Summary
Pseudomonas aeruginosa in the lungs of cystic fibrosis patients grows to high densities in mucopurulent material that is depleted in oxygen. Some have concluded that growth in these circumstances is dependent on anaerobic nitrate respiration. Here we present data in favour of the alternative hypothesis that microaerobic respiration is the predominant mode of P. aeruginosa growth in the cystic fibrosis lung. We found that P. aeruginosa strain PAO1 and a mucoid derivative of strain PAO1 each grew at dissolved oxygen concentrations of less than 3 µM. This is lower than the concentration of oxygen that has been measured in hypoxic cystic fibrosis mucous. A transcriptome analysis comparing cells grown under aerobic conditions (185 µM dissolved oxygen) with cells grown with 20 µM or 3 µM dissolved oxygen, or anaerobically with nitrate, revealed that overlapping sets of genes are expressed depending on oxygen availability. This suggests that P. aeruginosa responds to changes in oxygen concentration along a continuum rather than having a discrete low oxygen regulon. Any one of three high affinity terminal oxidases that P. aeruginosa encodes supported microaerobic growth. A triple mutant lacking all three of these oxidases failed to grow at low oxygen and formed abnormal biofilms.
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen that is especially problematic for patients with the inherited disease cystic fibrosis (CF) (Lyczak et al., 2000; 2002). Chronic P. aeruginosa infections eventually afflict almost all CF patients and are responsible for the progressive destruction of lung tissue that leads to most of the mortality of people with this disease. In CF infections P. aeruginosa grows in the stagnant mucus secretions that build up on lung airway epithelia to densities of 108-1010 cells ml−1 (Høiby, 1998).
Pseudomonas-infected mucus in the airways of CF patients is depleted in oxygen. Oxygen partial pressures as low as 333 Pa have been reported, equivalent to about 7 µM dissolved oxygen (Worlitzsch et al., 2002). This hypoxia is thought to be a consequence of CF-related increases in epithelial oxygen consumption and the proliferation of P. aeruginosa during chronic infections (Worlitzsch et al., 2002). This has raised the question of how P. aeruginosa, a facultative anaerobe that preferentially uses aerobic respiration, generates energy for growth and survival. Nitrate has been measured in CF and normal mucus at equivalent concentrations (c. 400 µM) sufficient to support some growth of P. aeruginosa by respiration using nitrate rather than oxygen as a terminal electron acceptor (Linnane et al., 1998). These and other observations have led some investigators to conclude that P. aeruginosa uses nitrate respiration as the primary mode of growth during chronic infections in the CF lung (Yoon et al., 2002). A problem with this conclusion is that it is unclear how the host generates sufficient nitrate to support the high numbers of bacterial cells that are found in the lung. In laboratory studies 100 mM nitrate is typically required for P. aeruginosa to grow to high cell densities (Filiatrault et al., 2006). In a recent study, CF sputum medium containing 400 µM nitrate supported a small increase in P. aeruginosa cell growth compared with what has been observed in CF lungs (Palmer et al., 2007).
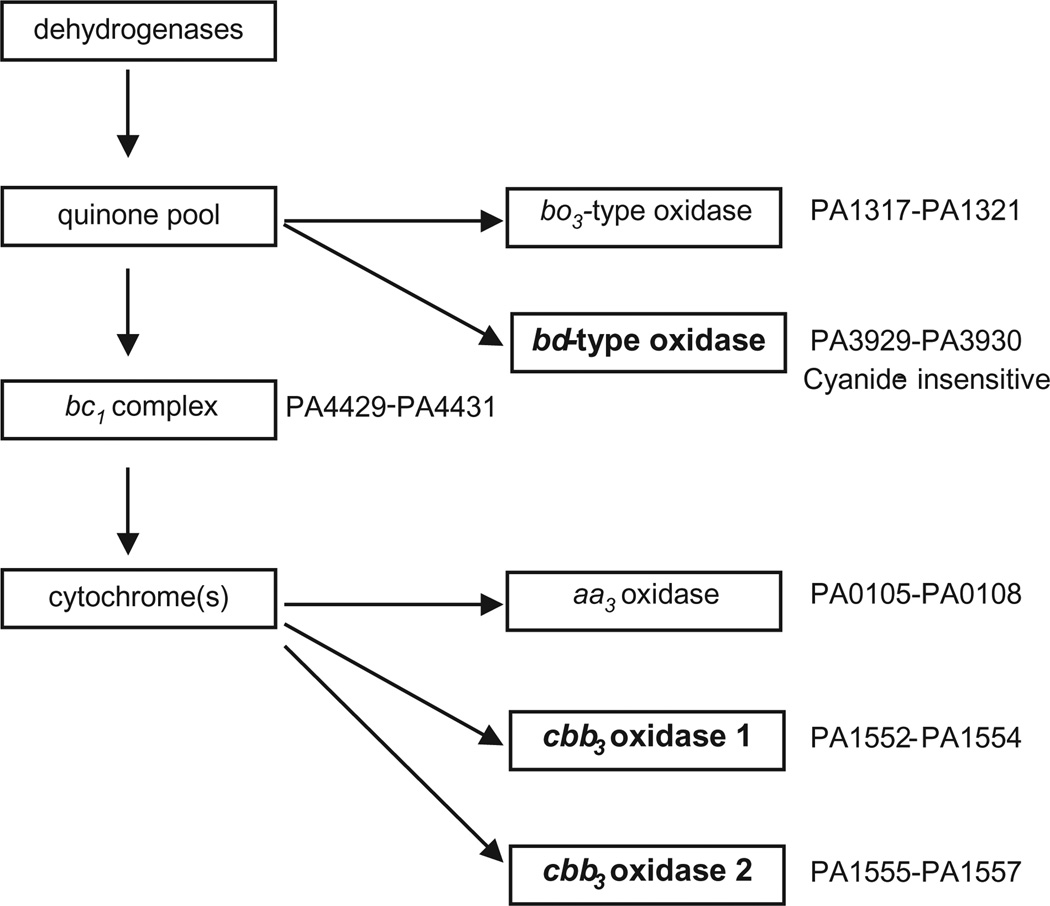
An alternative interpretation of the available data is that P. aeruginosa grows by respiring the oxygen that is continuously delivered to lung airway lumens as CF patients breath. The bacteria in the mucous secretions that line the airway lumens are typically at such high densities as to rapidly deplete available oxygen. If this is the case, we expect P. aeruginosa to be well adapted to growth under oxygen depleted, microaerobic conditions. P. aeruginosa is potentially well suited for microaerobic respiration because it possess a branched aerobic respiratory chain terminated by five predicted terminal oxidases (Matsushita et al., 1980; Fujiwara et al., 1992; Okamoto et al., 1995; Cunningham et al., 1997; Stover et al., 2000) (Fig. 1). Three of these, the cbb3-1 oxidase, the cbb3-2 oxidase and the cyanide insensitive bd-type oxidase, are predicted to have a high affinity for oxygen and therefore to sustain respiration when oxygen levels are low (Cunningham et al., 1997; Comolli and Donohue, 2004).
Fig. 1.
Diagram of Pseudomonas aeruginosa branched aerobic respiratory chain (adapted from Comolli and Donohue, 2002). The terminal oxidases predicted to have a high affinity for oxygen are the bd-type oxidase (cyanide insensitive), cbb3-1 and cbb3-2.
Here we show that P. aeruginosa strain PAO1 and a mutant strain of PAO1 that is mucoid, grow with dissolved oxygen at a concentration of 3 µM or less. We determine that three predicted high affinity terminal oxidases all contribute to planktonic and biofilm growth at low oxygen and use transcriptome analysis to identify genes that may be important for adaptation to a microaerobic mode of growth.
Results
Pseudomonas aeruginosa PAO1 grows well under microaerobic conditions
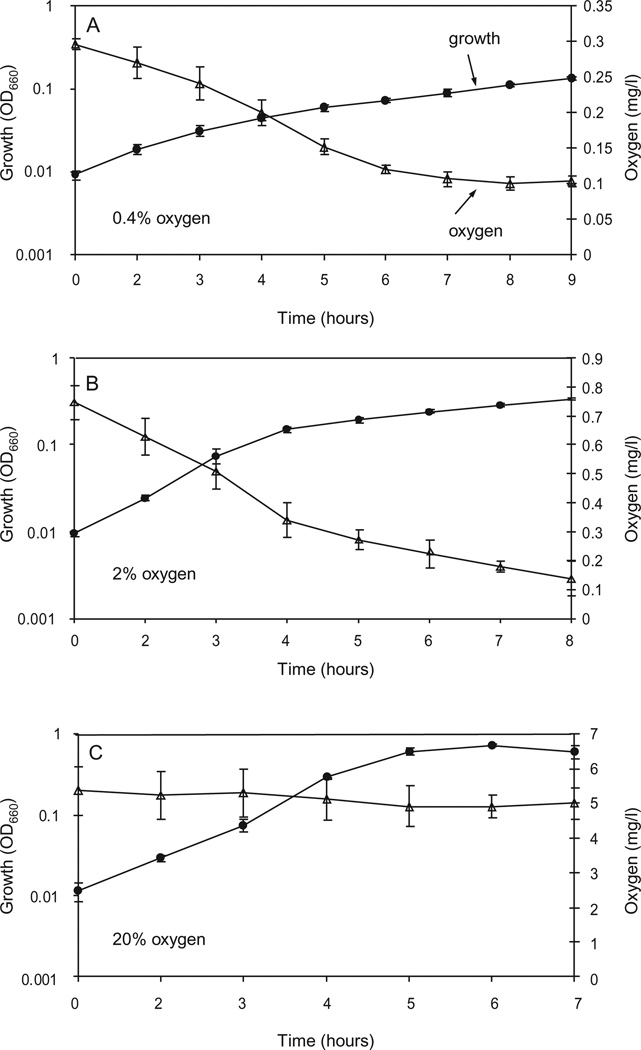
We tested the ability of P. aeruginosa to grow microaerobically using a synthetic CF sputum medium without added nitrate. This medium is formulated based on an analysis of small molecule components of CF sputum and consists of mineral salts and 19 amino acids supplied at concentrations ranging from about 0.02–2.0 millimolar. (Palmer, Aye and Whiteley, unpublished). We found that the lowest measurable concentration of dissolved oxygen that we could maintain in culture media during growth to a reasonable density was between 0.1 and 0.3 mg oxygen per l (3–10 mM) (Fig. 2A). This was achieved using an atmosphere of 0.4% oxygen and the mixing and sparging scheme described in the Experimental procedures. When cells reached an OD660 of about 0.1, the measured dissolved oxygen concentration dropped to 0.1 mg oxygen per l; the limit of sensitivity for the oxygen electrode that we used. Apparently the respiratory activity of cells depleted the medium of oxygen faster than we could replenish it. The same pattern of dissolved oxygen depletion during growth occurred in cultures supplied with 2% oxygen, but in this case the dissolved oxygen concentration did not fall to 0.1 mg per l until cells reached an OD660 of about 0.5 (Fig. 2B). By contrast we were able to maintain a constant dissolved oxygen concentration of 5.0 mg per l in cultures that were mixed and sparged with 20% oxygen (Fig. 2C). When we calculated doubling times from the early phase of growth, we found that strain PAO1 grew slightly more slowly with 2% oxygen compared to with 20% oxygen (Table 1). The growth rate at 0.4% oxygen, was much slower than at 2% oxygen, indicating that the corresponding dissolved oxygen concentration is growth limiting in our conditions. All cultures eventually reached a final OD660 of approximately 1.0 (data not shown). PAO1 did not grow as rapidly with 100 mM nitrate as with 20% or 2% oxygen; but it grew slightly faster than with 0.4% oxygen (Table 1). No growth was observed in N2 sparged medium to which no KNO3 had been added. This confirmed that all available oxygen was effectively removed from the medium and also showed that the amounts of fermentable substrates, such as arginine, that were present in synthetic CF sputum medium, were insufficient to support growth.
Fig. 2.
Growth of Pseudomonas aeruginosa PAO1 at different concentrations of oxygen. Oxygen was supplied in the gas phase at concentrations of 0.4% (A), 2% (B) or 20% (C). Culture optical density (circles) and dissolved oxygen concentrations (triangles) are plotted over time after inoculation into CF sputum medium. Dissolved oxygen is reported in mg per l; for uninoculated medium this is 5.88 ± 0.13 (20% oxygen) 0.81 ± 0.035 mg (2% oxygen) and 0.3 ± 0.01 (0.4% oxygen).
Table 1.
Effect of oxygen concentration on P. aeruginosa growth rates.
| Oxygen concentration | Doubling timea |
|---|---|
| 20% | 36 ± 0.8 |
| 2% | 46 ± 2 |
| 0.4% | 138 ± 20 |
| 0% | No growth |
| 0% + 100 mM KNO3 | 92 ± 11 |
Doubling time in min; determined from cultures grown in duplicate on three different days.
It has been argued that the conversion of non-mucoid P. aeruginosa to mucoid variants that overproduce the exopolysaccharide alginate, is a critical factor for persistence in the CF lung (Govan and Nelson, 1992; Pedersen, 1992). Mutations in the mucA gene, encoding an anti-sigma factor, cause activation of AlgT, an alternative RNA polymerase sigma factor that is essential for alginate production (Ramsey and Wozniak, 2005). We tested the growth of a mucoid mucA mutant strain at reduced oxygen to ascertain whether mucoidy might compromise microaerobic growth and found that the mucoid strain grew just as well as its wild-type parent, with a generation time of 122 ± 16 min, at 0.4% oxygen. The mucoid strain retained its mucoid phenotype throughout growth at low oxygen. We spread cells on Luria-Bertani (LB) solid medium at the end of the growth experiments and failed to observe any non-mucoid colonies out of several thousand that we screened.
Three terminal oxidases contribute to microaerobic growth
The cbb3-1, cbb3-2 and cyanide insensitive oxidases of P. aeruginosa are predicted to have high affinities for oxygen (Cunningham et al., 1997; Comolli and Donohue, 2004). To determine which of these enzymes might be important for microaerobic growth we grew cbb3-2, cioA and cbb3-1 mutants with 2% and 0.4% oxygen. The doubling time of each single mutant was similar to that of the wild type (Table 2), suggesting that more than one oxidase is capable of sustaining microaerobic growth. Consistent with this, a double cbb3-1 cbb3-2 mutant grew more slowly than the wild type at 2.0% oxygen and failed to grow at 0.4% oxygen. Growth of the double mutant at 2% oxygen is likely due to the activity of the cyanide insensitive oxidase. We found that a cioA cbb3-1 cbb3-2 triple mutant failed to grow with either 2% or 0.4% oxygen. The double and triple oxidase mutants each grew at the same rate as the wild type under anaerobic conditions with nitrate.
Table 2.
Effect of oxygen concentration on growth rates of terminal oxidase mutants.
| Doubling timea | ||||
|---|---|---|---|---|
| Oxygen % | ||||
| Strain | Genotype | 20 | 2 | 0.4 |
| PAO1 | Wild type | 36 ± 0.8 | 46 ± 2 | 138 ± 20 |
| PTL14859 | cbb3-1 oxidase | 48 ± 7 | 42 ± 5 | 104 ± 17 |
| PAO1371 | cbb3-2 oxidase | 36 ± 1 | 46 ± 5 | 116 ± 29 |
| PTL12436 | cioA, cyanide insensitive oxidase | 46 ± 1 | 43 ± 3 | 94 ± 6 |
| PAO1372 | cioA cbb3-2 | 35 ± 2 | 48 ± 6 | 108 ± 13 |
| PAO1373 | cbb3-1 cbb3-2 | 41 ± 0.4 | 123 ± 3 | No growth |
| PAO1387 | cioA cbb3-1 cbb3-2 | 70 ± 3 | No growth | No growth |
Doubling time, expressed in minutes plus or minus the standard deviation, were determined from cultures grown in duplicate on three different days.
Terminal oxidase genes have distinctive patterns of expression in response to oxygen concentration
In keeping with their role in contributing to microaerobic growth, we found in a transcriptome analysis that the cbb3-2 oxidase genes were highly expressed at low oxygen. This is consistent with previously reported results (Comolli and Donohue, 2004), as is our observation that the transcript for the cbb3-1 oxidase was present at high levels during fully aerobic growth (data not shown) and did not change much during growth at low oxygen tensions. Expression of the cbb3-1 oxidase genes did however, decrease under completely anaerobic growth conditions (Table 3). The expression of cioAB encoding the cyanide-insensitive terminal oxidase increased during growth with 0.4% oxygen compared to with 20% oxygen, but the absolute transcript levels of the cioAB genes were low, even under induced conditions. Expression of the predicted low–affinity bo3-type quinol oxidase genes encoded by the cyo operon decreased in all three oxygen-depleted conditions tested, suggesting that the encoded oxidase functions primarily with high oxygen. The transcript for the cox operon coding for the aa3-type low-affinity oxidase was not detected in any of the conditions tested. A previous study showed that these genes are preferentially expressed in the stationary phase of growth (Schuster et al., 2004).
Table 3.
Effect of oxygen on expression levels of aerobic oxidase and denitrification genes.
| Fold changea | ||||
|---|---|---|---|---|
| Oxygen % | ||||
| Gene no.b | Description | 2 | 0.4 | 0 (+ NO3)c |
| PA0105–PA0108 | coxBA, cytochrome c oxidase, aa3 type and associated proteins | A | A | A |
| PA1317–PA1321 | cyoABCDE, cytochrome o ubiquinol oxidase, cyo type | −8.5 | −9.6 | −17 |
| PA1552–PA1554 | ccoP1Q1O1N1, cytochrome c oxidase, cbb3 type | – | – | −4.2 |
| PA1555–PA1557 | ccoP2Q2O2N2, cytochrome c oxidase, cbb type | 7.2 | 6.2 | – |
| PA3929–PA3930 | cioAB, cyanide insensitive oxidase (CIO) | – | 7.1 | – |
| PA1172–PA1177 | napEFDABC, periplasmic nitrate reductase | – | 7.9 | – |
| PA3872–PA3875 | narK1K2GHJI, respiratory nitrate reductase | – | – | 71 |
| PA0509–PA0519 | nirSMCFDLGHJEN, nitrite reduction | 24 | – | 40 |
| PA0520–PA0522 | nirQOP, nitrite reduction | 11 | – | 86 |
| PA0523–PA0525 | norCBD, nitric-oxide reductase | 40 | – | 255 |
| PA3391–PA3396 | nosRZDFYL, nitrous-oxide reductase | 65 | – | 135 |
Fold changes are expressed in relation to transcript values during growth with 20% oxygen. The fold change shown is the highest change observed for a gene in the operon.
Gene number from the Pseudomonas genome project (http://www.pseudomonas.com).
Cells were grown with 100 mM nitrate.
A, the transcripts for these genes were absent; –, no change in expression.
Transcripts of the genes encoding for nitrite, nitric oxide and nitrous oxide reductases needed to mediate anaerobic respiration of nitrite to nitrogen gas, were elevated during growth with 2% oxygen, even though, as previously observed (Filiatrault et al., 2005), the highest levels of expression were achieved during anaerobic growth with nitrate. P. aeruginosa encodes a periplasmic nitrate reductase in addition to a membrane bound nitrate reductase that serves as the main mediator of anaerobic nitrate respiration (Palmer et al., 2007; Williams et al., 2007). Expression of the periplasmic nitrate reductase, encoded by the nap genes (Vollack et al., 1998) increased only during growth with 0.4% oxygen and was not elevated during anaerobic growth with nitrate. Thus, modest elevation of nitrate reductase genes is not indicative of growth by nitrate respiration.
A complicated pattern of gene expression occurred in response to low oxygen and anaerobiosis
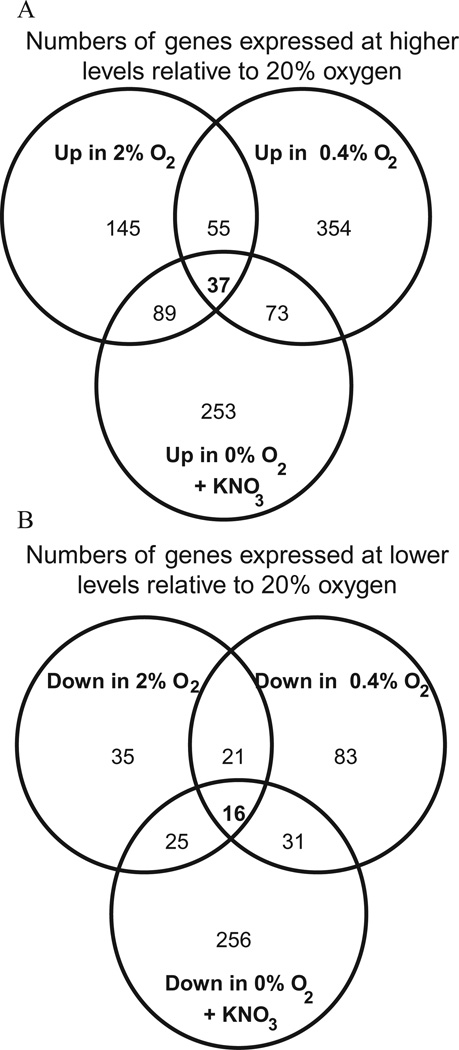
We scrutinized the transcriptomes of cells grown with low oxygen as well as by anaerobic nitrate respiration to get an impression of whether there might be a low oxygen regulon. Applying a cut-off of a 2.5-fold change in transcript level, we found that 180, 437 and 509 genes were differentially expressed during growth with 2% and 0.4% oxygen and anaerobically with nitrate, respectively (Fig. 3). We have listed in Table 4 those genes that were expressed at 10-fold or higher levels during growth in at least one of the three conditions compared with growth at 20% oxygen. We have also listed selected genes that did not meet the 10-fold cut-off criterion but that have known functions. We have excluded the terminal oxidase genes already listed in Table 3.
Fig. 3.
Numbers of genes that are differentially expressed in response to growth at different concentrations of oxygen. Gene expression profiles of cells grown at the indicated concentration of oxygen were compared with that of cells grown with 20% oxygen. A 2.5-fold cut-off and a P-value threshold of 0.001 were applied to transcriptome data.
A. Numbers of genes expressed at higher levels relative to 20% oxygen.
B. Numbers of genes expressed at lower levels relative to 20% oxygen.
Table 4.
Genes differentially expressed in response to a decrease in oxygen.
| Fold changeb | ||||
|---|---|---|---|---|
| Oxygen percentage | ||||
| Gene no.a | Descriptiona | 2 | 0.4 | 0 (+ NO3)c |
| PA0050 | Conserved hypothetical protein | 2.6 | 17 | 4.5 |
| PA0459 | Probable ClpA/B protease ATP binding subunit | 7.8 | 23 | 23 |
| PA0529 | Conserved hypothetical protein | – | – | 11 |
| PA0586-0588 | Conserved hypothetical and serine protein kinase | – | 15 | 11 |
| PA0617-0647 | Probable bacteriophage | – | – | 13 |
| PA0713 | Conserved hypothetical protein | 10 | 14 | 7.1 |
| PA0985 | Probable colcin-like toxin | – | – | 25 |
| PA1123 | Conserved hypothetical protein | 18 | 30 | 23 |
| PA1150 | Pyocin S2 | – | – | 13 |
| PA1323-1324 | Conserved hypothetical | – | 11 | 7.7 |
| PA1431 | rsaL | – | 27 | – |
| PA1432 | lasI, autoinducer synthase | – | 30 | – |
| PA1546 | hemN, O2-independent coproporphyrinogen III oxidase | 6.3 | 2.7 | 4.1 |
| PA1673 | Hemerythin | 5.8 | 2.3 | 4.2 |
| PA1746 | Predicted phosphatase | 11 | 20 | 3.4 |
| PA1789 | Universal stress protein, UspA | 5.1 | 2.7 | 3.2 |
| PA1891- 1897 | Conserved hypothetical | – | 25 | – |
| PA2128-2132 | Fimbrial protein synthesis and assembly (CupA) | 12 | 150 | 15 |
| PA2193-2195 | Hydrogen cyanide synthase | 4.0 | 6.0 | – |
| PA2365-2375 | Conserved hypothetical protein and probable Clp protease (PA2371) | 4.4 | 27 | 5.6 |
| PA2381 | Conserved hypothetical protein | 15 | 21 | 33 |
| PA2939 | Probable aminopeptidase | – | 21 | – |
| PA3049 | Ribosome modulation factor | – | 29 | 35 |
| PA3309 | Universal stress protein, UspA | 4.9 | – | – |
| PA3476 | rhlI, autoinducer synthase | – | 5.3 | – |
| PA3569-3560 | mmsBA, 3-OHisobutyrate/methylmalonate semiald DH | 3.5 | 10 | 18 |
| PA3904-3908 | Conserved hypothetical | – | 24 | – |
| PA3911-3913 | Conserved hypothetical protein | 13 | 3.4 | 26 |
| PA3914-3918 | Molybdenum cofactor biosynthesis | – | – | 209 |
| PA4131-4134 | Ferredoxin, CcoN homology, conserved hypotheticals | 8.2 | 19 | – |
| PA4236 | katA, catalase | 4.8 | 4.9 | – |
| PA4306 | Conserved hypothetical protein | – | 20 | – |
| PA4328 | Universal stress protein UspA | 11 | 2.2 | 6.4 |
| PA4352 | Universal stress protein, UspA | 7.4 | – | 4.1 |
| PA4587 | ccpR, cytochrome c551 peroxidase precursor | 15 | 17 | – |
| PA4738-4739 | Conserved hypothetical | – | 25 | 19 |
| PA5027 | Universal stress protein, UspA | 5.7 | 2.0 | 4.6 |
| PA5100 | hutU, urocanase | – | 12 | 3.1 |
| PA5427 | adhA, alcohol dehydrogenase | 9.8 | 7.6 | 12 |
| PA5475 | Conserved hypothetical protein | 9.9 | 6.4 | 10 |
| PA5481-5482 | Conserved hypothetical | – | 22 | 20 |
| PA5496-5497 | Ribonucleotide reductase | 7.8 | 3.1 | 46 |
Genes forming putative operons are grouped and the values shown represent the gene with the highest average expression ratio.
Fold change in gene expression when compared with a culture growing with 20% oxygen, P = 0.001.
The culture was supplemented with 100 mM KNO3.
–, no change in expression.
The expression levels of many genes changed in response to just one or two of the three conditions that we tested (Fig. 3). In general, we did not identify a large number of genes that were specific to growth at both reduced oxygen concentrations that we tested, as opposed to growth under anaerobic conditions. Besides the cbb3-2 terminal oxidase genes, PA4131–4134 and PA4587 were the only genes that were expressed at 10-fold or higher levels during growth under the two low oxygen conditions only. PA4131–4134 form a putative operon that encodes a predicted 4Fe-4S ferredoxin transmembrane protein (PA4131), a homologue of CcoN, the catalytic subunit of cbb3 oxidase subunit (PA4133) and two conserved hypotheticals. PA4587 encodes cytochrome c peroxidase. This enzyme is located in the periplasm and purified enzyme catalyses the reduction of hydrogen peroxide to water using cytochrome c as an electron donor (Williams et al., 2007). The role of cytochrome c peroxidase in P. aeruginosa physiology has not been characterized, but it is logical to expect that it functions in detoxification of toxic oxygen species in vivo. In addition to these highly expressed genes, PA4236, encoding catalase, and PA2193–2195, encoding hydrogen cyanide synthase, were expressed at moderately high levels under microaerobic conditions.
Some of the genes that were expressed at high levels under all three conditions resemble Clp proteases (PA0459 and PA2371) and may play a role in rapidly degrading and removing proteins that are no longer needed by cells as they switch to a low oxygen or anaerobic existence. Other genes that were induced as the oxygen concentration dropped to 2% were several with predicted universal stress protein (Usp) domains. PA4328 was most highly expressed at 2% oxygen but still had elevated levels of expression at 0.4% oxygen and under anaerobic nitrate-reducing conditions (Table 4). The Usp-type proteins PA3309 and PA4352 were also induced under microaerobic conditions. The precise biological function of Usp proteins is not known, but PA3309 and PA4352 have been shown to be required for survival of P. aeruginosa under anaerobic pyruvate fermentation conditions (Schreiber et al., 2006) and for survival under energy starvation conditions (Boes et al., 2006) respectively.
Slow growth at very low oxygen is associated with induction of CupA fimbrae and the quorum sensing regulon
The transcriptome data indicate that P. aeruginosa has a different response to growth with very low oxygen (0.4%) as opposed to growth with low oxygen (2.0%). At 0.4% oxygen, cells are probably responding to the slow growth that occurs as a consequence of oxygen limitation in addition to responding to low oxygen itself. Among the genes that were highly expressed during growth with 0.4% oxygen as compared with 2% oxygen or anaerobic nitrate respiration were genes for the synthesis of CupA fimbrae (Vallet et al., 2004).
A known gene that was expressed at elevated levels during growth with 0.4% oxygen only was lasI, which encodes N(3-oxo-dodecanoyl)-homoserine lactone synthase for the synthesis of one of the two acyl-HSL quorum signals used by P. aeruginosa. Expression of the rhlI gene, which encodes the synthase for N-butyrylhomoserine lactone, the second quorum signal used by this species, also increased exclusively during growth with 0.4% oxygen (Table 4). As might be predicted from this, we found that expression of about half of the quorum-regulated genes identified by Schuster et al. (2004) were increased in expression during growth with 0.4% oxygen (Supplementary Table S1). The quorum-sensing system is not essential for growth with 0.4% oxygen. The doubling time of a rhlI-lasI double mutant, was similar to that of PAO1 during growth with 0.4% oxygen.
Growth at low oxygen does not depend on the transcriptional regulators Anr or RoxR
A number of the genes that showed increased expression during growth at low oxygen, including the ccb3-2 oxidase, the cytochrome c peroxidase, hemN and the UspA protein PA3309, are regulated by the oxygen-responsive transcription factor Anr (Ray and Williams, 1997; Comolli and Donohue, 2004; Schreiber et al., 2006). In addition, RoxR, a homologue of the well-studied redox responsive global regulators PrrA and RegA from the photosynthetic bacteria Rhodobacter sphaeroides and Rhodobacter cap-sulatus, has been shown to regulate expression of the cyanide insensitive oxidase (Comolli and Donohue, 2002). This prompted us to test the growth of anr and roxR mutants at 0.4% oxygen. We found that the mutants grew at the same rate as the wild-type parent, indicating that these global regulators are not essential components of a low oxygen response.
Cells that are unable to grow microaerobically form abnormal biofilms
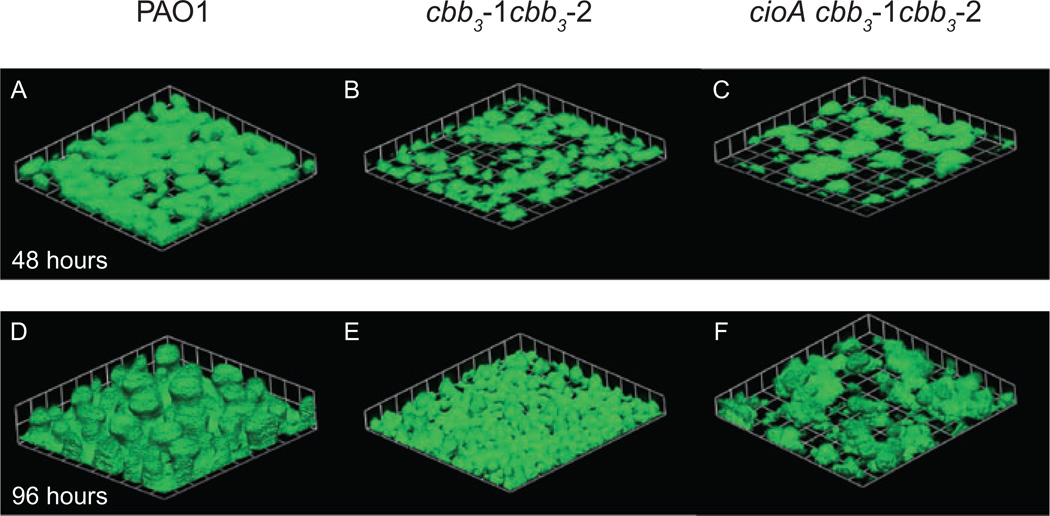
So far, our characterization of growth at low oxygen has been specific to actively growing planktonic cultures. It is also important to consider the contribution of aerobic respiration to biofilm formation and maintenance because evidence suggests that this is the primary way that P. aeruginosa grows in the CF lung (Singh et al., 2000; Høiby et al., 2001). Biofilms are surface-associated communities of bacteria encased in a self-produced extracellular matrix. Studies have suggested that the biofilm matrix can impede the diffusion of gases such that an oxygen gradient is established from the outside to the inside of the biofilm (Werner et al., 2004), much as has been observed in P. aeruginosa-infected CF mucous (Worlitzsch et al., 2002). To assess the contribution of microaerobic respiration to biofilm formation we grew strain PAO1 and terminal oxidase mutant strains expressing green fluorescent protein (GFP) in continuous flow chambers. After 96 h of growth cbb3-1, cbb3-2 and cioA single mutants formed biofilms with morphologies that were indistinguishable from that of the wild type. By contrast, the cbb3-1 cbb3-2 double mutant and the cioA cbb3-1 cbb3-2 triple mutant formed biofilms that were flatter and had less biomass than wild-type biofilms (Fig. 4, Table 5). In control experiments we amended the flow cell growth medium with 100 mM nitrate to allow for growth by anaerobic respiration. In this situation, the double and triple terminal oxidase mutants formed biofilms with morphologies very similar to that of the wild type (Supplementary Fig. S1). This suggests that the abnormal biofilms formed by the double and triple terminal oxidase mutants in aerobic flow cell chambers were caused by defective microaerobic respiration and not an unrelated indirect effect of the mutations.
Fig. 4.
Double and triple high affinity terminal oxidase mutants form defective biofilms. Images were obtained after 48 h and 96 h growth of the wild-type strain PAO1 (A and D), the cbb3-1 cbb3-2 double mutant PAO1373 (B and E) and the cioA cbb3-1 cbb3-2 triple mutant PAO1387 (C and F) in continuous flow chambers. Each square on the grid is 46 µM per side.
Table 5.
COMSTAT analysis of 96 h biofilm experiments.a
| Strainb | Genotype | Total biomass (µm3 µm−2) | Average thickness (µm) |
|---|---|---|---|
| PAO1 | Wild type | 32.5 ± 4.8 | 40.3 ± 7.4 |
| PAO1394 | cbb3-1 oxidase | 29.9 ± 2.8 | 36.4 ± 4.1 |
| PAO1391 | cbb −2 oxidase | 33.8 ± 7.3 | 43.2 ± 8.5 |
| PAO1378 | cioA, cyanide insensitive oxidase | 24.7 ± 8.2 | 32.3 ± 12.6 |
| PAO1375 | cbb3-1 cbb3-2 | 14.0 ± 1.8 | 14.2 ± 2.5 |
| PAO1390 | cioA cbb3-1 cbb3-2 | 13.7 ± 2.3 | 15.9 ± 2.8 |
Results of averages ± standard deviation of seven images from each flow cell.
All strains carry pMRP9-1 for GFP expression.
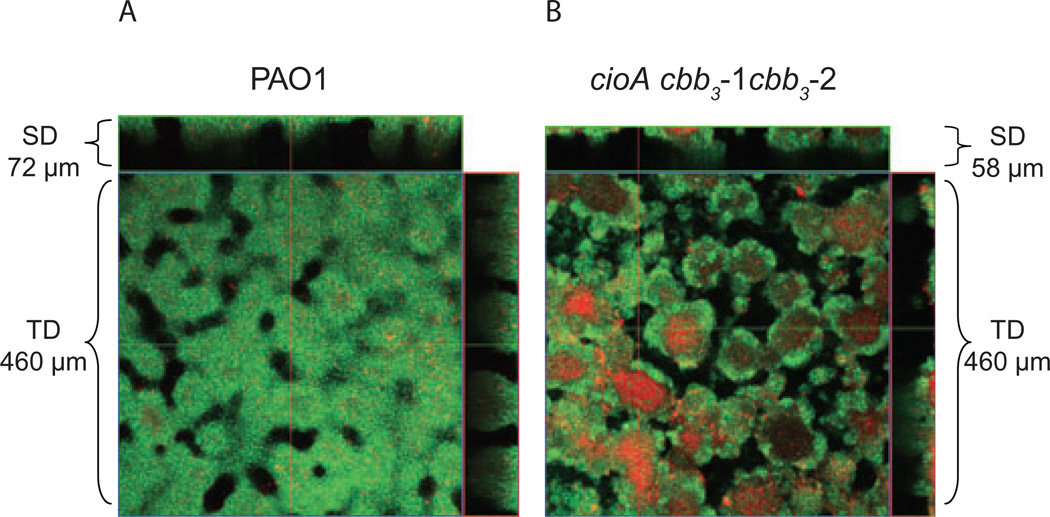
Our data suggest that the interior portions of a developing biofilm become highly depleted in oxygen and that the high affinity terminal oxidases are important for enabling cells to attain their maximum thickness and biomass. To determine the possible importance of microaerobic respiration to cell viability in biofilms we treated 96 h biofilms with propidium iodide, a stain that is commonly used to detect dead cells (Banin et al., 2006). Whereas most of the wild-type cells in 96 h biofilms were fully viable, the interior portions of biofilms formed by the triple high affinity oxidase mutant tended to be filled with dead cells (Fig. 5). Propidium iodide staining indicated that double cbb3-1 cbb3-2 mutant biofilms were fully viable, with very little evidence of dead cells, similar to the situation seen with the wild type. Thus, although the double terminal oxidase mutant forms abnormal biofilms similar in structure to the biofilms of the triple terminal oxidase mutant, it differs in that it retains its vitality.
Fig. 5.
The triple high affinity oxidase mutant loses viability in biofilms. Flow cell biofilms for wild type and the cioA cbb3-1 cbb3-2 triple mutant PAO1987. SD, side dimension (x,z-plane); TD, top down view (x,y-plane). GFP-labelled biofilms were grown for 96 h at which time dead cells were counterstained with propidium iodide. The combined green (GFP) and red (propidium iodide) channels are presented.
Discussion
Our results indicate that P. aeruginosa grows well in a medium with a nutrient content similar to that encountered in the CF lung at low concentrations of oxygen similar to that measured in P. aeruginosa-infected mucous from the CF lung (Worlitzsch et al., 2002) (Table 1). Cell cultures that were mixed and sparged with 0.4% oxygen were exposed to dissolved oxygen of between 3 and 10 µM until they grew to an OD660 of about 0.1. At this point, the dissolved oxygen dropped to below a concentration that we could measure accurately, but cells continued to grow and eventually reached an OD660 of 1.0. For comparison, cultures that were mixed and sparged with 20% oxygen, the percentage of oxygen in air, were exposed to about 185 µM dissolved oxygen throughout growth at 37°C until maximum cell densities were attained.
Pseudomonas aeruginosa has previously been reported to grow microaerobically with oxygen present as the sole electron acceptor (Castric, 1983; Sabra et al., 2002; Ju et al., 2005), but the dissolved oxygen concentrations that cells were exposed to during growth is often not reported. At odds with our results is a report by Sabra et al. that strain PAO1 grew at a faster rate at a dissolved oxygen tension of 1% of air saturation compared with when incubated in medium that had a dissolved oxygen tension of 50% of air saturation. Sabra et al. utilized minimal medium that had about half the amount of iron as the CF synthetic medium that we used. In a follow-up article, Kim et al. (2003) suggest that the concentration of the more readily utilizable reduced form of iron [Fe(II)] might be increased sufficiently in the low oxygen, low iron medium used by Sabra et al. to allow for improved rates of growth. Also at apparent odds with our findings is a report suggesting that alginate restricts the diffusion of oxygen to P. aeruginosa cells (Hassett, 1996). We found that a mucoid mutant strain that overproduces alginate grew just as well as its non-mucoid parent under microaerobic conditions. However, the incubation conditions that we used involved vigorous mixing and we recognize that alginate may be a barrier to the diffusion of oxygen to cells that are in microcolonies in the CF lung and therefore not constantly agitated and mixed.
Both of the cytochrome cbb3 oxidase isozymes that P. aeruginosa encodes were important for microaerobic growth at the lowest oxygen concentrations we tested. These enzymes are predicted to have a high affinity for oxygen; the homologous enzyme from Bradyrhizobium japonicum has a Km for oxygen of 7 nM (Preisig et al., 1996). A triple cioA cbb3-1 cbb3-2 mutant was unable to grow at a dissolved oxygen concentrations less than about 20 µM (2% oxygen supplied in gas phase), suggesting that the cyanide insensitive oxidase also contributes to microaerobic growth. Our transcriptome data showed that expression of the hydrogen cyanide synthase genes was activated about fivefold under microaerobic conditions, but not under nitrate-reducing conditions. Thus, the cyanide insensitive oxidase probably has the added attribute of protecting cells from hydrogen cyanide toxicity during microaerobic growth.
That P. aeruginosa can use any one of three of its five terminal aerobic oxidases to support microaerobic growth under either planktonic or biofilm conditions reinforces the notion that this bacterial species has evolved to thrive at low oxygen. The observation made here and previously by others that the cbb3-1 oxidase is expressed at high levels during growth at all concentrations of oxygen tested suggests that P. aeruginosa is ready to cope with and take advantage of sudden drops in oxygen without first having to mount to transcriptionally directed low oxygen response. This is not to say that P. aeruginosa does not express genes that may be important for adaptation to low oxygen. Our finding that cells adjust their profile of gene expression during growth at 2% or 0.4% oxygen suggests that the induction of Cup fimbrae, catalase, hemerythrin, Usps and many other proteins of unknown function, is likely part of a response to low oxygen. It is important to note, however, that a discrete set of genes was not expressed on exposure to low oxygen. Instead, overlapping sets of genes were expressed depending on the amount of oxygen available (2%, 0.4% or none). This raises the possibility that P. aeruginosa responds to low oxygen along a continuum and that it may also be well-suited to respiring combinations of terminal electron acceptors that it may encounter. Consistent with this, P. aeruginosa has been shown to carry out microaerobic respiration and nitrate respiration simultaneously (Chen et al., 2006).
Many of the gene expression changes that we observed in cells grown with 0.4% oxygen may be due to the slower growth rate that occurred as a consequence of oxygen deprivation rather than to oxygen deprivation per se. The genes encoding quorum signal synthesis as well as the large number of genes of the quorum sensing regulon that were differentially expressed during growth with 0.4% oxygen exclusively (Supplementary Table S1) may fall into this category. Our observation that the quorum sensing regulon was expressed at low oxygen was surprising given that our cultures were harvested at a relatively low optical density and induction of the quorum sensing regulon requires accumulation of the acyl-HSL signals; which happens as a culture reaches a critical cell density (Whiteley et al., 1999; Wagner et al., 2003; Schuster et al., 2004). It is possible that the slower growth rate with 0.4% oxygen allowed basal levels of signal to accumulate over time to a concentration sufficient to stimulate the quorum sensing regulon. In future work it will be of interest to apply techniques such as continuous culture to separate low oxygen effects from growth rate effects. In the meantime it is important to keep in mind that the growth rate changes that occur as a consequence of oxygen deprivation, are part of a general response to low oxygen and thus are important in considering potential effects of low oxygen on the physiology of P. aeruginosa in the CF lung.
Understanding the physiology of P. aeruginosa during chronic infections may be key to the development of new antimicrobial therapies. Here we have shown that P. aeruginosa grows at low concentrations of oxygen and that microaerobic respiration therefore has the potential to contribute to growth in the CF lung. Our results are compatible with a scenario whereby microaerobically respiring P. aeruginosa cells near the edges of mucous layers in CF lungs rapidly deplete oxygen and create a gradient. Oxygen, continuously supplied from the airway lumen should allow cells to grow to high densities along this gradient. Microaerobic respiration probably occurs in concert with nitrate respiration in microenvironments in the CF lung where nitrate and oxygen are both present. In this study we defined the terminal oxidases required for growth at low oxygen concentrations and our transcriptome analyses revealed genes that might be recruited for survival and adaptation in microaerobic conditions.
Experimental procedures
Bacterial strains and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 6. P. aeruginosa and Escherichia coli strains were routinely grown in LB medium at 37°C for routine strain manipulations. E. coli strains carrying the pEX19Gm and pEX19Ap plasmids and their derivatives were grown at 30°C. Where appropriate, antibiotics were used at the following concentrations: gentamicin (Gm), 50 µg ml −1 and carbenicillin (Cb), 300 µg ml −1 (P. aeruginosa); gentamicin, 20 µg ml −1 and ampicillin (Ap), 100 µg ml-1 (E. coli).
Table 6.
Bacterial strains and plasmids used for this study.
| Strains or plasmid | Genotype and phenotype | Reference or origin |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 | Wild-type strain | Jacobs et al. (2003) |
| PTL14859 | ccoN1::ISlacZ/hah, Tcr | Jacobs et al. (2003) |
| PTL12436 | cioA::ISlacZ/hah, Tcr | Jacobs et al. (2003) |
| PTL13321 | anr::ISlacZ/hah, Tcr | Jacobs et al. (2003) |
| PAO1371 | ΔccoNOQP-2; 3088 bp deleted from PA1555–PA1557 | This study |
| PAO1372 | cioA::ISlacZ/hah ΔccoNOQP-2; 3088 bp deleted from PA1555–PA1557, Tcr | This study |
| PAO1373 | ΔccoNOQP-2 ΔccoNOQP-1; 6680 bp deleted from PA1552–PA1557 | This study |
| PAO1387 | cioA::ISlacZ/hah ΔccoNOQP-2 ΔccoNOQP-1; 6680 bp deleted from PA1552–PA1557, Tcr | This study |
| PAO1374 | ΔmucA; mucoid | Starkey and Parsek |
| PAO1-MW1 | rhlI::Tn501 lasI::tetA, Hgr Tc r | Whiteley et al. (1999) |
| PAK | Wild-type strain | Comolli and Donohue (2002) |
| RoxR1 | PAK, roxR ΔSmaI–NsiI::ΩGmr | Comolli and Donohue (2002) |
| E. coli strains | ||
| DH5a | F− λ− recA1 Δ(lacZYA-argF) U169 hsdR17 thi-1 gyrA96 supE44 endA relA1 Φ80dLacZΔM15 | Gibco-BRL |
| S17-1 | thi pro hdsR hdsM+ recA; chromosomal insertion of RP4-2 (Tc::Mu Km::Tn7) | Simon et al. (1983) |
| Plasmids | ||
| pUC19 | High-copy-number cloning vector; Apr | Yanisch-Perron et al. (1985) |
| pEX19Gm | oriT+ sacB+; gene replacement vector with MCS from pUC19; Gmr | Hoang et al. (1998) |
| pPS858 | FRT cassette vector for insertion of selectable markers; Apr, Gmr | Hoang et al. (1998) |
| pFLP2 | sacB+; Flp recombinase-expressing bhr vector; Apr | Hoang et al. (1998) |
| pEX19Ap | oriT+ sacB+; gene replacement vector with MCS from pUC19; Apr | Hoang et al. (1998) |
| pUC-Δcbb3-2 | In-frame deletion of ccoNOPQ-2 cloned into BamHI/XbaI sites of pUC19; Apr |
This study |
| pUC-Δcbb31–2 | In-frame deletion of ccoNOPQ-2 and ccoNOPQ-1 cloned into BamHI/XbaI sites of pUC19; Apr | This study |
| pUC-Δcbb31–2 BamHI | In-frame deletion of ccoNOPQ-2 and ccoNOPQ-1 including an engineered BamHI site cloned into SacI/XbaI sites of pUC19; Apr |
This study |
| pEX-Δcbb3-2 | BamHI/XbaI fragment from pUC-Δcbb3-2 cloned into pEX19Gm; Gmr | This study |
| pEX-Δcbb31–2 | BamHI/XbaI fragment from pUC-Δcbb31–2 cloned into pEX19Gm; Gmr | This study |
| pEX-Δcbb31–2 Gm | SacI/XbaI fragment from pUC Δcbb31–2 BamHI cloned into pEX19Ap; Gmr cassette from pPS858 cloned into the engineered BamHI site of Δcbb31–2; Apr Gmr | This study |
Growth at defined oxygen concentrations
For experiments carried out at defined oxygen concentrations, P. aeruginosa strains were grown at 37°C in synthetic CF sputum medium (Palmer, Aye and Whiteley, unpublished) without added nitrate. The medium consisted of a buffered base (1.3 mM NaH2PO4, 1.25 mM Na2HPO4, 0.271 mM K2SO4, 0.122 g l−1 NH4Cl, 1.114 g l −1 KCl and 3.03 g l−1 NaCl) and 19 mM total L-amino acids at the following concentrations: aspartate, 0.827 mM; threonine, 1.072 mM; serine, 1.446 mM; glutamine, 1.549 mM; proline, 1.661 mM; glycine, 1.203 mM; alanine, 1.78 mM; cysteine, 0.160 mM; valine, 1.117 mM; methionine, 0.633 mM; isoleucine, 1.121 mM; leucine, 1.609 mM; tyrosine, 0.802 mM; phenylalanine, 0.53 mM; ornithine, 0.676 mM; lysine, 2.128 mM; histidine, 0.519 mM; tryptophan, 0.013 mM; and 0.306 mM arginine. The pH was adjusted to 6.8 and the medium was filter sterilized. After sterilization metals were added at the following concentrations: 1.754 mM CaCl2, 0.606 mM MgCl2 and 3.597 mM FeSO4·7H2O.
A thermal mass flow meter/controller (PEGAS 4000 MF gas mixer, Columbus Instruments, Columbus, Ohio) was used to achieve different dissolved oxygen concentrations in culture media. The gas mixer was connected to a nitrogen tank and an oxygen tank. Cells were grown in baffled 300 ml Erlenmeyer flasks containing 50 ml of growth medium that were sealed with rubber stoppers through which tubing from the gas mixer was inserted. Flasks were placed on a magnetic stirrer operating at ~100 r.p.m. to allow mixing of the culture and gas was bubbled through the medium. The media in flasks were bubbled with the appropriate gas mixture for at least 12 h before inoculation. Bubbling was continued throughout growth. An Accumet benchtop dissolved oxygen meter was used to measure the dissolved oxygen in the medium. For uninoculated medium this was 5.88 ± 0.13 mg per l for 20% oxygen in the gas mixture, 0.81 ± 0.035 mg per l for 2% oxygen in the gas mixture and 0.3 ± 0.01 mg per l for 0.4% oxygen in the gas mixture. In the case of the anaerobic experiments, media were bubbled with nitrogen gas alone.
For growth rate determinations, P. aeruginosa strains were initially grown overnight in test tubes at 37°C in 5 ml of synthetic CF sputum medium, except for the cioA cbb3-1 cbb3-2 mutant, which was grown in 5 ml of tryptic soy broth (TSB; Becton Dickinson, Sparks, Maryland). These cultures were used to inoculate previously gas-sparged CF sputum medium to an OD660 of 0.010. Growth was monitored by removing a 1 ml sample and determining its optical density (OD660). For anaerobic experiments, overnight cultures were grown as described above in the presence of 100 mM KNO3 (equivalent to 1% KNO3) and used to inoculate anaerobic medium containing 100 mM KNO3. The culture was allowed to grow to exponential phase and used to inoculate fresh medium from which the growth curve was constructed. For all conditions, doubling times were determined from cultures grown in duplicate on three different days.
Construction of P. aeruginosa mutant strains
In-frame deletions of ccoNOPQ-2 and ccoNOPQ-1 were constructed by overlap extension polymerase chain reaction (PCR) (Ho et al., 1989; Horton et al., 1993) as described previously (Ferrández et al., 2002). Generally, PCR products were first cloned into pUC19 and then excised as restriction fragments and cloned into the pEX19Gm suicide vector (Hoang et al., 1998). Plasmids were mobilized from E. coli S17-1 into P. aeruginosa by conjugation and recombinants were obtained as described previously (Ferrández et al., 2002) and confirmed by colony PCR. An additional set of primers internal to deleted regions was used for final confirmation of mutant genotypes. For construction of PAO1387 (cioA cbb3-2 cbb3-1), an in-frame deletion of the ccoNOPQ-2 ccoNOPQ-1 genes was constructed by overlap extension PCR. An engineered BamHI site was included in the in-frame deletion and an FRT-flanked gentamicin cassette excised as BamHI restriction fragment from the pPS858 vector (Hoang et al., 1998) was cloned into this site. This construct, cloned in pEX19Amp (Hoang et al., 1998), was mobilized into PTL12436 (a cioA Tn insertion mutant). The cioA cbb3-2 cbb3-1 GmrP. aeruginosa integrant was transformed with pFLP2 to allow Flp-catalysed excision of the gentamicin marker (Hoang et al., 1998) to generate the final PAO1387 strain. pFLP2 was cured from PAO1387 as indicated by Hoang et al. (1998). PAO1371 (cbb3-2) was used as the recipient in the construction of PAO1373 (cbb3-1 cbb3-2). Transposon insertion mutations in the strains obtained from the University of Washington P. aeruginosa PAO1 mutant collection were verified as recommended (http://www.genome.washington.edu/UWGC/pseudomonas/).
Transcriptome experiments and data analysis
For the transcriptome experiments, P. aeruginosa PAO1 was grown at 37°C in synthetic CF sputum medium at the following oxygen concentrations: 20%, 2%, 0.4% and 0% with 100 mM KNO3.The cells were subcultured once and harvested for RNA extraction at an OD660 of 0.080–0.090. RNA isolation, cDNA synthesis and cDNA fragmentation were performed as described (Schuster et al., 2003). End-labelling was carried out with GeneChip Labeling Reagent (Affymetrix) following the manufacturer’s recommendations. Each experiment was done in duplicate with independently prepared samples. Hybridization and processing of P. aeruginosa GeneChips were done at the University of Washington Center for Expression Arrays. The Affymetrix GeneChip Operating Software Version 1.4 was used for initial data acquisition and processing. Transcript data were further analysed with by using CYBER-T program (http://visitor.ics.uci.edu/genex/cybert/) to identify genes whose expression exhibited a statistically significant change (Schuster et al., 2003). The P-value threshold was 0.001, resulting in a posterior probability of differential gene expression greater than 0.97. The transcriptome data have been deposited at http://www.ncbi.nlm.nih.gov/geo under Accession number GSE6741.
Biofilm experiments
Biofilms were cultivated in a sterilized flow cell system and were examined by confocal microscopy (Parsek and Green-berg, 1999). Strains that expressed GFP constitutively were constructed by introduction of the plasmid pMRP9-1 (Davies et al., 1998) for use in the biofilm experiments. The flow cells were inoculated with a stationary phase culture that was diluted in 1% TSB to an OD660 of 0.1 as previously described (Banin et al., 2006). The biofilms were continuously fed with 1% TSB at a flow rate of 0.17 ml per min and incubated at room temperature (24–25°C). Where indicated, KNO3 was added at a final concentration of 100 mM. For these sets of experiments Tygon® tubing (Saint-Gobain Performance Plastics, Fisher) was used and the medium was constantly sparged with N2 gas. Biofilm images were captured with an LSM 510 confocal microscope (Carl Zeiss, Germany). Propidium iodide (Invitrogen, Carlsbad, California) was used at a concentration of 30 µM to stain non-viable cells. The excitation wavelength for GFP and propidium iodide was 488 nm. The emission wavelength was 515 nm and 660 nm, respectively, for GFP and propidium iodide. After acquisition the images were processed with Volocity software (Improvision, Lexington, Massachusetts).
Image analysis
Characteristics of images series were quantified using COMSTAT analysis software (Heydorn et al., 2000). Seven images per strain per day were analysed.
Supplementary Material
Acknowledgements
We thank Marvin Whiteley for sharing the recipe for CF sputum medium with us prior to its publication and E. P. Greenberg for critically reading the manuscript. We also thank Michael Jacobs, Colin Manoil and Maynard Olson for providing us with the P. aeruginosa mutants from the University of Washington Genome Center P. aeruginosa PAO1 transposon mutant library, Timothy Donohue for providing us with the roxR1 mutant and its parent strain, and Matthew Parsek for sharing strain PA01374. This work was supported by Public Health Service Grant GM56665 from the National Institute of General Medical Sciences and by the Cystic Fibrosis Research Development Program.
Footnotes
Supplementary material
The following supplementary material is available for this article:
Fig. S1. Double and triple high affinity oxidase mutants form normal biofilms when respiring nitrate.
Table S1. Quorum-sensing genes that are differentially expressed by growth with 0.470 oxygen.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2958.2007.05772.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Banin E, Brady KM, Greenberg EP. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol. 2006;72:2064–2069. doi: 10.1128/AEM.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes N, Schreiber K, Hartig E, Jaensch L, Schobert M. The Pseudomonas aeruginosa universal stress protein PA4352 is essential for surviving anaerobic energy stress. J Bacteriol. 2006;188:6529–6538. doi: 10.1128/JB.00308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castric PA. Hydrogen cyanide production by Pseudomonas aeruginosa at reduced oxygen levels. Can J Microbiol. 1983;29:1344–1349. doi: 10.1139/m83-209. [DOI] [PubMed] [Google Scholar]
- Chen F, Xia Q, Ju LK. Competition between oxygen and nitrate respirations in continuous culture of Pseudomonas aeruginosa performing aerobic denitrification. Biotechnol Bioeng. 2006;93:1069–1078. doi: 10.1002/bit.20812. [DOI] [PubMed] [Google Scholar]
- Comolli JC, Donohue TJ. Pseudomonas aeruginosa RoxR, a response regulator related to Rhodobacter sphaeroides PrrA, activates expression of the cyanide-insensitive terminal oxidase. Mol Microbiol. 2002;45:755–768. doi: 10.1046/j.1365-2958.2002.03046.x. [DOI] [PubMed] [Google Scholar]
- Comolli JC, Donohue TJ. Differences in two Pseudomonas aeruginosa cbb3 cytochrome oxidases. Mol Microbiol. 2004;51:1193–1203. doi: 10.1046/j.1365-2958.2003.03904.x. [DOI] [PubMed] [Google Scholar]
- Cunningham L, Pitt M, Williams HD. The cioAB genes from Pseudomonas aeruginosa code for a novel cyanide-insensitive terminal oxidase related to the cytochrome bd quinol oxidases. Mol Microbiol. 1997;24:579–591. doi: 10.1046/j.1365-2958.1997.3561728.x. [DOI] [PubMed] [Google Scholar]
- Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- Ferrández A, Hawkins AC, Summerfield DT, Harwood CS. Cluster II che genes from Pseudomonas aeruginosa are required for an optimal chemotactic response. J Bacteriol. 2002;184:4374–4383. doi: 10.1128/JB.184.16.4374-4383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiatrault MJ, Wagner VE, Bushnell D, Haidaris CG, Iglewski BH, Passador L. Effect of anaerobiosis and nitrate on gene expression in Pseudomonas aeruginosa . Infect Immun. 2005;73:3764–3772. doi: 10.1128/IAI.73.6.3764-3772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiatrault MJ, Picardo KF, Ngai H, Passador L, Iglewski BH. Identification of Pseudomonas aeruginosa genes involved in virulence and anaerobic growth. Infect Immun. 2006;74:4237–4245. doi: 10.1128/IAI.02014-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Fukumori Y, Yamanaka T. A novel terminal oxidase, cytochrome baa3 purified from aerobically grown Pseudomonas aeruginosa: it shows a clear difference between resting state and pulsed state. J Biochem (Tokyo) 1992;112:290–298. doi: 10.1093/oxfordjournals.jbchem.a123893. [DOI] [PubMed] [Google Scholar]
- Govan JR, Nelson JW. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992;48:912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- Hassett DJ. Anaerobic production of alginate by Pseudomonas aeruginosa: alginate restricts diffusion of oxygen. J Bacteriol. 1996;178:7322–7325. doi: 10.1128/jb.178.24.7322-7325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Høiby N. Pseudomonas in Cystic Fibrosis: Past, Present, and Future. London: Cystic Fibrosis Trust; 1998. [Google Scholar]
- Høiby N, Krogh Johansen H, Moser C, Song Z, Ciofu O, Kharazmi A. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 2001;3:23–35. doi: 10.1016/s1286-4579(00)01349-6. [DOI] [PubMed] [Google Scholar]
- Horton RM, Ho SN, Pullen JK, Hunt HD, Cai Z, Pease LR. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa . Proc Natl Acad Sci USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju LK, Chen F, Xia Q. Monitoring microaerobic denitrification of Pseudomonas aeruginosa by online NAD (P) H fluorescence. J Ind Microbiol Biotechnol. 2005;32:622–628. doi: 10.1007/s10295-005-0035-6. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Sabra W, Zeng AP. Iron deficiency leads to inhibition of oxygen transfer and enhanced formation of virulence factors in cultures of Pseudomonas aeruginosa PAO1. Microbiology. 2003;149:2627–2634. doi: 10.1099/mic.0.26276-0. [DOI] [PubMed] [Google Scholar]
- Linnane SJ, Keatings VM, Costello CM, Moynihan JB, O’Connor CM, Fitzgerald MX, McLoughlin P. Total sputum nitrate plus nitrite is raised during acute pulmonary infection in cystic fibrosis. Am J Respir Crit Care Med. 1998;158:207–212. doi: 10.1164/ajrccm.158.1.9707096. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Yamada M, Shinagawa E, Adachi O, Ameyama M. Membrane-bound respiratory chain of Pseudomonas aeruginosa grown aerobically. J Bacteriol. 1980;141:389–392. doi: 10.1128/jb.141.1.389-392.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A, Fujiwara T, Fukumori Y, Yamanaka T. Reactivity of the co-type and baa3-type cytochrome c oxidases from Pseudomonas aeruginosa with different endogenous cytochromes c . Curr Microbiol. 1995;30:123–126. doi: 10.1007/BF00296195. [DOI] [PubMed] [Google Scholar]
- Palmer KL, Brown SA, Whiteley M. The membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J Bacteriol. 2007 doi: 10.1128/JB.00162-07. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsek MR, Greenberg EP. Quorum sensing signals in development of Pseudomonas aeruginosa biofilms. Methods Enzymol. 1999;310:43–55. doi: 10.1016/s0076-6879(99)10005-3. [DOI] [PubMed] [Google Scholar]
- Pedersen SS. Lung infection with alginateproducing, mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS Suppl. 1992;28:1–79. [PubMed] [Google Scholar]
- Preisig O, Zufferey R, Thony-Meyer L, Appleby CA, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum . J Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey DM, Wozniak DJ. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol Microbiol. 2005;56:309–322. doi: 10.1111/j.1365-2958.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Ray A, Williams HD. The effects of mutation of the anr gene on the aerobic respiratory chain of Pseudomo-nas aeruginosa . FEMS Microbiol Lett. 1997;156:227–232. doi: 10.1111/j.1574-6968.1997.tb12732.x. [DOI] [PubMed] [Google Scholar]
- Sabra W, Kim E-J, Zeng A-P. Physiological responses of Pseudomonas aeruginosa PAO1 to oxidative stress in controlled microaerobic and aerobic cultures. Microbiology. 2002;148:3195–3202. doi: 10.1099/00221287-148-10-3195. [DOI] [PubMed] [Google Scholar]
- Schreiber K, Boes N, Eschbach M, Jaensch L, Wehland J, Bjarnsholt T, et al. Anaerobic survival of Pseudomonas aeruginosa by pyruvate fermentation requires an Usp-type stress protein. J Bacteriol. 2006;188:659–668. doi: 10.1128/JB.188.2.659-668.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M, Lostroh CP, Ogi T, Greenberg EP. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M, Hawkins AC, Harwood CS, Greenberg EP. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol Microbiol. 2004;51:973–985. doi: 10.1046/j.1365-2958.2003.03886.x. [DOI] [PubMed] [Google Scholar]
- Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/ Technology. 1983;1:784–791. [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Vallet I, Diggle SP, Stacey RE, Camara M, Ventre I, Lory S, et al. Biofilm formation in Pseudomonas aeruginosa: fimbrial cup gene clusters are controlled by the transcriptional regulator MvaT. J Bacteriol. 2004;186:2880–2890. doi: 10.1128/JB.186.9.2880-2890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollack K, Xie J, Hartig E, Romling U, Zumft W. Localization of denitrification genes on the chromosomal map of Pseudomonas aeruginosa . Microbiology. 1998;144:441–448. doi: 10.1099/00221287-144-2-441. [DOI] [PubMed] [Google Scholar]
- Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol. 2003;185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E, Roe F, Bugnicourt A, Franklin MJ, Heydorn A, Molin S, et al. Stratified growth in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2004;70:6188–6196. doi: 10.1128/AEM.70.10.6188-6196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa . Proc Natl Acad Sci USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HD, Zlosnik JE, Ryall B. Oxygen, cyanide and energy generation in the cystic fibrosis pathogen Pseudomonas aeruginosa . Adv Microb Physiol. 2007;52:1–71. doi: 10.1016/S0065-2911(06)52001-6. [DOI] [PubMed] [Google Scholar]
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev Cell. 2002;3:593–603. doi: 10.1016/s1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.