Abstract
The consumption of cacao-derived products, particularly in the form of dark chocolate is known to provide beneficial cardiovascular effects in normal individuals and in those with vascular dysfunction (reduced nitric oxide [NO] bioavailability and/or synthesis). Upstream mechanisms by which flavonoids exert these effects are poorly understood and may involve the participation of cell membrane receptors. We previously demonstrated that the flavanol (-)-epicatechin (EPI) stimulates NO production via Ca+2-independent eNOS activation/phosphorylation. We wished to investigate the plausible participation of a cell surface receptor using a novel cell-membrane impermeable EPI-Dextran conjugate (EPI-Dx). Under Ca2+-free conditions, human coronary artery endothelial cells (HCAEC) were treated for 10 min with EPI or EPI-Dx at equimolar concentrations (100 nM). Results demonstrate that both EPI and EPI-Dx induced the phosphorylation/activation of PI3K, PDK-1, AKT and eNOS. Interestingly, EPI-Dx effects were significantly higher in magnitude than those of EPI alone. The capacity of EPI-Dx to stimulate cell responses supports the existence of an EPI cell membrane receptor mediating eNOS activation.
Keywords: eNOS, PI3K/AKT, epicatechin, endothelial cells
Flavonoids are a class of plant secondary compounds present in fruits, tea and cacao that are known for their healthy effects.1 The consumption of cacao-derived products, particularly in the form of dark chocolate (referred to herein as cocoa) is known to provide beneficial cardiovascular effects in normal individuals and in those with endothelial (i.e., vascular) dysfunction such as smokers, diabetics and postmenopausal women.2 The vascular actions of cocoa are related to its capacity to activate endothelial nitric oxide synthase (eNOS) and thus, stimulate nitric oxide (NO) production.2-3 These actions can be reproduced by the administration of pure (-)-epicatechin (EPI), which is the most abundant flavanol present in cacao.3 Recently, we demonstrated in human coronary artery endothelial cells (HACEC), that in the presence of Ca2+, EPI is capable of acutely inducing the synthesis of NO through eNOS activation via the PI3K/AKT/PKA and Ca2+-CaM/CaMKII pathways.4 Using pharmacological approaches (inhibition of phospholipase [PLC]) we also provided evidence for the presence of a possible receptor like molecule on the plasmalemma for EPI.
We have previously anchored biologically active molecules to macromolecular entities such as dextran (Dx) (250-750 KDa) to restrict the effect of such molecules to the vascular lumen.5 Blocking the internalization of EPI by its anchoring to Dx can thus, be used as a strategy to trigger biological responses solely at the plasmalemma. Of interest is the observation that under Ca2+-free conditions, EPI is uniquely capable of inducing NO production secondary to eNOS phosphorylation via AKT activation independently of the translocation of the enzyme from the plasmalemma.6-6 However, no studies have examined the differential effects of EPI restricting its presence to the plasmalemma.
The objective of this study was to examine cell membrane effects of EPI-Dx on upstream signaling including PI3K, PDK-1, AKT and eNOS in the absence of intracellular Ca2+. Documenting such effects would provide further evidence as to the possible existence of cell membrane receptors that mediates such actions.
Dx’s glucose residues were oxidized to aldehydes with NaIO4, as suggested by J. Maia et al. NaIO4 predominantly attacks Dx’s C3-C4 (Fig. 1).7 The aldehyde groups were then reacted with the amine of the spacer AC, which underwent reductive amination after the addition of NaBH3CN. EPI’s phenols were then esterified by reacting with the carboxylic acids of AC (Fig. 1). It is hypothesized that esterification occurred through the phenols from ring B, due to their higher reactivity (lower pka) compared to phenols from ring A 8, although we do not exclude the possibility that phenols from ring A were involved in the binding. Furthermore, it has been suggested that only one aldehyde of oxidized Dx undergoes reaction with amine group-containing compounds (e.g. carbazates), suggesting that EPI can bind in the same manner 7.
Fig.1.
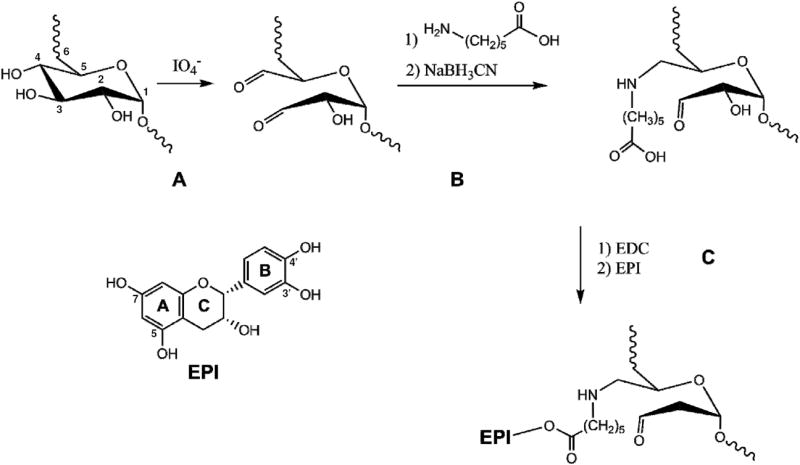
Schematic representation of dextran oxidation by sodium periodate.(A) Oxidation and formation of aldehyde groups at positions C3 and C4. (B) Dextran coupling to 6-aminocaproic acid. (C) (-)-epicatechin coupling to 6-aminocaproic acid. IO4 -; sodium periodate, NaBH3CN; sodium cyanoborohydride, EDC; N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide, EPI; (-)-epicatechin.
The amount of EPI bound to Dx was determined by means of cleaving the ester bond using rabbit esterase. The amount of EPI bound to Dx calculated by HPLC-MS was 0.630 μg/mg of EPI-Dx conjugate. We did not detect EPI in the extract in the absence of enzyme (see supplemental data).
We incubated HCAEC with EPI-Dx for 10 min to study the stability of the conjugate. We used this incubation time because we examine the effects of EPI-Dx only within this time frame. HPLC-MS results indicate that EPI is not released from EPI-Dx during the incubation time as determined from media or cell lysate samples derived from EPI-Dx-treated cells. However, when media from EPI-Dx treated cells was incubated with esterase we detected EPI at the predicted concentrations (see supplemental data). On the other hand, when the cell lysate is treated with esterase (searching for any cellular uptake of EPI-Dx by the endothelial cells), there were no detectable levels of EPI in EPI-Dx treated cells. Furthermore, when we incubated EPI-Dx in the absence of cells the same results were obtained. EPI was only detected after the solution was treated with esterase.
In order to evaluate the efficacy of EPI-Dx on NO production, we performed a concentration response curve (the concentration used was equivalent to that of free EPI). We measured NO levels in the supernatant after 10 min of incubation. Fig. 2 results show a concentration-dependent increase in NO production with EPI-Dx evidencing a calcium independent membrane initiated stimulation of eNOS activation. There was a significant increase in NO production at the lowest concentration used of EPI-Dx (50 nM) that peaked at 150 nM (no statistical differences between 100 vs. 150 nM EPI-Dx). There were no responses to Dx-AC alone (control-1) or Dx-AC with possible adsorbed EPI (control-2). Thus, in subsequent experiments we used EPI-Dx at a concentration of 100 nM. EPI was also used at the same concentration.
Fig.2.
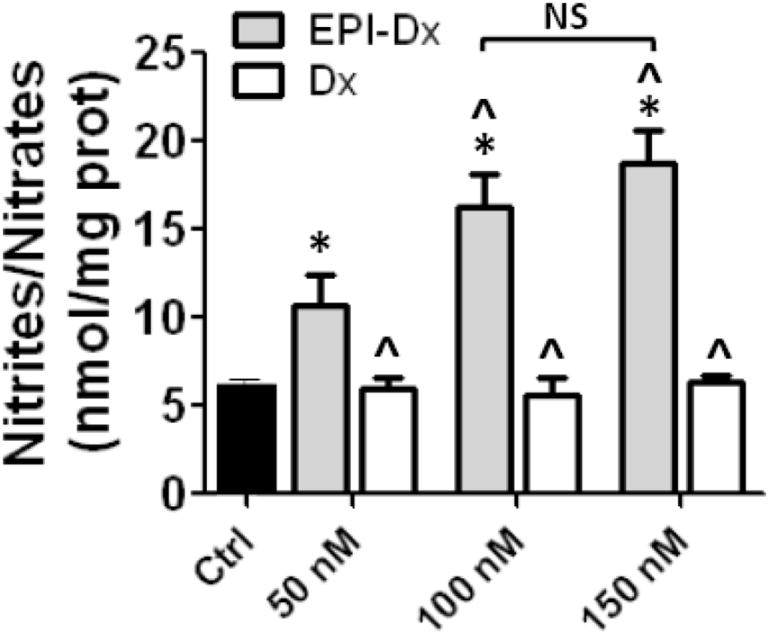
Concentration-response curve of EPI-Dx on nitric oxide (NO) production.
Ca2+-free HCAEC were treated for 10 min with increasing concentrations of EPI-Dx or dextran (Dx). Results show increases in NO production when cells were treated with EPI-Dx achieving a maximal effect at 100 nM (no significant difference between 100 vs 150 nM). No effects were observed when cells were treated only with dextran. The concentrations 50, 100 and 150 nM corresponds to the concentration achieved by the amount of EPI bound of dextran. Data are expressed as mean ± SD (n=3). * p<0.05 vs control, ˆ p<0.05 vs 50 nM EPI-Dx.
To examine EPI and EPI-Dx (100 nM) capacity to stimulate cell membrane associated signaling events in the absence of intracellular Ca2+, we evaluated the effects of treatments on PI3K, PDK-1, AKT and eNOS phosphorylation levels (i.e. activation) and PIP3 production. As shown in Fig. 3A, both agents stimulated PI3K phosphorylation. However, EPI-Dx induced a significantly higher increase in the levels of phosphorylated PI3K vs. EPI. This event triggers increases in PIP3 levels (Fig. 3B), with EPI-Dx being more efficacious. Both responses were blocked by the use of the PI3K inhibitor wortmannin. Fig. 3C demonstrates that the phosphorylation of PDK1 (which is downstream from PI3K) can be activated by both agents and also blocked by wortmannin. Fig. 3D illustrates the phosphorylation of AKT as induced by EPI and EPI-Dx. EPI-Dx was also more efficacious than EPI and both are blocked by wortmannin.
Fig. 3.
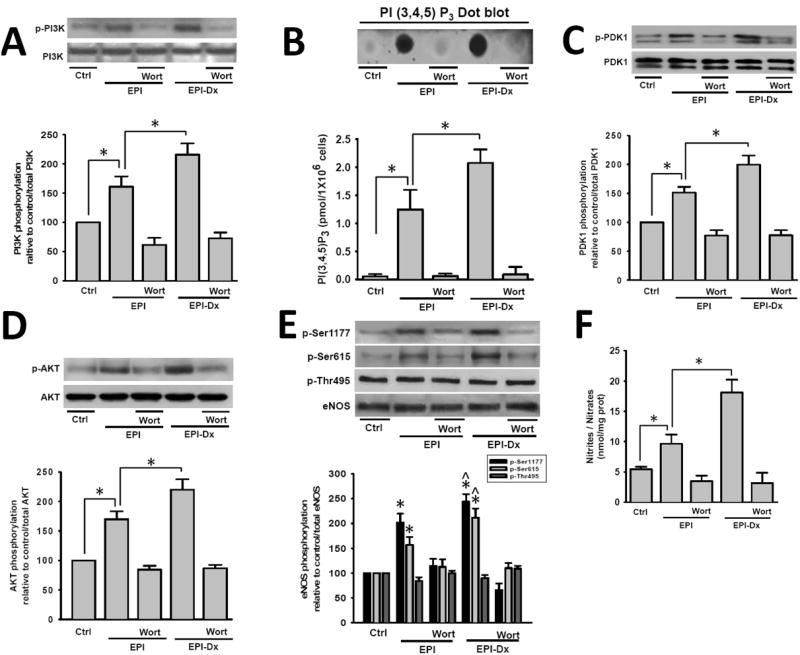
Acute effects of EPI and EPI-Dx on upstream kinases activation
(A) Ca2+-free HCAEC treated for 10 min with 100 nM EPI or EPI-Dx show increases in (A) PI3K, (C) PDK1, (D) AKT and (E) eNOS protein phosphorylation levels, and the production of PIP3 (B). These effects were blocked by the PI3K inhibitor, wortmannin as shown in the representative blots (each Western Blot is representative of three independent experiments). Data are expressed as mean ± SD (n=3). *P<0.05.
We evidenced eNOS phosphorylation on the activation residues Ser 1177 and Ser 615 as well as, in the inactivation residue Thr 495. Treatment led to the phosphorylation on Ser 1177 and Ser 615 (Fig. 3E), which were blocked by wortmannin. There were no changes in the phosphorylation levels of Thr 495 with treatment. However, at the concentrations used, EPI-Dx led to greater levels of phosphorylation on Ser 11777 and Ser 615 vs. EPI. Fig. 3F documents changes in NO levels in EPI- and EPI-Dx treated cells which were more prominent with EPI-Dx. The effects were also blocked by wortmannin.
A very limited number of studies have explored the possible participation of cell membrane receptors in mediating natural product effects on cells. Han et al, revealed [3H]resveratrol binding sites in rat brain enriched cell membrane pellets.9 Binding was sensitive to trypsin digestion and protein denaturation, which suggest resveratrol-membrane protein interaction. Saturation binding experiments revealed a single class of sites. Interestingly, the tea catechin gallates (epigallocatechin gallate and epicatechin gallate) displayed the highest affinities (Ki=25-45 nM) followed by resveratrol (Ki=102 nM) while EPI failed to compete for binding even at high doses (10 μM). The authors concluded that the neuroprotective action of various polyphenols and resveratrol analogs could be mediated by the activation of common “receptor” binding sites particularly enriched at the level of the cellular plasma membrane of the rat brain. A study using surface plasmon resonance has also provided evidence for the binding of epigallocatechin gallate at physiological concentrations to the 67-kDa laminin receptor which has been associated with its anticancer activity.10 EPI (at 5μM) failed to bind to such receptor. EPI’s well-known ability to trigger physiological effects strongly suggests the involvement of receptors, which are likely distinct from those described above.
We recently reported that EPI is capable of stimulating NO production secondary to the activation of plasmalemma bound PLC which is known to be linked to transmembrane receptors.4 We have also observed that (+)-catechin (an EPI stereoisomer) only evokes a partial stimulation of HCAEC NO production vs. EPI when compared at the same concentrations.4 In concentration response experiments, the co-incubation of fixed concentrations of (+)-catechin with EPI displaces the EPI concentration-response curve to the right. These observations strongly hint at the existence of an EPI receptor, which harbors relative specificity given that (+)-catechin only triggers a partial stimulation of NO production. To further evidence the possible participation of cell membrane receptors, we synthesized an impermeable form of the flavanol, a novel EPI-Dx macro conjugate (270 KDa). EPI was bound to Dx by an ester bond through AC. We have used AC in order to provide spatial flexibility to the bound EPI and to allow for physical interactions with the cell membrane. Given the nature of the experimental design multiple controls were necessary. Due to the ester bond created between EPI and AC, esterases bound to the cell membrane may cleave EPI from Dx, releasing EPI that can then diffuse into the cell. We thus, performed a set of control experiments to exclude these possibilities. Results indicate that EPI bound to Dx was not released into the media (neither by spontaneous or esterase cleavage activity) during the 10 min incubation period. The macromolecule Dx and/or the EPI-Dx complex can also alter the density and viscosity of cell media, which in turn may modify endothelial cells responses (although we used Dx as low as 0.006 % w/v in the media).11 We therefore, evaluated NO production in the presence of Dx alone (controls-1 and -2) and no effect was observed. Another possibility is related with the probable uptake of EPI-Dx into endothelial cells. Pore theory modeling studies using macromolecular Dx or microspheres in dog myocardium and limb muscle have defined an upper limit of the capillary large-pore diameter to be 0.024-0.032 microns for myocardium 7 and 0.04 microns for the leg.8 The size of Dx (≈0.05 microns) used in our work exceeds the upper limit of capillary diameter and we thus, believe it is to be excluded from the cells. In order to validate this affirmation, we performed experiments incubating EPI-Dx with cells and after several washes cultures were homogenized and samples analyzed for possible EPI traces inside the cells. Results indicate that EPI-Dx does not permeate the cell membrane since we were unable to detect it in the lysates. Interestingly, the greater levels of stimulation evoked by EPI-Dx vs EPI in all the endpoints examined hint at the importance that either a preservation of outside EPI concentrations or longer stimulation of the receptor may have in triggering the noted effects. In consequence, we speculate that the acute EPI effects on endothelial cells are principally mediated by stimulating a surface entity and not by activating an intracellular target.
In summary, we demonstrate that under Ca2+ free conditions, the signaling pathway that leads to eNOS activation in EPI-Dx-treated cells is dependent on the PI3K pathway which appears to couple to a cell membrane receptor (Fig. 4). Future studies will need to focus on the isolation, characterization and cloning of such receptors as a means to develop novel and more potent therapeutics aimed at preserving health.
Fig. 4.
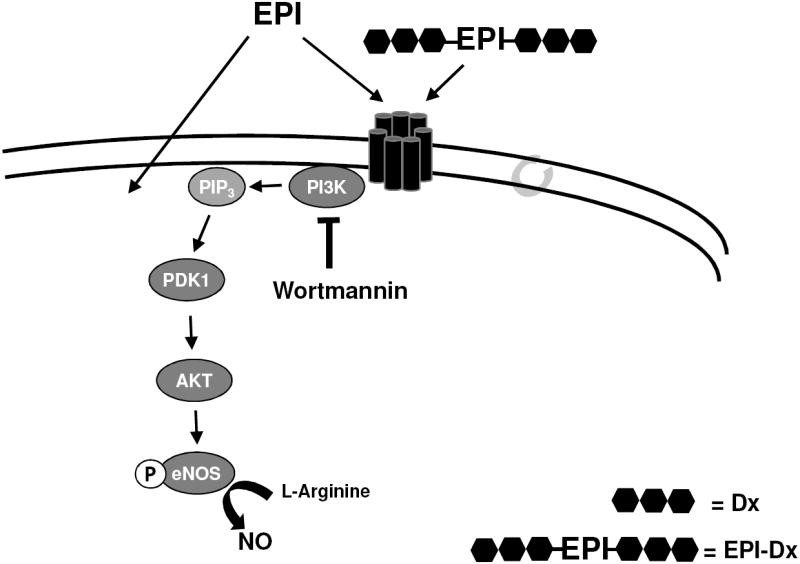
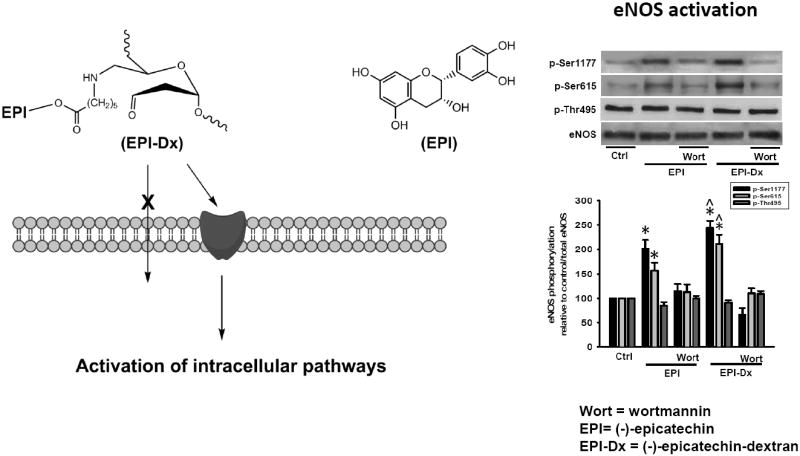
EPI-Dx-induced eNOS activation in Ca+2 free HCAEC
Schematic representation of signaling pathways involved in EPI-Dx induced eNOS activation in Ca2+ free conditions. The activation of eNOS is presumably mediated by a receptor located at the plasma membrane.
Supplementary Material
Acknowledgments
Sources of Funding:
The work was partially funded by Cardero Therapeutics, Inc., NIH HL-43617, AT004277 and NIH/NIMHD-sponsored (P60 MD000220) to F. Villarreal. Aldo Moreno Ulloa (Ph.D. fellowship #388585), I. Ramirez-Sanchez, (post-doctoral fellowship #93759) and G. Ceballos (sabbatical fellowship #129889) from CONACYT, Mexico.
Footnotes
Disclosures: F. Villarreal is a Co-founder of Cardero Therapeutics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. The American journal of clinical nutrition. 2001;74(4):418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 2.Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119(10):1433–41. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 3.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. 2006;103(4):1024–9. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (-)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55(6):1398–405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Hernandez A, Rubio-Gayosso I, Ramirez I, Ita-Islas I, Meaney E, Gaxiola S, Meaney A, Asbun J, Figueroa-Valverde L, Ceballos G. Intraluminal-restricted 17 beta-estradiol exerts the same myocardial protection against ischemia/reperfusion injury in vivo as free 17 beta-estradiol. Steroids. 2008;73(5):528–38. doi: 10.1016/j.steroids.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.(a) Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (-)-Epicatechin induces calcium and translocation independent eNOS activation in arterial endothelial cells. Am J Physiol Cell Physiol. 2011;300(4):C880–7. doi: 10.1152/ajpcell.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ramirez-Sanchez I, Aguilar H, Ceballos G, Villarreal F. (-)-Epicatechin-induced calcium independent eNOS activation: roles of HSP90 and AKT. Mol Cell Biochem. 2012;370(1-2):141–50. doi: 10.1007/s11010-012-1405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor A, Granger D. Exchange of macromolecules across the microcirculation. Hanbook of Physiology. 1984;4:488–499. [Google Scholar]
- 8.Grotte G. Passage of dextran molecules across the blood-lymph barrier. Acta Chir Scand Suppl. 1956;211:1–84. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


