Abstract
Objective
To determine the reliability of a new scale for the clinical assessment of essential tremor.
Background
The Essential Tremor Rating Assessment Scale contains nine performance items that rate action tremor in the head, face, voice, limbs and trunk 0–4 in half-point intervals. Head and limb tremor ratings are defined by specific amplitude ranges in centimeters.
Design/Methods
Videos of 44 patients and 6 controls were rated by 10 specialists on two occasions, 1–2 months apart. Inter- and intra-rater reliabilities were assessed with a two-way random effects intraclass correlation, using an absolute agreement definition.
Results
The inter- and intra-rater intraclass correlations for head and upper limb tremor ranged from 0.86 to 0.96, and the intraclass correlations for the total score were 0.94 and 0.96. The intraclass correlations for voice, face, trunk and leg were less robust.
Conclusions
This scale is an exceptionally reliable tool for the clinical assessment of essential tremor.
Keywords: essential tremor, rating scale, reliability
Introduction
Before 1993, there were no rating scales for essential tremor (ET) with documented reliability and validity. Many different ad hoc scales had been used in numerous small clinical trials of essential tremor, making the results of these trials difficult or impossible to compare.1
The Fahn-Tolosa-Marín scale has been used extensively in clinical trials of ET, at times with modifications to enhance relevance to ET.2 There is still no comprehensive item-by-item analysis of this scale, and inter-rater reliability has been fair to poor, especially for writing and drawing.3–5 Furthermore, severe extremity tremor is defined as > 4 cm, which is much less than advanced ET.6
The tremor rating scales of Bain and coworkers assess tremor in the head, limbs, Archimedes spirals, and handwriting using 0–10 ratings.7, 8 Ratings are defined subjectively or by examples in a published manual, making good reliability difficult to achieve.7, 8
The original tremor rating scale of Louis and coworkers9 was subsequently revised “to broaden the applicability of this scale to clinical trials”.10 This scale measures only upper extremity tremor and requires video training to achieve high inter-rater reliability.10
Recognizing the limitations of existing scales, the Tremor Research Group (TRG) met several times over a period of nine years to develop the TRG Essential Tremor Rating Assessment Scale (TETRAS) (Appendix 1). TETRAS has a 12-item activities of daily living (ADL) subscale that addresses many of the activities assessed in the ADL scales of Fahn,6 Louis,11 Bain,7 and their coworkers, and TETRAS also has a 9-item performance subscale that quantifies tremor in the head, face, voice, limbs and trunk. TETRAS was developed with three mandates: 1) rapid clinical assessment of ET, 2) no equipment other than pen and paper, and 3) objective metric anchors for each 0–4 rating whenever possible, so as to reduce experiential rating bias and uncertainty. The performance subscale takes less than 10 minutes. Ratings of head tremor and limb tremor are defined by specific amplitude ranges in centimeters (Table 1), so raters must first estimate the maximum amplitude of tremor and then assign the corresponding rating. The performance subscale allows 0.5-point increments in scoring. The 0.5-point increments in upper limb tremor ratings are defined by specific ranges of tremor amplitude. For all other items, the 0.5 increments may be used when the 0–4 integer rating is uncertain.
Table.
Metric anchors for TETRAS performance measures of head and extremity tremor
| Head tremor | Upper limb tremor | Lower limb tremor |
|---|---|---|
| 0 = no tremor | 0 = no tremor | 0 = no tremor |
| 1 = < 0.5 cm | 1 = barely visible | 1 = barely visible |
| 2 = 0.5– < 2.5 cm | 1.5 = < 1 cm | 2 = <1 |
| 3 = 2.5–5 cm | 2 = 1– < 3 cm | 3 = 1 – 5 |
| 4 = > 5 cm | 2.5 = 3– < 5 cm | 4 = > 5 cm |
| 3 = 5– < 10 cm | ||
| 3.5 = 10– < 20 cm | ||
| 4 = > 20 cm |
In a preliminary study, 10 members of TRG simultaneously rated 10 ET patients during the live administration of the TETRAS performance subscale. Excellent inter-rater reliabilities were found for head and upper limb tremor.12 In another preliminary study, ten members of TRG simultaneously rated three patients with mild, moderate and severe ET and rated the videos of these exams one month later. The correlations between video scores and live exam scores were greater than 0.87 for all items except face (0.67) and voice (0.63) tremor.13 We now estimate the inter- and intra-rater reliabilities of the TETRAS performance subscale and the correlation of this subscale with the ADL subscale, using 50 videotaped exams.
Methods
All studies were performed with the signed written informed consent of the patients and controls, approved by the institutional review board of each institution. Patients with ET and controls with no history of tremor were recruited from the authors’ clinics. The patients were diagnosed using Tremor Investigation Group criteria.14
Nine of the authors, all movement disorder specialists, videotaped TETRAS exams of one control and at least four patients. Each specialist was asked to video patients with mild, moderate and severe ET so that the videos were evenly distributed over these levels of severity. The TETRAS ADL and performance subscales were performed during each videotaping. Fifty videos (44 patients and 6 controls) were compiled in random order to a set of DVDs and mailed to the same specialists and one other. Each specialist rated all 50 videos. The same videos in different order were rated by the same specialists one to two months after the first rating.
Due to omissions in some of the videos, some of the test items could not be scored for every video. Four of the ten video raters had no experience or training in TETRAS, and nearly all video omissions came from these four raters. Nevertheless, all raters scored 31 to 46 videos for each item, except the standing item for which only 19 were scored.
Inter- and intra-rater reliability of the performance subscale were assessed with two-way random effects intraclass correlations (ICC), using an absolute agreement definition.
Results
The patients (mean 67, range 35–80) and controls (mean 50; range 27–82) had comparable ages, and 27 of 50 participants were men. The average duration of tremor in the 44 patients was 30 years (range 6–72). The distribution of total TETRAS performance scores for the 50 participants was fairly uniform (Appendix 2).
The inter- and intra-rater ICCs were greater than 0.85 for all items except face tremor, voice tremor, lower limb tremor and trunk (standing) tremor (see Appendices 3 and 4 for details). The six experienced and four inexperienced raters did not differ statistically (repeated measures ANOVA) in their inter- and intra-rater reliabilities for any of the test items, but the biggest differences were for the face, lower extremity and trunk (standing) items.
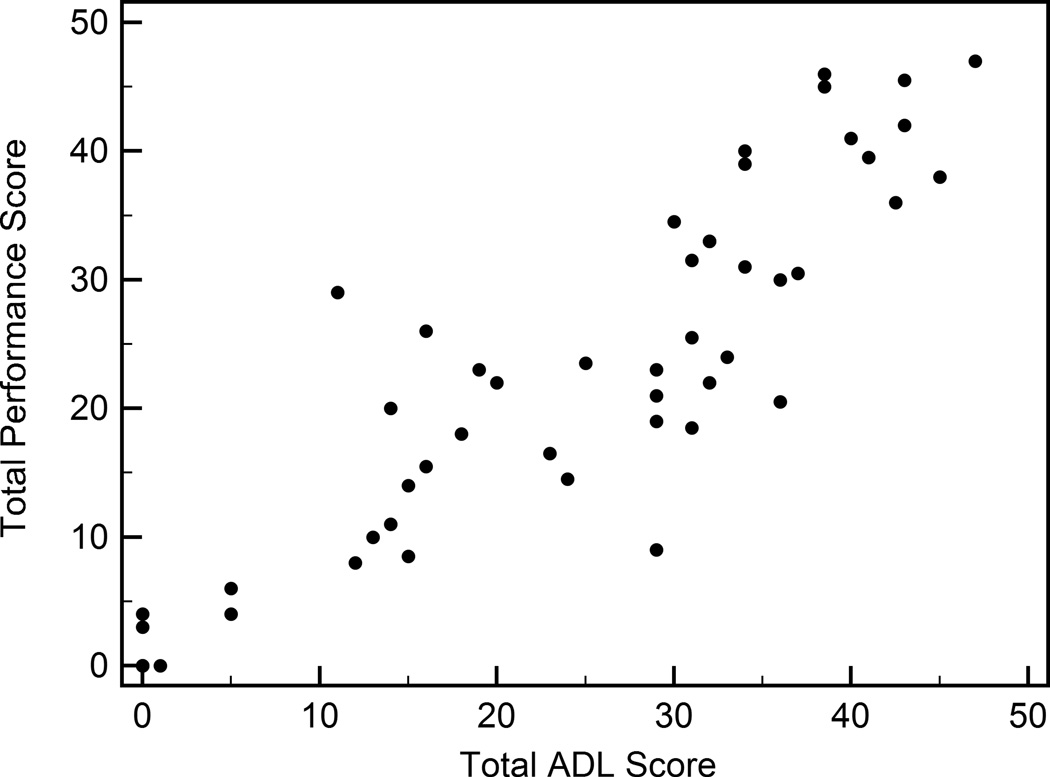
The raters performed live TETRAS assessments during the videotaping of the 50 TETRAS exams. The Pearson correlation between the total ADL scores and the total performance scores was 0.887 (p < 0.0001) (Figure).
Figure.
Total ADL and performance scores for the 50 patients and controls
Cronbach alpha for the live exams performed during the video tapings was 0.951, and it was 0.968 after removal of the face, lower limb and trunk items. Statistically identical results were obtained when Cronbach alpha was computed using the video ratings of each rater. The item-to-total score correlations ranged from 0.88 to 0.95 (mean 0.91) for all upper limb items except right upper limb postural tremor with the limb extended forward (0.75). Item-to-total correlations for the head (0.69), face (0.45), voice (0.68), lower limb (0.60) and trunk (0.46) were lower.
Discussion
Our use of performance ratings defined in terms of specific amplitude ranges (cm) resulted in exceptional inter- and intra-rater reliabilities, even for raters without prior experience or training with this scale. However, all raters were experienced movement disorder specialists, and it remains to be determined if raters with less expertise perform as well. Comparable inter-rater reliabilities were found in our earlier preliminary study in which ten TRG members, all developers of TETRAS, simultaneously rated ten ET patients during the live administration of the TETRAS performance subscale.12
The TETRAS ADL and performance subscales have obvious content validity for ET, and there is also evidence of strong construct validity. The TETRAS ADL and performance scores are highly correlated, and the TETRAS performance items of upper extremity function correlate strongly with transducer measures of upper limb tremor.15–17
TETRAS was designed specifically for ET and has not been tested in children or other patient populations. Its metric anchors are too large for small children. TETRAS does not include an assessment of rest tremor because rest tremor is usually not present in ET.18 Furthermore, distinguishing rest tremor in ET from postural tremor in the presence of incomplete relaxation is difficult and is best avoided when the principal goal is assessment of ET severity rather than the diagnosis or complete characterization of ET in a particular patient.
TETRAS is heavily weighted by upper extremity tremor and is arguably not ideal when the principal interest is tremor elsewhere. The least reliable items of the TETRAS performance subscale were face tremor, lower limb tremor and trunk tremor while standing. This has been the experience with other tremor scales.7 These items could be deleted with little loss of content validity since the current clinical definition of ET focuses on upper extremity tremor and head tremor.14 Exclusion of the face, lower limb and trunk (standing) items would reduce the maximum total performance score from 64 to 52.
Finally, most of us thought that the anchor “barely perceptible” for grade 1 upper and lower extremity tremor could be improved by adding “< 0.5 cm” or “a few mm”. Most also thought that lower extremity tremor was difficult to distinguish from other irregularities of movement and posture. Some raters performed heel-knee-shin testing with the patient seated, and others performed this test with the patient supine, which seems preferable for the specific assessment of tremor. Training would improve consistency and quality of administering the lower extremity exam, but it is unclear whether training can reduce the uncertainty in rating tremor.
In conclusion, TETRAS was designed specifically for the clinical measurement of ET severity, requiring no instruments other than a pen and paper. The scale is brief, valid and highly reliable, and we believe it is ideal for ET clinical trials.
Supplementary Material
Acknowledgment
This work was funded by a grant from GlaxoSmithKline.
Financial disclosures for the previous 12 months:
Rodger Elble has received consulting fees from the Kinetics Foundation, and he receives research grant support from GlaxoSmithKline, Phytopharm, TEVA, the National Institutes of Health (NINDS), and the Spastic Paralysis Research Foundation of Kiwanis International, Illinois-Eastern Iowa District.
Cynthia Comella has received consulting fees from Allergan, Merz, Ipsen, and Nupathe, and she receives research grant support from the Parkinson Disease Foundation, NIH, Dystonia Medical Research Foundation, Allergan, Merz and Ipsen. She receives royalties from Wolters Kluwer, Inc and Cambridge University Press.
Stanley Fahn has received consulting fees from AstraZeneca Pharmaceuticals, IMPAX Pharmaceuticals, RJG Foundation, Green Cross Corporation, Civitas, Genervon Laboratories, Intec Pharma, and Merz. He receives grant support from the Parkinson’s Disease Foundation and the Smart Family Foundation. He has received lecture honoraria from the American Academy of Neurology, Columbia University, and Sun Pharmaceuticals India. He has received editor and author honoraria from Springer for serving as co-editor of Current Neurology and Neurosurgery Report and from Elsevier for co-author of book Principles and Practice of Movement Disorders.
Mark Hallett is employed at the NIH. He serves as Chair of the Medical Advisory Board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He received royalties from publishing from Cambridge University Press, Oxford University Press, John Wiley & Sons, Wolters Kluwer, and Elsevier. He has received honoraria for lecturing from Columbia University and the Parkinson and Aging Research Foundation. Dr. Hallett's research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds came from Ariston Pharmaceutical Company via a Cooperative Research and Development Agreement (CRADA) with NIH for treatment studies of essential tremor, and the Kinetics Foundation, for studies of instrumental methods to monitor Parkinson’s disease, BCN Peptides, S.A., for treatment studies of blepharospasm, and Medtronics, Inc., for studies of deep brain stimulation, via Clinical Trials Agreements (CTA) with NIH.
Joseph Jankovic has received research funding from Allergan, Inc; Allon Therapeutics; Ceregene, Inc; Chelsea Therapeutics; Diana Helis Henry Medical Research Foundation; EMD Serono; Huntington’s Disease Society of America; Huntington Study Group; Impax Pharmaceuticals; Ipsen Limited; Lundbeck Inc; Michael J Fox Foundation for Parkinson Research; Medtronic; Merz Pharmaceuticals; National Institutes of Health; National Parkinson Foundation; Neurogen; St. Jude Medical; Teva Pharmaceutical Industries Ltd; University of Rochester; Parkinson Study Group. During the past year Dr. Jankovic has been compensated for his services as a consultant or an advisory committee member by Allergan, Inc; Auspex Pharmaceuticals, Inc; EMD Serono; Lundbeck Inc; Merz Pharmaceuticals; Michael J Fox Foundation for Parkinson Research; Neurocrine Biosciences; Neurotoxin Institute; Teva Pharmaceutical Industries Ltd. During the past year Dr. Jankovic has served on the following editorial boards: Medlink: Neurology; Expert Review of Neurotherapeutics, Neurology in Clinical Practice; Associate editor of The Botulinum Journal; Therapeutic Advances in Neurological Disorders; Neurotherapeutics; Tremor and Other Hyperkinetic Movements; Journal of Parkinson’s Disease; UpToDate. During the past year Dr. Jankovic has received royalties from the following publishers: Elsevier and Wiley-Blackwell.
Jorge Juncos has research support from Chelsea, Covance and the National Institutes of Health.
Peter LeWitt has received lecture fees from Chelsea, Lundbeck, and Novartis, consulting fees from Depomed, ExpressScripts, Knopp Biosciences, Glaxo SmithKline, Impax, Intec, Ipsen, NeuroDerm, Merck, Noven Pharmaceuticals, Orient Pharma, Tercica, Teva, UCB, and XenoPort; and clinical research grant support (for clinical trials and other research) from Adamas, Addex, Biotie, Great Lakes Neurotechnologies, The Michael J. Fox Foundation for Parkinson’s Research, Merz, Neurologix, and Novartis, Phytopharm, and UCB. He serves with compensation as editor-in-chief of Clinical Neuropharmacology and as an uncompensated editorial board member for Translational Neuroscience and Journal of Neural Transmission.
Kelly Lyons has received consulting fees from St Jude Medical and Teva Neuroscience. She has received royalties from Informa Healthcare and Oxford University Press.
William Ondo has received speaker fees from GSK, Teva, Allergan, Ipsen, Lundbeck, Avanir, and Merz.
Rajesh Pahwa received personal compensation from Teva Neuroscience, Merck Serono, Novartis, Medtronic, GE Healthcare, Impax, Ceregene, Noven, Adamas, St. Jude Medical for consulting. Dr. Pahwa has received personal compensation from Informa Healthcare for being Co-Editor of the International Journal of Neuroscience. Dr. Pahwa has received research support as PI of clinical trials sponsored by Novartis, Impax, Merck Serono, BI, Schering, Adamas, Biotie, Phytopharm, Allon, Acadia, Xenoport, GlaxoSmithKline and NINDS.
Kapil Sethi is a consultant for Abbott, Synosia and Veloxis. He has part time employment as a Senior Medical Expert Merz Pharmaceuticals. He is a speaker for Teva, Ipsen and Merz and receives research support from the National Institutes of Health, National Parkinson Foundation, Impax, Acadia, Synosia, Adamas, BI Pharmaceuticals, and Teva. He has provided expert testimony for welding defense and metoclopramide litigation. He owns stock in Elan Pharma.
Natividad Stover receives research grant support from GSK, Allergan, NINDS, Biotie, EMD Serono, Impax Laboratories and Teva.
Daniel Tarsy has received grant support from Phytopharm PLC and Michael J. Fox, patient education grants from Allergan, UCB, Teva, and Ipsen, unrestricted grants for fellowship support from Allergan and Teva, personal compensation as a consultant for Neurocrine Biosciences and Genzyme, royalties from UpToDate and Springer, foundation support from National Parkinson Foundation, and honoraria from the Movement Disorders Society.
Claudia Testa received research support from the Huntington Society of Canada (principal investigator), as well as the Tremor Research Group, HighQ Foundation, and Huntington Study Group (site principal investigator subcontracts). Huntington Study Group subcontract sponsors were NIH/NINDS or Medivation. She was a co-investigator on NIH/NCRR 2R24RR018827-05A1 and NIH/NIA P50 AG025688. She received Emory institutional support via NIH PHS UL1 RR025008 and NIH PHS M01-RR00039. She received an honorarium from Virginia Commonwealth University, Department of Neurology.
Ron Tintner received personal compensation from BI Pharmaceuticals for speaking, Allergan for speaking and Advisory Board, and Merz Pharmaceuticals for Advisory Board.
Theresa Zesiewicz has received speaker fees from Teva, UCB Pharma, and General Electric.
Dr. Watts has received honoraria for serving on the International Parkinson Disease Scientific Advisory Board of UCB Pharma.
Footnotes
Author roles: 1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique.
Rodger Elble: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Cynthia Comella: 1A, 1C, 2C, 3B
Stanley Fahn: 1A, 1C, 2C, 3B
Mark Hallett: 1A, 1C, 2C, 3B
Joseph Jankovic: 1A, 1C, 2C, 3B
Jorge Juncos: 1A, 1C, 2C, 3B
Peter LeWitt: 1A, 1C, 2C, 3B
Kelly Lyons: 1A, 1B, 1C, 2C, 3B
William Ondo: 1A, 1B, 1C, 2C, 3B
Rajesh Pahwa: 1A, 1B, 1C, 2C, 3B
Kapil Sethi: 1A, 1B, 1C, 2C, 3B
Natividad Stover: 1C, 2C, 3B
Daniel Tarsy: 1A, 1B, 1C, 2C, 3B
Claudia Testa: 1A, 1C, 2C, 3B
Ron Tintner: 1A, 1B, 1C, 2C, 3B
Theresa Zesiewicz: 1A, 1C, 2C, 3B
Ray Watts: 1A, 1C, 2C, 3B
The authors have no conflicts of interest or financial disclosures relevant to this paper.
Appendices for on-line publication
Appendix 1: Tremor Research Group Essential Tremor Rating Assessment Scale
Appendix 2: Distribution of total TETRAS performance scores
Appendix 3: Summary of round 1 and round 2 inter-rater ICCs
Appendix 4: Intra-rater ICCs
Contributor Information
Rodger Elble, Southern Illinois University School of Medicine, Springfield IL.
Cynthia Comella, Rush University Medical Center, Chicago IL.
Stanley Fahn, Columbia University Medical Center, New York NY.
Mark Hallett, National Institute of Neurological Disorders and Stroke, Bethesda MD.
Joseph Jankovic, Baylor College of Medicine, Houston TX.
Jorge L. Juncos, Emory University, Atlanta GA.
Peter LeWitt, Henry Ford Health Systems, Bloomfield MI.
Kelly Lyons, University of Kansas Medical Center, Kansas City KS.
William Ondo, University of Texas Health Science Center, Houston TX.
Rajesh Pahwa, University of Kansas Medical Center, Kansas City KS.
Kapil Sethi, Georgia Health Sciences University, Augusta GA.
Natividad Stover, University of Alabama at Birmingham, Birmingham, AL.
Daniel Tarsy, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston MA.
Claudia Testa, Virginia Commonwealth University, Richmond, VA.
Ron Tintner, The Methodist Hospital, Houston TX.
Ray Watts, University of Alabama at Birmingham, Birmingham, AL.
Theresa Zesiewicz, University of South Florida, Tampa FL.
References
- 1.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10:148–161. doi: 10.1016/S1474-4422(10)70322-7. [DOI] [PubMed] [Google Scholar]
- 2.Zesiewicz TA, Elble RJ, Louis ED, et al. Evidence-based guideline update: Treatment of essential tremor: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2011;77:1752–1755. doi: 10.1212/WNL.0b013e318236f0fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elble RJ, Lyons KE, Pahwa R. Levetiracetam is Not Effective for Essential Tremor. Clin Neuropharmacol. 2007;30:350–356. doi: 10.1097/WNF.0b013E31807A32C6. [DOI] [PubMed] [Google Scholar]
- 4.Hooper J, Taylor R, Pentland B, Whittle IR. Rater reliability of Fahn's tremor rating scale in patients with multiple sclerosis. Arch Phys Med Rehabil. 1998;79:1076–1079. doi: 10.1016/s0003-9993(98)90174-5. [DOI] [PubMed] [Google Scholar]
- 5.Stacy MA, Elble RJ, Ondo WG, Wu SC, Hulihan J. Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov Disord. 2007;22:833–838. doi: 10.1002/mds.21412. [DOI] [PubMed] [Google Scholar]
- 6.Fahn S, Tolosa E, Marín C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson's Disease and Movement Disorders. 2nd ed. Baltimore: Williams & Wilkins; 1993. pp. 225–234. [Google Scholar]
- 7.Bain PG, Findley LG, Atchison P, et al. Assessing tremor severity. J Neurol Neurosurg Psychiatry. 1993;56:868–873. doi: 10.1136/jnnp.56.8.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bain PG, Findley LJ. Assessing Tremor Severity: A Clinical Handbook. London: Smith-Gordon; 1993. [Google Scholar]
- 9.Louis ED, Ford B, Bismuth B. Reliability between two observers using a protocol for diagnosing essential tremor. Mov Disord. 1998;13:287–293. doi: 10.1002/mds.870130215. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Barnes L, Wendt KJ, et al. A teaching videotape for the assessment of essential tremor. Mov Disord. 2001;16:89–93. doi: 10.1002/1531-8257(200101)16:1<89::aid-mds1001>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Louis ED, Barnes LF, Wendt KJ, et al. Validity and test-retest reliability of a disability questionnaire for essential tremor. Mov Disord. 2000;15:516–523. doi: 10.1002/1531-8257(200005)15:3<516::AID-MDS1015>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Elble R, Comella C, Fahn S, et al. The essential tremor rating assessment scale (TETRAS) Mov Disord. 2008;23(Suppl. 1):S357. [Google Scholar]
- 13.Tintner R, Group TR. The Tremor Rating Scale (TRS) Mov Disord. 2004;19:1131–1132. [Google Scholar]
- 14.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13:2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 15.Mostile G, Giuffrida JP, Adam OR, Davidson A, Jankovic J. Correlation between Kinesia system assessments and clinical tremor scores in patients with essential tremor. Mov Disord. 2010;25:1938–1943. doi: 10.1002/mds.23201. [DOI] [PubMed] [Google Scholar]
- 16.Elble RJ, Pullman SL, Matsumoto JY, Raethjen J, Deuschl G, Tintner R. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain. 2006;129:2660–2666. doi: 10.1093/brain/awl190. [DOI] [PubMed] [Google Scholar]
- 17.Mostile G, Fekete R, Giuffrida JP, et al. Amplitude fluctuations in essential tremor. Parkinsonism & related disorders. 2012 doi: 10.1016/j.parkreldis.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Quinn NP, Schneider SA, Schwingenschuh P, Bhatia KP. Tremor-some controversial aspects. Mov Disord. 2011;26:18–23. doi: 10.1002/mds.23289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.