cells (CD3− CD16+ CD56+) from both HIV-positive and -negative donors. Whereas IL-7 enhances NK function through upregulation of Fas ligand, the effect of IL-15 is mediated through upregulation of tumor necrosis factor-related apoptosis-inducing ligand. The difference in these effector mechanisms is reflected by the ability of IL-15-treated but not IL-7-treated NK cells to reduce the burden of replication-competent HIV in autologous peripheral blood mononuclear cells (PBMC) (infectious units per million for control NK cells, 6.79; for IL-7-treated NK cells, 236.17; for IL-15-treated cells, 1.01; P = 0.01 versus control). In addition, the treatment of PBMC with IL-15-treated but not IL-7-treated NK cells causes undetectable HIV p24 (five of five cases), HIV RNA (five of five cases), or HIV DNA (three of five cases). These results support the concept of adjuvant immunotherapy of HIV infection with either IL-7 or IL-15 but suggest that the NK-mediated antiviral effect of IL-15 may be superior.
The successes of present antiretroviral therapies (ART) are underscored by the apparent inability of such therapies to augment either innate or acquired human immunodeficiency virus (HIV)-specific immunity or to effect viral eradication (70). Accordingly, adjuvant approaches have been added to standard antiretroviral agents in attempts to enhance the acquisition of innate or acquired HIV-specific immunity and/or to enhance viral clearance. Two such approaches that have been widely tested include scheduled therapeutic interruptions (STI) and interleukin-2 (IL-2). The effectiveness of STI has been proven in patients with acute HIV disease where intermittent pharmacologic control of viral replication results in enhanced anti-HIV cytolytic T-cell (CTL) activity and partial control of viral replication (61). However, although STI in patients with chronic HIV infection may impact HIV-specific CTL activity, such changes do not meaningfully impact viral replication (3). IL-2 coadministered with ART causes nearly universal increases in CD4 T-cell and natural killer (NK) cell numbers (38). Yet despite increased T-cell numbers, vaccination responses were unchanged in a cohort of patients treated with ART and IL-2 compared to results with ART alone (71), suggesting that acquired immunity is not enhanced by IL-2 cotherapy. Furthermore, despite initial optimism that IL-2 would activate HIV from its latent reservoir and thereby enhance the ability of ART to cause a decline in the size of the HIV latent pool (15), this effect has not translated to viral eradication, even after 2 years of intense ART plus IL-2 therapy (59, 66, 78). Other approaches towards enhancing HIV-specific immunity and enhancing viral clearance are therefore needed, especially for patients with chronic HIV infection.
IL-7 and IL-15 have both been suggested as potential immunomodulators for use in HIV infection. Experimental disruption of either IL-7 (74) or IL-7 receptor (IL-7R) (58) results in severe leukopenia and impaired thymopoesis, indicating a necessary role for this cytokine in T-cell development. However, unlike IL-7R gamma chain deficiency, NK cell numbers and function were preserved in IL-7R alpha chain mutants (60), suggesting that perturbations in IL-7 and IL-7R signaling may have more important effects on T-cell survival than on NK cell biology when compared to the loss of the IL-15 cytokine signaling pathway. While it is clear that IL-7 has a strong positive effect on T cells, less is known about its effect on NK cell survival and function.
Exogenous administration of IL-7 enhances T-cell reconstitution following myeloablation in murine models (9, 48) and stimulates the proliferation and maturation of peripheral T cells (13). In patients with HIV infection, IL-7 levels are inversely correlated with CD4 T-cell lymphopenia, raising the possibility that IL-7 is a naturally occurring homeostatic response (52), a suggestion which is supported by a similar inverse correlation between IL-7 levels and CD4 T-cell numbers in patients with idiopathic CD4 lymphopenia and chemotherapy-induced myeloablation (26). IL-7 augments T-cell numbers by two distinct means: the expansion of existing T cells (2) and enhanced de novo T-cell synthesis, as indicated by increased T-cell receptor excision circle frequency (53).
IL-15-deficient mice demonstrate impaired CD8 and NK cell function (36, 68), suggesting a necessary role of IL-15 in the generation of both innate and acquired effector arms of immunity. Consistent with this suggestion, IL-15 administration in murine models enhances CD8 T-cell number and survival (79), and the presence of IL-15 is absolutely required for the generation of antigen-specific CD8 T cells (62). On a molecular level this requirement may be explained by the ability of IL-15 to enhance the expression of perforin, granzymes A and B, and gamma interferon (IFN-γ) in human CD8 T cells treated in vitro (39). IL-15 also directly activates and enhances the survival of NK cells (75). In patients with HIV infection, the diminished peripheral blood mononuclear cell (PBMC) production of IL-15 correlates with uncontrolled levels of viral replication (21), and in vitro treatment of PBMCs with IL-15 enhances neutrophil chemotaxis and fungicidal activity (46), enhances NK cell survival and proliferation (51), and enhances survival, activation, and IFN-γ production of HIV-specific CD8 T cells (50).
NK cell function is impaired in untreated HIV-infected patients, and consequently NK cells are unable to lyse or suppress viral replication in autologous HIV-infected T lymphocytes (10, 37). Given the failure of NK cell function in HIV-infected patients and the proposed use of IL-7 and IL-15 as immunotherapeutic approaches to HIV infection, we have evaluated the effect of IL-7 and IL-15 on NK cell function by using cells from both HIV-infected and non-HIV-infected donors. While both IL-7 and IL-15 augment NK function, they do so through distinct pathways, which in turn impacts the ability of NK cells stimulated with these cytokines to kill autologous targets which harbor latent HIV.
MATERIALS AND METHODS
Cell lines, cultures, and cytokines.
All cell lines were purchased from American Type Culture Collection (Rockville, Md.) and maintained in complete medium, RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U of penicillin per ml, and 100 mg of streptomycin (Canadian Life Technologies Products, Montreal, Quebec, Canada) per ml. IL-2, IL-7, and IL-15 were purchased from R&D Systems (Minneapolis, Minn.), reconstituted in phosphate-buffered saline (PBS), and frozen at −20°C until use.
Detection of TRAIL and TRAIL receptors by RT-PCR and flow cytometry.
Total RNA was extracted (QIAGEN, Mississauga, Ontario, Canada) from NK cells and quantitated by spectrophotometry (Becton Dickinson). Fifty nanograms of template RNA was used in each reverse transcription (RT)-PCR. Semiquantitative RT-PCR for tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor, Fas ligand (FasL), and β-actin was performed by using previously described conditions and primers (45, 64). RT-PCR products were visualized by 1.5% ethidium bromide-stained agarose gels. The levels of TRAIL and TRAIL receptor and of FasL expression were quantitated by using phosphorimaging, and expression was normalized to the amount of β-actin message. For detection of the surface expression of FasL (NOK-1; Research Diagnostics Inc., Flanders, N.J.) and TRAIL and TRAIL receptors (Immunex Corp., Seattle, Wash.), 5 × 105 cells were stained with monoclonal primary antibodies, as previously described (45). For receptor detection on resting memory cells, peripheral blood lymphocytes (PBL) were isolated from non-HIV-infected donors and sorted by magnetic antibody cell sorting by using CD4, CD45RO, and HLA-DR antibodies. Cells were subsequently stained for TRAIL receptors as described above.
Isolation of primary human NK cells.
PBMCs were isolated by Ficoll-Hypaque (Amersham, Uppsala, Sweden) from HIV-infected or non-HIV-infected donors following informed consent. Monocyte-depleted PBLs (plastic adherence for 3 h) were incubated with anti-CD3-labeled beads (Miltenyi Biotec, Auburn, Calif.) to remove T cells, followed by positive NK cell selection by using anti-CD16 and anti-CD56 beads (Miltenyi Biotec) according to the manufacturer's instructions. NK cell purity (CD3− CD16+ CD56+) was consistently ≥90% as determined by flow cytometry (data not shown).
Cytotoxicity and cell death assay.
Apoptotic assays on PBLs were performed by treatment with control protein or 1 μg of recombinant TRAIL (Immunex) for 16 to 18 h, followed by detection with Hoechst 33482 staining. PBLs were gated by forward and side light scatter. Four-color flow cytometry was used to analyze CD4+ CD45RO HLA-DR− cells (CD4, Texas red; CD45RO, allophycocyanin; and HLA-DR, phycoerythrin) combined with Hoechst staining for apoptotic cells. A determination of cell-mediated cytotoxicity was performed in triplicate by using a flow cytometry-based assay (31). Only if variation between triplicate samples was <10% were the results accepted. NK effector cells (106) were stimulated for 72 h with 10 ng of IL-2, IL-7, or IL-15 (R&D Systems) per ml in complete medium and washed extensively with PBS to ensure that no residual cytokine was present at the time of coculture. K562 target cells were grown as described above, collected, and washed twice in PBS. Cells were resuspended in phenol red-free RPMI medium to a final density of 106 cells/ml. K562 target cells were labeled with 10 mM 3,3-dioctadecyloxacarbocyanine (DiO; Molecular Probes, Eugene, Ore.) for 20 min at 37°C and washed three times with PBS. Target and effector cells were mixed together at effector/target ratios of 50:1, 25:1, 12.5:1 and 6.25:1 (12) or with the indicated effector/target ratios. Cells were coincubated for 3 h. Propidium iodide (PI; 1 μg/ml) (Sigma, St. Louis, Mo.) was added to the cells and incubated for an additional 20 min. To block ligand- and receptor-induced cell death, 1 μg of antibodies specific for TRAIL (M181; Immunex Corp.) or FasL (NOK-1; Research Diagnostics, Inc.) per ml was added prior to adding the targets cells. Percent specific cytotoxicity was determined by the following formula: (the number of PI+ plus DiO+ cells/total number of DiO+ cells) × 100%. Prior to the assays, both effector and target cell viabilities were determined by PI staining to be less than 10% PI+.
Isolation of HIV by limiting-dilution quantitative micrococulture in PBL from infected patients.
NK cells were isolated from HIV-infected donors by using magnetic bead separation as described above. NK cells were stimulated with IL-2, IL-7, or IL-15 for 72 h, washed extensively, and cocultured with autologous PBL for 16 h at an effector/target ratio of 5:1. After the coculture, NK cells were depleted from the coculture by positive selection by using CD16 and CD56 magnetic beads. The remaining PBL were then subjected to quantitative micrococulture (16, 23, 45) to determine the frequency of replication-competent HIV within the PBMCs. The amount of viral replication was quantitated by using (i) p24 enzyme-linked immunosorbent assay (Perkin-Elmer, Boston, Mass.), (ii) viral RNA (Bayer Diagnostics, Markham, Ontario, Canada), and (iii) proviral DNA by semiquantitative PCR, as previously described (45), and maximum likelihood analysis calculated to estimate infectious units per million (IUPM) cells.
Statistical analysis.
Assays of NK cell cytotoxicity were all compared by using a paired t test. Maximum likelihood analysis was used to determine the frequency of replication-competent HIV cells within the PBMC population, as described previously (16, 23, 45). Student's t tests were used in cytotoxicity assays on control versus cytokine treatments in both HIV-infected and non-HIV-infected groups, with a Bonferroni correction for multiple comparisons.
RESULTS
NK cell activity in patients with suppressed viremia is enhanced by in vitro treatment with IL-7 and IL-15.
Recent data have suggested two distinct populations of NK cells, CD56+ (bright) and CD56+ (dim), that appear to differ functionally as well as developmentally (19, 22). Since we were interested in the activity of the entire NK cell pool, we chose to study all CD56+ CD16+ CD3− cells. Henceforth, we will define total NK cells as CD56+ CD16+ CD3−. To assess what effect HIV infection has on NK cell function, we assayed NK cell activity in cells from patients with detectable levels of plasma viremia. In NK cells from infected patients with unsuppressed viral replication, NK cell activity was reduced compared to levels of activity in cells from noninfected controls (Fig. 1A), which is consistent with previous reports that untreated HIV infection perturbs NK cell activity (56). When we performed a similar analysis on NK cells from HIV-infected patients with suppressed levels of plasma viremia, we observed no significant difference in the cytolytic activity of K562 target cells compared to activity in noninfected controls, confirming our prior observation that suppression of viremia by effective highly active antiretroviral therapy leads to normalization of NK cell activity (56) (Fig. 1B).
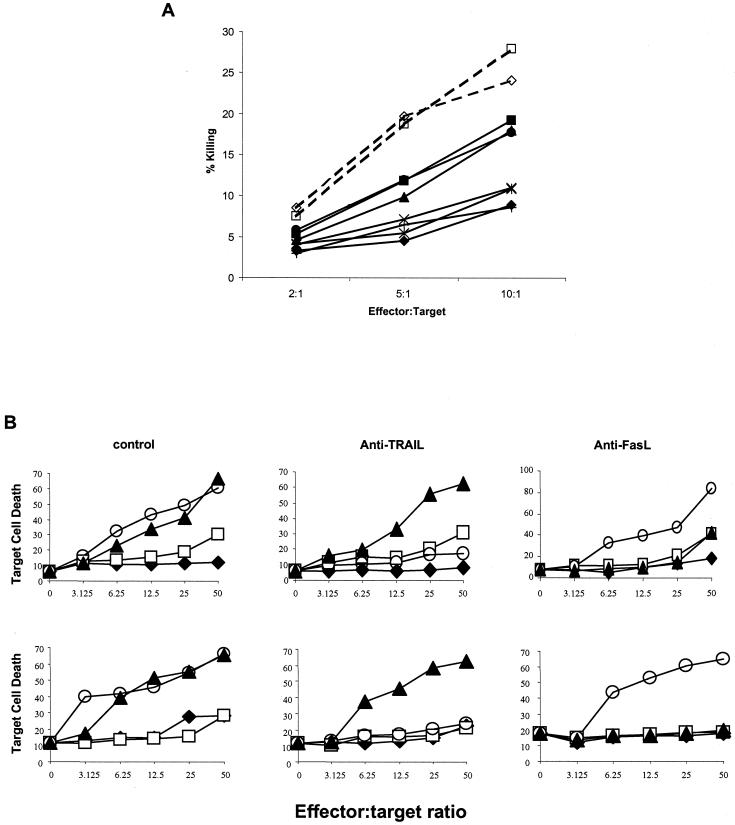
FIG. 1.
(A) NK cells from HIV patients with detectable viremia have decreased NK cell killing activity against target cells. CD3− CD16+ CD56+ NK cells from patients with unsuppressed viral replication (solid lines) or from noninfected donors (dashed lines) were isolated and subjected to cytolytic assay using K562 target cells in triplicate (P < 0.05 for noninfected versus infected donors). Each symbol represents a different patient sample. (B) IL-7 and IL-15 enhance cytolytic activity of NK cells from HIV-infected patients with suppressed viral loads. CD3− CD16+ CD56+ NK cells from noninfected donors (upper panel) or HIV-infected patients with suppressed viral replication (lower panel) were either untreated (control, ♦) or stimulated with 10 ng each of IL-2 (□), IL-7 (▴), or IL-15 (○) per ml for 72 h. NK cell cytolytic activity was assayed for 3 h in the presence or absence of 1.0 μg of neutralizing TRAIL or FasL Abs per ml, as indicated, in triplicate. Results are representative of four independent experiments.
Next, we determined whether IL-7 or IL-15 could further enhance NK cell cytolytic activity in purified NK cells from HIV-infected patients with suppressed viremia or in non-HIV-infected donors. Isolated NK cells were left untreated or treated for 72 h with 10 ng of IL-7, IL-15, or IL-2 per ml and used as effectors in our K562 cytotoxicity assay. Following 72-h culture in the absence of stimulation, control cells had minimal cytotoxicity against the K562 target cells. As expected, treatment with low-dose IL-2 increased NK cell cytotoxicity against K562, particularly at the higher effector/target ratios. Treatment of purified NK cells from HIV-infected (Fig. 1B, lower panels) or non-HIV-infected donors (Fig. 1B, upper panels) with IL-7 significantly enhanced NK cell-mediated K562 target cell killing (P = 0.04 for noninfected donors, and P = 0.04 for infected donors). IL-7-treated NK cell-mediated killing of K562 target cells was reduced by neutralizing FasL antibodies (Abs) in cells from both HIV-infected (P = 0.03) and non-HIV-infected (P = 0.04) patients but not by neutralizing TRAIL Abs. IL-15 treatment of NK cells from HIV-infected or non-HIV-infected individuals had significantly enhanced levels of K562 target cell killing (for noninfected donors, P = 0.03; for infected donors, P = 0.01). The enhanced killing by IL-15-treated NK cells was inhibited by TRAIL-neutralizing Abs (for noninfected donors, P = 0.04; for infected donors, P = 0.03). In contrast, FasL-neutralizing Abs had no effect on the cytolytic activity of IL-15-treated NK cells.
IL-7 and IL-15 differentially modulate TRAIL and FasL expression in CD3− CD16+ CD56+ human NK cells.
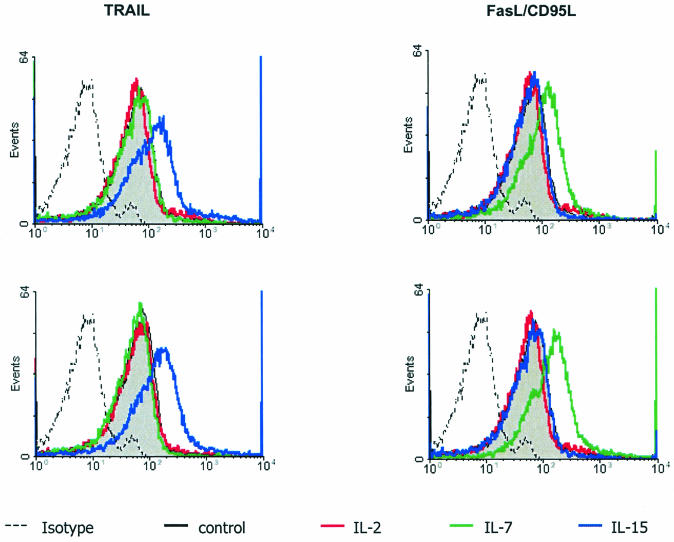
In light of the effects of IL-7 and IL-15 on NK cell function, we assessed whether IL-2-, IL-7-, or IL-15-treated CD3− CD16+ CD56+ human NK cells alter the expression of death-inducing ligands as assessed by semiquantitative PCR for TRAIL and TRAIL receptors and for FasL. Compared to untreated cultures, treatment with IL-2, IL-7, and IL-15 caused no change in TRAIL receptors 1, 2, 3, or 4 mRNA in NK cells from healthy subjects or HIV-infected individuals (data not shown). IL-15 treatment of NK cells did, however, increase TRAIL transcripts by 4.9-fold in NK cells from healthy subjects and by 4.5-fold in NK cells from infected patients (data not shown). No changes in FasL mRNA expression were detected in IL-15-treated cultures. FasL mRNA was increased 2-fold by IL-7 in cells from healthy individuals and 3.2-fold in cells from HIV-infected patients (data not shown), yet IL-7 had no effect on TRAIL mRNA expression. Consistent with RT-PCR data, IL-2, IL-7, and IL-15 did not affect surface expression of TRAIL receptor 1, 2, 3, or 4 (data not shown), while TRAIL was upregulated 1.5-fold by IL-15 in HIV-negative patients (mean fluorescence intensity of control was 9.4 versus 14.1 for IL-15-treated patients; P = 0.03) and 2.8-fold in cells from HIV-positive patients (mean fluorescence intensity of control was 7.4 versus 20.4 for IL-15-treated patients; P = 0.03) (Fig. 2). IL-7 increased FasL expression 3.4-fold in NK cells from HIV-negative patients (mean fluorescence intensity of control was 10.2 versus 35.1 for IL-7-treated patients; P = 0.03) and 2-fold in HIV-positive patients (mean fluorescence intensity of control was 7.7 versus 15 for IL-7-treated patients; P = 0.04). These data suggest that IL-7 and IL-15 have distinct effects on the regulation of death-inducing ligands, and this differential effect on ligand expression provides a potential mechanism for the differential NK cell cytolytic activity following IL-7 or IL-15 treatment.
FIG. 2.
IL-7 and IL-15 treatments of NK cells from both HIV-infected and non-HIV-infected individuals have differential enhancement of the death receptor FasL and TRAIL. CD3− CD16+ CD56+ NK cells from non-HIV-infected donors (top panels) or HIV-infected donors (bottom panels) were treated with control or 10 ng of IL-2, IL-7, or IL-15 per ml and analyzed by flow cytometry for TRAIL or FasL/CD95L expression. All histograms are representative of four independent analyses (n = 4), and background mean fluorescence intensity was normalized by using isotype control Abs.
PBL and resting memory cells from HIV-infected patients are sensitive to killing by TRAIL.
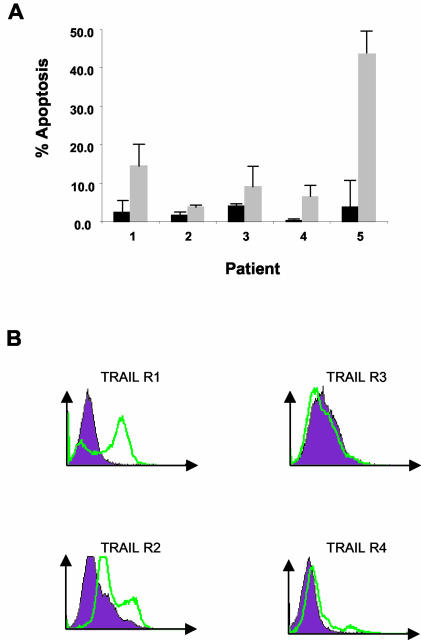
To determine whether cells from HIV-infected patients are sensitive to TRAIL-mediated killing, we treated cells from individual patients with a vehicle control or TRAIL for 16 h. In all cases and in agreement with other previously published studies, PBL from HIV-infected patients with suppressed viral replication underwent apoptosis as determined by Hoechst staining (data not shown) (29, 33, 45). We further examined whether the resting memory subset within these PBL was also sensitive to TRAIL-mediated killing. PBL were stained and analyzed by flow cytometry, focusing specifically on the CD4+ CD45RO+ HLA-DR− population which contains the latently infected resting memory T-cell pool. TRAIL treatment induces apoptosis of this resting memory subset of cells within the total PBL population (Fig. 3B). When we analyzed the expression of TRAIL receptors in the same resting memory cells, we found high levels of the death receptors TRAIL-R1 and TRAIL-R2 compared to low levels of TRAIL-R3 and TRAIL-R4 (Fig. 3B) (45). Collectively, this finding indicates that PBL as well as the resting memory cell subset from HIV-infected patients express TRAIL death receptors and are sensitive to TRAIL-induced apoptosis.
FIG. 3.
Resting memory cells from HIV-infected individuals have increased TRAIL-R1 and TRAIL-R2 expression and are susceptible to TRAIL killing in vitro. (A) CD4+ CD45RO+ HLA-DR− cells undergo apoptosis following treatment with 1.0 μg of TRAIL per ml when compared to control treatments. (B) Resting memory cells have high levels of endogenous expression of surface TRAIL-R1 and TRAIL-R2 and low levels of expression of TRAIL-R3 and TRAIL-R4. The purple area represents isotype control staining; the green lines represent staining with the indicated anti-TRAIL antibody.
IL-7- and IL-15-stimulated NK cells from HIV-infected patients demonstrate enhanced killing of autologous peripheral blood reservoirs of HIV.
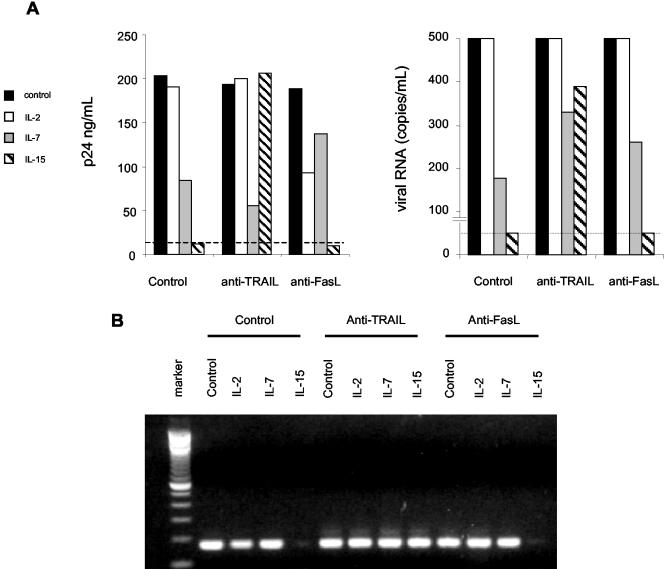
We have recently described that in vitro treatment of resting memory CD4 T cells and/or PBMCs with recombinant TRAIL can reduce the burden of replication-competent HIV within these cell populations (45). Therefore, we assessed whether NK cells treated with IL-7 or IL-15 could impact the viral burden of cells from HIV-infected patients. NK effector cells from HIV-positive patients were stimulated with IL-2, IL-7, or IL-15 for 72 h and subsequently used in a cytotoxicity assay against fresh autologous PBL. Following a 16-h coculture of NK effector cells and PBL targets, the NK cells were depleted, and the remaining PBL were analyzed for residual burden by limiting-dilution quantitative micrococulture (16, 23). In all five subjects, PBL treated with IL-15-stimulated NK cells had undetectable HIV p24 antigen and viral RNA (Fig. 4A and Table 1). Neutralizing anti-TRAIL Abs but not anti-FasL Abs eliminated the ability of IL-15-stimulated NK cells to reduce measurable HIV production from microculture. NK cells stimulated with IL-7 also reduced the level of virus present in cocultures, although not to undetectable levels (Fig. 4A and Table 1; P = 0.01 by t test). The addition of anti-FasL Abs but not anti-TRAIL Abs blocked the ability of IL-7-stimulated NK cells to reduce HIV p24 and RNA production from microcultures. Results obtained with p24 antigen and RNA were confirmed by IUPM estimates. In control cultures, the average IUPM estimates in our five patients was 5.06, while IL-15-stimulated NK cells resulted in an average IUPM of 2.48 (Table 1; P = 0.01). The addition of anti-TRAIL but not anti-FasL to IL-15-stimulated NK cell cocultures negated the reduction in IUPM (P value was not significant versus control). By contrast, the IUPM of IL-7-stimulated NK cell cocultures was consistently higher (P < 0.001) than that of controls.
FIG. 4.
Autologous PBL cocultured IL-15-treated NK cells have undetectable levels of HIV replication, viral RNA, and, in some cases, viral DNA. NK cells from HIV-infected patients were incubated with the indicated cytokines for 72 h, followed by coculture with autologous PBL for 3 h in the presence or absence of neutralizing TRAIL or FasL/CD95L antibodies. (A) CD56+ CD16+ NK cells were depleted, and the remaining PBL were subjected to limiting-dilution quantitative micrococulture and assayed for HIV p24 or HIV RNA (103 copies/ml). A dashed line indicates a p24 antigen limit of detection of 11.3 pg/ml (left panel) or a viral RNA limit of detection of 50 copies/ml (right panel). (B) Treated PBL were also assayed for HIV DNA. Molecular weight markers are included for reference.
TABLE 1.
Clinical profiles of HIV patients on suppressive highly active antiretroviral therapy and their responses following in vitro coculture of autologous PBL with cytokine-treated NK cells
| Patient no. | Patient characteristics
|
Viral load following in vitro NK cell coculture
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 (cells/ml) | Viral load (copies/ml) | Therapya | Treatment | IUPM
|
Viral RNA (copies/ml)
|
|||||||
| Control | IL-2 | IL-7 | IL-15 | Control | IL-2 | IL-7 | IL-15 | |||||
| 1 | 126.5 | <50 | RTV, SQV | Control | 8.08 | 8.08 | 236.17 | 1.01b | >500 | 436 | 133 | <50 |
| Anti-TRAIL | 1.61 | 1.61 | 236.17 | 8.08 | >500 | 389 | 110 | 376 | ||||
| Anti-FasL | 8.08 | 1.61 | 236.17 | 1.61 | >500 | 445 | 361 | >50 | ||||
| 2 | 877 | <50 | RTV, SQV | Control | 1.61 | 1.61 | 236.17 | 1.01 | >500 | >500 | 179 | >50 |
| Anti-TRAIL | 1.61 | 1.61 | 236.17 | 1.61 | >500 | >500 | 327 | 379 | ||||
| Anti-FasL | 1.61 | 1.61 | 236.17 | 1.01 | >500 | >500 | 279 | <50 | ||||
| 3 | 866 | <50 | RTV, SQV, 3TC, d4T | Control | 8.08 | 1.61 | 236.17 | 1.01b | >500 | >500 | 198 | <50 |
| Anti-TRAIL | 1.61 | 1.61 | 236.17 | 1.61 | >500 | >500 | 364 | 455 | ||||
| Anti-FasL | 1.61 | 1.61 | 236.17 | 1.01 | >500 | >500 | 122 | <50 | ||||
| 4 | 573 | <50 | RTV, SQV, 3TC, d4T | Control | 8.08 | 8.08 | 236.17 | 1.01b | >500 | >500 | 300 | <50 |
| Anti-TRAIL | 8.08 | 8.08 | 236.17 | 8.08 | >500 | >500 | 168 | 462 | ||||
| Anti-FasL | 8.08 | 8.08 | 236.17 | 1.01 | >500 | >500 | 110 | <50 | ||||
| 5 | 907 | <50 | RTV, SQV | Control | 8.08 | 8.08 | 236.17 | 1.01 | >500 | >500 | 155 | <50 |
| Anti-TRAIL | 1.61 | 1.61 | 236.17 | 8.08 | >500 | >500 | 196 | >500 | ||||
| Anti-FasL | 8.08 | 8.08 | 236.17 | 1.01 | >500 | >500 | 258 | <50 | ||||
RTV, ritonavir; SQV, saquinavir; 3TC, lamivudine; d4T, stavudine.
Viral DNA undetectable by PCR.
To further assess the impact of the IL-7 and IL-15 stimulation of NK cells, we directly assessed the viral DNA content of treated PBL by using a nested PCR assay with a sensitivity limit of one copy of DNA provirus (25, 28). In these experiments, three of five IL-15 cultures contained undetectable levels of HIV DNA (Fig. 4B; Table 1, values marked with asterisks). As with our results obtained by using p24, RNA, and IUPM estimates, coadministration of anti-TRAIL but not anti-FasL Abs eliminated the antiviral effects of IL-15-stimulated NK cells. In contrast, in IL-7-treated cultures, all samples contained HIV DNA (Table 1).
IL-15-treated NK cells are nontoxic to cells from HIV-negative patients.
The ability of NK cells stimulated by IL-15 to affect viral clearance from infected cell populations suggests a potential therapeutic strategy. However, in order for such an approach to be clinically applicable, it is necessary that such IL-15-stimulated NK cells do not exert nonspecific cytotoxicity against noninfected targets. Since recombinant TRAIL and/or selective TRAIL agonists are sufficiently nontoxic in preclinical models that phase 1 studies in patients with cancer are under way, such nonspecific toxicity is unlikely. Nonetheless, NK cells were incubated with control vehicle, IL-2, or IL-15 and used as effectors against PBL from noninfected donors. Over a range of effector/target ratios, NK cell killing was similar between groups; at an effector/target ratio of 2:1, control NK cell killing was 6.2%, IL-2-stimulated NK cell killing was 6.5%, and IL-15-stimulated NK cell killing was 7.1% (P value was not significant). Similar differences were seen at effector/target ratios of 5:1 and 10:1, indicating that IL-15-stimulated NK cells do not exert nonspecific target cell killing above and beyond that of control cells.
DISCUSSION
In HIV-infected patients, anti-HIV NK cell responses are inadequate to eliminate reservoirs, and global NK cell functions are impaired in patients with unsuppressed viral replication. In the present report, we describe augmentation of NK cell function by in vitro therapy with either IL-7 or IL-15, yet IL-15 therapy but not IL-7 augments NK cell activity against HIV reservoirs.
NK cells are effectors of the innate immune response that, once activated, can exert antiviral (4, 7, 8, 24, 55) and antitumor (20, 67) host defenses. Target cell death mediated by NK cells is mediated by two predominant mechanisms: the Ca2+-dependent release of perforin and granzymes (77) and production of members of the TNF family of death-inducing ligands, such as FasL (42, 57), TNF alpha (77), and TRAIL. Recently, it has been shown that TRAIL contributes to both human (30, 32, 77) and mouse (20, 32, 35, 65, 67) NK cell protection from tumor metastasis and provides evidence for the central role of death receptor-mediated apoptosis in tumor rejection. Moreover, the expression of death-inducing ligands on other immune-relevant cells (e.g., CTL or activated monocytes/macrophages) represents the principal effector mechanisms of defense against transformed and virally infected cells (40).
During HIV infection the loss of NK cell function may contribute to the loss of immune-mediated viral control and progression to AIDS (43, 47). A significantly lower proportion of CD3− CD16+ CD56+ NK cells are found in HIV-infected patients with uncontrolled HIV replication (6), in comparison to patients with therapeutically suppressed HIV viremia. Moreover, NK cell activity is impaired by both a reduction of NK cell number and a reduction in NK cell cytolytic function (27, 44, 69). Data in our present report confirm that NK cell function in patients with uncontrolled HIV replication have impaired cytolytic activity. Mechanisms for reduced NK cell function are obscure; however, one intriguing possibility is that CD56+ CD3− NK cells are capable of expressing both CD4 and CCR5/CXCR4, which renders them susceptible to HIV infection, resulting in both quantitative and qualitative impairments in NK cell function (49, 72). It has alternatively been proposed that modifications in target cells can alter their susceptibility to immune clearance; for example, downregulation of HLA-A and HLA-B on HIV-infected target cells may provide a means of escape from CTL-mediated killing, and high expression of HLA-C and HLA-E stimulates NK cell inhibitory signals which prevent NK cell-mediated lysis (10, 18, 69). In addition, NK cells from HIV-infected patients have decreased IFN-γ production and impaired killing of the NK target cell K562 (14). More recently, NK cell production of CC-chemokines has been documented and shown to exert anti-HIV effects (37). Whatever the mechanism, the NK cell effector arm of the innate immune response may contribute to the ability of HIV to escape immune clearance and allow for the establishment of viral reservoirs.
Our analysis found that both TRAIL and TRAIL receptors were expressed on the surface of NK cells, yet these cells do not appear to undergo spontaneous forms of death. PBL from noninfected individuals as well as murine T cells, macrophages, and NK cells exhibit similar levels of TRAIL and TRAIL receptors but also do not self-destruct (32, 67). This may be the result of a threshold switch between the redundant death receptors and decoy receptors. Indeed, NK cells from noninfected individuals express high levels of TRAIL-R1 and lower levels of TRAIL-R2 compared to levels in NK cells from infected patients. However, TRAIL-R2 levels increase while TRAIL-R1 levels decrease in NK cells from infected donors compared to levels in noninfected donors. Clearly, our understanding of the molecular mechanism of acquiring TRAIL sensitivity is lacking, and future work in this area will be important in our knowledge of TRAIL biology.
The findings that (i) NK cells can mediate cytotoxicity by TRAIL (7, 32, 35, 54, 63, 65, 77), (ii) that IL-15 potentiates NK cell cytolytic activity, and (iii) that TRAIL has intrinsic activity against a variety of virally infected targets (5, 11, 14, 17, 21, 34, 41, 45, 46, 73, 76) suggest a role for IL-15 to potentiate TRAIL-mediated NK cell viricidal activity. We demonstrate that PBL and resting memory cells from infected individuals express high levels of the TRAIL death receptors and undergo cell death following TRAIL treatments (29, 33, 45), suggesting that these cells are potential targets for TRAIL killing. Indeed, we demonstrate that IL-15-stimulated NK cells from HIV-infected patients have enhanced TRAIL expression and acquire a cytotoxicity phenotype against autologous PBL, causing a significant decrease in detectable replication-competent HIV and HIV proviral DNA. Most notably, in three of five cases, HIV proviral DNA becomes undetectable following coculture with IL-15-stimulated NK cells. This effect is eliminated by antagonists of TRAIL and occurs following overnight coculture, suggesting that the effect is mediated by an inhibitor of replication (e.g., chemokines) and, specifically, by the cytotoxic ligand TRAIL. While cultures of IL-7-stimulated NK cells have enhanced FasL expression and function, IL-7-stimulated NK cells do not impact HIV levels. Such data are consistent with a selective sensitivity of HIV-infected cells towards TRAIL but not FasL-mediated lysis (45). A somewhat unexpected observation is that in some cases IL-7-stimulated NK cells increase the amount of virus present in autologous cocultured PBL. Although the possible reasons for this increase are speculative, these data are consistent with the known ability of FasL to induce proliferation, particularly in resting cells (such as latently HIV-infected resting memory T cells) (1).
In the present report, we confirm the induction of TRAIL expression in NK (CD3− CD56+ CD16+) cells cultured with IL-15 (35, 67, 77); we demonstrate further that this effect is also seen in NK cells from HIV-infected donors and that TRAIL expression is not modulated by IL-2 or IL-7. Finally, given that CD4 cells from HIV-infected patients, including the resting memory subset, are sensitive to TRAIL-mediated killing (29, 33, 45) and that killing of these cells in vitro (along with the virus they contain) can reduce viral burden, our data indicate a novel approach towards augmenting innate anti-HIV activity and provide an additional rationale supporting the immunotherapeutic role of IL-15 for HIV infection.
Acknowledgments
A.D.B. is supported by grants from the National Institutes of Health (R01 AI40384-06 and 1 R21 AI054187-01-A1) and the Doris Duke Charitable Foundation (grant 19980002).
We also thank Teresa Hoff for her secretarial assistance in preparation of the manuscript.
REFERENCES
- 1.Alderson, M. R., R. J. Armitage, E. Maraskovsky, T. W. Tough, E. Roux, K. Schooley, F. Ramsdell, and D. H. Lynch. 1993. Fas transduces activation signals in normal human T lymphocytes. J. Exp. Med. 178:2231-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Harthi, L., and A. Landay. 2002. Immune recovery in HIV disease: role of the thymus and T cell expansion in immune reconstitution strategies. J. Hematother. Stem Cell Res. 11:777-786. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld, M., J. van Lunzen, N. Frahm, X. G. Yu, C. Schneider, R. L. Eldridge, M. E. Feeney, D. Meyer-Olson, H. J. Stellbrink, and B. D. Walker. 2002. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Investig. 109:837-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atedzoe, B. N., A. Ahmad, and J. Menezes. 1997. Enhancement of natural killer cell cytotoxicity by the human herpesvirus-7 via IL-15 induction. J. Immunol. 159:4966-4972. [PubMed] [Google Scholar]
- 5.Azimi, N., Y. Tagaya, J. Mariner, and T. A. Waldmann. 2000. Viral activation of interleukin-15 (IL-15): characterization of a virus-inducible element in the IL-15 promoter region. J. Virol. 74:7338-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzoni, L., E. Papasavvas, J. Chehimi, J. R. Kostman, K. Mounzer, J. Ondercin, B. Perussia, and L. J. Montaner. 2002. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J. Immunol. 168:5764-5770. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A. 1997. Natural killer cell regulation during viral infection. Biochem. Soc. Trans. 25:687-690. [DOI] [PubMed] [Google Scholar]
- 8.Biron, C. A., H. C. Su, and J. S. Orange. 1996. Function and regulation of natural killer (NK) cells during viral infections: characterization of responses in vivo. Methods 9:379-393. [DOI] [PubMed] [Google Scholar]
- 9.Bolotin, E., M. Smogorzewska, S. Smith, M. Widmer, and K. Weinberg. 1996. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood 88:1887-1894. [PubMed] [Google Scholar]
- 10.Bonaparte, M. I., and E. Barker. 2003. Inability of natural killer cells to destroy autologous HIV-infected T lymphocytes. AIDS 17:487-494. [DOI] [PubMed] [Google Scholar]
- 11.Carson, W. E., T. A. Fehniger, S. Haldar, K. Eckhert, M. J. Lindemann, C. F. Lai, C. M. Croce, H. Baumann, and M. A. Caligiuri. 1997. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Investig. 99:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, L., G. A. Gusewitch, D. B. Chritton, J. C. Folz, L. K. Lebeck, and S. L. Nehlsen-Cannarella. 1993. Rapid flow cytometric assay for the assessment of natural killer cell activity. J. Immunol. Methods 166:45-54. [DOI] [PubMed] [Google Scholar]
- 13.Chazen, G. D., G. M. Pereira, G. LeGros, S. Gillis, and E. M. Shevach. 1989. Interleukin-7 is a T-cell growth factor. Proc. Natl. Acad. Sci. USA 86:5923-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chehimi, J., J. D. Marshall, O. Salvucci, I. Frank, S. Chehimi, S. Kawecki, D. Bacheller, S. Rifat, and S. Chouaib. 1997. IL-15 enhances immune functions during HIV infection. J. Immunol. 158:5978-5987. [PubMed] [Google Scholar]
- 15.Chun, T. W., D. Engel, S. B. Mizell, C. W. Hallahan, M. Fischette, S. Park, R. T. Davey, Jr., M. Dybul, J. A. Kovacs, J. A. Metcalf, J. M. Mican, M. M. Berrey, L. Corey, H. C. Lane, and A. S. Fauci. 1999. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat. Med. 5:651-655. [DOI] [PubMed] [Google Scholar]
- 16.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke, P., S. M. Meintzer, S. Gibson, C. Widmann, T. P. Garrington, G. L. Johnson, and K. L. Tyler. 2000. Reovirus-induced apoptosis is mediated by TRAIL. J. Virol. 74:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 19.Cooper, M. A., T. A. Fehniger, and M. A. Caligiuri. 2001. The biology of human natural killer-cell subsets. Trends Immunol. 22:633-640. [DOI] [PubMed] [Google Scholar]
- 20.Cretney, E., K. Takeda, H. Yagita, M. Glaccum, J. J. Peschon, and M. J. Smyth. 2002. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J. Immunol. 168:1356-1361. [DOI] [PubMed] [Google Scholar]
- 21.d'Ettorre, G., G. Forcina, M. Lichtner, F. Mengoni, C. D'Agostino, A. P. Massetti, C. M. Mastroianni, and V. Vullo. 2002. Interleukin-15 in HIV infection: immunological and virological interactions in antiretroviral-naive and -treated patients. AIDS 16:181-188. [DOI] [PubMed] [Google Scholar]
- 22.Fehniger, T. A., and M. A. Caligiuri. 2001. Ontogeny and expansion of human natural killer cells: clinical implications. Int. Rev. Immunol. 20:503-534. [DOI] [PubMed] [Google Scholar]
- 23.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 24.Flamand, L., I. Stefanescu, and J. Menezes. 1996. Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J. Clin. Investig. 97:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fransen, K., P. Zhong, H. De Beenhouwer, G. Carpels, M. Peeters, J. Louwagie, W. Janssens, P. Piot, and G. van der Groen. 1994. Design and evaluation of new, highly sensitive and specific primers for polymerase chain reaction detection of HIV-1 infected primary lymphocytes. Mol. Cell. Probes 8:317-322. [DOI] [PubMed] [Google Scholar]
- 26.Fry, T. J., E. Connick, J. Falloon, M. M. Lederman, D. J. Liewehr, J. Spritzler, S. M. Steinberg, L. V. Wood, R. Yarchoan, J. Zuckerman, A. Landay, and C. L. Mackall. 2001. A potential role for interleukin-7 in T-cell homeostasis. Blood 97:2983-2990. [DOI] [PubMed] [Google Scholar]
- 27.Hu, P. F., L. E. Hultin, P. Hultin, M. A. Hausner, K. Hirji, A. Jewett, B. Bonavida, R. Detels, and J. V. Giorgi. 1995. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56− cells with low lytic activity. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:331-340. [PubMed] [Google Scholar]
- 28.Janssens, W., K. Fransen, I. Loussert-Ajaka, L. Heyndrickx, T. Ivens, J. Eberle, and J. Nkengasong. 1995. Diagnosis of HIV-1 group O infection by polymerase chain reaction. Lancet 346:451-452. [DOI] [PubMed] [Google Scholar]
- 29.Jeremias, I., I. Herr, T. Boehler, and K. M. Debatin. 1998. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur. J. Immunol. 28:143-152. [DOI] [PubMed] [Google Scholar]
- 30.Johnsen, A. C., J. Haux, B. Steinkjer, U. Nonstad, K. Egeberg, A. Sundan, A. Ashkenazi, and T. Espevik. 1999. Regulation of APO-2 ligand/trail expression in NK cells—involvement in NK cell-mediated cytotoxicity. Cytokine 11:664-672. [DOI] [PubMed] [Google Scholar]
- 31.Kane, K. L., F. A. Ashton, J. L. Schmitz, and J. D. Folds. 1996. Determination of natural killer cell function by flow cytometry. Clin. Diagn. Lab. Immunol. 3:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashii, Y., R. Giorda, R. B. Herberman, T. L. Whiteside, and N. L. Vujanovic. 1999. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J. Immunol. 163:5358-5366. [PubMed] [Google Scholar]
- 33.Katsikis, P. D., M. E. Garcia-Ojeda, J. F. Torres-Roca, I. M. Tijoe, C. A. Smith, and L. A. Herzenberg. 1997. Interleukin-1 beta converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J. Exp. Med. 186:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz, J. D., R. Mitsuyasu, M. S. Gottlieb, L. T. Lebow, and B. Bonavida. 1987. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. II. Normal antibody-dependent cellular cytotoxicity (ADCC) mediated by effector cells defective in natural killer (NK) cytotoxicity. J. Immunol. 139:55-60. [PubMed] [Google Scholar]
- 35.Kayagaki, N., N. Yamaguchi, M. Nakayama, K. Takeda, H. Akiba, H. Tsutsui, H. Okamura, K. Nakanishi, K. Okumura, and H. Yagita. 1999. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J. Immunol. 163:1906-1913. [PubMed] [Google Scholar]
- 36.Kennedy, M. K., M. Glaccum, S. N. Brown, E. A. Butz, J. L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C. R. Willis, K. Brasel, P. J. Morrissey, K. Stocking, J. C. Schuh, S. Joyce, and J. J. Peschon. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191:771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kottilil, S., T. W. Chun, S. Moir, S. Liu, M. McLaughlin, C. W. Hallahan, F. Maldarelli, L. Corey, and A. S. Fauci. 2003. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 187:1038-1045. [DOI] [PubMed] [Google Scholar]
- 38.Lalezari, J. P., J. A. Beal, P. J. Ruane, C. J. Cohen, E. L. Jacobson, D. Sundin, W. P. Leong, S. P. Raffanti, D. A. Wheeler, R. D. Anderson, P. Keiser, S. R. Schrader, J. C. Goodgame, C. R. Steinhart, R. L. Murphy, M. J. Wolin, and K. A. Smith. 2000. Low-dose daily subcutaneous interleukin-2 in combination with highly active antiretroviral therapy in HIV+ patients: a randomized controlled trial. HIV Clin. Trials 1:1-15. [DOI] [PubMed] [Google Scholar]
- 39.Liu, K., M. Catalfamo, Y. Li, P. A. Henkart, and N. P. Weng. 2002. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc. Natl. Acad. Sci. USA 99:6192-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 41.Loubeau, M., A. Ahmad, E. Toma, and J. Menezes. 1997. Enhancement of natural killer and antibody-dependent cytolytic activities of the peripheral blood mononuclear cells of HIV-infected patients by recombinant IL-15. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:137-145. [DOI] [PubMed] [Google Scholar]
- 42.Lowin, B., M. C. Peitsch, and J. Tschopp. 1995. Perforin and granzymes: crucial effector molecules in cytolytic T lymphocyte and natural killer cell-mediated cytotoxicity. Curr. Top. Microbiol. Immunol. 198:1-24. [DOI] [PubMed] [Google Scholar]
- 43.Lucey, D. R., L. A. Pinto, F. R. Bethke, J. Rusnak, G. P. Melcher, F. N. Hashemi, A. L. Landay, H. A. Kessler, R. J. Paxton, K. Grabstein, and G. M. Shearer. 1997. In vitro immunologic and virologic effects of interleukin-15 on peripheral blood mononuclear cells from normal donors and human immunodeficiency virus type 1-infected patients. Clin. Diagn. Lab. Immunol. 4:43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucia, B., C. Jennings, R. Cauda, L. Ortona, and A. L. Landay. 1995. Evidence of a selective depletion of a CD16+ CD56+ CD8+ natural killer cell subset during HIV infection. Cytometry 22:10-15. [DOI] [PubMed] [Google Scholar]
- 45.Lum, J. J., A. A. Pilon, J. Sanchez-Dardon, B. N. Phenix, J. E. Kim, J. Mihowich, K. Jamison, N. Hawley-Foss, D. H. Lynch, and A. D. Badley. 2001. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2L. J. Virol. 75:11128-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastroianni, C. M., G. d'Ettorre, G. Forcina, M. Lichtner, F. Mengoni, C. D'Agostino, A. Corpolongo, A. P. Massetti, and V. Vullo. 2000. Interleukin-15 enhances neutrophil functional activity in patients with human immunodeficiency virus infection. Blood 96:1979-1984. [PubMed] [Google Scholar]
- 47.Meyaard, L., S. A. Otto, I. P. Keet, M. T. Roos, and F. Miedema. 1994. Programmed death of T cells in human immunodeficiency virus infection. No correlation with progression to disease. J. Clin. Investig. 93:982-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrissey, P. J., P. Conlon, S. Braddy, D. E. Williams, A. E. Namen, and D. Y. Mochizuki. 1991. Administration of IL-7 to mice with cyclophosphamide-induced lymphopenia accelerates lymphocyte repopulation. J. Immunol. 146:1547-1552. [PubMed] [Google Scholar]
- 49.Motsinger, A., D. W. Haas, A. K. Stanic, L. Van Kaer, S. Joyce, and D. Unutmaz. 2002. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J. Exp. Med. 195:869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mueller, Y. M., P. M. Bojczuk, E. S. Halstead, A. H. Kim, J. Witek, J. D. Altman, and P. D. Katsikis. 2003. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood 101:1024-1029. [DOI] [PubMed] [Google Scholar]
- 51.Naora, H., and M. L. Gougeon. 1999. Enhanced survival and potent expansion of the natural killer cell population of HIV-infected individuals by exogenous interleukin-15. Immunol. Lett. 68:359-367. [DOI] [PubMed] [Google Scholar]
- 52.Napolitano, L. A., R. M. Grant, S. G. Deeks, D. Schmidt, S. C. De Rosa, L. A. Herzenberg, B. G. Herndier, J. Andersson, and J. M. McCune. 2001. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7:73-79. [DOI] [PubMed] [Google Scholar]
- 53.Okamoto, Y., D. C. Douek, R. D. McFarland, and R. A. Koup. 2002. Effects of exogenous interleukin-7 on human thymus function. Blood 99:2851-2858. [DOI] [PubMed] [Google Scholar]
- 54.Oliva, A., A. L. Kinter, M. Vaccarezza, A. Rubbert, A. Catanzaro, S. Moir, J. Monaco, L. Ehler, S. Mizell, R. Jackson, Y. Li, J. W. Romano, and A. S. Fauci. 1998. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J. Clin. Investig. 102:223-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orange, J. S., B. Wang, C. Terhorst, and C. A. Biron. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parato, K. G., A. Kumar, A. D. Badley, J. L. Sanchez-Dardon, K. A. Chambers, C. D. Young, W. T. Lim, S. Kravcik, D. W. Cameron, and J. B. Angel. 2002. Normalization of natural killer cell function and phenotype with effective anti-HIV therapy and the role of IL-10. AIDS 16:1251-1256. [DOI] [PubMed] [Google Scholar]
- 57.Perez, C., I. Albert, K. DeFay, N. Zachariades, L. Gooding, and M. Kriegler. 1990. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell 63:251-258. [DOI] [PubMed] [Google Scholar]
- 58.Peschon, J. J., P. J. Morrissey, K. H. Grabstein, F. J. Ramsdell, E. Maraskovsky, B. C. Gliniak, L. S. Park, S. F. Ziegler, D. E. Williams, C. B. Ware, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180:1955-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prins, J. M., S. Jurriaans, R. M. van Praag, H. Blaak, R. van Rij, P. T. Schellekens, I. J. ten Berge, S. L. Yong, C. H. Fox, M. T. Roos, F. de Wolf, J. Goudsmit, H. Schuitemaker, and J. M. Lange. 1999. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS 13:2405-2410. [DOI] [PubMed] [Google Scholar]
- 60.Roifman, C. M., J. Zhang, D. Chitayat, and N. Sharfe. 2000. A partial deficiency of interleukin-7R alpha is sufficient to abrogate T-cell development and cause severe combined immunodeficiency. Blood 96:2803-2807. [PubMed] [Google Scholar]
- 61.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 62.Schluns, K. S., K. Williams, A. Ma, X. X. Zheng, and L. Lefrancois. 2002. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168:4827-4831. [DOI] [PubMed] [Google Scholar]
- 63.Screpanti, V., R. P. Wallin, H. G. Ljunggren, and A. Grandien. 2001. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J. Immunol. 167:2068-2073. [DOI] [PubMed] [Google Scholar]
- 64.Sedger, L. M., D. M. Shows, R. A. Blanton, J. J. Peschon, R. G. Goodwin, D. Cosman, and S. R. Wiley. 1999. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J. Immunol. 163:920-926. [PubMed] [Google Scholar]
- 65.Smyth, M. J., E. Cretney, K. Takeda, R. H. Wiltrout, L. M. Sedger, N. Kayagaki, H. Yagita, and K. Okumura. 2001. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J. Exp. Med. 193:661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stellbrink, H. J., F. T. Hufert, K. Tenner-Racz, J. Lauer, C. Schneider, H. Albrecht, P. Racz, and J. van Lunzen. 1998. Kinetics of productive and latent HIV infection in lymphatic tissue and peripheral blood during triple-drug combination therapy with or without additional interleukin-2. Antivir. Ther. 3:209-214. [PubMed] [Google Scholar]
- 67.Takeda, K., Y. Hayakawa, M. J. Smyth, N. Kayagaki, N. Yamaguchi, S. Kakuta, Y. Iwakura, H. Yagita, and K. Okumura. 2001. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 7:94-100. [DOI] [PubMed] [Google Scholar]
- 68.Tanchot, C., F. A. Lemonnier, B. Perarnau, A. A. Freitas, and B. Rocha. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science 276:2057-2062. [DOI] [PubMed] [Google Scholar]
- 69.Tarazona, R., J. G. Casado, O. Delarosa, J. Torre-Cisneros, J. L. Villanueva, B. Sanchez, M. D. Galiani, R. Gonzalez, R. Solana, and J. Pena. 2002. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J. Clin. Immunol. 22:176-183. [DOI] [PubMed] [Google Scholar]
- 70.Valdez, H. 2002. Immune restoration after treatment of HIV-1 infection with highly active antiretroviral therapy (HAART). AIDS Rev. 4:157-164. [PubMed] [Google Scholar]
- 71.Valdez, H., R. Mitsuyasu, A. Landay, A. D. Sevin, E. S. Chan, J. Spritzler, S. A. Kalams, R. B. Pollard, J. Fahey, L. Fox, A. Namkung, S. Estep, R. Moss, D. Sahner, and M. M. Lederman. 2003. Interleukin-2 increases CD4+ lymphocyte numbers but does not enhance responses to immunization: results of A5046s. J. Infect. Dis. 187:320-325. [DOI] [PubMed] [Google Scholar]
- 72.Valentin, A., M. Rosati, D. J. Patenaude, A. Hatzakis, L. G. Kostrikis, M. Lazanas, K. M. Wyvill, R. Yarchoan, and G. N. Pavlakis. 2002. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 99:7015-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vidalain, P. O., O. Azocar, B. Lamouille, A. Astier, C. Rabourdin-Combe, and C. Servet-Delprat. 2000. Measles virus induces functional TRAIL production by human dendritic cells. J. Virol. 74:556-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Freeden-Jeffry, U., P. Vieira, L. A. Lucian, T. McNeil, S. E. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waldmann, T. A., and Y. Tagaya. 1999. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17:19-49. [DOI] [PubMed] [Google Scholar]
- 76.Washburn, B., M. A. Weigand, A. Grosse-Wilde, M. Janke, H. Stahl, E. Rieser, M. R. Sprick, V. Schirrmacher, and H. Walczak. 2003. TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus. J. Immunol. 170:1814-1821. [DOI] [PubMed] [Google Scholar]
- 77.Zamai, L., M. Ahmad, I. M. Bennett, L. Azzoni, E. S. Alnemri, and B. Perussia. 1998. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 188:2375-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zanussi, S., M. T. Bortolin, U. Tirelli, G. Nasti, E. Vaccher, M. Giacca, and P. De Paoli. 2000. Effects of 2-year antiretroviral combination therapies on HIV-1 DNA levels. J. Acquir. Immune Defic. Syndr. 23:439-441. [DOI] [PubMed] [Google Scholar]
- 79.Zhang, X., S. Sun, I. Hwang, D. F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8:591-599. [DOI] [PubMed] [Google Scholar]