Abstract
Mitral regurgitation (MR) is common with coronary artery disease (CAD), as altered myocardial substrate can impact valve performance. SPECT myocardial perfusion imaging (MPI) enables assessment of myocardial perfusion alterations. This study examined perfusion pattern in relation to MR. 2377 consecutive patients with known or suspected CAD underwent stress MPI and echocardiography (echo) within 1.6±2.3 days. MR was present on echo in 34% of patients, among whom 13% had advanced (≥moderate) MR. MR prevalence was higher among patients with abnormal MPI (44% vs. 29%, p<0.001), corresponding to increased global ischemia (p<0.001). Regional perfusion varied in left ventricular (LV) segments adjacent to each papillary muscle: Adjacent to the anterolateral papillary muscle, magnitude of baseline and stress-induced anterior/anterolateral perfusion abnormalities was greater among patients with MR (both p<0.001). Adjacent to the posteromedial papillary muscle, baseline inferior/inferolateral perfusion abnormalities were greater with MR (p<0.001), whereas stress inducibility was similar (p=0.39). In multivariate analysis, stress-induced anterior/anterolateral and rest inferior/inferolateral perfusion abnormalities were independently associated with MR (both p<0.05) even after controlling for perfusion in reference segments not adjacent to the papillary muscles. MR severity increased in relation to magnitude of perfusion abnormalities in each territory adjacent to the papillary muscles, as evidenced by greater prevalence of advanced MR among patients with ≥moderate anterior/anterolateral stress perfusion abnormalities (10.7% vs. 3.6%), with similar results when MR was stratified based on rest inferior/inferolateral perfusion (10.4% vs. 3.0%, both p<0.001). In conclusion, findings demonstrate that myocardial perfusion pattern in LV segments adjacent to the papillary muscles influences presence and severity of MR.
Keywords: mitral regurgitation, myocardial perfusion, SPECT
Introduction
This study examined myocardial perfusion pattern in relation to mitral regurgitation (MR) among a consecutive cohort of 2377 patients with known or suspected coronary artery disease (CAD) undergoing stress myocardial perfusion imaging (MPI) and echo. The goal was to test the interaction between altered myocardial perfusion and both presence and severity of MR.
Methods
The study population consisted of consecutive patients who underwent single photon emission computed tomography (SPECT) MPI and transthoracic echo within a 1-week interval at Weill Cornell Medical College. Imaging was performed between December 2010 and December 2013. To test the impact of myocardial perfusion pattern on MR, patients with primary mitral valve disorders (mitral valve prolapse, rheumatic disease) or prior mitral valve surgery (prosthesis, annuloplasty) were excluded. This study was conducted with approval of the Weill Cornell Medical College Institutional Review Board.
MPI was performed in accordance with a previously described protocol.1,2 In brief, thallium-201 (Tl-201; ~3 mCi) or technetium-99m (Tc-99m; ~10 mCi) sestamibi was injected intravenously; baseline (i.e. rest) perfusion images were acquired approximately 10 minutes after Tl-201 injection, and 60 minutes after Tc-99m sestamibi injection. Following baseline imaging, patients capable of exercise underwent treadmill testing using a Bruce protocol: Tc-99m (~30 mCi) sestamibi was intravenously administered at peak stress following achievement of target heart rate response to exercise (≥85% age-predicted maximum heart rate). Serial 12-lead electrocardiograms (ECGs) were obtained at baseline and at each stage of the exercise treadmill protocol. In patients unable to exercise or to achieve adequate exercise heart rate response, pharmacologic protocols were employed using either intravenous adenosine-based agents or dobutamine. Post-stress images were acquired approximately 30 minutes following exercise, and 1–2 hours following pharmacologic stress.
SPECT imaging was performed using a dual headed scintillation camera system with a low-energy high-resolution collimator. Images were acquired using a 180° arc of rotation along a circular orbit encompassing a total of 64 projections. For Tl-201 imaging, 2 photopeaks of 70 keV and 167 keV were used. For Tc-99m imaging, a photopeak of 140 keV was used. Stress images were ECG-gated for assessment of contractile function; left ventricular (LV) ejection fraction was quantitatively measured (Cedars-Sinai AutoQuant).
Echoes were performed by experienced sonographers using commercially available equipment (e.g. General Electric Vivid-7, Philips IE33). Images were acquired in parasternal, as well as apical 2-, 3-, and 4- chamber orientations. LV ejection fraction and chamber size were quantified using linear dimensions in parasternal views.3 Color and pulsed wave Doppler were used to presence and severity of MR.
MPI was interpreted by American Heart Association/American College of Cardiology (AHA/ACC) level III trained readers utilizing a 17-segment model.4 Perfusion defect severity on a per-segment basis was graded using a 5-point scoring system (0 = normal perfusion, 1 = equivocal or mildly reduced, 2 = moderately reduced, 3 = severely reduced, 4 = absence of detectable radioisotope).5 Summed stress and rest scores were calculated by adding per-segment defect severity for all segments. Inducible defect severity (summed difference score) was assessed as the difference between rest and stress images.
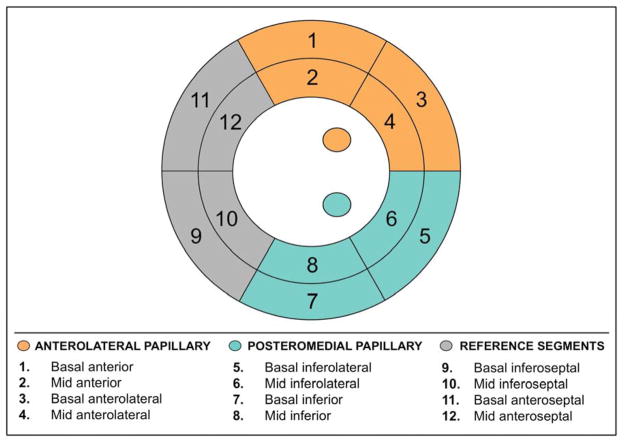
To test the relation between mitral apparatus ischemia and MR, regional perfusion was assessed within myocardial segments subtending the anterolateral and posteromedial papillary muscles (Figure 1): For the anterolateral papillary muscle, LV perfusion was assessed within the basal to mid anterior and anterolateral segments. For the posteromedial papillary muscle, LV perfusion was assessed within the basal to mid inferior and inferolateral segments. As a reference for LV regions not adjacent to the papillary muscles, perfusion was assessed within the basal to mid antero- and inferoseptal segments. Perfusion to each region was additionally graded based on the maximal perfusion score within the region: Regions with completely normal perfusion were scored as normal, regions with a maximum perfusion score of 1 among the constituent segments were scored as mildly abnormal, and regions with a maximum perfusion score of ≥2 among the constituent segments were scored as ≥moderately abnormal.
Figure 1. Mitral Apparatus Perfusion Categories.
Bullseye plot illustrating regional LV perfusion categories. Each category was comprised of 4 segments, such that total myocardium subtended by each was equivalent. For the anterolateral papillary muscle, LV perfusion was assessed within the basal to mid anterior and anterolateral segments. For the posteromedial papillary muscle, LV perfusion was assessed within the within the basal to mid inferior and inferolateral segments. As a reference for LV segments not adjacent to the papillary muscles, perfusion was assessed within the basal to mid antero- and inferoseptum.
Echoes were interpreted by experienced (ACC/AHA level III) readers in a high volume laboratory, for which methods of measurement of chamber volumes and MR have been previously reported.6 MR severity was graded using a 5-point scale, as primarily determined based on the distance reached from the mitral orifice by the regurgitant jet (mild [1+]; ≤1.5 cm | moderate [2+]; 1.5–3.0 cm | moderately-severe [3+]; 3.0–4.5 cm | severe [4+]; ≥4.5 cm). Additional criteria used to confirm MR severity included jet area and vena contracta, as well as mitral and pulmonary vein flow pattern.7 Pulmonary artery systolic pressure was calculated from tricuspid regurgitant velocity and inferior vena cava caliber.
Comparisons between groups with and without MR were made using Student’s t test for continuous variables (expressed as mean value±standard deviation). Indices were tested for normality of distribution; non-normally distributed data (i.e. perfusion scores) were compared after logarithmic transformation for which results are expressed as the antilog of the mean and 95% confidence intervals. Categorical variables were compared using chi-square or, when fewer than 5 expected outcomes per cell, Fisher’s exact test. Multivariable logistic regression analysis was performed to evaluate associations between MR and SPECT perfusion pattern. Binary logistic regression analysis was used to examine the association between MR, clinical variables and imaging parameters. Two-sided p<0.05 was considered indicative of statistical significance. Calculations were performed using SPSS Version 20 (IBM, Armonk, N.Y.).
Results
The study population comprised 2377 consecutive patients without primary mitral valve disease who underwent MPI and echo within a 1-week (1.6±2.3 day) interval. A total of 80 otherwise eligible patients were excluded based on echo-evidenced primary mitral valve disease (57% prolapse, 13% rheumatic) or prior mitral valve surgery (30% prosthesis or annuloplasty).
MR was present in one-third (34%) of the study population (87% mild, 8% moderate, 3% moderate-severe, 2% severe). Table 1 details population clinical and imaging characteristics, stratified based on presence or absence of MR. As shown, MR was strongly associated with clinically established CAD, as evidenced by a near 2-fold increase in prevalence of prior MI or CABG among MR-affected patients (both p<0.001). Regional LV contractile dysfunction on echo was also more common among patients with MR, who manifested increased prevalence of regional wall motion abnormalities in all coronary vascular territories (all p<0.001). Accordingly, LV ejection fraction was lower among patients with MR, whether measured via baseline echo or post-stress SPECT imaging (both p<0.001).
Table 1.
Clinical and Functional Imaging Characteristics
| Parameter | Overall (n=2377) | Mitral Regurgitation | p | |
|---|---|---|---|---|
| Yes (n=812) | No (n=1565) | |||
| Age (years) | 64 ± 13 | 68 ± 13 | 62 ± 13 | <0.001 |
| Men | 1271 (54%) | 418 (52%) | 853 (55%) | 0.16 |
| Hypertension* | 1707 (72%) | 629 (78%) | 1078 (69%) | <0.001 |
| Hypercholesterolemia* | 1414 (60%) | 483 (60%) | 931 (60%) | 0.99 |
| Diabetes mellitus | 759 (32%) | 246 (30%) | 513 (33%) | 0.22 |
| Family history of coronary artery disease | 563 (24%) | 169 (21%) | 394 (25%) | 0.02 |
| Known coronary artery disease | 552 (23%) | 241 (30%) | 311 (20%) | <0.001 |
| Prior myocardial infarction (MI) | 177 (7%) | 82 (10%) | 95 (6%) | <0.001 |
| Prior coronary revascularization | ||||
| Percutaneous coronary intervention (PCI) | 344 (15%) | 136 (17%) | 208 (13%) | 0.02 |
| Coronary artery bypass grafting (CABG) | 176 (7%) | 96 (12%) | 80 (5%) | <0.001 |
| Medications | ||||
| Beta-blocker | 1148 (48%) | 459 (57%) | 689 (44%) | <0.001 |
| ACE inhibitor or angiotensin receptor blocker | 940 (40%) | 364 (45%) | 576 (37%) | <0.001 |
| HMG-CoA reductase inhibitor | 1237 (52%) | 469 (58%) | 768 (49%) | <0.001 |
| Aspirin | 1164 (49%) | 435 (54%) | 729 (47%) | 0.001 |
| Thienopyridine | 289 (12%) | 110 (14%) | 179 (11%) | 0.14 |
| Indication for stress perfusion testing | ||||
| Chest pain | 984 (41%) | 298 (37%) | 686 (44%) | 0.001 |
| Dyspnea | 679 (29%) | 242 (30%) | 437 (28%) | 0.34 |
| SPECT myocardial perfusion imaging | ||||
| Exercise stress | 907 (38%) | 245 (30%) | 662 (42%) | <0.001 |
| Adenosine/regadenoson stress | 1465 (62%) | 564 (70%) | 901 (58%) | <0.001 |
| Dipyridamole stress | 2 (0.1%) | 2 (0.2%) | — | 0.12 |
| Dobutamine stress | 3 (0.1%) | 1 (0.1%) | 2 (0.1%) | 1.00 |
| Post-stress ejection fraction (%) | 63 ± 13 | 60 ± 15 | 64 ± 12 | <0.001 |
| Post-stress ejection fraction <50% | 270 (11%) | 159 (20%) | 111 (7%) | <0.001 |
| Echocardiography | ||||
| LV ejection fraction (%) | 61 ± 11 | 58 ± 14 | 62 ± 9 | <0.001 |
| LV ejection fraction <50% | 320 (14%) | 167 (21%) | 153 (10%) | <0.001 |
| LV end-diastolic diameter (cm) | 5.0 ± 0.8 | 5.1 ± 0.9 | 5.0 ± 0.8 | <0.001 |
| LV regional wall motion abnormality | 299 (13%) | 157 (19%) | 142 (9%) | <0.001 |
| Anterior wall motion abnormality | 117 (5%) | 71 (9%) | 46 (3%) | <0.001 |
| Lateral wall motion abnormality | 175 (7%) | 104 (13%) | 71 (5%) | <0.001 |
| Inferior wall motion abnormality | 219 (9%) | 133 (16%) | 86 (6%) | <0.001 |
| LV myocardial mass (g/m2) | 92 ± 32 | 100 ± 35 | 87 ± 30 | <0.001 |
| Left atrial diameter (cm) | 3.9 ± 1.4 | 4.1 ± 0.8 | 3.8 ± 1.6 | <0.001 |
| Left atrial volume (ml/m2) | 34 ± 14 | 40 ± 16 | 30 ± 11 | <0.001 |
Numbers in boldface indicate p values < 0.05
Defined based on self-reported history, confirmed via medical record review at time of MPI
MR was more common among patients with, compared to those without, abnormal myocardial perfusion on SPECT (44% vs. 29%, p<0.001); differences were more marked with respect to advanced (≥moderate) MR (8% vs. 2%, p<0.001). Among patients who underwent exercise stress (n=907), ECG stress response was more frequently abnormal among those with MR, as evidenced by higher prevalence of exercise-induced horizontal or downsloping (≥1.0mm) ST segment depression (20% vs. 14%, p=0.03).
As shown in Table 2A, patients with MR had increased severity of impaired perfusion on rest imaging, and greater magnitude of stress-induced perfusion abnormalities (both p<0.001). While ancillary SPECT findings were uncommon, prevalence of stress-induced transient ischemic dilation was similar between patients with and without MR (2.0% vs. 1.3%; p=0.19) whereas increased lung uptake – a marker of elevated LV filling pressure – was more frequent with MR (3.8% vs. 0.4%, p=0.001).
Table 2.
Myocardial Perfusion Patterns
| Mitral Regurgitation | ≥Moderate Mitral Regurgitation | |||||
|---|---|---|---|---|---|---|
| Present (n=812) | Absent (n=1565) | p | Present (n=104) | Absent (n=2273) | p | |
|
2A. LEFT VENTRICULAR GLOBAL PERFUSION |
||||||
| Summed Stress Score | 2.68 (2.47, 2.92) | 1.83 (1.74, 1.93) | <0.001 | 4.99 (3.85, 6.47) | 2.00 (1.92, 2.09) | <0.001 |
| Summed Rest Score | 2.14 (1.99, 2.32) | 1.54 (1.48, 1.61) | <0.001 | 3.65 (2.82, 4.71) | 1.67 (1.60, 1.73) | <0.001 |
| Summed Difference Score | 1.55 (1.46, 1.64) | 1.33 (1.29, 1.37) | <0.001 | 2.04 (1.70, 2.45) | 1.38 (1.34, 1.42) | <0.001 |
|
2B. MITRAL APPARATUS PERFUSION |
||||||
| Anterolateral | ||||||
| Summed Stress Score | 1.35 (1.29, 1.41) | 1.18 (1.15, 1.21) | <0.001 | 1.61 (1.39, 1.87) | 1.22 (1.19, 1.24) | <0.001 |
| Summed Rest Score | 1.14 (1.11, 1.17) | 1.07 (1.05, 1.08) | <0.001 | 1.25 (1.12, 1.40) | 1.08 (1.07, 1.10) | 0.01 |
| Summed Difference Score | 1.23 (1.19, 1.27) | 1.12 (1.10, 1.14) | <0.001 | 1.41 (1.25, 1.58) | 1.14 (1.12, 1.16) | 0.001 |
| Posteromedial | ||||||
| Summed Stress Score | 1.78 (1.68, 1.90) | 1.41 (1.36, 1.46) | <0.001 | 2.50 (2.05, 3.04) | 1.49 (1.45, 1.54) | <0.001 |
| Summed Rest Score | 1.64 (1.55, 1.74) | 1.31 (1.26, 1.35) | <0.001 | 2.30 (1.90, 2.79) | 1.38 (1.34, 1.42) | <0.001 |
| Summed Difference Score | 1.15 (1.11, 1.18) | 1.13 (1.11, 1.15) | 0.39 | 1.16 (1.07, 1.26) | 1.14 (1.12, 1.16) | 0.60 |
Comparisons based on log-transformation of (non-normally) distributed data; presented as antilog of mean (95% confidence interval)
Table 2B reports severity of myocardial perfusion abnormalities in LV segments adjacent to each of the papillary muscles. For segments adjacent to the anterolateral papillary muscle, both baseline and stress-induced perfusion abnormalities were greater among patients with MR (both p<0.001). For LV segments adjacent to the posteromedial papillary muscle, magnitude of baseline perfusion abnormalities was greater with MR (p<0.001), whereas inducibility during stress was similar (p=0.39). MR was associated with inferior/inferolateral rest (OR=1.32 [95% CI 1.09–1.59], p=0.004) and anterior/anterolateral stress (OR=1.19 [95% CI 1.05–1.36], p=0.007) perfusion defects even after controlling for echo-quantified LV end-diastolic diameter (OR=1.32 per cm [95% CI 1.16–1.50], p<0.001), age (OR 1.37 per decade [95% CI 1.29–1.45], p<0.001) and hypertension (OR 1.22 [95% CI 0.99–1.49], p=0.07).
Multivariate analysis was performed to further test MR severity in relation to myocardial perfusion pattern. As shown in Table 3, inferior/inferolateral baseline perfusion and anterior/anterolateral inducible perfusion abnormalities were each independently associated with MR, after controlling for magnitude of LV perfusion abnormalities in septal reference segments not adjacent to the papillary muscles. Regression results demonstrate MR severity to be associated with magnitude of SPECT-evidenced defect severity, such that increasing defect scores would be expected to confer increased risk for MR. Applied clinically, a mild rest perfusion abnormality within the basal to mid inferolateral or inferior wall would be expected to confer a slight increase in likelihood of any (≥mild) MR, but nearly a 2-fold increase in likelihood of advanced (≥moderate) MR, with incremental risk conferred by stress-induced perfusion abnormalities within the basal to mid anterior or anterolateral wall. Inducible perfusion defect severity within reference (septal) segments was associated with advanced MR, consistent with MR-associated increases in global summed difference score shown in Table 2. In multivariate regression models including both regional and global LV perfusion indices, rest inferior/inferolateral defects were independently associated with MR (OR 1.07 per point [95% CI 1.01–1.13], p=0.02) after controlling for global summed rest score (OR 1.03 [95% CI 1.001–1.06], p=0.02); results were similar when stress-induced anterior/anterolateral defects (OR 1.13 [95% CI 1.003–1.28], p=0.04) were tested together with global summed difference score (OR 1.02 [95% CI 0.98–1.07], p=0.37).
Table 3.
Multivariate Regression
| Variable* | Odds Ratio (95% Confidence Interval) | p |
|---|---|---|
| Mild Mitral Regurgitation | ||
|
| ||
| Inferior/inferolateral rest perfusion defect severity | 1.32 (1.17 – 1.50) | <0.001 |
| Anterior/anterolateral inducible perfusion defect severity | 1.29 (1.07 – 1.56) | 0.009 |
| Septal rest perfusion defect severity | 0.88 (0.68 – 1.15) | 0.21 |
| Septal inducible perfusion defect severity | 1.32 (0.86 – 2.03) | 0.36 |
|
| ||
| ≥Moderate Mitral Regurgitation | ||
|
| ||
| Inferior/inferolateral rest perfusion defect severity | 1.87 (1.47 – 2.37) | <0.001 |
| Anterior/anterolateral inducible perfusion defect severity | 1.46 (1.05 – 2.04) | 0.03 |
| Septal rest perfusion defect severity | 1.43 (1.00 – 2.05) | 0.051 |
| Septal inducible perfusion defect severity | 2.23 (1.27 – 3.89) | 0.005 |
|
| ||
| model χ2 = 92.4, p < 0.001 | ||
Odds ratios reported in relation to maximal myocardial perfusion severity within respective territories as graded on a 3-point scale (normal perfusion [0], mildly abnormal perfusion [1], and ≥moderately abnormal perfusion [2])
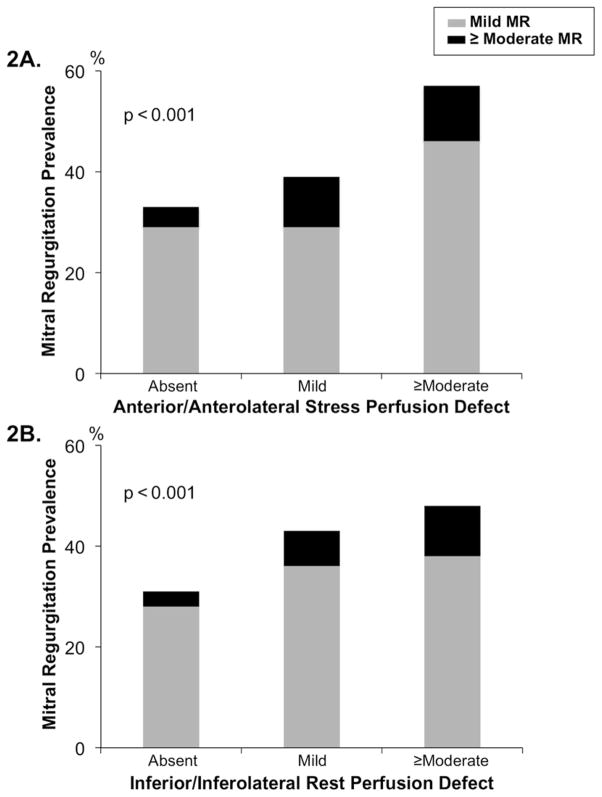
Figure 2 provides a breakdown of MR severity in relation to mitral apparatus perfusion abnormalities. As shown, MR severity increased in relation to magnitude of perfusion defects within each LV territory: Advanced (≥moderate) MR was 3-fold more common among patients with ≥moderate stress perfusion defects within the anterior/anterolateral wall, compared to those with normal perfusion in these territories (10.7% vs. 3.6%, p<0.001). Results were similar when prevalence of advanced MR was compared between groups with or without ≥moderate rest perfusion defects within the inferior/inferolateral wall (10.4% vs. 3.0%, p<0.001).
Figure 2. Mitral Regurgitation Severity in Relation to Mitral Apparatus Perfusion.
Prevalence and graded severity of MR in relation to mitral apparatus perfusion. Note stepwise increase in MR among patients with increasing magnitude of SPECT-evidenced anterior/anterolateral stress (2A) and inferior/inferolateral rest (2B) perfusion defects.
Discussion
This study provides new insights concerning the relationship between myocardial substrate and MR. Findings, obtained in a broad patient cohort with known or suspected CAD undergoing stress MPI and echo, demonstrate perfusion pattern within LV segments supporting each papillary muscle to influence MR. In myocardium adjacent to the anterolateral papillary muscle, inducible ischemia was associated with both presence and severity of MR. Conversely, resting perfusion abnormalities in segments adjacent to the posteromedial papillary were greater among patients with (as compared to those without) MR, with no difference in magnitude of stress-induced ischemia. For each myocardial territory, MR severity was proportional to magnitude of perfusion abnormalities, an association that remained significant even after controlling for perfusion to LV segments not associated with the papillary muscles.
Our results build upon prior research that has used animal models to examine myocardial ischemia in relation to MR. Using a canine model, Kono et al reported that inducible ischemia within the left circumflex artery territory produced MR in all animals studied.8 MR uniformly resolved after resolution of ischemia, supporting the notion that MR can be effectively treated by revascularization. Myocardial ischemia has also been shown to impact mitral valve performance. Using a sheep model in which targeted coronary occlusion was performed, Messas et al demonstrated that LV chamber wall perfusion deficits (in the absence of papillary muscle ischemia) yielded acute MR.9 Our data provide clinical support for this concept, demonstrating that impaired LV perfusion impacts MR in patients with known or suspected CAD, a setting in which atherosclerosis is often diffuse in nature and of varying anatomic severity.
Our observed link between resting inferior/inferolateral perfusion defects and MR suggests that infarction, even in the absence of ischemia, can contribute to MR. Of course, it is important to recognize that equivalence between resting perfusion abnormalities and infarction cannot be fully established in the absence of dedicated viability imaging, which was not performed as part of our study and is thus a limitation of our imaging protocol. On the other hand, prior studies have demonstrated a link between similarly located infarction and MR. For example, among a post–myocardial infarction cohort, our group reported MR to be more common among patients with lateral wall infarction, with MR severity proportional to lateral wall infarct size.10 Similarly, among subjects with advanced LV dysfunction, Srichai et al found that MR was associated with infarction within LV territories underlying the papillary muscles.11 Regarding mechanisms responsible for our finding of association between inferior/inferolateral perfusion defects and MR, it should be noted that inferior wall MI has been recognized as a frequent cause of MR due to geometric distortion within LV segments supporting the papillary muscles.12,13 Regional remodeling after inferior or posterior MI has also been linked to chronic MR, due to impaired LV basal rotational mechanics.14 Taken together, these prior studies suggest that our observed association between MR and rest inferior/inferolateral perfusion defects is due to the impact of localized LV remodeling on mitral apparatus geometry or function.
Our finding that perfusion pattern is related to MR may explain the results of clinical studies that have reported heterogeneity in MR response to therapeutic interventions. MR has been reported to improve or resolve in approximately half of patients undergoing CABG, and persist or worsen in the remainder.15 Even when mitral annuloplasty is performed adjunctively with CABG, nearly one-third of patients manifest recurrent MR within 1 year.16 Our data support the notion that MR therapeutic response varies based on underlying tissue substrate. For example, a patient with profound inducible perfusion defects adjacent to the anterolateral papillary muscle would be expected to manifest persistent MR if coronary graft targets are insufficient to redress underlying ischemia. Conversely, for a patient with fixed perfusion defects adjacent to the posteromedial papillary muscle, revascularization alone would not be expected to reduce MR in the absence of myocardial viability.
Several limitations should be noted. First, invasive angiography was not performed in all patients with abnormal MPI, and thus perfusion defects could not be verified based on an independent standard of anatomic CAD. It is also important to recognize that SPECT imaging assesses relative radiotracer uptake rather than absolute blood flow, and that current results are based on semi-quantitative scoring of myocardial perfusion. However, the analytic approaches used in the current research are encompassed in consensus guidelines,17 consistent with widespread clinical practice, and have been validated in prior outcomes studies demonstrating magnitude of abnormal myocardial perfusion to predict adverse prognosis.18 Second, this study measured MR via an established grading system used in prior population-based studies, rather than a quantitative method such as effective regurgitant orifice area. While our approach did not quantify absolute magnitude of MR, it is unlikely that our grading system would have disproportionately affected MR assessment in relation to a particular LV perfusion territory so as to bias study results. Finally, SPECT does not provide adequate spatial resolution to directly visualize papillary muscles. Accordingly, whereas current research demonstrates MR to vary based on perfusion pattern within LV territories adjacent to the papillary muscles, one outstanding question concerns the magnitude to which actual papillary muscle ischemia exacerbates or attenuates MR.
Acknowledgments
Sources of Funding: K23 HL102249-01 (JWW)
Footnotes
Conflicts of Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinsaft JW, Gade CL, Wong FJ, Kim HW, Min JK, Manoushagian SJ, Okin PM, Szulc M. Diagnostic impact of SPECT image display on assessment of obstructive coronary artery disease. J Nucl Cardiol. 2007;14:659–668. doi: 10.1016/j.nuclcard.2007.06.115. [DOI] [PubMed] [Google Scholar]
- 2.Weinsaft JW, Wong FJ, Walden J, Szulc M, Okin PM, Kligfield P. Anatomic distribution of myocardial ischemia as a determinant of exercise-induced ST-segment depression. Am J Cardiol. 2005;96:1356–1360. doi: 10.1016/j.amjcard.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 3.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 5.Berman DS, Kiat H, Friedman JD, Wang FP, van Train K, Matzer L, Maddahi J, Germano G. Separate acquisition rest thallium-201/stress technetium-99m sestamibi dual-isotope myocardial perfusion single-photon emission computed tomography: a clinical validation study. J Am Coll Cardiol. 1993;22:1455–1464. doi: 10.1016/0735-1097(93)90557-h. [DOI] [PubMed] [Google Scholar]
- 6.Jones EC, Devereux RB, Roman MJ, Liu JE, Fishman D, Lee ET, Welty TK, Fabsitz RR, Howard BV. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study) Am J Cardiol. 2001;87:298–304. doi: 10.1016/s0002-9149(00)01362-x. [DOI] [PubMed] [Google Scholar]
- 7.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 8.Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Stein PD, Goldstein S. Mechanism of functional mitral regurgitation during acute myocardial ischemia. J Am Coll Cardiol. 1992;19:1101–1105. doi: 10.1016/0735-1097(92)90302-4. [DOI] [PubMed] [Google Scholar]
- 9.Messas E, Guerrero JL, Handschumacher MD, Chow CM, Sullivan S, Schwammenthal E, Levine RA. Paradoxic decrease in ischemic mitral regurgitation with papillary muscle dysfunction: insights from three-dimensional and contrast echocardiography with strain rate measurement. Circulation. 2001;104:1952–1957. doi: 10.1161/hc4101.097112. [DOI] [PubMed] [Google Scholar]
- 10.Chinitz JS, Chen D, Goyal P, Wilson S, Islam F, Nguyen T, Wang Y, Hurtado-Rua S, Simprini L, Cham M, Levine RA, Devereux RB, Weinsaft JW. Mitral apparatus assessment by delayed enhancement CMR: relative impact of infarct distribution on mitral regurgitation. JACC Cardiovasc Imaging. 2013;6:220–234. doi: 10.1016/j.jcmg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srichai MB, Grimm RA, Stillman AE, Gillinov AM, Rodriguez LL, Lieber ML, Lara A, Weaver JA, McCarthy PM, White RD. Ischemic mitral regurgitation: impact of the left ventricle and mitral valve in patients with left ventricular systolic dysfunction. Ann Thorac Surg. 2005;80:170–178. doi: 10.1016/j.athoracsur.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 12.Kumanohoso T, Otsuji Y, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A, Minagoe S, Levine RA, Tei C. Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg. 2003;125:135–143. doi: 10.1067/mtc.2003.78. [DOI] [PubMed] [Google Scholar]
- 13.Chaput M, Handschumacher MD, Guerrero JL, Holmvang G, Dal-Bianco JP, Sullivan S, Vlahakes GJ, Hung J, Levine RA. Mitral leaflet adaptation to ventricular remodeling: prospective changes in a model of ischemic mitral regurgitation. Circulation. 2009;120:S99–103. doi: 10.1161/CIRCULATIONAHA.109.844019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zito C, Cusma-Piccione M, Oreto L, Tripepi S, Mohammed M, Di Bella G, Falanga G, Oreto G, Lentini S, Carerj S. In patients with post-infarction left ventricular dysfunction, how does impaired basal rotation affect chronic ischemic mitral regurgitation? J Am Soc Echocardiogr. 2013;26:1118–1129. doi: 10.1016/j.echo.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Penicka M, Linkova H, Lang O, Fojt R, Kocka V, Vanderheyden M, Bartunek J. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation. 2009;120:1474–1481. doi: 10.1161/CIRCULATIONAHA.108.842104. [DOI] [PubMed] [Google Scholar]
- 16.Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, Smith PK, Hung JW, Blackstone EH, Puskas JD, Argenziano M, Gammie JS, Mack M, Ascheim DD, Bagiella E, Moquete EG, Ferguson TB, Horvath KA, Geller NL, Miller MA, Woo YJ, D’Alessandro DA, Ailawadi G, Dagenais F, Gardner TJ, O’Gara PT, Michler RE, Kron IL. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, Ficaro EP, Freeman MR, Hendel RC, Jain D, Leonard SM, Nichols KJ, Polk DM, Soman P. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17:941–973. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 18.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]